Structural Design of Dry-Processed Lithium-Rich Mn-Based Materials with High Loading for Enhanced Energy Density
Abstract
1. Introduction
2. Materials and Methods
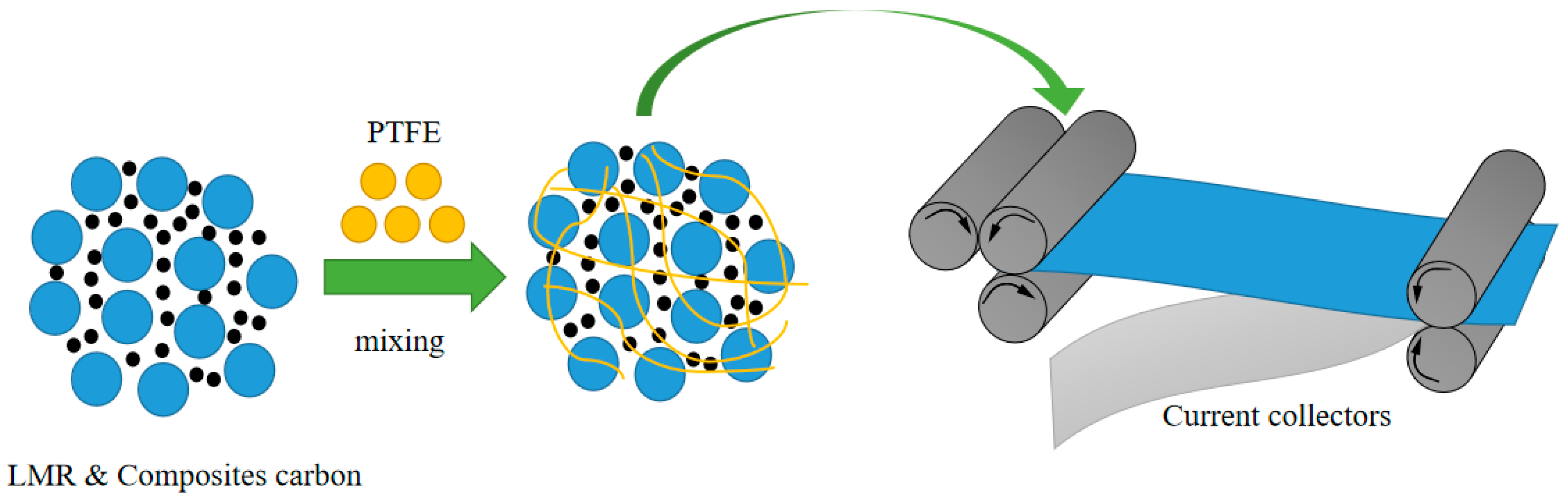
2.1. Preparation of Electrode
2.2. Material Characterization
2.3. Electrochemical Measurements
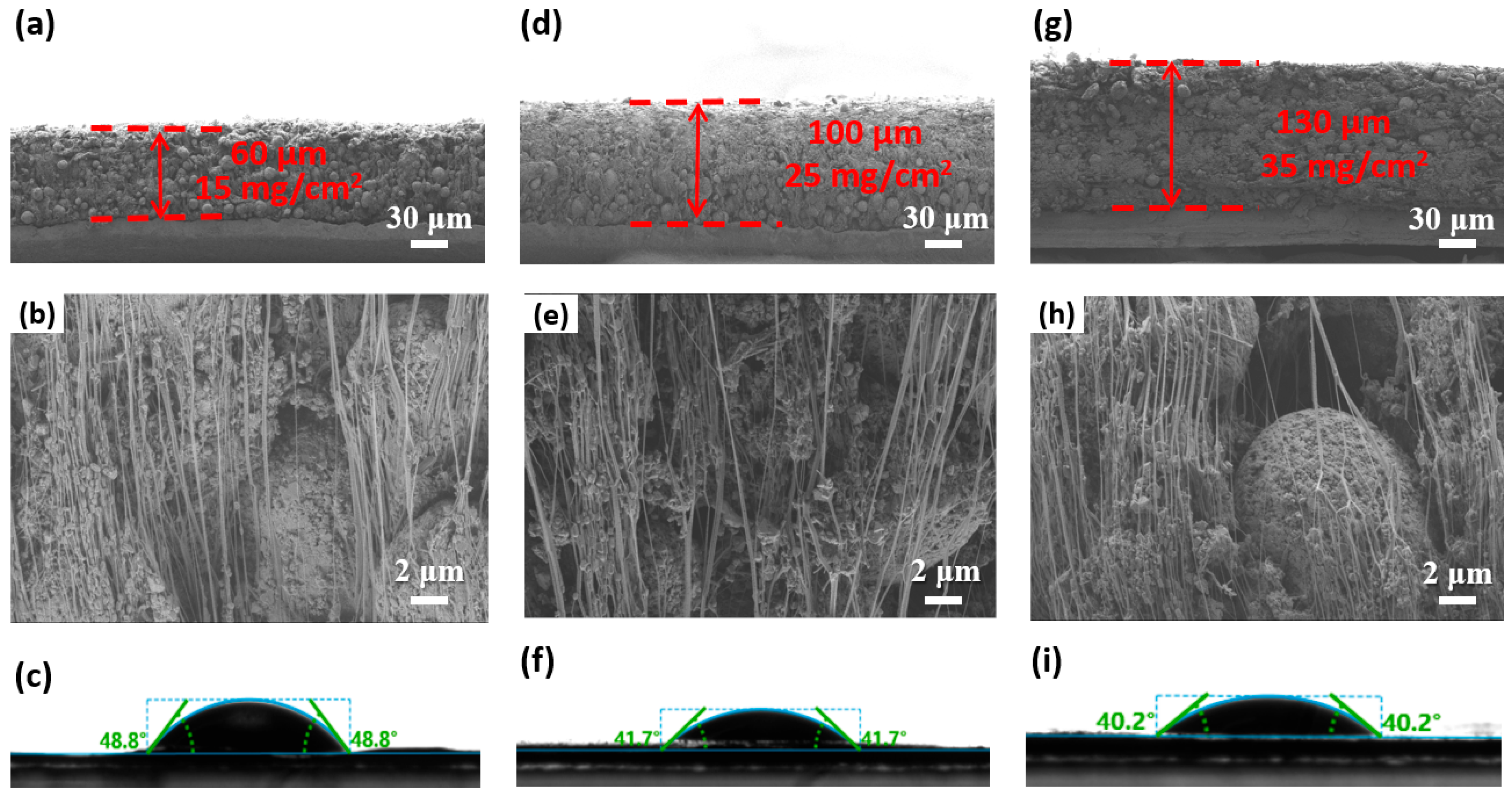
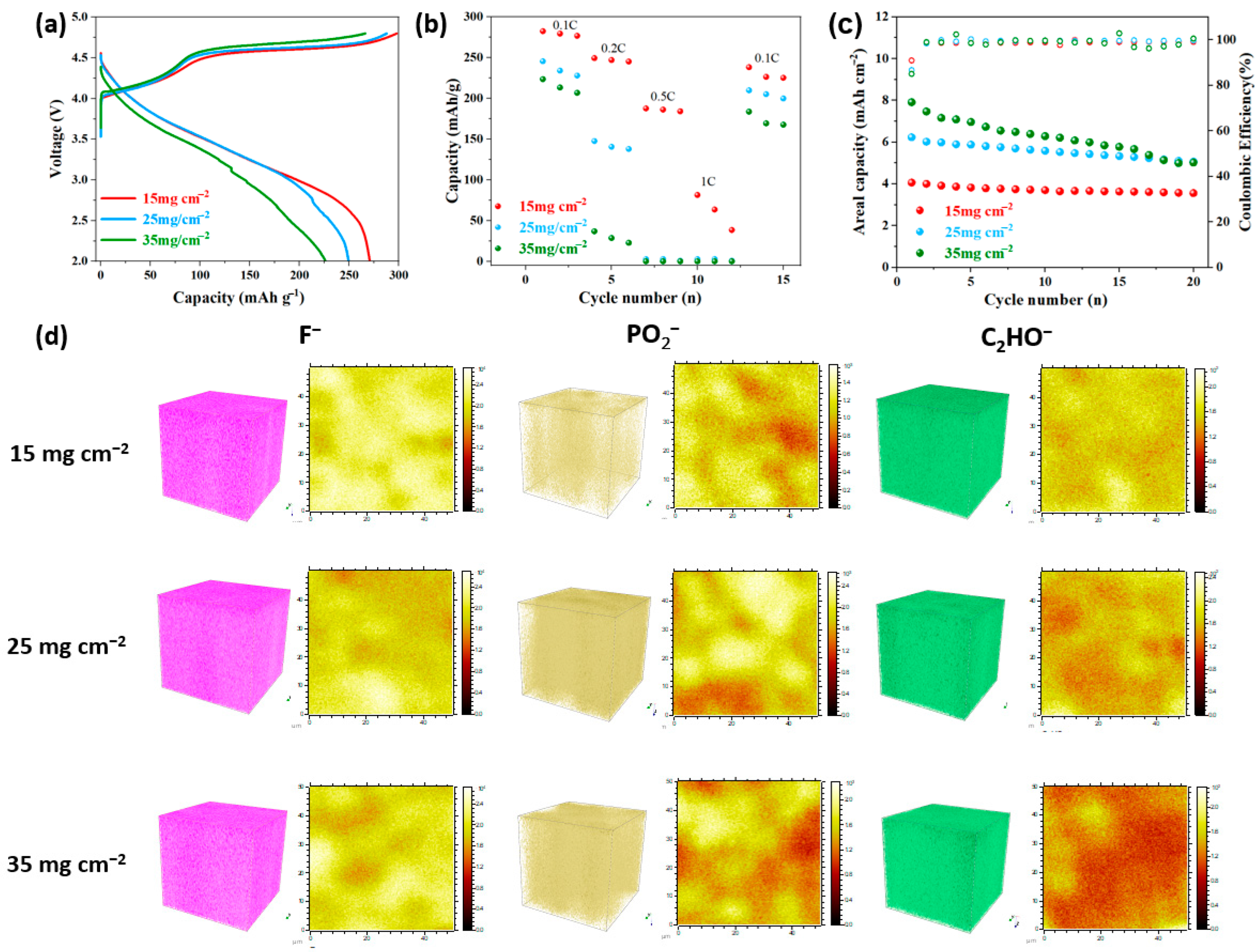
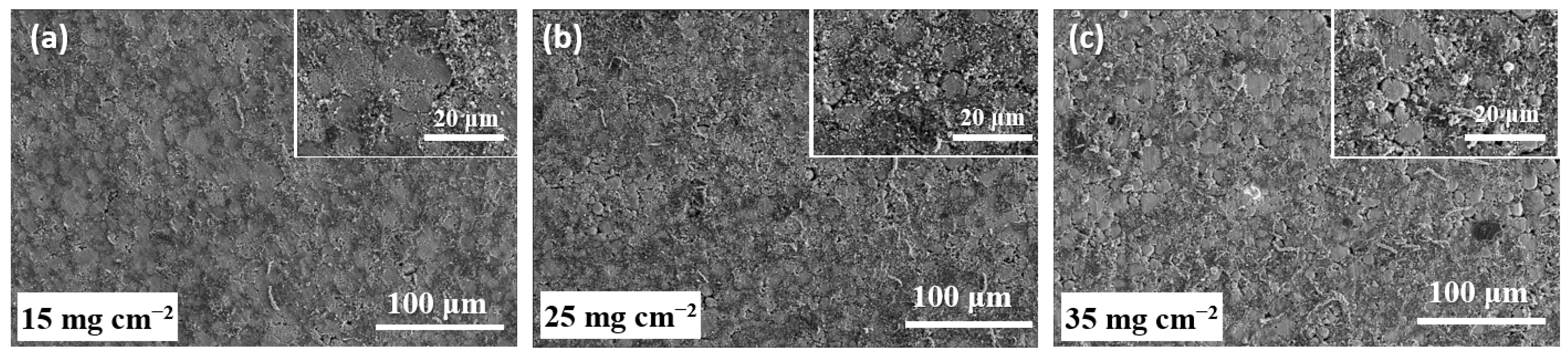
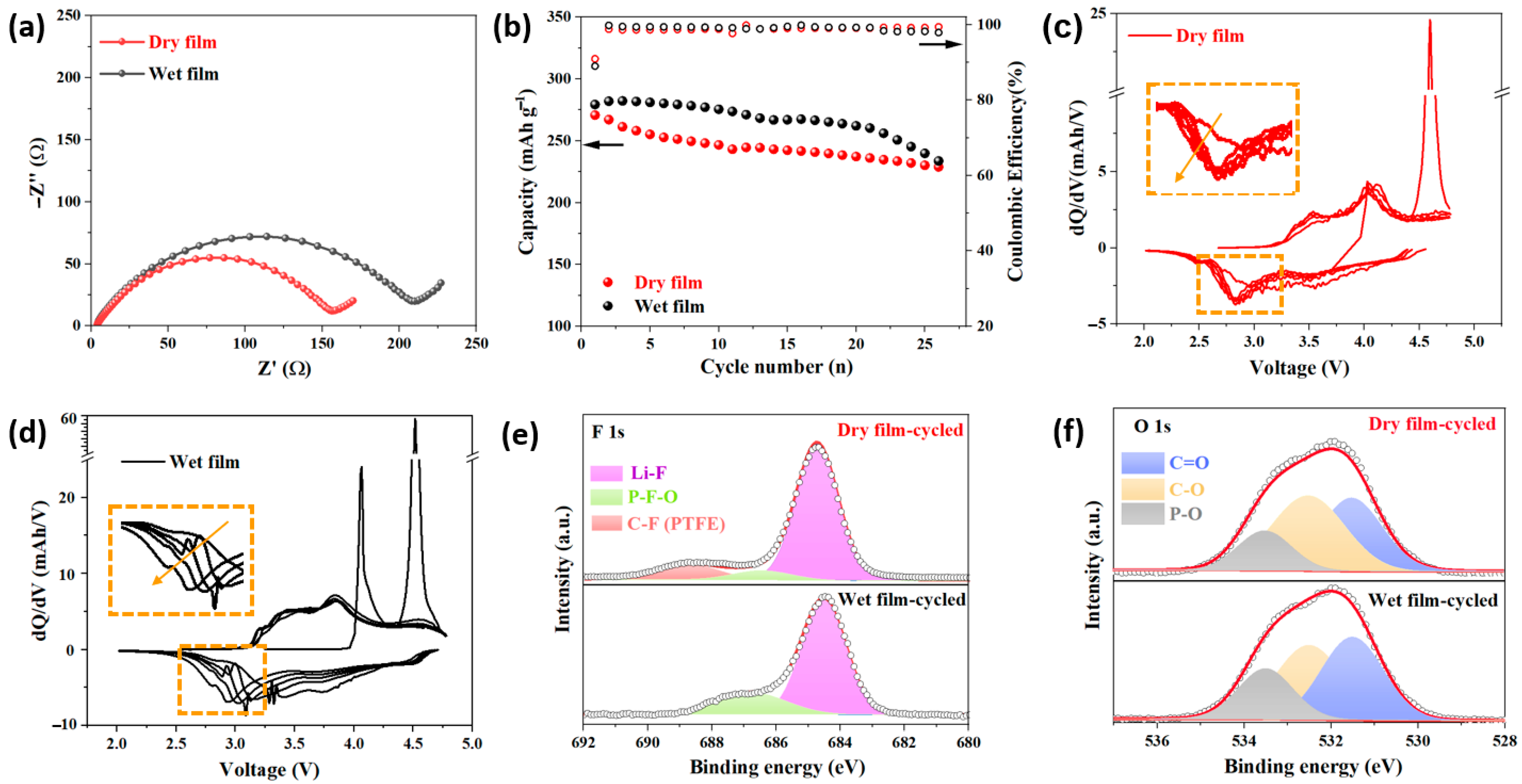
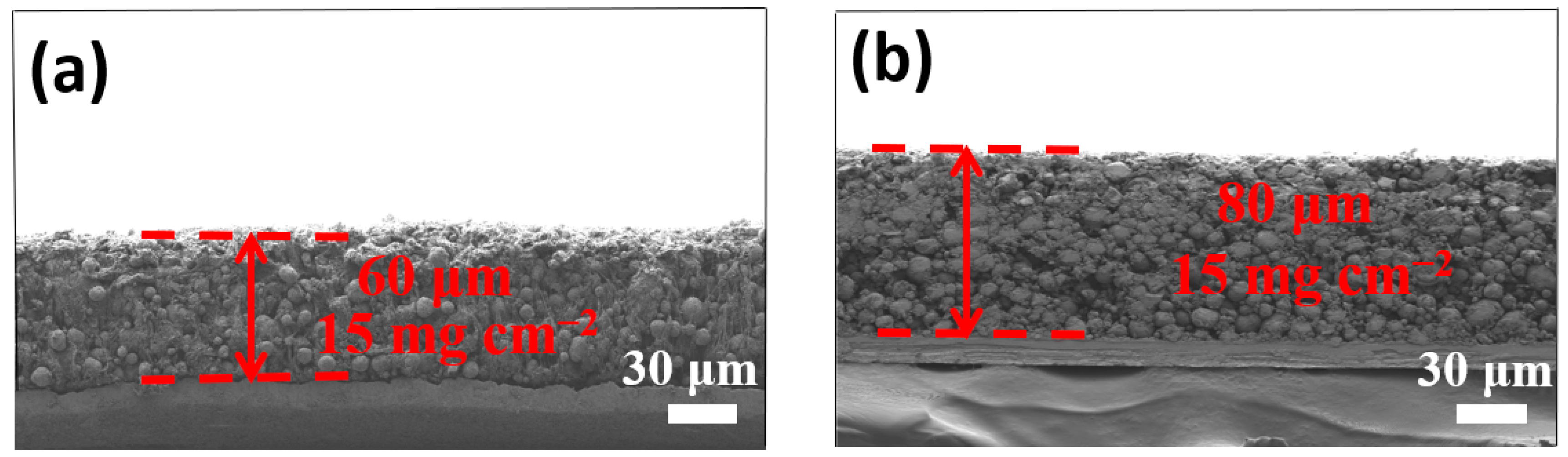
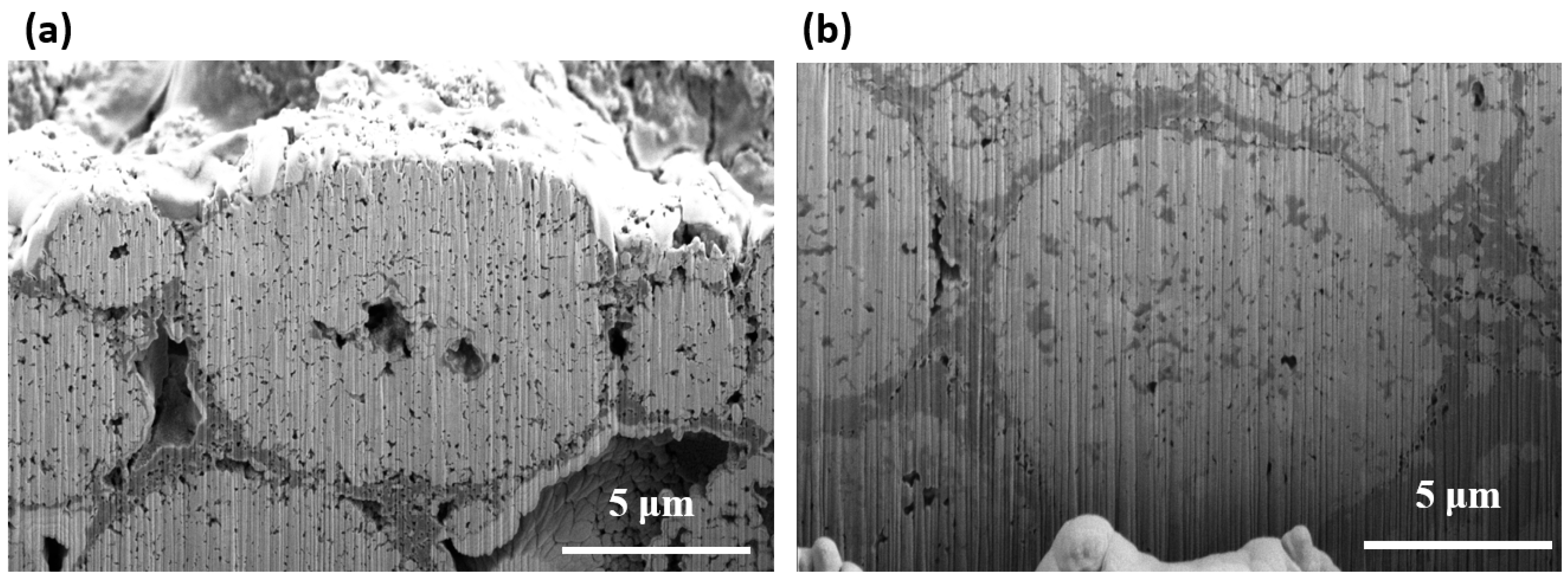
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Qin, C.L.; Zhao, B.Y.; Jia, S.F.; Wang, Z.F.; Yang, T.Z.; Liu, X.C.; Pan, L.N.; Zheng, L.L.; Luo, D.; et al. A cation and anion dual-doping strategy in novel Li-rich Mn-based cathode materials for high-performance Li metal batteries. Energy Storage Mater. 2024, 70, 103559. [Google Scholar] [CrossRef]
- Xu, J.J.; Cai, X.Y.; Cai, S.M.; Shao, Y.X.; Hu, C.; Lu, S.R.; Ding, S.J. High-energy lithium-ion batteries: Recent progress and a promising future in applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yan, Y.J.; Zhang, Y.G.; Chen, Y.X.; Peng, X.Y.; Wang, X.; Zhao, W.M.; Qin, C.L.; Liu, Q.; Liu, X.J.; et al. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium-sulfur batteries. Carbon Energy 2023, 5, e306. [Google Scholar] [CrossRef]
