Novel Test Procedure for Assessing Lead–Acid Batteries for Partial-State-of-Charge Duty Using Internal Resistance Charge Acceptance Technique
Abstract
1. Introduction
2. Test Design and Setup
3. Single-Cell Test Results
4. Results for 12 V Battery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ah | Ampere hours |
| AGM | Absorbed glass mat |
| BESS | Battery energy storage system |
| PSOC | Partial state of charge |
| SOC | State of charge |
| UPS | Uninterruptible power supply |
| VRLA | Valve-regulated lead–acid |
References
- Worku, M.Y. Recent Advances in Energy Storage Systems for Renewable Source Grid Integration: A Comprehensive Review. Sustainability 2022, 14, 5985. [Google Scholar] [CrossRef]
- Ulbig, A.; Borsche, T.S.; Andersson, G. Impact of low rotational inertia on power system stability and operation. Ifac Secr. 2014, 47, 7290–7297. [Google Scholar] [CrossRef]
- Quoilin, S.; Kavvadias, K.; Mercier, A.; Pappone, I.; Zucker, A. Quantifying self-consumption linked to solar home battery systems: Statistical analysis and economic assessment. Appl. Energy 2016, 182, 58–67. [Google Scholar] [CrossRef]
- Sharma, V.; Haque, M.H.; Aziz, S.M. Energy cost minimization for net zero energy homes through optimal sizing of battery storage system. Renew. Energy 2019, 141, 278–286. [Google Scholar] [CrossRef]
- Rappaport, R.D.; Miles, J. Cloud energy storage for grid scale applications in the UK. Energy Policy 2017, 109, 609–622. [Google Scholar] [CrossRef]
- Chalmers, S.M.; Hitt, M.M.; Underhill, J.T.; Anderson, P.M.; Vogt, P.L.; Ingersoll, R. The effect of photovoltaic power generation on utility operation. IEEE Trans. Power Appar. Syst. 1985, PAS-104, 524–530. [Google Scholar] [CrossRef]
- Jewell, W.T.; Unruh, T.D. Limits on cloud-induced fluctuation i photovoltaic generation. IEEE Trans. Energy Convers. 1990, 5, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Faaborg, A. IEA PVPS International Energy Agency Implementing Agreement on Photovoltaic Power Systems TASK V Grid Interconnection of Building Integrated and Other Dispersed Photovoltaic Power Systems Impacts of Power Penetration from Photovoltaic Power Systems in Distribution Networks 2002. IEA PVPS. Available online: https://hme.ca/gridconnect/IEA_PVPS_Task_5-10_Impacts_of_PV_Power_Penetration.pdf (accessed on 14 January 2023).
- Tomazic, G.; Skyllas-Kazacos, M. Redox Flow Batteries; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 309–336. [Google Scholar] [CrossRef]
- McMahon, R.; Logan, T.; Miles, J.; McMahon, N.; Ridge, A. Operational study of domestic battery energy storage system. J. Eng. 2019, 2019, 3479–3483. [Google Scholar] [CrossRef]
- King, S.; Boxall, N.J. Lithium battery recycling in Australia: Defining the status and identifying opportunities for the development of a new industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y. An overview of recycling and treatment of spent LiFePO4 batteries in China. Resour. Conserv. Recycl. 2017, 127, 233–243. [Google Scholar] [CrossRef]
- Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L.; Song, W.; You, L.; Wu, Z.S.; Liu, J.W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. Rsc Adv. 2017, 7, 4783–4790. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Q.; Liu, Y.; Xu, J.; Sun, J.; Xiao, H.; Wang, Q. Experimental investigation of water spray on suppressing lithium-ion battery fires. Fire Saf. J. 2020, 120, 103117. [Google Scholar] [CrossRef]
- Insurance Risks of Li-ion | Fire Protection Association—Thefpa.co.uk. Available online: https://www.thefpa.co.uk/news/insurance-risks-of-li-ion (accessed on 6 December 2024).
- AIG. Lithium-ion Battery Energy Storage Systems AIG Energy Industry Group. 2018. Available online: https://www.aig.co.uk/content/dam/aig/emea/united-kingdom/documents/Insights/battery-storage-systems-energy.pdf (accessed on 25 March 2023).
