Analysis and Investigation of Diffusion-Induced Stress in Lithium-Ion Particle Through Elastic-Viscoplastic Model of Binder
Abstract
1. Introduction
2. Finite Element Modeling
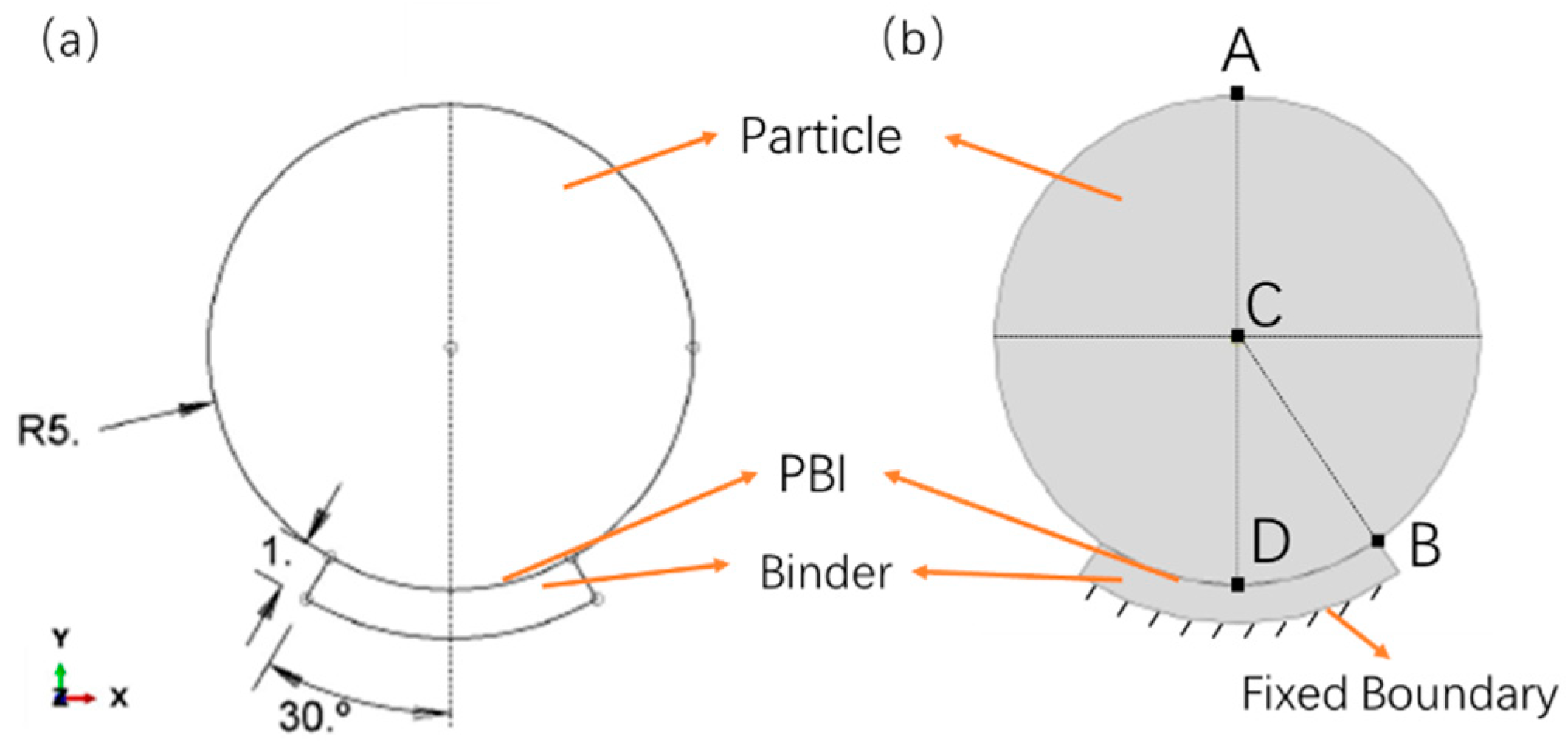
2.1. Geometric Model Diagram
2.2. Electrochemical-Mechanical Model
