Molten Salt Electrolyte for Na-ZnCl2 All-Liquid Battery for Grid Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. FactSageTM Thermodynamic Simulation
- The CalPhaD methodology was introduced in the 1960s by Larry Kaufman. The essence is the optimization of the Gibbs energy functions to reproduce the known phase diagrams and utilize it to make the prediction of unknown phase equilibria.
- CalPhaD relies on extensive databases containing thermodynamic properties (like Gibbs energy functions). FactSage gives access to databases of thermodynamic data for thousands of compounds, as well as to evaluated and optimized databases for hundreds of liquid and solid metals, liquid and solid oxides, molten and solid salts, and aqueous solutions [28]. These databases are built through a combination of experimental data (e.g., calorimetry, phase equilibrium measurements) and theoretical modeling.
- For solution phases (e.g., molten salt phases in this work), CalPhaD employs thermodynamic models to describe how the Gibbs energy of the phase changes with composition. Common models include: Ideal Solution Model, Regular Solution Model, Subregular Solution Model, Compound Energy Formalism (CEF), etc.
- To find the minimum Gibbs energy, FactSage employs sophisticated numerical optimization algorithms. These algorithms iteratively adjust the amounts and compositions of different phases until the system reaches its lowest G. Common methods include Newton–Raphson methods, linear programming techniques, etc.
2.2. Sample Preparation for DSC and Solubility Analysis
2.3. Differential Scanning Calorimetry (DSC) for Thermal Analysis
2.4. Solubility Measurement and Salt-Metal Post-Analysis
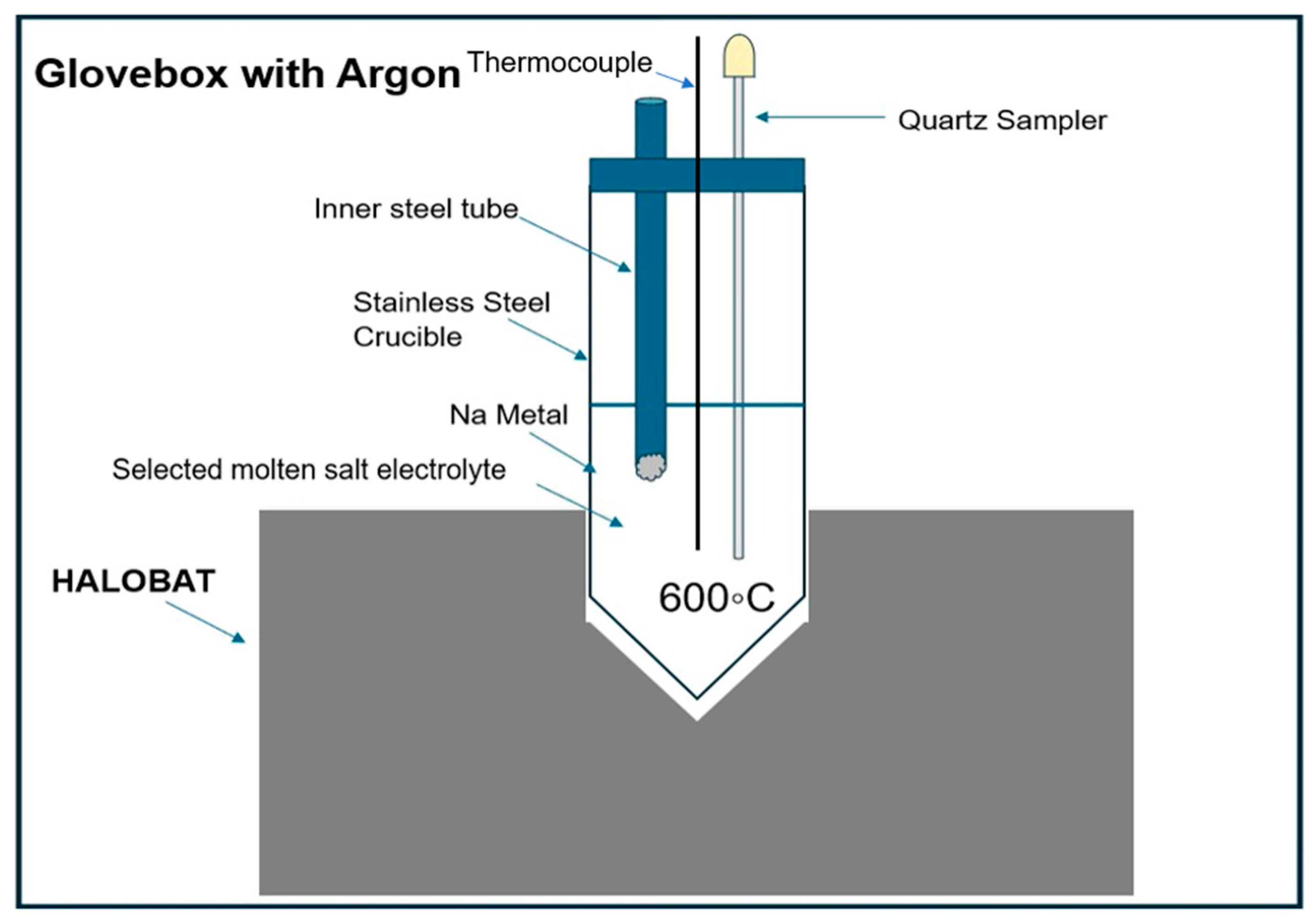
2.4.1. Na Solubility Measurement
- represents the concentration of dissolved Na (mol%);
- is the consumed volume of HCl in L;
- is the amount of the salt sample in mol.