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Association, I.L. Lead Recycling Sustainability in Action. 2015. Available online: https://hammondglobal.com/wp-content/uploads/2022/06/ILA9927-FS_Recycling_V08.pdf (accessed on 25 March 2023).
- International, B.C. NATIONAL RECYCLING RATE STUDY Vault Consulting for Battery Council International. 2023. Available online: https://batterycouncil.org/resource/national-recycling-rate-study/ (accessed on 25 March 2023).
- Battery Council International Comparative Life Cycle Assessment of Lead and LFP Batteries for Automotive Applications 2 of 92 Client: Battery Council International and International Lead Association Title: Comparative Life Cycle Assessment of Batteries for Automotive Applications. 2023. Available online: https://batterycouncil.org/wp-content/uploads/2023/09/BCI_Comparative_LCA_Automotive-Batteries_Lead_and_LFP_5.9.23.pdf (accessed on 25 March 2023).
- Schaeck, S.; Stoermer, A.O.; Hockgeiger, E. Micro-hybrid electric vehicle application of valve-regulated lead-acid batteries in absorbent glass mat technology: Testing a partial-state-of-charge operation strategy. J. Power Sources 2009, 190, 173–183. [Google Scholar] [CrossRef]
- Kaushik, R.; Mawston, I. Coulombic efficiency of lead/acid batteries, particularly in remote-area power-supply (RAPS) systems. J. Power Sources 1991, 35, 377–383. [Google Scholar] [CrossRef]
- Stevens, J.; Corey, G. A study of lead-acid battery efficiency near top-of-charge and the impact on PV system design. In Proceedings of the Conference Record of the Twenty Fifth IEEE Photovoltaic Specialists Conference—1996, Washington, DC, USA, 13–17 May 1996; IEEE: New York, NY, USA, 1996; pp. 1485–1488. [Google Scholar] [CrossRef]