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Inoue, G.; Matsunaga, T. Effects of dry powder mixing on electrochemical performance of lithium-ion battery electrode using solvent-free dry forming process. J. Power Sources 2023, 581, 233466. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C.; et al. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li-S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Arnot, D.J.; Mayilvahanan, K.S.; Hui, Z.; Takeuchi, K.J.; Marschilok, A.C.; Bock, D.C.; Wang, L.; West, A.C.; Takeuchi, E.S. Thick electrode design for facile electron and ion transport: Architectures, advanced characterization, and modeling. Acc. Mater. Res. 2022, 3, 472–483. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick electrode batteries: Principles, opportunities, and challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Chen, A.X.; Sayahpour, B.; Shimizu, R.; Raghavendran, G. A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating. Energy Environ. Sci. 2023, 16, 1620–1630. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.K.; Lee, S.Y.; Park, J.H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.H.; Lee, T.; An, J.; Kim, H.; Song, H.; Lee, H.; Choi, J.W.; Kang, J.H. Ozone-treated carbon nanotube as a conductive agent for dry-processed lithium-ion battery cathode. ACS Energy Lett. 2023, 8, 3460–3466. [Google Scholar] [CrossRef]
- Tao, R.M.; Steinhoff, B.; Sun, X.G.; Sardo, K.; Skelly, B.; Meyer, H.M., III; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.J.; et al. High-throughput and high-performance lithium-ion batteries via dry processing. Chem. Eng. J. 2023, 471, 144300. [Google Scholar] [CrossRef]
- Sul, H.; Lee, D.; Manthiram, A. High-loading lithium-sulfur batteries with solvent-free dry-electrode processing. Small 2024, 20, 2400728. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.N.; Yang, Y.; Yang, T.Z.; Zhang, Z.; Tang, L.; Mao, Z.Y.; Zhang, Y.G.; Luo, D.; Chen, Z.W. Design lithium exchanged zeolite based multifunctional electrode additive for ultra-high loading electrode toward high energy density lithium metal battery. Adv. Energy Mater. 2024, 15, 2403063. [Google Scholar] [CrossRef]
- Lv, Z.W.; Liu, J.; Li, C.; Peng, J.X.; Zheng, C.X.; Zheng, X.F.; Wu, Y.Q.; Xia, M.; Zhong, H.Y.; Gong, Z.L.; et al. High-areal-capacity all-solid-state Li-S battery enabled by dry process technology. Etransportation 2024, 19, 100298. [Google Scholar] [CrossRef]