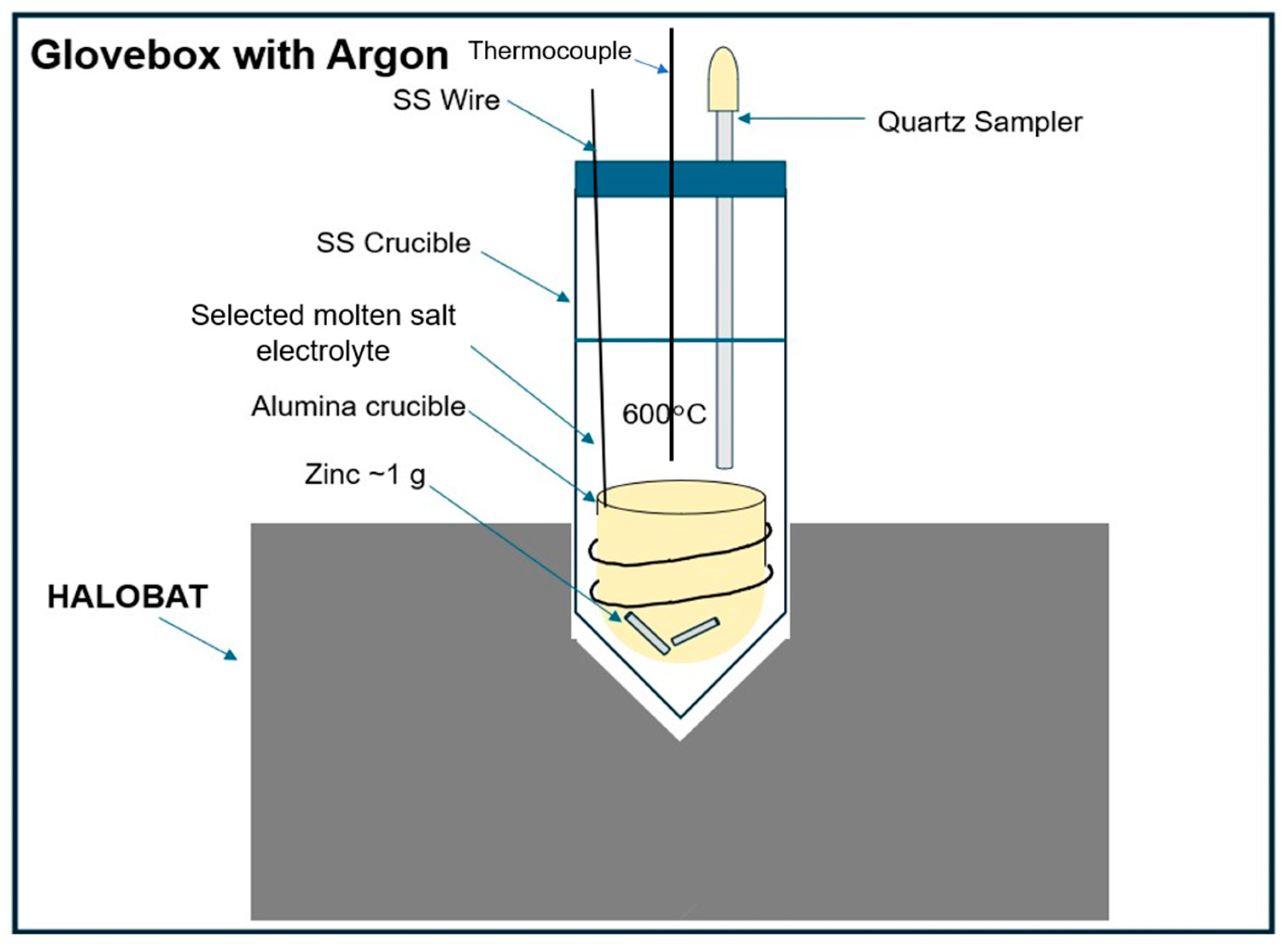
2.4.2. Zn Solubility Measurement
3. Results and Discussion
3.1. FactSage Simulation
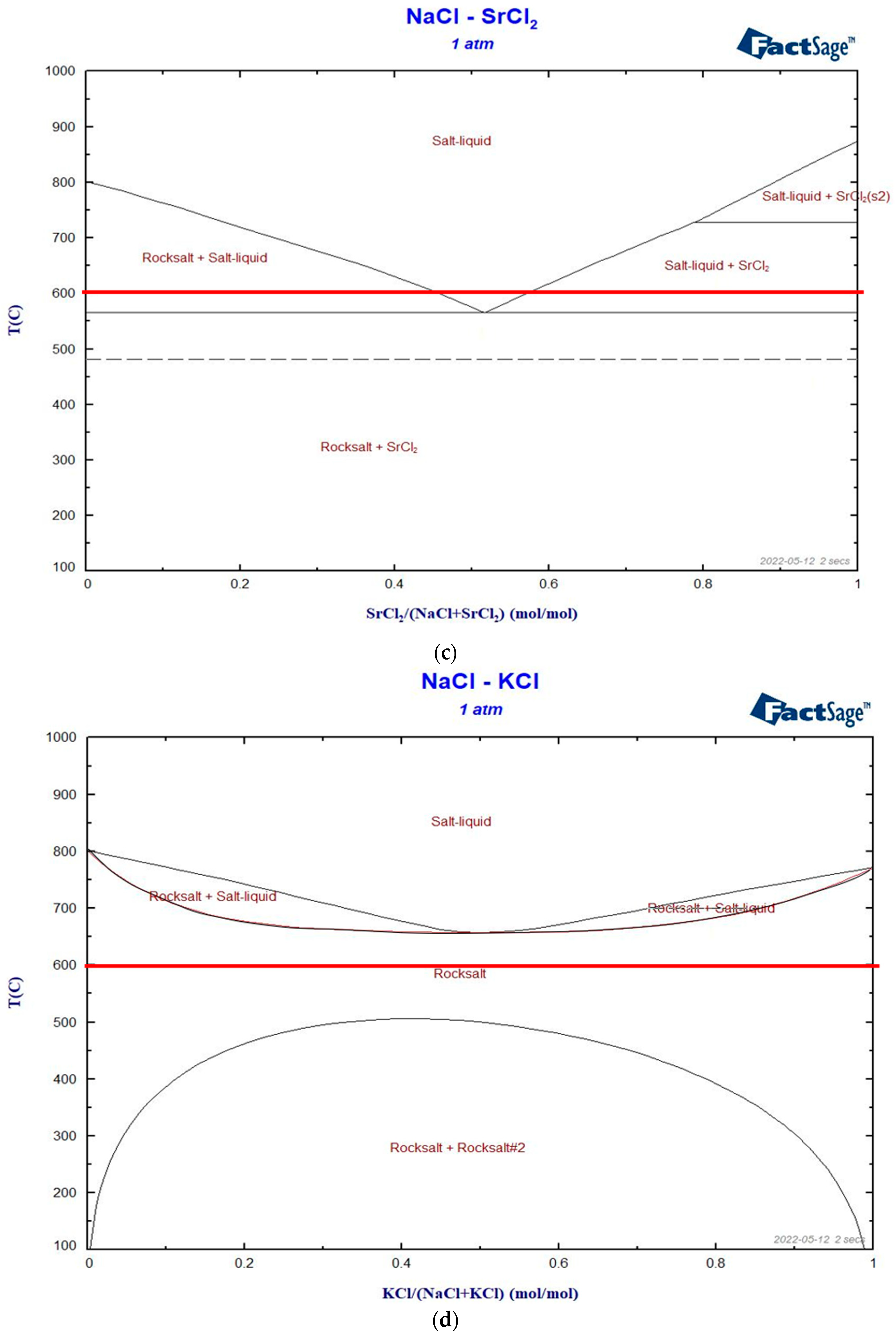
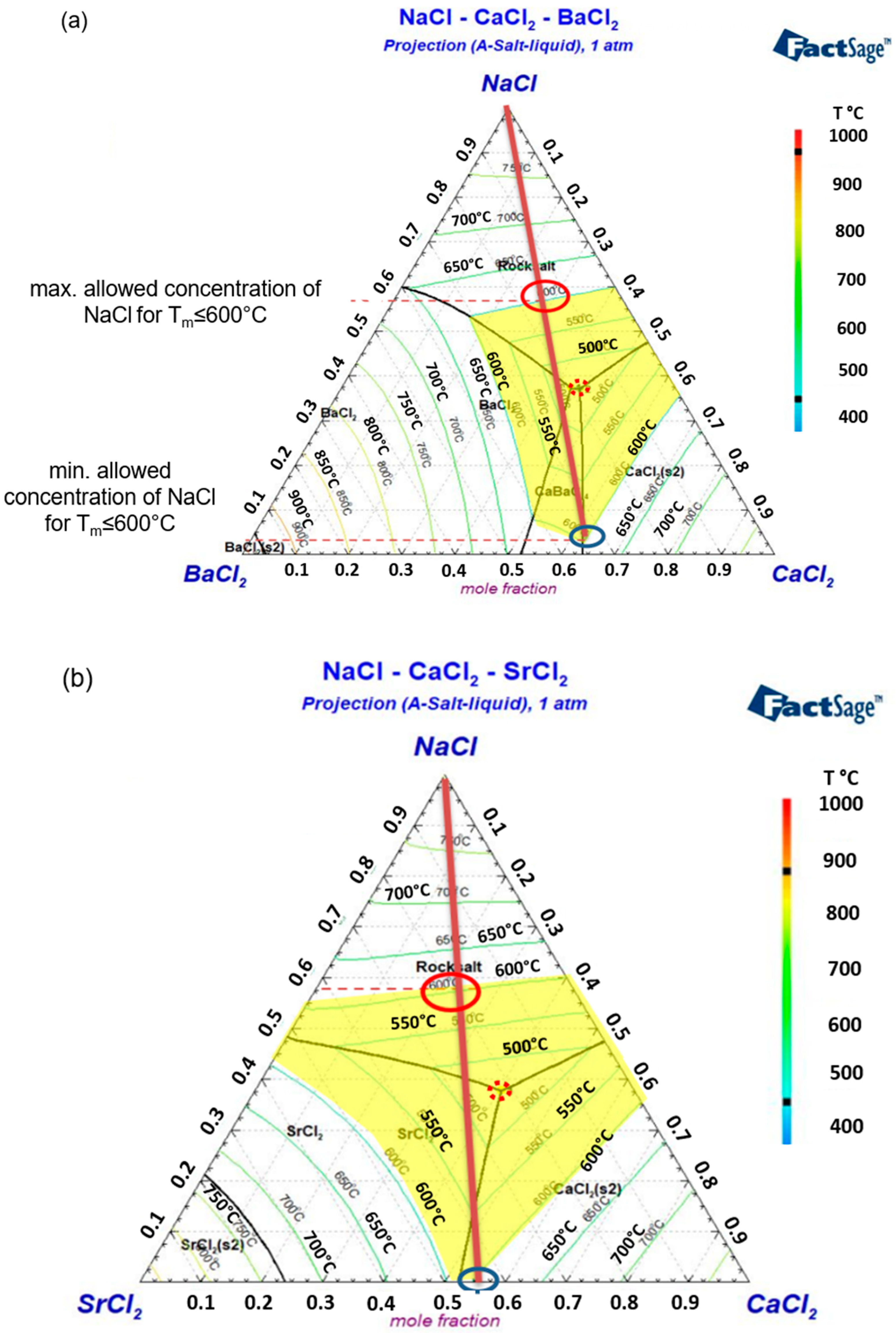
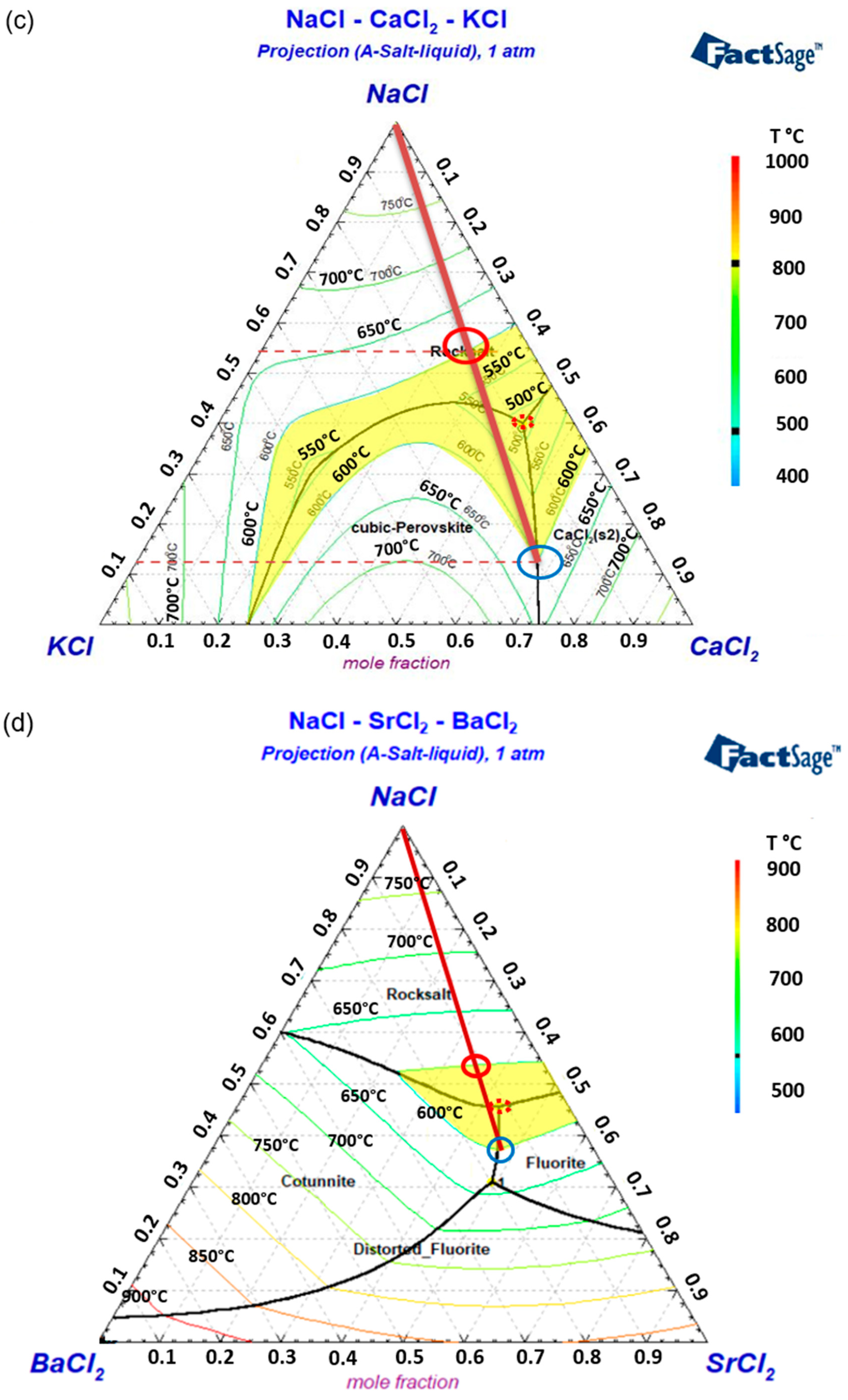
3.1.1. Binary Phase Diagrams
3.1.2. Ternary Phase Diagrams
3.2. Comparison of EMFs (Co-Reduction) and Material Costs
3.3. DSC Measurements and Simulated cp vs. T
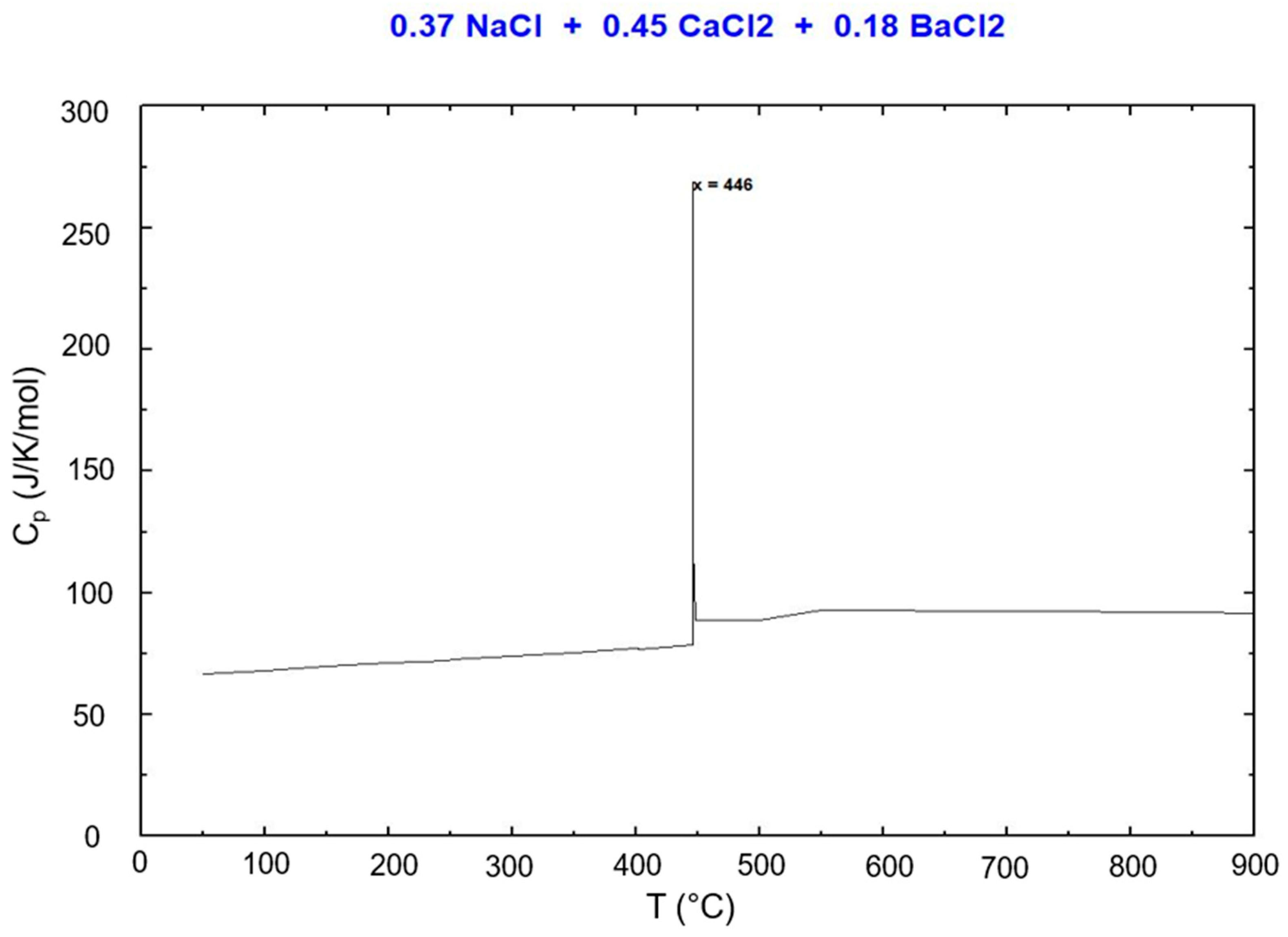
3.3.1. NaCl-CaCl2-BaCl2
3.3.2. NaCl-SrCl2-KCl
3.4. Solubilities of Metal Electrodes in Selected Molten Salt Electrolytes
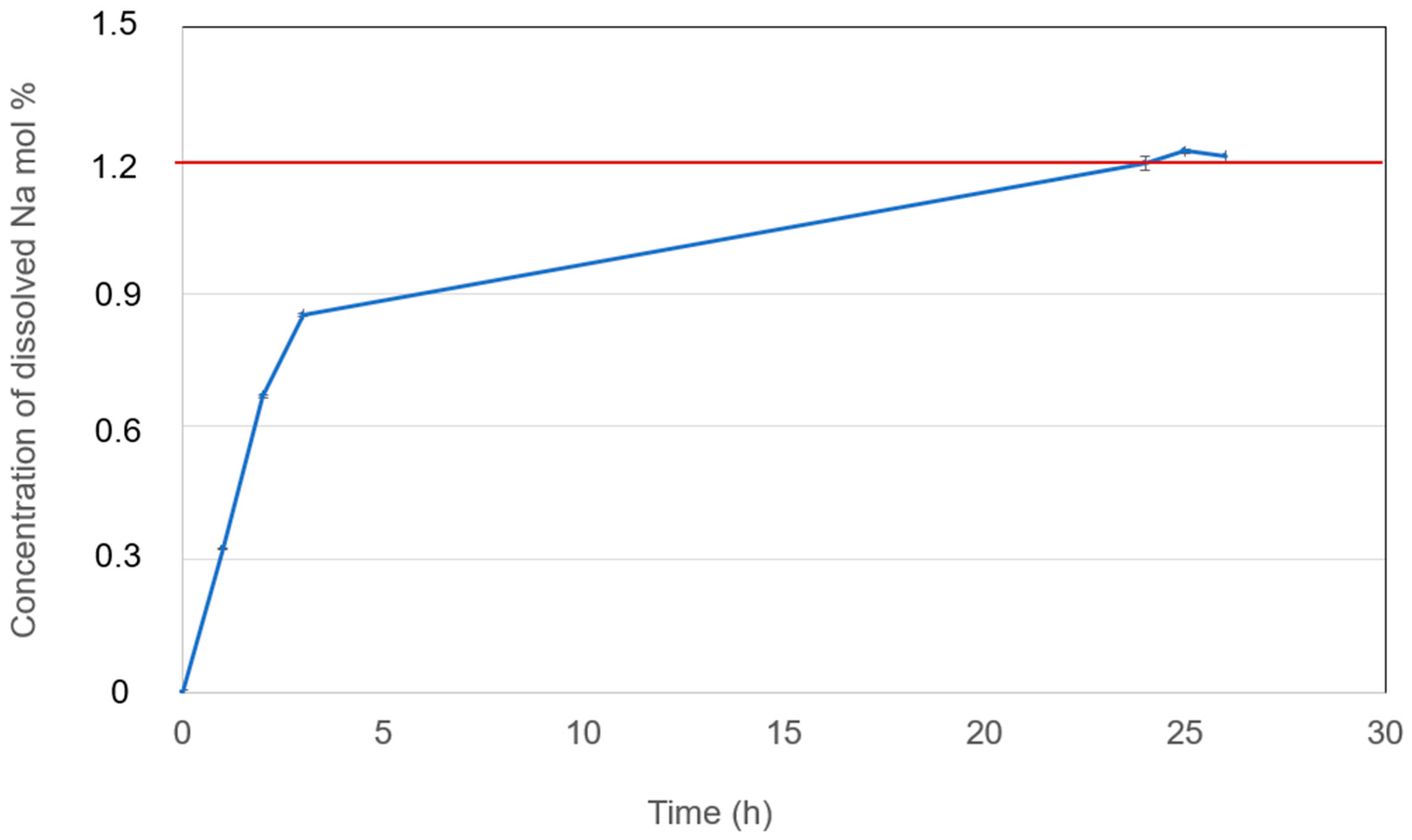
3.4.1. Solubilities of Na Metal
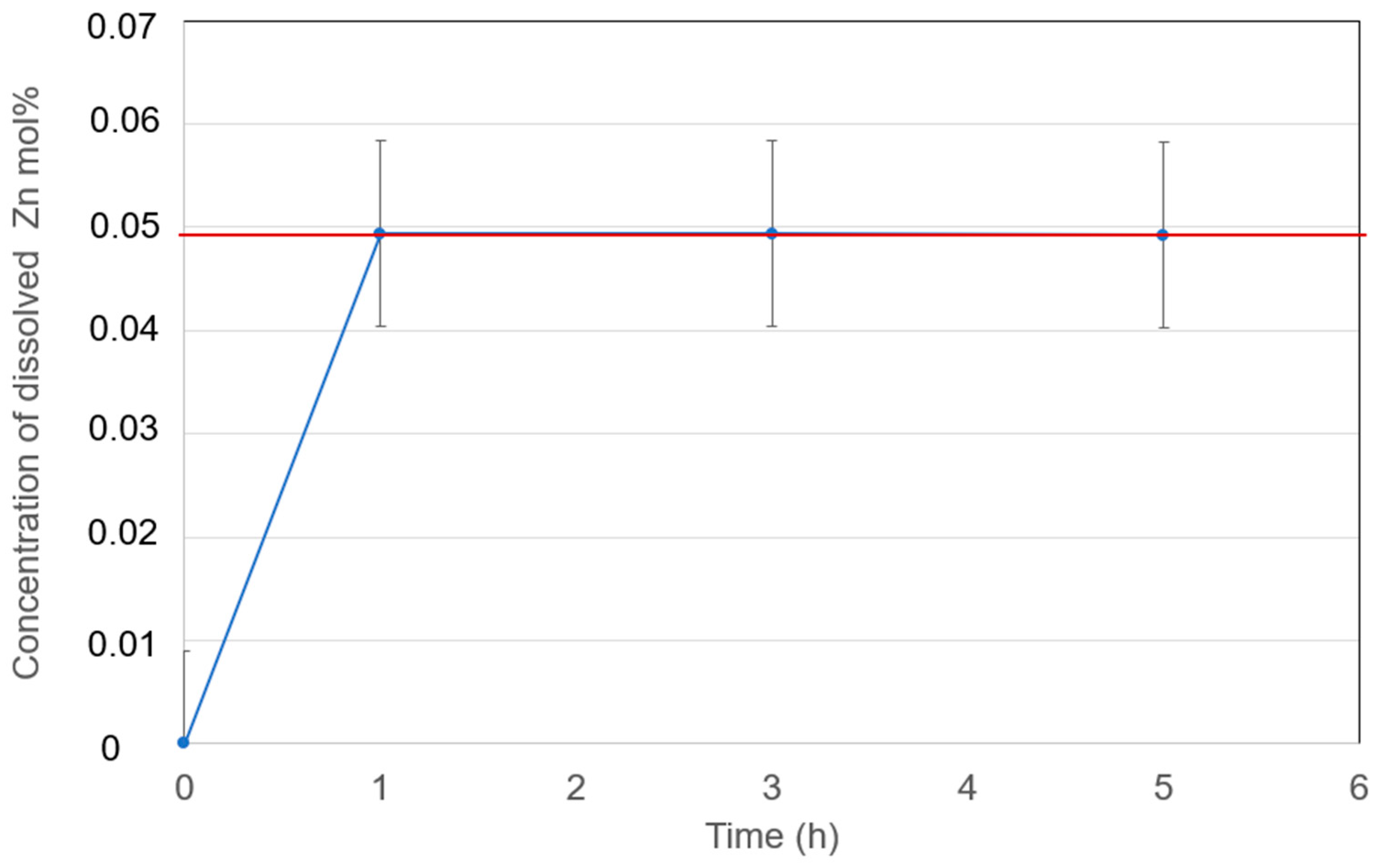
3.4.2. Solubilities of Zn Metal
4. Conclusions