- Nakarnura, K.; Shiomi, M.; Takahashi, K.; Tsubota, M. PWg Failure modes of valve-regulated lead/acid batteries. J. Power Sources Geneva 1996, 59, 153–157. [Google Scholar]
- Lam, L.; Haigh, N.; Phyland, C.; Urban, A. Failure mode of valve-regulated lead-acid batteries under high-rate partial-state-of-charge operation. J. Power Sources Geneva 2004, 133, 126–134. [Google Scholar] [CrossRef]
- Büngeler, J.; Cattaneo, E.; Riegel, B.; Sauer, D.U. Advantages in energy efficiency of flooded lead-acid batteries when using partial state of charge operation. J. Power Sources Geneva 2018, 375, 53–58. [Google Scholar] [CrossRef]
- Catherino, H.A.; Feres, F.F.; Trinidad, F. Sulfation in lead–acid batteries. J. Power Sources 2004, 129, 113–120. [Google Scholar] [CrossRef]
- Wong, Y.; Hurley, W.; Wölfle, W. Charge regimes for valve-regulated lead-acid batteries: Performance overview inclusive of temperature compensation. J. Power Sources 2008, 183, 783–791. [Google Scholar] [CrossRef]
- Ebner, E.; Wark, M.; Börger, A. Passive Mischelemente zur Elektrolytkonvektion in Blei-Säure- Nassbatterien. Chemie-Ingenieur-Technik 2011, 83, 2051–2058. [Google Scholar] [CrossRef]
- Sauer, D.U.; Karden, E.; Fricke, B.; Blanke, H.; Thele, M.; Bohlen, O.; Schiffer, J.; Gerschler, J.B.; Kaiser, R. Charging performance of automotive batteries—An underestimated factor influencing lifetime and reliable battery operation. J. Power Sources 2007, 168, 22–30. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shiota, M.; Nakayama, Y.; Hirai, N.; Hara, S. In situ analysis of electrochemical reactions at a lead surface in sulfuric acid solution. J. Power Sources 2000, 85, 22–28. [Google Scholar]