- Fu, J.Z.; Gong, X.T.; Jin, W.T.; Podder, C.; Liu, Y.T.; Yang, Z.Z.; Sultanov, M.; Pan, H.; Wang, Y. Enable superior performance of ultra-high loading electrodes through the cost-efficient solvent-free electrode manufacturing technology. Energy Storage Mater. 2024, 69, 103423. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Hu, J.K.; Huang, J.Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Tang, W.H.; Zhu, J.P.; Chen, C.; Ye, Q.; Zeng, F.H.; Ma, Z.P. Modification strategies and challenges of high-performance lithium-rich manganese-based cathode materials. Energy Technol. 2024, 12, 2301254. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Yu, X.Q.; L, H. A 700 W·h·kg−1 rechargeable pouch type lithium battery. Chin. Phys. Lett. 2023, 40, 048201. [Google Scholar] [CrossRef]
- Yuan, X.D.; Dong, T.; Liu, J.X.; Cui, Y.Y.; Dong, H.T.; Yuan, D.; Zhang, H.T. Biaffinity electrolyte optimizing high-voltage lithium-rich manganese oxide battery via interface modulation strategy. Angew. Chem. Int. Ed. 2023, 62, e202304121. [Google Scholar] [CrossRef]
- Zhang, B.D.; Wu, X.H.; Luo, H.Y.; Yan, H.; Chen, Y.L.; Zhou, S.Y.; Yin, J.H.; Zhang, K.; Liao, H.G.; Wang, Q.S.; et al. Gradient interphase engineering enabled by anionic redox for high-voltage and long-life Li-ion batteries. J. Am. Chem. Soc. 2024, 146, 4557–4569. [Google Scholar] [CrossRef]
- Ge, B.C.; Deng, J.J.; Wang, Z.J.; Liang, Q.H.; Hu, L.; Ren, X.Y.; Li, R.M.; Lin, Y.X.; Li, Y.S.; Wang, Q.R.; et al. Aggregate-dominated dilute electrolytes with low-temperature-resistant ion-conducting channels for highly reversible Na plating/stripping. Adv. Mater. 2024, 36, 2409161. [Google Scholar] [CrossRef] [PubMed]
- Steven, L.; Su, L.S.; Alex, M.; Cui, Z.H.; Arumugam, M. Cracking vs. surface reactivity in high-nickel cathodes for lithium-ion batteries. Joule 2023, 7, 2430–2444. [Google Scholar]
- Wade, A.; Llewellyn, A.V.; Heenan, T.M.M.; Tan, C.; Brett, D.J.L.; Jervis, R.; Shearing, P.R. First cycle cracking behaviour within Ni-rich cathodes during high-voltage charging. J. Electrochem. Soc. 2023, 170, 070513. [Google Scholar] [CrossRef]
- Gao, T.H.; Andrew, K.; Lu, W. Modeling electrode-level crack and quantifying its effect on battery performance and impedance. Electrochim. Acta 2020, 363, 137197. [Google Scholar] [CrossRef]