- Binary and ternary phase diagrams of these molten salt mixtures were simulated via FactSage, and some selected salt compositions were verified by DSC. Based on the melting temperatures obtained from the binary phase diagrams, CaCl2 and SrCl2 are selected as the primary additives for NaCl. CaCl2 could have a co-reduction issue with the Na electrode due to a similar EMF as NaCl. SrCl2 is more expensive than other chlorides. BaCl2 and KCl are the third salt mixed to further reduce the co-reduction issue, melting temperature, and/or material costs.
- Regarding the low material cost and melting temperature, the NaCl-CaCl2-BaCl2 (0.32 USD/kg) is one of the most promising ALB molten salt electrolytes, but the co-reduction of Ca in the Na electrode could be an issue that should be examined in future work. The NaCl-SrCl2-KCl is expected to have the least co-reduction issue due to high EMFs. However, it has a higher melting temperature and higher cost (0.61 USD/kg) due to the utilization of SrCl2. Thus, the eutectic mixtures of these two ternary systems were selected for further study via DSC and melting behavior.
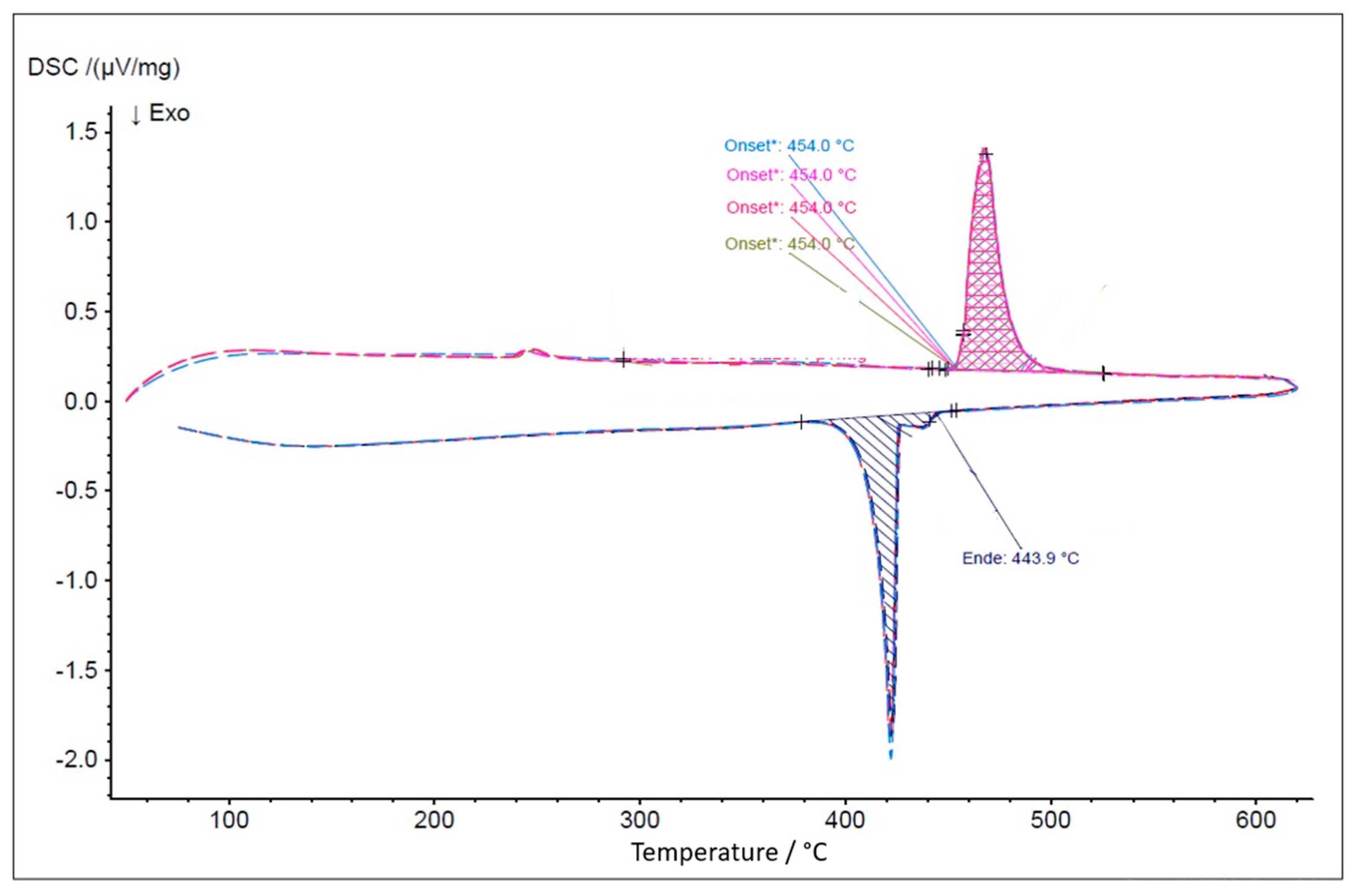
- Melting temperatures and melting behaviors of eutectic NaCl-CaCl2-BaCl2 (eutectic 37.1-44.9-18.0 mol%, Teut = 446 °C) and NaCl-SrCl2-KCl (eutectic 30.0-26.5-43.5 mol%, Teut = 503°C) were verified by DSC measurement and cp vs. T simulation of FactSage. The measured melting temperatures agree with the simulated values well. This means that FactSage simulation could be used to assist further optimization of the molten salt electrolyte (e.g., optimized salt composition) and the overall cell designs. In future work, DSC measurements on more salt compositions at various states of charge of the cell will be performed based on the results from the cell tests, such as electrochemical performance, and from neutron imaging of the operating cell, such as salt phase changes.
- Na- and Zn solubilities in these two selected molten salt electrolytes (NaCl-CaCl2-BaCl2 37-45-18 mol%, NaCl-SrCl2-KCl 30.0-26.5-43.5 mol%) at 600 °C were investigated with a homemade setup in the glovebox and an analysis method based on titration. To the best of our knowledge, these data are available in the literature for the first time.