- Lach, J.; Wróbel, K.; Wróbel, J.; Podsadni, P.; Czerwiński, A. Applications of carbon in lead-acid batteries: A review. J. Solid State Electrochem. 2019, 23, 693–705. [Google Scholar] [CrossRef]
- Smith, M.J.; Gladwin, D.T.; Stone, D.A. Experimental analysis of the influence of high-frequency ripple currents on dynamic charge acceptance in lead-acid batteries. In Proceedings of the IECON 2017—43rd Annual Conference of the IEEE Industrial Electronics Society, Beijing, China, 29 October–1 November 2017; IEEE: New York, NY, USA, 2017; pp. 7140–7145. [Google Scholar] [CrossRef]
- Swogger, S.W.; Everill, P.; Dubey, D.P.; Sugumaran, N. Discrete carbon nanotubes increase lead acid battery charge acceptance and performance. J. Power Sources 2014, 261, 55–63. [Google Scholar] [CrossRef]
- Bozkaya, B.; Bauknecht, S.; Settelein, J.; Kowal, J.; Karden, E.; Giffin, G.A. Comparison of Dynamic Charge Acceptance Tests on Lead–Acid Cells for Carbon Additive Screening. Energy Technol. 2022, 10, 2101051. [Google Scholar] [CrossRef]
- Bauknecht, S.; Kowal, J.; Bozkaya, B.; Settelein, J.; Karden, E. The Influence of Cell Size on Dynamic Charge Acceptance Tests in Laboratory Lead-Acid Cells. Energy Technol. 2022, 10, 2101053. [Google Scholar] [CrossRef]
- Kumar, S.; Babu, N.; Sumanth, V.K.B.; Siva, P.K.; Balaji, G.; Jagadish, M. Influence of Carbon and Interaction of Carbon and Lignosulfonateon Dynamic Charge Acceptance of Flooded (Enhanced) Lead Acid Batteries. Int. J. Sci. Res. 2018, 7. [Google Scholar] [CrossRef]