- Xu, M.; Reichman, B.; Wang, X. Modeling the effect of electrode thickness on the performance of lithium-ion batteries with experimental validation. Energy 2019, 186, 115864. [Google Scholar] [CrossRef]
- An, F.Q.; Zhou, W.N.; Li, P. A comparison of model prediction from P2D and particle packing with experiment. Electrochim. Acta 2021, 370, 137775. [Google Scholar]
- Kwon, K.; Kim, K.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T.; et al. Low-resistance LiFePO4 thick film electrode processed with dry electrode technology for high-energy-density lithium-ion batteries. Small Sci. 2024, 4, 2300302. [Google Scholar] [CrossRef]
- Liang, J.W.; Zhu, Y.M.; Li, X.N.; Luo, J.; Deng, S.X.; Zhao, Y.; Sun, Y.P.; Wu, D.J.; Hu, Y.F.; Li, W.H.; et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 2023, 14, 146. [Google Scholar] [CrossRef]
- Chen, D.C.; Kan, W.H.; Chen, G.Y. Understanding performance degradation in cation-disordered rock-salt oxide cathodes. Adv. Energy Mater. 2019, 9, 1901255. [Google Scholar]
- Bai, P.X.; Ji, X.; Zhang, J.X.; Zhang, W.R.; Hou, S.; Su, H.; Li, M.J.; Deng, T.; Cao, L.S.; Liu, S.F.; et al. Formation of LiF-rich cathode-electrolyte interphase by electrolyte reduction. Angew. Chem. Int. Ed. 2022, 61, e202202731. [Google Scholar] [CrossRef]