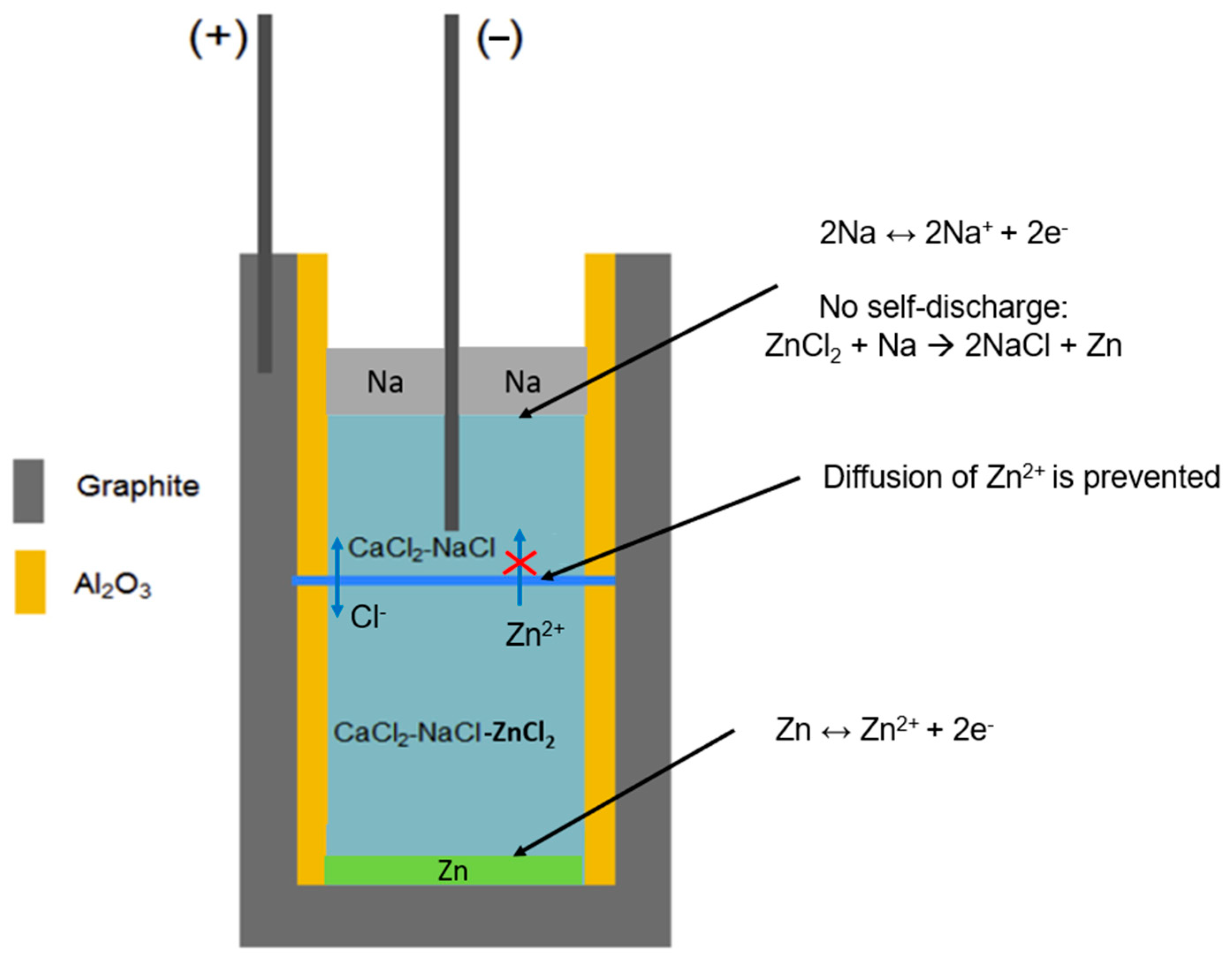
- The Na solubilities in the eutectic NaCl-CaCl2-BaCl2 and NaCl-SrCl2-KCl molten salt electrolyte at 600 °C is 1.2 mol% and 0.22 mol%, respectively. Such high Na solubility in NaCl-CaCl2-BaCl2 can enhance the self-discharge of ALBs, which deserves special attention. For NaCl-SrCl2-KCl, the Na solubility is low.
- Zn solubilities in the eutectic NaCl-CaCl2-BaCl2 and NaCl-SrCl2-KCl molten salt electrolyte at 600 °C are 0.04 mol% and 0.05 mol%, respectively. Compared to Na, Zn has much lower solubility in the examined molten chlorides. Thus, the Zn solubility is likely not an important factor for self-discharge.
- It is suggested to pay more attention to the reaction of Na with ZnCl2 in the molten salt at the interface between Na metal and the molten salt electrolyte, which has been reported in the literature [7] and can lead to fast self-discharge. It is suggested for future work to find the solutions to prevent fast self-discharge, e.g., finding and using a good-performance diaphragm.
- Overall, the NaCl-CaCl2-BaCl2 and NaCl-SrCl2-KCl salt mixtures are selected in this work and are considered the two most promising electrolytes for Na-ZnCl2 ALB. The NaCl-CaCl2-BaCl2 (eutectic 37.1-44.9-18.0 mol%) and NaCl-SrCl2-KCl (eutectic 30.0-26.5-43.5 mol%) molten salt electrolytes are suggested to be tested in the ALB demonstrators.
- Future work is suggested on optimization of the molten salt electrolyte by adding some NaCl in these two selected molten salt electrolytes for a larger operation range of the ALB cell based on the ternary phase diagrams obtained in this work and the cell tests.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Website of HORIEN (Former FZSONICK). Available online: https://saltbattery.horien.com/company-profile/ (accessed on 11 December 2024).
- European Association for Storage of Energy (EASE). Energy Storage Technology Descriptions: Sodium-Nickel-Chloride Battery. 2016. Available online: https://ease-storage.eu/wp-content/uploads/2016/07/EASE_TD_Electrochemical_NaNiCl2.pdf (accessed on 17 April 2025).
- Parthasarathy, P.; Weber, N.; Virkar, A.V. High Temperature Sodium-Zinc Chloride Batteries with Sodium Beta—Alumina Solid Electrolyte (BASE). ECS Trans. 2007, 6, 67–76. [Google Scholar] [CrossRef]
- FZSONICK. Sodium Metal Chloride Battery System—Technical Overview. Available online: https://drive.google.com/file/d/1s4fwmsG_RT1mji_wQDWNyZIM05Rp090J/view (accessed on 11 December 2024).