- Smith, M.; Gladwin, D.; Stone, D. An analysis of the influence of high-frequency ripple currents on dynamic charge acceptance in lead-acid batteries. J. Energy Storage 2019, 22, 27–35. [Google Scholar] [CrossRef]
- BSI. Lead-Acid Starter Batteries - Batteries for Micro-Cycle Applications. BSI. 2015. Available online: https://knowledge.bsigroup.com/products/lead-acid-starter-batteries-batteries-for-micro-cycle-applications (accessed on 25 March 2023).
- (SBA) Battery Industry Association Standards. S0101 Lead-Acid Battery for Idling Stop Vehicles. SBA. 2014. Available online: https://www.baj.or.jp/publication/books02.html (accessed on 25 March 2023).
- Moseley, P.T.; Rand, D.A. Partial State-of-Charge Duty: A Challenge but Not a Show-Stopper for Lead-Acid Batteries! Electrochem. Soc. 2012, 41, 3–16. [Google Scholar] [CrossRef]
- Cooper, A.; Moseley, P. Progress in overcoming the failure modes peculiar to VRLA batteries. J. Power Sources 2003, 200–208. [Google Scholar] [CrossRef]
- Bouabidi, A.; Ayadi, S.; Kossentini, M.; Driss, Z.; Abid, M.S. Cycling Performances and Failure Modes for AGM and Standard Flooded Lead Acid Batteries under Partial State of Charge Mode. J. Energy Eng. 2016, 142, 04015027. [Google Scholar] [CrossRef]
- Misra, S.S.; Mraz, S.L.; Dillon, J.D.; Swanson, D.B. VRLA Battery with AGM-Gel Hybrid for Superior Performance; IEEE: New York City, NY, USA, 2003; pp. 378–382. [Google Scholar]
- Nelson, R. Overview Lead-Acid Batteries. Memb. J. Miner. Met. Mater. Soc. 2001, 53, 28–33. [Google Scholar]