2.3. Particle-Binder Interface (PBI) Model
2.4. PVDF Binder Model
2.5. Numerical Simulation
3. Results and Discussion
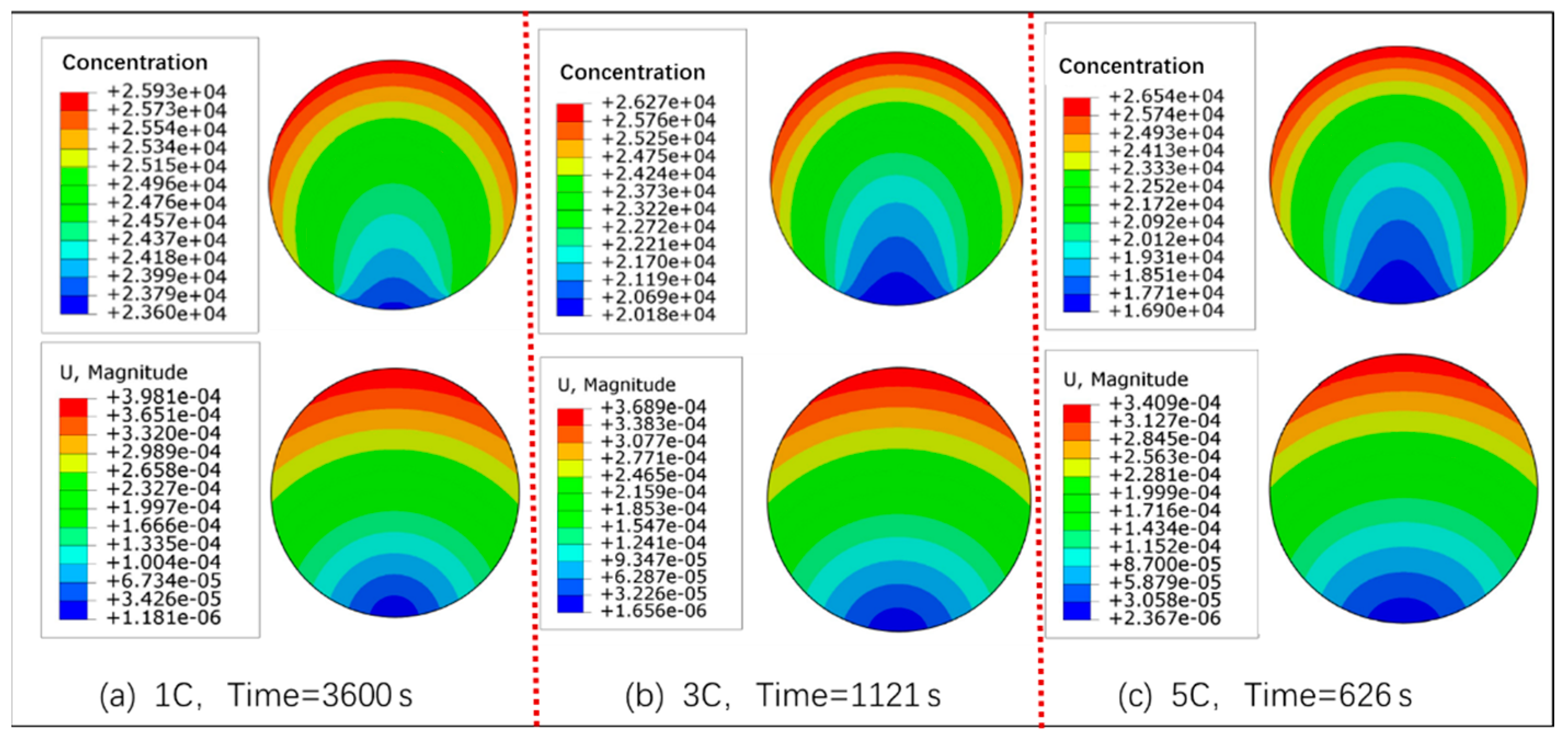
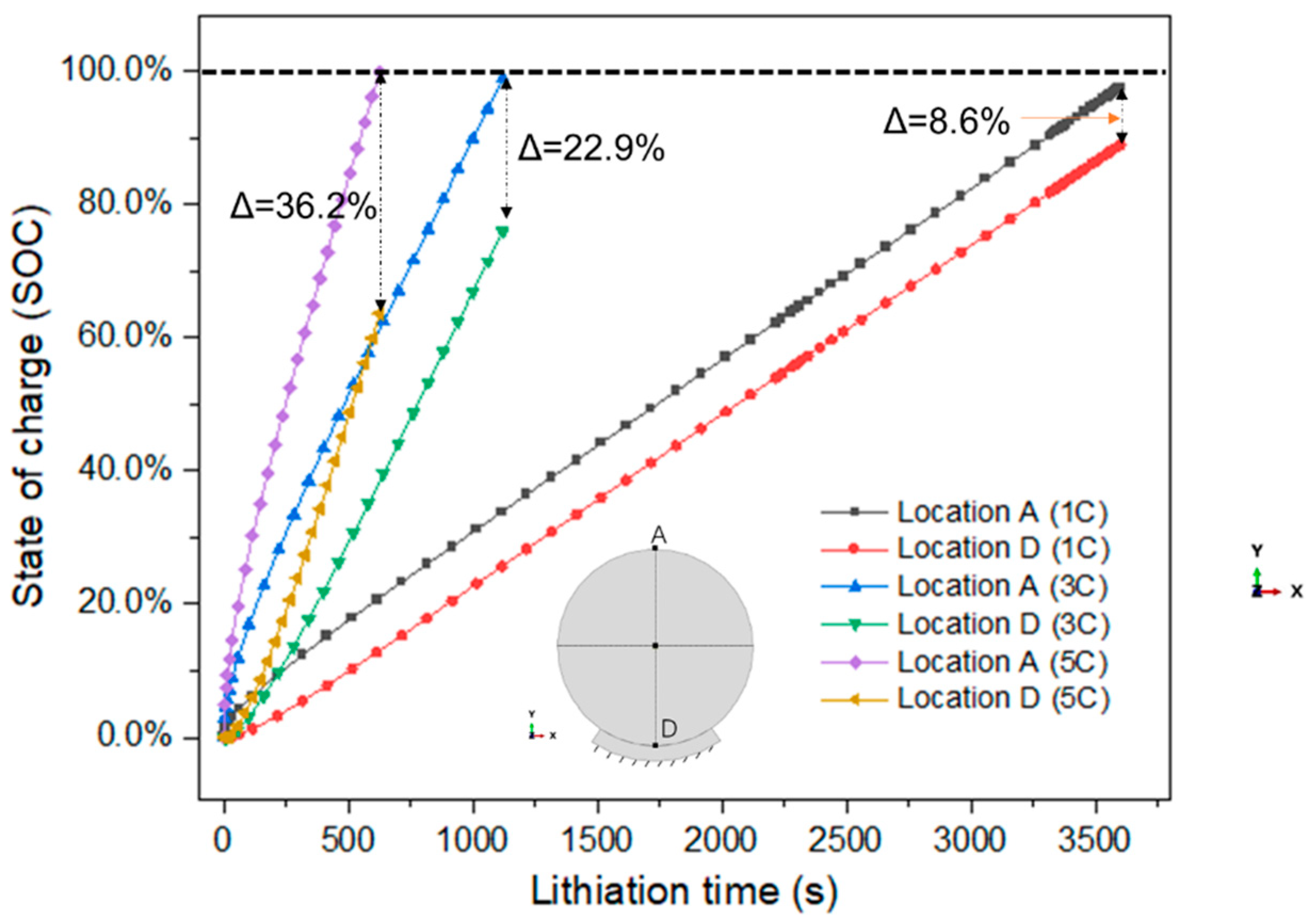
3.1. Concentration, Volume, and Stress of Active Particle