- Feng, Z.R.; Guo, L.Y.; Liu, X.F.; Li, W.W.; Zhang, R.P.; Wang, D.; Zhang, W.; Zheng, W.T. Stabilizing the lithium-rich manganese-based oxide cathode via regulating a CEI film. ACS Appl. Energy Mater. 2024, 7, 2791–2799. [Google Scholar] [CrossRef]
- Li, A.L.; Li, G.H.; Lu, S.G.; Ren, Z.M.; Wang, J.T.; Zhuo, H.X.; Quan, W.; Zhang, G.N.; Han, F.J.; Xia, Y.M.; et al. Interface stabilization of 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether to high-voltage Li-rich Mn-based layered cathode materials. Rare Met. 2022, 41, 822–829. [Google Scholar] [CrossRef]
- Beuse, T.; Fingerle, M.; Wagner, C.; Winter, M.; Boerner, M. Comprehensive Insights into the Porosity of Lithium-Ion Battery Electrodes: A Comparative Study on Positive Electrodes Based on LiNi0.6Mn0.2Co0.2O2 (NMC622). Batteries 2021, 7, 70. [Google Scholar] [CrossRef]
- Li, Q.H.; Wang, Y.; Wang, X.L.; Sun, X.R.; Zhang, J.N.; Yu, X.Q.; Li, H. Investigations on the fundamental process of cathode electrolyte interphase formation and evolution of high-voltage cathode. ACS Appl. Mater. Interfaces 2019, 12, 2319–2326. [Google Scholar] [CrossRef]