- Doraswamy, S.; Dama, N.; Kurivella, S.M.; Mandava, J.; Veeredhi, V.R. EIS and Electrical Investigations on (1D) Multiwall Carbon Nanotubes as NAM Additive for Automotive Lead-Acid Battery. Curr. Appl. Sci. Technol. 2022, 1–7. [Google Scholar] [CrossRef]
- Vermesan, H.; Hirai, N.; Shiota, M.; Tanaka, T. Effect of barium sulfate and strontium sulfate on charging and discharging of the negative electrode in a lead-acid battery. J. Power Sources 2004, 133, 52–58. [Google Scholar] [CrossRef]
- Franke, M.; Kowal, J. Empirical sulfation model for valve-regulated lead-acid batteries under cycling operation. J. Power Sources 2018, 380, 76–82. [Google Scholar] [CrossRef]
- Parker, M.; McMahon, R. Investigations into the Charge Times of Lead–Acid Cells under Different Partial-State-of-Charge Regimes. Batteries 2024, 10, 201. [Google Scholar] [CrossRef]
- Křivík, P.; Bača, P.; Kazelle, J. Effect of ageing on the impedance of the lead-acid battery. J. Energy Storage 2021, 36, 102382. [Google Scholar] [CrossRef]
- Nelson, R.; Sexton, E.; Olson, J.; Keyser, M.; Pesaran, A. Search for an optimized cyclic charging algorithm for valve-regulated lead–acid batteries. J. Power Sources 2000, 88, 44–52. [Google Scholar] [CrossRef]
- Berndt, D.; Meissner, E.; Rusch, W. Aging effects in valve-regulated lead-acid batteries. In Proceedings of the Intelec 93: 15th International Telecommunications Energy Conference, Paris, France, 27–30 September 1993; Volume 2, pp. 139–145. [Google Scholar] [CrossRef]