- Galloway, R.C.; Dustmann, C.-H. ZEBRA Battery-Material Cost, Availability and Recycling; EVS 20; MES-DEA GmbH: Stabio, Switzerland, 2003. [Google Scholar]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. A novel low-cost sodium–zinc chloride battery. Energy Environ. Sci. 2013, 6, 1837–1843. [Google Scholar] [CrossRef]
- Xu, J.; Kjos, O.S.; Osen, K.S.; Martinez, A.M.; Kongstein, O.E.; Haarberg, G.M. Na-Zn liquid metal battery. J. Power Sources 2016, 332, 274–280. [Google Scholar] [CrossRef]
- Xu, J.; Martinez, A.M.; Osen, K.S.; Kjos, O.S.; Kongstein, O.E.; Haarberg, G.M. Electrode behaviors of Na-Zn liquid metal battery. J. Electrochem. Soc. 2017, 164, A2335–A2340. [Google Scholar] [CrossRef]
- Leonardi, S.G.; Samperi, M.; Frusteri, L.; Antonucci, V.; D’Urso, C. A review of sodium-metal chloride batteries: Materials and cell design. Batteries 2023, 9, 524. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, M.; Wu, X.; Wen, Z. Improvement of rate capability and cycle life of Na-NiCl2 battery via designing a graphene anchoring porous nickel nanostructures as cathode. Chem. Eng. J. 2024, 497, 154444. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, X.; Wen, Z. Rational structure design of metal-based cathode for high-rate and long-cycling sodium nickel chloride batteries. Compos. B Eng. 2024, 283, 111636. [Google Scholar] [CrossRef]
- Sieuw, L.; Lan, T.; Svaluto-Ferro, E.; Vagliani, F.; Kumar, S.; Ding, W.; Turconi, A.; Basso, D.; Pozzi, A.; Battaglia, C.; et al. Influence of precursor morphology and cathode processing on performance and cycle life of sodium-zinc chloride (Na-ZnCl2) battery cells. Energy Storage Mater. 2024, 64, 103077. [Google Scholar] [CrossRef]
- Kumar, S.; Ding, W.; Hoffmann, R.; Sieuw, L.; Heinz, M.V.; Weber, N.; Bonk, A. AlCl3-NaCl-ZnCl2 Secondary electrolyte in next-generation ZEBRA (Na-ZnCl2) battery. Batteries 2023, 9, 401. [Google Scholar] [CrossRef]
- Heinz, M.V.F.; Sieuw, L.; Lan, T.; Turconi, A.; Basso, D.; Vagliani, F.; Pozzi, A.; Battaglia, C. A cell design strategies for sodium-zinc chloride (Na-ZnCl2) batteries, and first demonstration of tubular cells with 38 Ah capacity. Electrochim. Acta 2023, 464, 142881. [Google Scholar] [CrossRef]
- Lu, X.; Chang, H.J.; Bonnett, J.F.; Canfield, N.L.; Jung, K.; Sprenkle, V.L.; Li, G. An intermediate-temperature high-performance Na–ZnCl2 battery. ACS Omega 2018, 3, 15702–15708. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Svaluto-Ferro, E.; Kovalska, N.; Graeber, G.; Vagliani, F.; Basso, D.; Turconi, A.; Makowska, M.; Blugan, G.; Battaglia, C.; et al. Scaling of planar sodium-nickel chloride battery cells to 90 cm2 active area. Batter. Supercaps 2025, 8, e202400447. [Google Scholar] [CrossRef]
- Ding, W.; Gong, Q.; Liang, S.; Hoffmann, R.; Zhou, H.; Li, H.; Wang, K.; Zhang, T.; Weisenburger, A.; Müller, G.; et al. A Multicationic molten salt electrolyte of high-performance sodium liquid metal battery for grid storage. J. Power Sources 2023, 553, 232254. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.; Gong, Q.; Yan, S.; Zhou, X.; Liang, S.; Ding, W.; He, Y.; Jiang, K.; Wang, K. A sodium liquid metal battery based on the multi-cationic electrolyte for grid energy storage. Energy Storage Mater. 2022, 50, 572–579. [Google Scholar] [CrossRef]
- Hamer, W.J.; Malmberg, M.S.; Rubin, B. Theoretical electromotive forces for cells containing a single solid or molten chloride electrolyte. J. Electrochem. Soc. 1956, 103, 8. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, J.; Xu, J.; Shi, Z. Anode reaction mechanisms of Na|NaCl-CaCl2|Zn liquid metal battery. J. Ener. Chem. 2022, 72, 81–87. [Google Scholar] [CrossRef]
- Reis, B.H. Development of a Novel Thermodynamic Database for Salt Systems with Potential as Phase Change Materials. Ph.D. Thesis, Brandenburg University of Technology, Cottbus, Germany, 2021. [Google Scholar] [CrossRef]
- Sarma, M.; Lee, J.; Nash, W.; Lappan, T.; Shevchenko, N.; Landgraf, S.; Monrrabal, G.; Trtik, P.; Weber, N.; Weier, T. Reusable cell design for high-temperature (600 °C) liquid metal battery cycling. J. Electrochem. Soc. 2024, 171, 040531. [Google Scholar] [CrossRef]
- Godinez-Brizuela, O.E.; Duczek, C.; Weber, N.; Nash, W.; Sarma, M.; Einarsrud, K.E. A continuous multiphase model for liquid metal batteries. J. Energy Storage 2023, 73, 109147. [Google Scholar] [CrossRef]
- International Standard ISO 12100. Safety of Machinery—General Principles for Design—Risk Assessment and Risk Reduction. 2010. Available online: https://www.iso.org/standard/51528.html (accessed on 4 February 2025).
- Weber, N.; Duczek, C.; Monrrabal, G.; Nash, W.; Sarma, M.; Weier, T. Risk assessment for Na-Zn liquid metal batteries. Open Res. Eur. 2024, 4, 236. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Molten chloride salts for next generation CSP plants: Selection of promising chloride salts & study on corrosion of alloys in molten chloride salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar]
- Kim, H.; Boysen, D.A.; Newhouse, J.M.; Spatocco, B.L.; Chung, B.; Burke, P.J.; Bradwell, D.J.; Jiang, K.; Tomaszowska, A.A.; Wang, K.; et al. Liquid metal batteries: Past, present, and future. Chem Rev. 2013, 113, 2075–2099. [Google Scholar] [CrossRef] [PubMed]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. CALPHAD: Calculation of Phase Diagrams: A Comprehensive Guide; Pergamon: Oxford, UK, 1998. [Google Scholar]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Villada, C.; Ding, W.; Bonk, A.; Bauer, T. Simulation-assisted determination of the minimum melting temperature composition of MgCl2–KCl–NaCl salt mixture for next-generation molten salt thermal energy storage. Front. Energy Res. 2022, 10, 809663. [Google Scholar] [CrossRef]
- Igarashi, K.; Ohtani, H.; Mochinaga, J. Phase diagram of the system LaCl3-CaCl2-NaCl. Z. Naturforschung 1987, 42a, 1421–1424. [Google Scholar] [CrossRef]
- Janz, G.J.; Allen, C.B.; Bansal, N.P.; Murphy, R.M.; Tomkins, R.P.T. Physical Properties Data Compilations Relevant to Energy Storage. II. Molten Salts: Data on Single and Multi-Component Systems; U.S. Government Printing Office: Washington, DC, USA, 1979.
- Janz, G.J.; Tomkins, R.P.T. Physical Properties Data Compilations Relevant to Energy Storage. IV. Molten Salts: Data on Additional Single and Multi-Component Salt Systems; U.S. Government Printing Office: Washington, DC, USA, 1981.