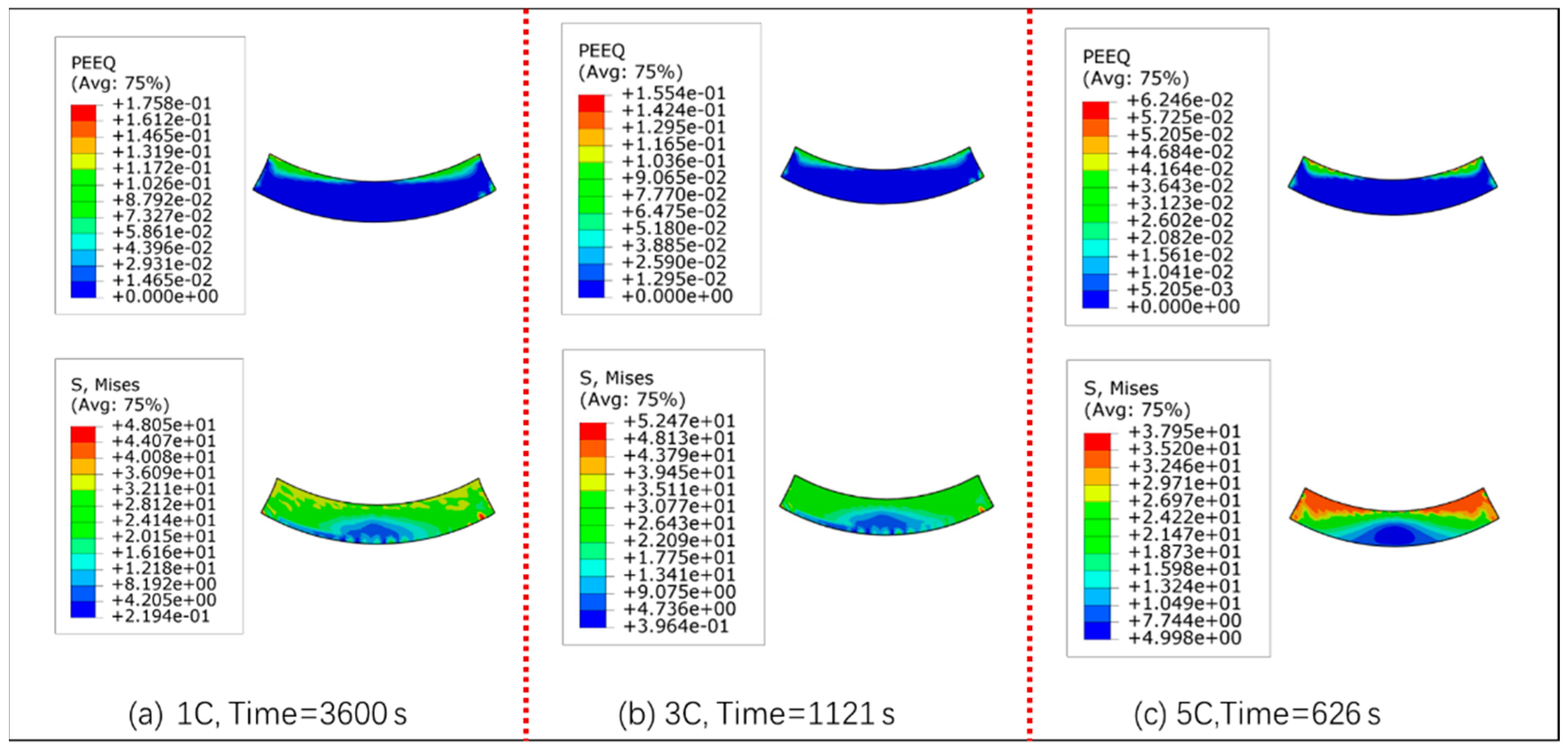
3.2. Stress and Strain Analysis of Binder
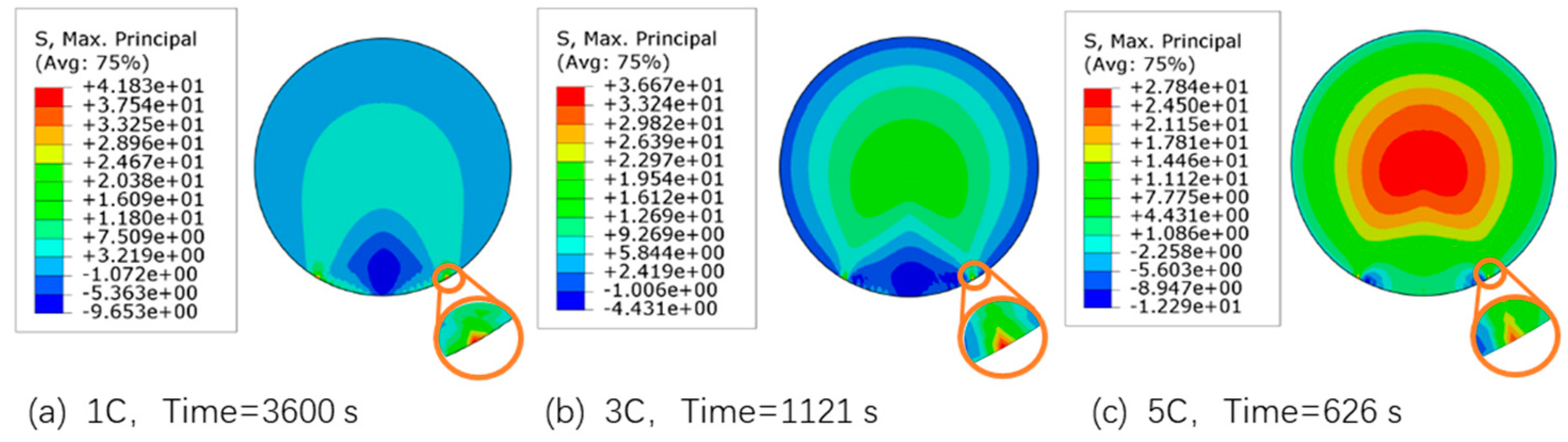
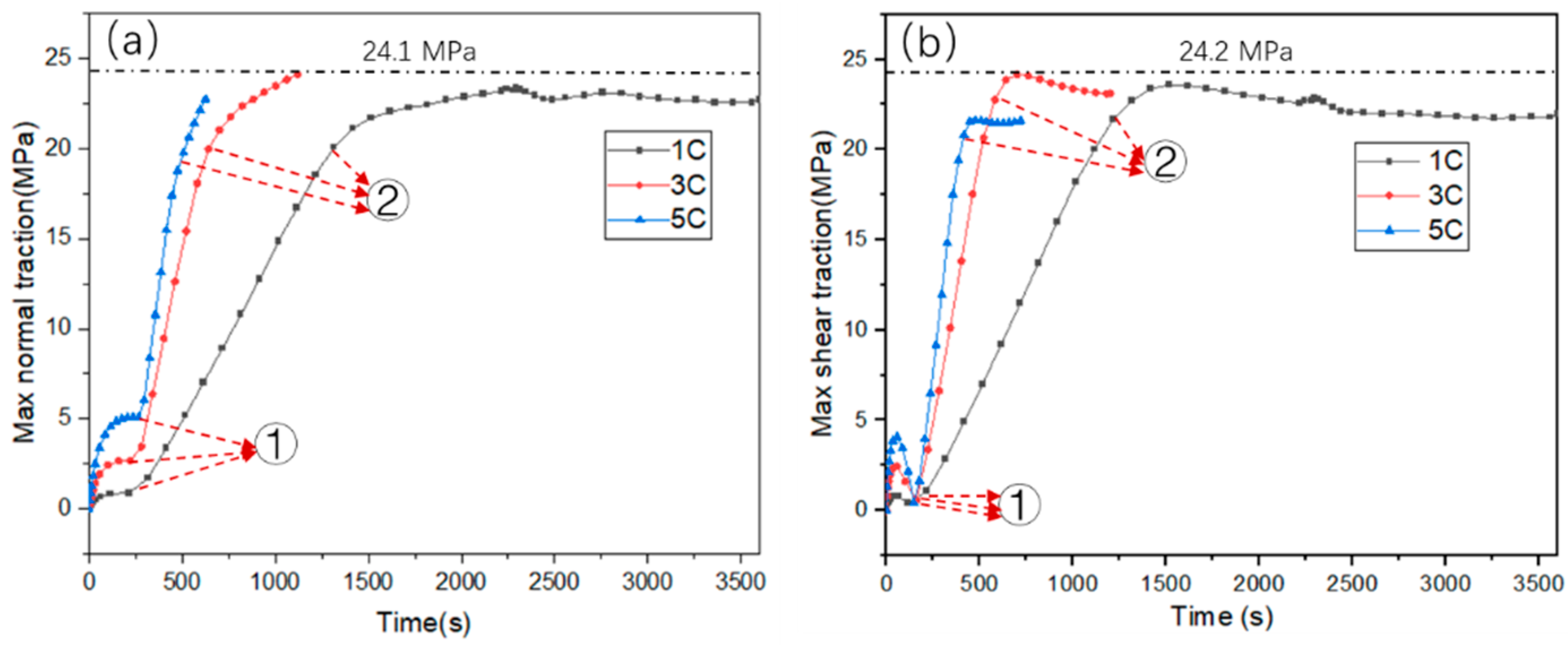
3.3. PBI Interface Stress Analysis
4. Conclusions
- (1)
- As the charge rate increases, the concentration gradient of lithium-ion in particles increases, but the volume expansion ratio decreases. At the 1 C, 3 C, and 5 C charge rates, the volume expansion ratios of the particle are 8.1%, 7.5%, and 7.0%, respectively.
- (2)
- Under 1 C and 3 C charge rates, the maximum principal stress occurs at the edge of the particle and binder interface; thus, the particle is most likely to crack from the edge of the PBI. While under a higher rate of 5 C, the maximum principal stress occurs inside the particle; this may cause particle fracture from the center area.