- Wu, F.; Shi, Q.; Chen, L.; Dong, J.; Zhao, J.; Wang, H.; Gao, F.; Liu, J.; Zhang, H.; Li, N.; et al. New insights into dry-coating-processed surface engineering enabling structurally and thermally stable high-performance Ni-rich cathode materials for lithium ion batteries. Chem. Eng. J. 2023, 470, 144045. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Guo, H.; Yang, T.; Wang, Z. Structural Design of Dry-Processed Lithium-Rich Mn-Based Materials with High Loading for Enhanced Energy Density. Batteries 2025, 11, 146. https://doi.org/10.3390/batteries11040146
Ma Y, Guo H, Yang T, Wang Z. Structural Design of Dry-Processed Lithium-Rich Mn-Based Materials with High Loading for Enhanced Energy Density. Batteries. 2025; 11(4):146. https://doi.org/10.3390/batteries11040146
Chicago/Turabian StyleMa, Yujie, Haojin Guo, Tai Yang, and Zhifeng Wang. 2025. "Structural Design of Dry-Processed Lithium-Rich Mn-Based Materials with High Loading for Enhanced Energy Density" Batteries 11, no. 4: 146. https://doi.org/10.3390/batteries11040146
APA StyleMa, Y., Guo, H., Yang, T., & Wang, Z. (2025). Structural Design of Dry-Processed Lithium-Rich Mn-Based Materials with High Loading for Enhanced Energy Density. Batteries, 11(4), 146. https://doi.org/10.3390/batteries11040146