- Qiao, Z.; Mo, W.; Wu, S.; Zhu, Y.; Hillert, M. Measurement and calculation of the phase diagram of the NaCl CaCl2 SrCl2 system. Calphad 1985, 9, 143–151. [Google Scholar] [CrossRef]
- Haarberg, G.M.; Thonstad, J. Electrochemical properties of metal-molten salt mixtures. J. Appl. Electrochem. 1989, 19, 789–801. [Google Scholar] [CrossRef]
- Corbett, J.D.; von Winbush, S.; Albers, F.C. The solubility of the post-transition metals in their molten halides. J. Am. Chem. Soc. 1957, 79, 3020–3024. [Google Scholar] [CrossRef]







| Binary System | Eutectic Temperature (°C) | Eutectic Composition (NaCl mol%) | Source |
|---|---|---|---|
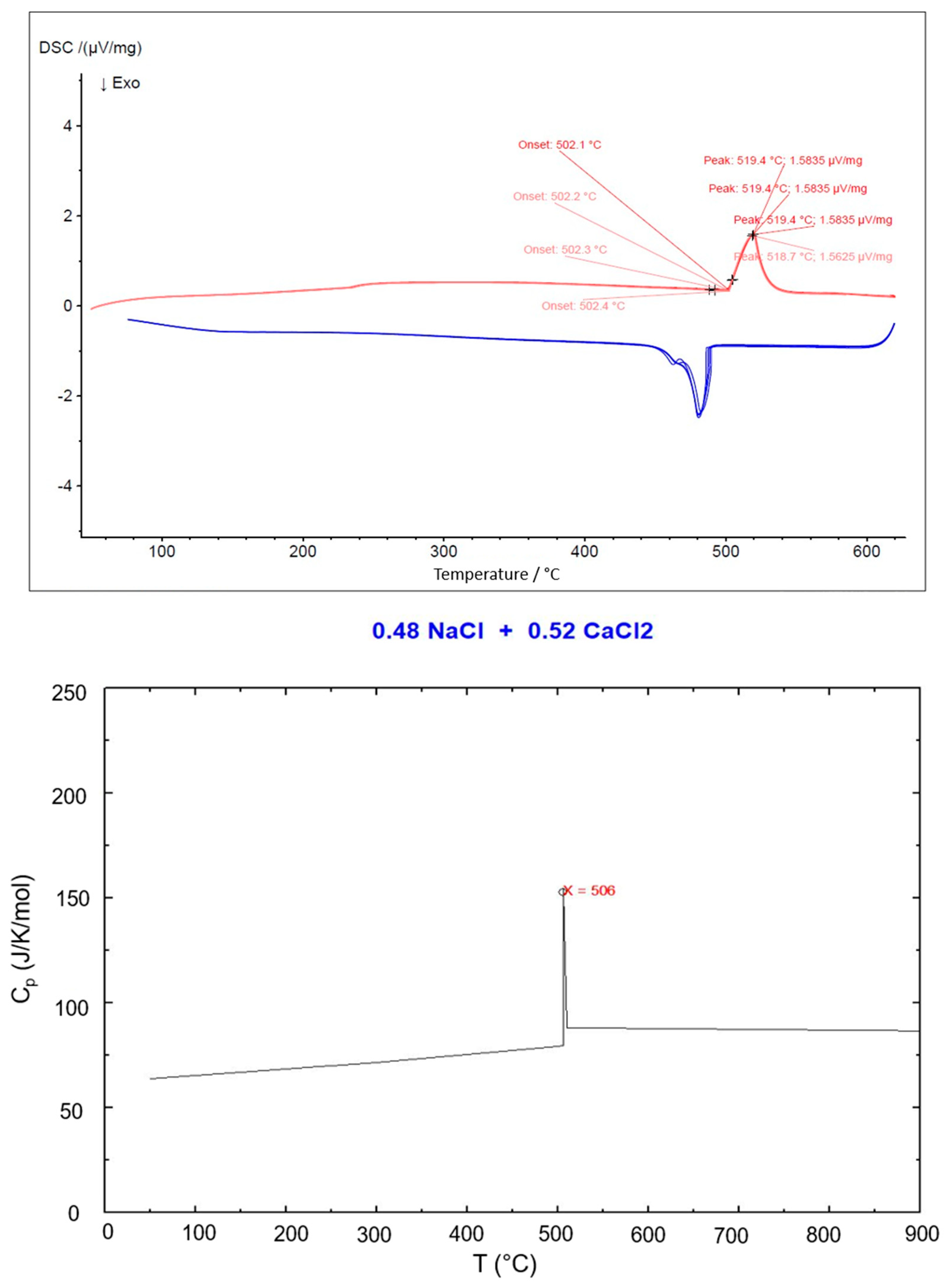
| NaCl-CaCl2 | 506 | 48% | This work |
| NaCl-CaCl2 | 508 | 50% | [32] |
| NaCl-CaCl2 | 500 | 48% | [33] |
| NaCl-BaCl2 | 650 | 60% | This work |
| NaCl-BaCl2 | 654 | 61% | [34] |
| NaCl-SrCl2 | 560 | 48% | This work |
| NaCl-SrCl2 | 560 | 48% | [35] |
| NaCl-KCl | 660 | 50% | This work |
| NaCl-KCl | 685 | 50% | [33] |
| Ternary Systems | Min. Melting Temperature (°C) | Eutectic Composition (mol%) | Source |
|---|---|---|---|
| NaCl-CaCl2-BaCl2 | 446 | 37.1-44.9-18.0 | This work, FactSage |
| NaCl-CaCl2-BaCl2 | 454 | 37.1-44.9-18.0 | This work, DSC |
| NaCl-CaCl2-SrCl2 | 448 | 37.7-40.4-21.9 | This work, FactSage |
| NaCl-CaCl2-SrCl2 | 471 | 41.9-40.6-18.1 | [35] |
| NaCl-CaCl2-KCl | 481 | 40.0-51.4-8.6 | This work, FactSage |
| NaCl-CaCl2-KCl | 504 | 42-52-6 | [34] |
| NaCl-SrCl2-BaCl2 | 555 | 45.6-43.0-11.4 | This work, FactSage |
| NaCl-SrCl2-KCl | 503 | 30.0-26.5-43.5 | This work, FactSage |
| NaCl-SrCl2-KCl | 503–505 | 30.0-26.5-43.5 | This work, DSC |
| Metal Chlorides | EMF (V) at 600 °C | Cost (USD/kg) |
|---|---|---|
| BaCl2 | 3.728 | ~0.5 |
| KCl | 3.658 | ~0.4 |
| SrCl2 | 3.612 | ~1 * |
| CaCl2 | 3.462 | ~0.3 |
| NaCl | 3.424 | ~0.06 |
| Molten Chlorides | Na Solubility (mol%) | Source |
|---|---|---|
| NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) | 1.2 (600 °C) | This work |
| NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) | 0.22 (600 °C) | This work |
| NaCl-CaCl2 (eutectic) | ~3.3 (600 °C) | [36] |
| NaCl | 2.1 (795 °C) | [27] |
| LiCl-NaCl-KCl (59-5-36 mol%) | 0.09 (450 °C), 0.15 (560 °C), 0.18 (600 °C) | [17] |
| Metal in Molten Salts | Metal Solubility (mol%) | Source |
|---|---|---|
| Zn in NaCl-CaCl2-BaCl2 (37.1-44.9-18.0 mol%) | 0.04 (600 °C) | This work |
| Zn in NaCl-SrCl2-KCl (30.0-26.5-43.5 mol%) | 0.05 (600 °C) | This work |
| Zn in ZnCl2 | 0.187 (498 °C), 0.61 (600 °C) | [37] |
| Zn in ZnI2 | 0.28 (498 °C), 0.87 (600 °C) | [37] |
| Mg in MgCl2 | 0.20–1.2 (714–900 °C), 0.07 (600 °C) | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Hoffmann, R.; Barge, A.; Kjos, O.S.; Weber, N.; Weier, T.; Bauer, T. Molten Salt Electrolyte for Na-ZnCl2 All-Liquid Battery for Grid Storage. Batteries 2025, 11, 177. https://doi.org/10.3390/batteries11050177
Ding W, Hoffmann R, Barge A, Kjos OS, Weber N, Weier T, Bauer T. Molten Salt Electrolyte for Na-ZnCl2 All-Liquid Battery for Grid Storage. Batteries. 2025; 11(5):177. https://doi.org/10.3390/batteries11050177
Chicago/Turabian StyleDing, Wenjin, Ralf Hoffmann, Akshata Barge, Ole S. Kjos, Norbert Weber, Tom Weier, and Thomas Bauer. 2025. "Molten Salt Electrolyte for Na-ZnCl2 All-Liquid Battery for Grid Storage" Batteries 11, no. 5: 177. https://doi.org/10.3390/batteries11050177
APA StyleDing, W., Hoffmann, R., Barge, A., Kjos, O. S., Weber, N., Weier, T., & Bauer, T. (2025). Molten Salt Electrolyte for Na-ZnCl2 All-Liquid Battery for Grid Storage. Batteries, 11(5), 177. https://doi.org/10.3390/batteries11050177