- (3)
- When the binder material PVDF adopts an elastic-viscoplastic model, the maximum normal stress and shear stress are at the edge of PBI. Moreover, simulation analysis results show that there is no delamination damage at the particle and binder interface due to the plastic deformation behavior of the PVDF, which reduced the stress concentration at the PBI.
- (4)
- The plastic deformation of PVDF decreases with the increase of charge rate. However, the plastic deformation of PVDF may lead to the fatigue failure of the binder under repeated charging and discharging processes.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar]
- Liu, J.; Zhou, L.; Zhang, Y.; Wang, J.; Wang, Z. Aging behavior and mechanisms of lithium-ion battery under multi-aging path. J. Clean. Prod. 2023, 423, 138678. [Google Scholar] [CrossRef]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Liu, J.; Yadav, S.; Salman, M.; Chavan, S.; Kim, S.C. Review of thermal coupled battery models and parameter identification for lithium-ion battery heat generation in EV battery thermal management system. Int. J. Heat Mass Transf. 2024, 218, 124748. [Google Scholar] [CrossRef]
- Harris, S.J.; Deshpande, R.D.; Qi, R.; Dutta, R.; Cheng, R.T. Mesopores inside electrode particles can change the Li-ion transport mechanism and diffusion-induced stress. J. Mater. Res. 2010, 25, 1433–1440. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Verbrugge, M.W. Evolution of stress within a spherical insertion electrode particle under potentiostatic and galvanostatic operation. J. Power Sources 2009, 190, 453–460. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Wolfenstine, J. Critical grain size for microcracking during lithium insertion. J. Power Sources 1999, 79, 111–113. [Google Scholar] [CrossRef]
- Aifantis, K.E.; Deinpsey, J.P. Stable crack growth in nanostructured Li-batteries. J. Power Sources 2005, 143, 203–211. [Google Scholar] [CrossRef]
- Clerici, D. Diffusion-induced stress amplification in phase-transition materials for electrodes of lithium-ion batteries. Int. J. Mech. Sci. 2024, 281, 109541. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Cheng, Y.T. Stress and Strain-Energy Distributions within Diffusion-Controlled Insertion-Electrode Particles Subjected to Periodic Potential Excitations. J. Electrochem. Soc. 2009, 156, A927–A937. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Chen, H.; Luan, W. The diffusion induced stress and cracking behaviour of primary particle for Li-ion battery electrode. Int. J. Mech. Sci. 2020, 178, 105608. [Google Scholar]
- Korsunsky, A.M.; Sui, T.; Song, B. Explicit formulae for the internal stress in spherical particles of active material within lithium ion battery cathodes during charging and discharging. Mater. Des. 2015, 69, 247–252. [Google Scholar]
- Woodford, W.H.; Chiang, Y.M.; Carter, W.C. “Electrochemical Shock” of Intercalation Electrodes: A Fracture Mechanics Analysis. J. Electrochem. Soc. 2010, 157, A1052–A1059. [Google Scholar]
- Klinsmann, M.; Rosato, D.; Kamlah, M.; McMeeking, R.M. Modeling crack growth during Li insertion in storage particles using a fracture phase field approach. J. Mech. Phys. Solids 2016, 92, 313–344. [Google Scholar] [CrossRef]
- Klinsmann, M.; Rosato, D.; Kamlah, M.; Mcmeeking, R.M. Modeling crack growth during Li extraction and insertion within the second half cycle. J. Power Sources 2016, 331, 32–42. [Google Scholar]
- Jiang, S.; Zhou, J.; Yang, H.; Tan, S.; Wu, Y.; Wang, C. Ionic liquid fabricated PVDF binder for cathode toward stable and high-rate lithium-ion batteries. J. Power Sources 2025, 633, 236439. [Google Scholar]
- Chen, S.; Guo, J.; Zang, H.; Liu, C.; Yu, N.; Geng, B. Oxygen vacancy-enriched TiO2 nanosheets filled PVDF electrolyte for semi-solid-state batteries: Synergistic effects of conformational transition and defect sites. J. Alloys Compd. 2025, 1020, 179357. [Google Scholar]