- Misra, S.; Williamson, A. Impact of grid corrosion in valve regulated lead-acid battery on standby float service. In Proceedings of the INTELEC 95. 17th International Telecommunications Energy Conference, Hague, The Netherlands, 29 October–1 November 1995; IEEE: New York, NY, USA, 1995; pp. 360–363. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Handbook of Batteries; McGraw-Hill Education: New York, NY, USA, 2002. [Google Scholar]
- Cárdenas, B.; Swinfen-styles, L.; Rouse, J.; Garvey, S.D. Short-, medium-, and long-duration energy storage in a 100% Renewable Electricity Grid: A UK Case Study. Energies 2021, 14, 8524. [Google Scholar] [CrossRef]
- Moseley, P.T. High rate partial-state-of-charge operation of VRLA batteries. J. Power Sources 2004, 127, 27–32. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P. Lead–Carbon Electrode with Inhibitor of Sulfation for Lead-Acid Batteries Operating in the HRPSoC Duty. J. Electrochem. Soc. 2012, 159, A1215–A1225. [Google Scholar] [CrossRef]
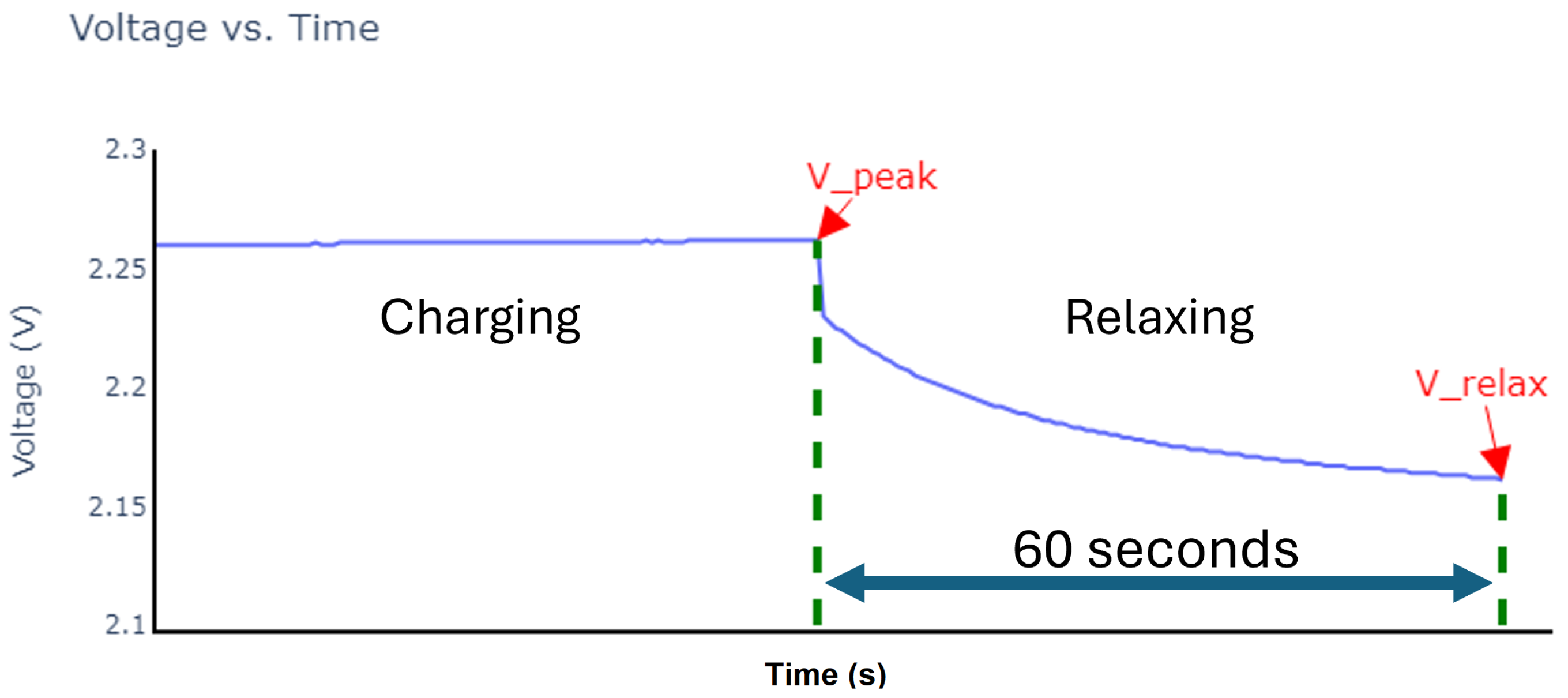

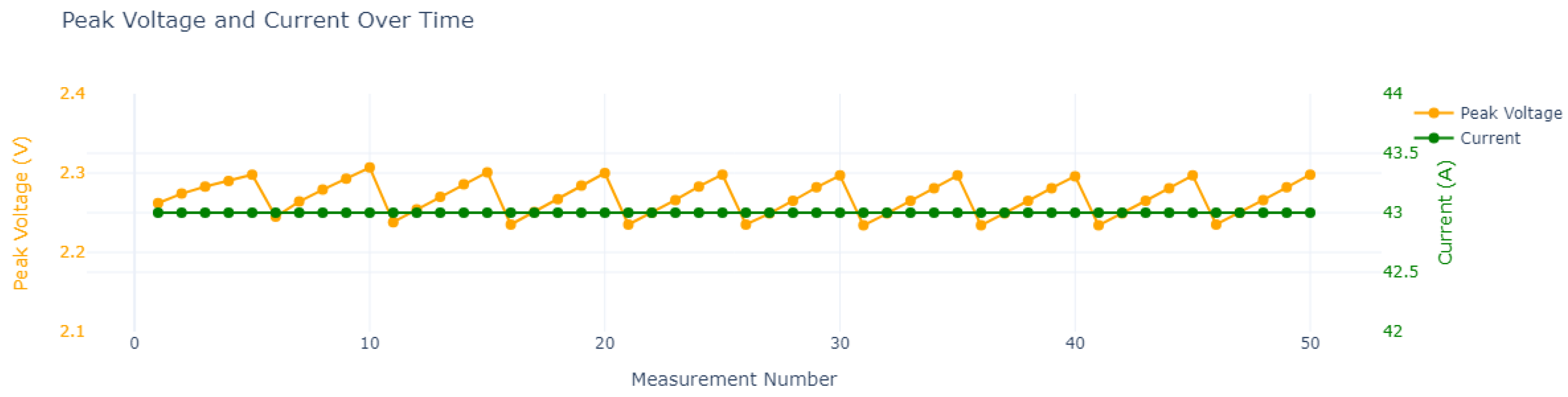
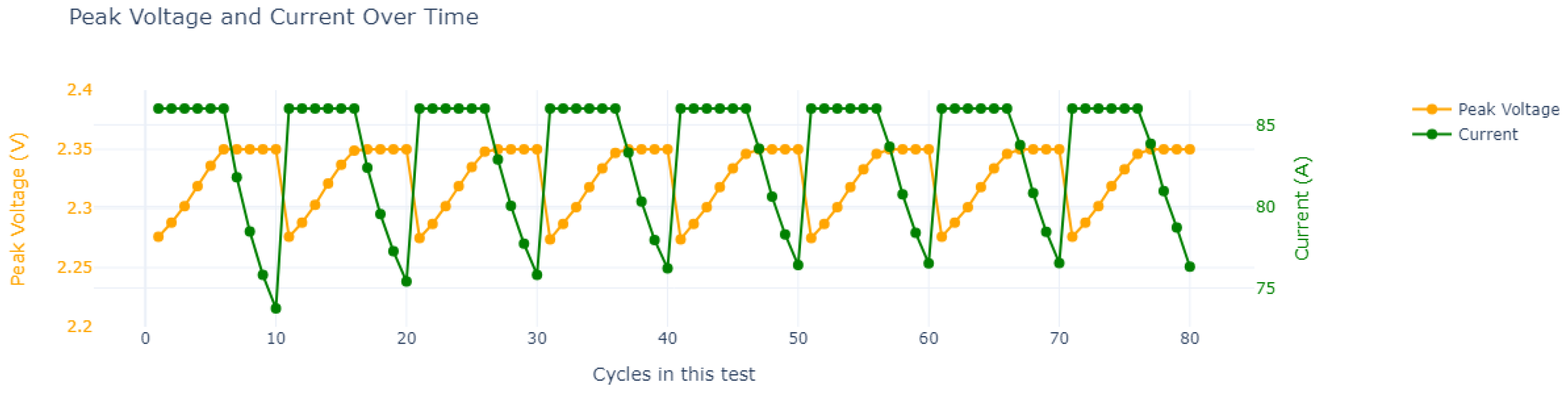
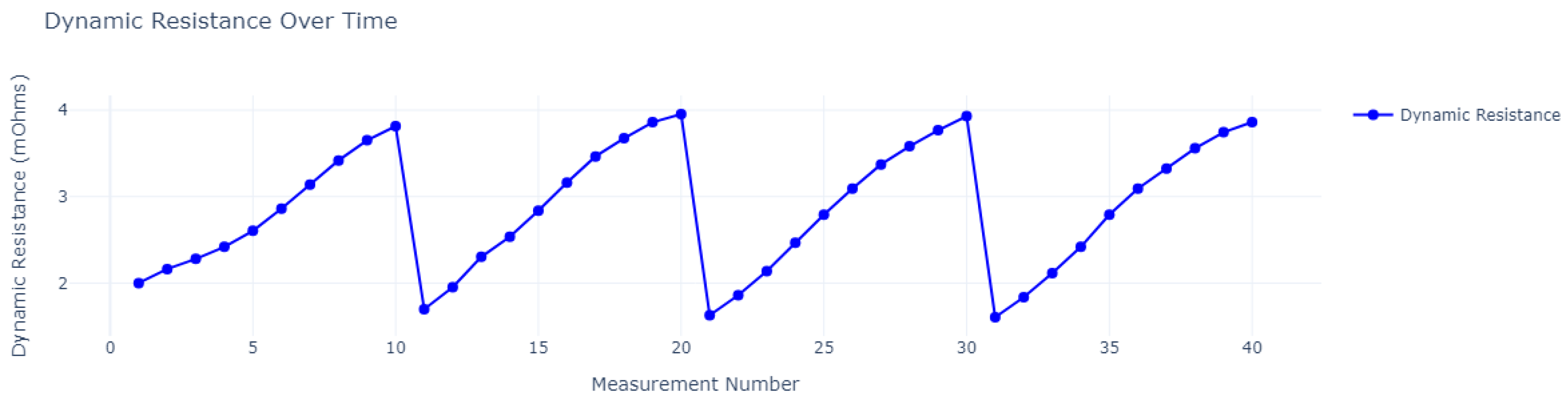
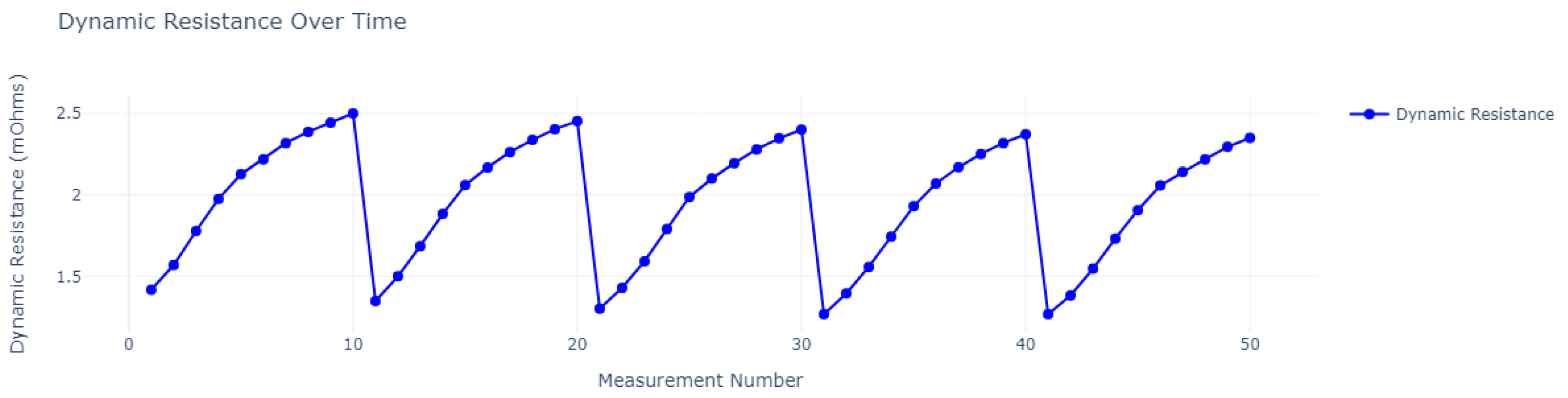

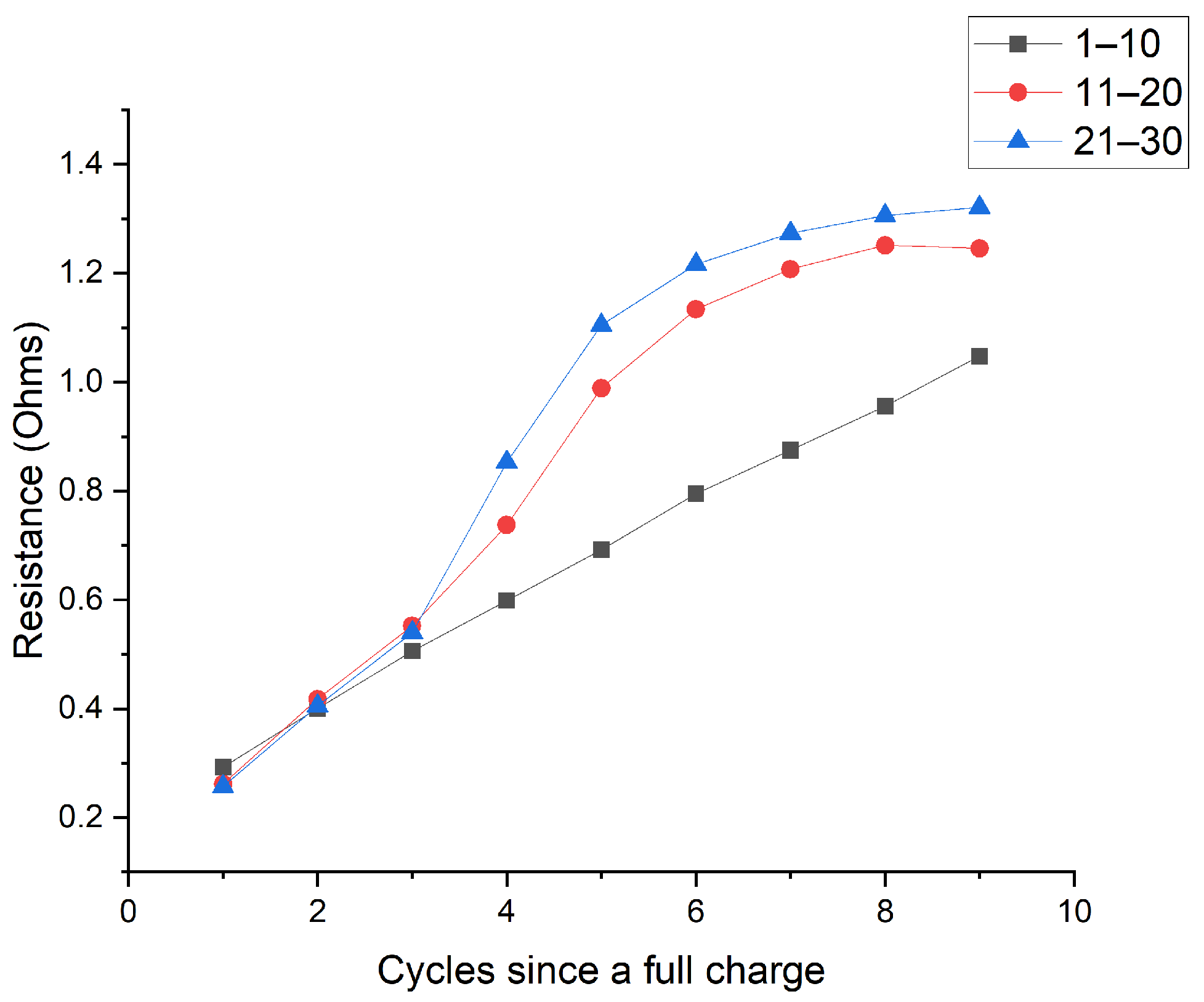
| Cycle | Charge Time in Seconds (h) | ||||
|---|---|---|---|---|---|
| Ten-Cycle Interval | Five-Cycle Interval | ||||
| Cell 1 43A | Cell 1 86 A | Cell 1 High Rate Extended | Cell 2 43A | Cell 2 86 A | |
| - | - | - | 293,277 (81.5) | 127,431 (35.4) | |
| 10 | 374,441 (104.0) | 270,278 (75.1) | 832,971 (231.4) | 199,376 (55.4) | 130,121 (36.1) |
| 15 | - | - | - | 171,362 (47.6) | 128,323 (35.6) |
| 20 | 373,196 (103.7) | 271,742 (75.5) | 237,477 (66.0) | 156,167 (43.4) | 121,266 (33.7) |
| 25 | - | - | - | 146,680 (40.7) | 117,669 (32.7) |