- Wang, H.; Nadimpalli, S.P.V.; Shenoy, V.B. Inelastic shape changes of silicon particles and stress evolution at binder/particle interface in a composite electrode during lithiation/delithiation cycling. Extrem. Mech. Lett. 2016, 9, 430–438. [Google Scholar]
- Xu, R.; Yang, Y.; Yin, F.; Liu, P.; Cloetens, P.; Liu, Y.; Lin, F.; Zhao, K. Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling. J. Mech. Phys. Solids 2019, 129, 160–183. [Google Scholar]
- Lee, S.; Yang, J.; Lu, W. Debonding at the interface between active particles and PVDF binder in Li-ion batteries. Extrem. Mech. Lett. 2016, 6, 37–44. [Google Scholar]
- Iqbal, N.; Ali, Y.; Lee, S. Mechanical degradation analysis of a single electrode particle with multiple binder connections: A comparative study. Int. J. Mech. Sci. 2020, 188, 105943. [Google Scholar]
- Wu, Y.; Guo, Z.-S. Modeling Li-ion concentration distribution and stress of porous electrode particles considering binder and direct particle contact. J. Energy Storage 2021, 44, 103315. [Google Scholar]
- Trembacki, B.L.; Noble, D.R.; Brunini, V.E.; Ferraro, M.E.; Roberts, S.A. Mesoscale Effective Property Simulations Incorporating Conductive Binder. J. Electrochem. Soc. 2017, 164, E3613. [Google Scholar]
- Huang, P.; Gao, L.T.; Guo, Z.-S. Elastoplastic model for chemo-mechanical behavior of porous electrodes using image-based microstructure. Int. J. Solids Struct. 2022, 254, 111903. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Wang, M.; Huang, X. A Microstructural Resolved Model for the Stress Analysis of Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A803. [Google Scholar] [CrossRef]
- Santimetaneedol, A.; Tripuraneni, R.; Chester, S.A.; Nadimpalli, S.P.V. Time-dependent deformation behavior of polyvinylidene fluoride binder: Implications on the mechanics of composite electrodes. J. Power Sources 2016, 332, 118–128. [Google Scholar]
- Rahani, E.K.; Shenoy, V.B. Role of Plastic Deformation of Binder on Stress Evolution during Charging and Discharging in Lithium-Ion Battery Negative Electrodes. J. Electrochem. Soc. 2013, 160, A1153. [Google Scholar]
- Hund, J.; Granum, H.M.; Olufsen, S.N.; Holmström, P.H.; Johnsen, J.; Clausen, A.H. Impact of stress triaxiality, strain rate, and temperature on the mechanical response and morphology of PVDF. Polym. Test. 2022, 114, 107717. [Google Scholar] [CrossRef]
- André-Castagnet, S.; Tencé-Girault, S. Relationships Between Mechanical Tensile Behavior and Micro-mechanisms in Poly(Vinylidene Fluoride) at High Temperatures: Influence of the Molecular Weight Distribution. J. Macromol. Sci. Part B 2002, 41, 957–976. [Google Scholar]
- Sethuraman, V.A.; Nguyen, A.; Chon, M.J.; Nadimpalli, S.P.V.; Wang, H.; Abraham, D.P.; Bower, A.F.; Shenoy, V.B.; Guduru, P.R. Stress Evolution in Composite Silicon Electrodes during Lithiation/Delithiation. J. Electrochem. Soc. 2013, 160, A739–A746. [Google Scholar] [CrossRef]
- Chaurasiya, S.P.; Ghosh, R. A new mathematical model of compressive stress-strain behaviour of low viscosity and high viscosity bone cement with different strain rates. Med. Eng. Phys. 2023, 117, 104001. [Google Scholar]
- Zhang, X.; Shyy, W.; Sastry, A.M. Numerical Simulation of Intercalation-Induced Stress in Li-Ion Battery Electrode Particles. J. Electrochem. Soc. 2007, 154, A910–A916. [Google Scholar] [CrossRef]
- Iqbal, N.; Ali, Y.; Haq, I.U.; Lee, S. Progressive interface debonding in composite electrodes of Li-ion batteries via mixed-mode cohesive zone model: Effects of binder characteristics. Compos. Struct. 2020, 259, 113173. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Luan, W. Shakedown, ratcheting and fatigue analysis of cathode coating in lithium-ion battery under steady charging-discharging process. J. Mech. Phys. Solids 2021, 150, 104366. [Google Scholar]
- Kapidžić, Z.; Ansell, H.; Schön, J.; Simonsson, K. Quasi-static bearing failure of CFRP composite in biaxially loaded bolted joints. Compos. Struct. 2015, 125, 60–71. [Google Scholar] [CrossRef]
- Benzeggagh, M.L.; Kenane, M. Measurement of Mixed-Mode Delamination Fracture Toughness of Unidirectional Glass/Epoxy Composites with Mixed-Mode Bending Apparatus. Compos. Sci. Technol. 1996, 56, 439–449. [Google Scholar]
- Díaz, A.; Alegre, J.M.; Cuesta, I.I. Coupled hydrogen diffusion simulation using a heat transfer analogy. Int. J. Mech. Sci. 2016, 115, 360–369. [Google Scholar]
- Iqbal, N.; Lee, S. Mechanical Failure Analysis of Graphite Anode Particles with PVDF Binders in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1961–A1970. [Google Scholar]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Iqbal, N.; Ali, Y.; Lee, S. Analysis of mechanical failure at the interface between graphite particles and polyvinylidene fluoride binder in lithium-ion batteries. J. Power Sources 2020, 457, 228019. [Google Scholar] [CrossRef]
- Iqbal, N.; Lee, S. Stress-regulated pulse charging protocols via coupled electrochemical-mechanical model for the mechanical stability of electrode materials in lithium-ion batteries. J. Power Sources 2022, 536, 231376. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Yang, J.; Lu, W. Molecular Dynamics Simulations of the Traction-Separation Response at the Interface between PVDF Binder and Graphite in the Electrode of Li-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1218–A1223. [Google Scholar] [CrossRef]
- Zhao, K.; Pharr, M.; Vlassak, J.J.; Suo, Z. Fracture of electrodes in lithium-ion batteries caused by fast charging. J. Appl. Phys. 2010, 108, 473. [Google Scholar] [CrossRef]





| Parameter | Units | Values | References |
|---|---|---|---|
| Faraday’s constant (F) | C/mol | 96,487 | |
| Absolute temperature (T) | K | 300 | |
| Specific Capacity (a) | mAhg−1 | 372 | [39] |
| Partial molar volume of lithium(Ω) | m3/mol | 3.645 × 10−6 | [39] |
| Diffusion coefficient of lithium (D) | m2s−1 | 1 × 10−15 | [12] |
| Gas constant (R) | J/(mol × K) | 8.314 | |
| Charge rate (Crate) | h−1 | 1, 3, 5 | |
| Young’s modulus of graphite (E) | GPa | 15 | [40,41] |
| Poisson’s ratio of graphite (υ) | 0.3 | [40,41] | |
| Maximum lithium concentration (cmax) | mol/m3 | 26,580 | [39] |
| Nominal normal traction () | MPa | 300 | [42,43] |
| Nominal shear traction () | MPa | 50 | [42,43] |
| Boltzmann’s constant (kB) | J/K | 1.38 × 10−23 | |
| Activation volume (V) | nm3 | 2.69 | [29] |
| Activation enthalpy (ΔH) | kJ/mol | 248.4 | [29] |
| Reference strain rate () | s−1 | 9.56 × 1029 | [29] |
| Charge Rate | Unit Volume Value (mm3) | Expansivities |
|---|---|---|
| 1 C | 8.49 × 10−5 | 8.10% |
| 3 C | 8.44 × 10−5 | 7.50% |
| 5 C | 8.40 × 10−5 | 7.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, Y. Analysis and Investigation of Diffusion-Induced Stress in Lithium-Ion Particle Through Elastic-Viscoplastic Model of Binder. Batteries 2025, 11, 132. https://doi.org/10.3390/batteries11040132
Cao J, Zhang Y. Analysis and Investigation of Diffusion-Induced Stress in Lithium-Ion Particle Through Elastic-Viscoplastic Model of Binder. Batteries. 2025; 11(4):132. https://doi.org/10.3390/batteries11040132
Chicago/Turabian StyleCao, Juanhua, and Yafang Zhang. 2025. "Analysis and Investigation of Diffusion-Induced Stress in Lithium-Ion Particle Through Elastic-Viscoplastic Model of Binder" Batteries 11, no. 4: 132. https://doi.org/10.3390/batteries11040132
APA StyleCao, J., & Zhang, Y. (2025). Analysis and Investigation of Diffusion-Induced Stress in Lithium-Ion Particle Through Elastic-Viscoplastic Model of Binder. Batteries, 11(4), 132. https://doi.org/10.3390/batteries11040132