| 30 | 326,372 (90.7) | 245,628 (68.2) | 233,750 (64.9) | 137,751 (38.3) | 118,747 (33.0) |
| 35 | - | - | - | 133,273 (37.0) | 113,492 (31.5) |
| 40 | - | 237,571 (66.0) | 228,304 (63.4) | 127,250 (35.3) | 113,156 (31.4) |
| 45 | - | - | - | 125,554 (34.9) | 113,468 (31.5) |
| 50 | - | 231,245 (64.2) | 224,956 (62.5) | 120,038 (33.3) | 114,289 (31.7) |
| 60 | - | - | 219,660 (61.0) | - | - |
| 70 | - | - | 219,506 (61.0) | - | - |
| 80 | - | - | 186,275 (51.7) | - | - |
| Charge Following Cycle | Charge Time in Seconds (h) |
|---|---|
| 10 | 70,411 (19.6) |
| 20 | 72,001 (20.0) |
| 30 | 68,101 (18.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, M.; McMahon, R. Novel Test Procedure for Assessing Lead–Acid Batteries for Partial-State-of-Charge Duty Using Internal Resistance Charge Acceptance Technique. Batteries 2025, 11, 131. https://doi.org/10.3390/batteries11040131
Parker M, McMahon R. Novel Test Procedure for Assessing Lead–Acid Batteries for Partial-State-of-Charge Duty Using Internal Resistance Charge Acceptance Technique. Batteries. 2025; 11(4):131. https://doi.org/10.3390/batteries11040131
Chicago/Turabian StyleParker, Max, and Richard McMahon. 2025. "Novel Test Procedure for Assessing Lead–Acid Batteries for Partial-State-of-Charge Duty Using Internal Resistance Charge Acceptance Technique" Batteries 11, no. 4: 131. https://doi.org/10.3390/batteries11040131
APA StyleParker, M., & McMahon, R. (2025). Novel Test Procedure for Assessing Lead–Acid Batteries for Partial-State-of-Charge Duty Using Internal Resistance Charge Acceptance Technique. Batteries, 11(4), 131. https://doi.org/10.3390/batteries11040131







