A Patent Landscape Analysis on the Recycling of Lithium-Ion Battery Positive Electrode Materials: Trends, Technologies, and the Future
Abstract
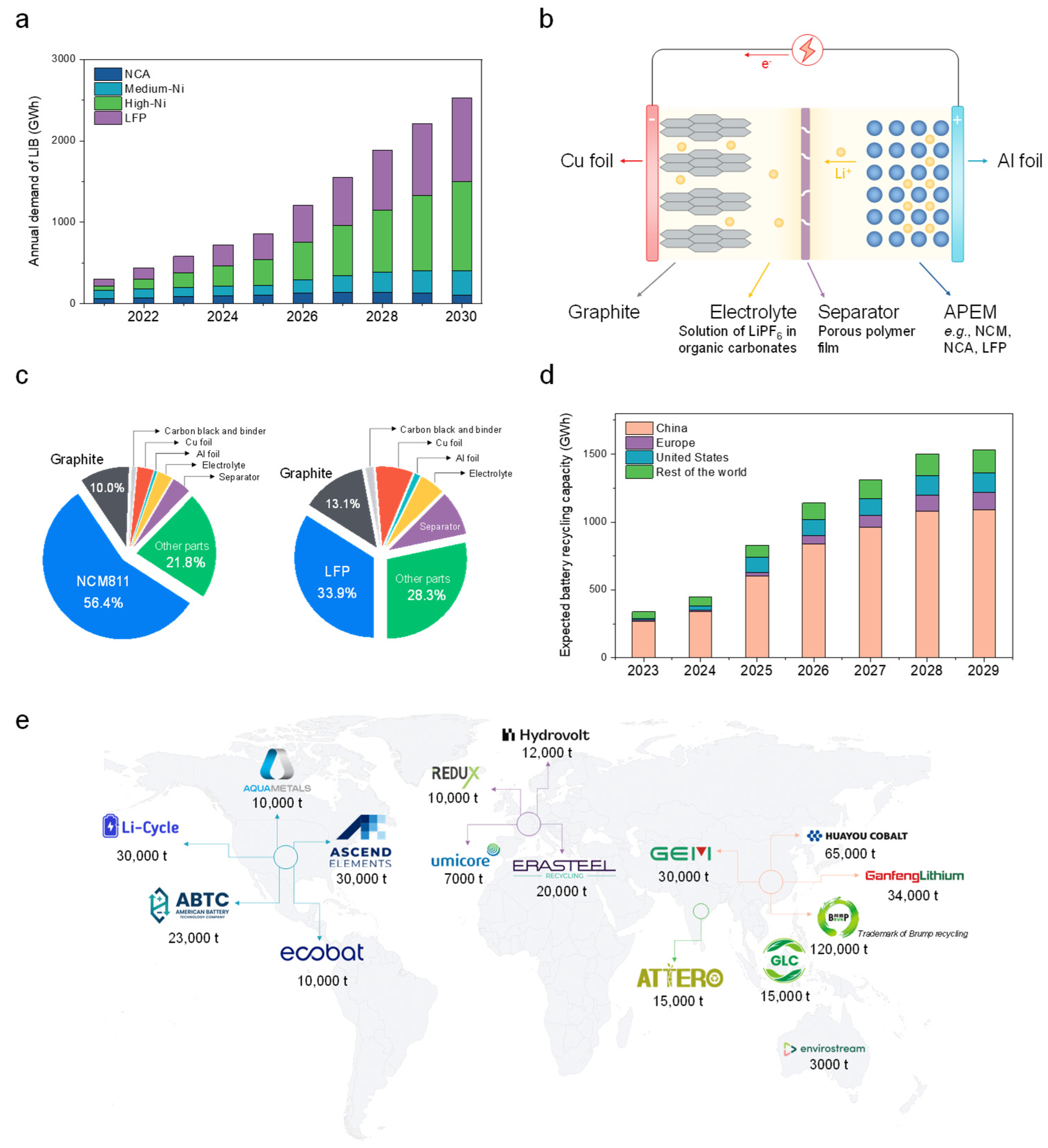
1. Introduction
2. Materials and Methods
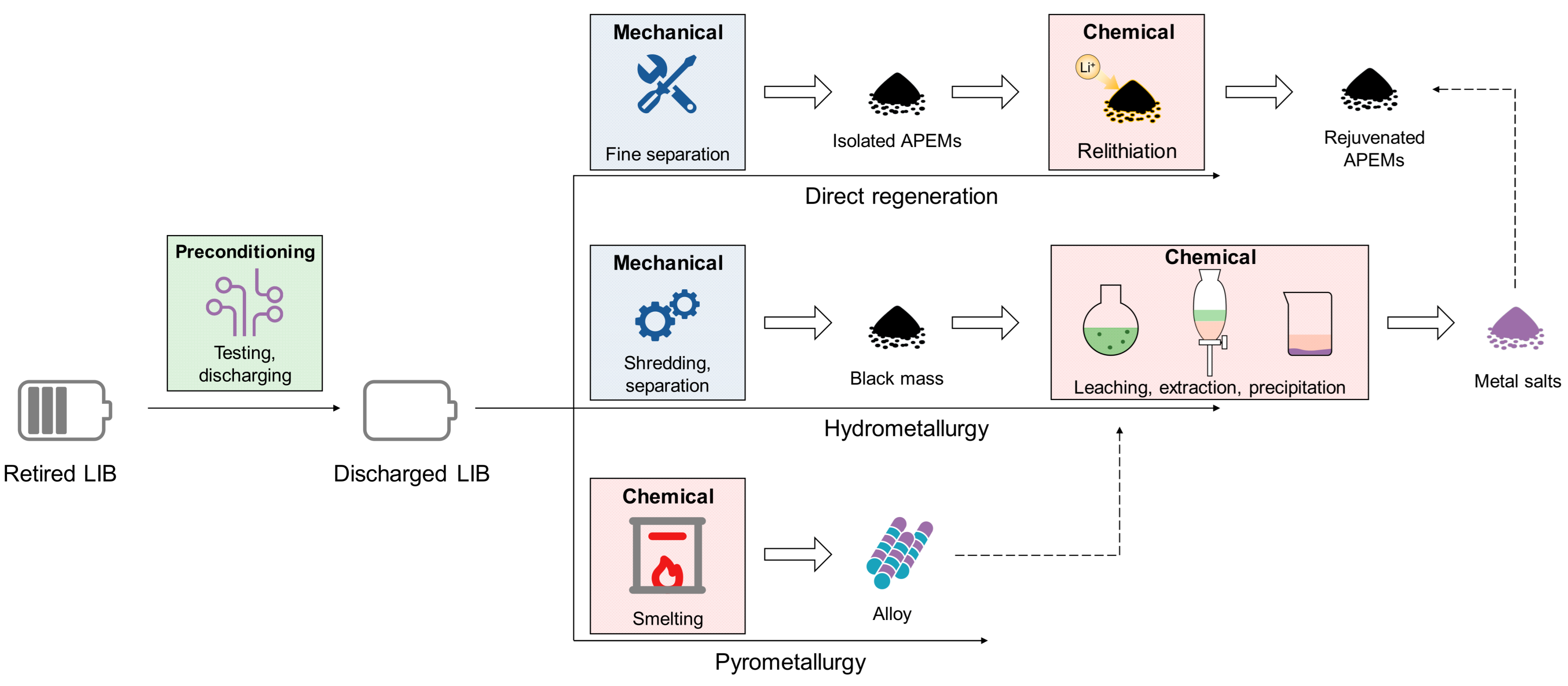
3. Overview of LIB Recycling Technologies and Industry
- Preconditioning stage: This initial phase involves steps that do not alter the internal structure of cells. It includes measures such as state-of-health testing, battery sorting based on the APEM chemistry, and discharging to ensure safety. A deep discharging also ensures the return of Li ions to APEM lattices for later recovery.
- Mechanical stage: In this phase, the discharged cells are subject to mechanical treatments to separate APEMs from the spent LIBs. Depending on the battery type, following the chemical route, and other parameters, the requirements of the mechanical process are different [16,25,26,27,28]. In the case of pyrometallurgy, the mechanical stage can be largely simplified or even skipped, while for hydrometallurgical leaching, shredding and separation are necessary to obtain “black mass”, a powder mixture of APEMs, graphite, and other electrode components. If direct regeneration is targeted, more sophisticated separation is required to isolate APEMs from other electrode components like binder and conductive carbon.
- Chemical processing stage: After the mechanical process, the dismantled cells or crushed materials are subjected to the core chemical process of pyrometallurgy, hydrometallurgy, or direct regeneration. Pyrometallurgy uses high temperatures to smelt and to recover valuable metals [29]. As mentioned above, pyrometallurgy can smelt entire battery packs without mechanical pretreatment, yielding alloys of cobalt, nickel, and copper, while lithium and manganese typically remain in unrecovered slag. The recent EU Battery Regulation, however, mandates 50% lithium recovery from spent LIBs by 2027 and 80% by 2031, necessitating effective lithium and manganese extraction from slag [30]. In contrast, hydrometallurgy starts by dissolving the black mass through leaching, followed by purification and selective precipitation of the dissolved metals using specific reagents [9,31,32,33]. Direct regeneration aims to preserve the structural integrity of the APEM, allowing it to be reused without breaking it down into individual elements [34,35,36]. Note that these chemical processes are frequently combined. For example, the metal alloy produced through pyrometallurgy needs to be further processed using hydrometallurgical techniques to produce individual battery-grade salts.
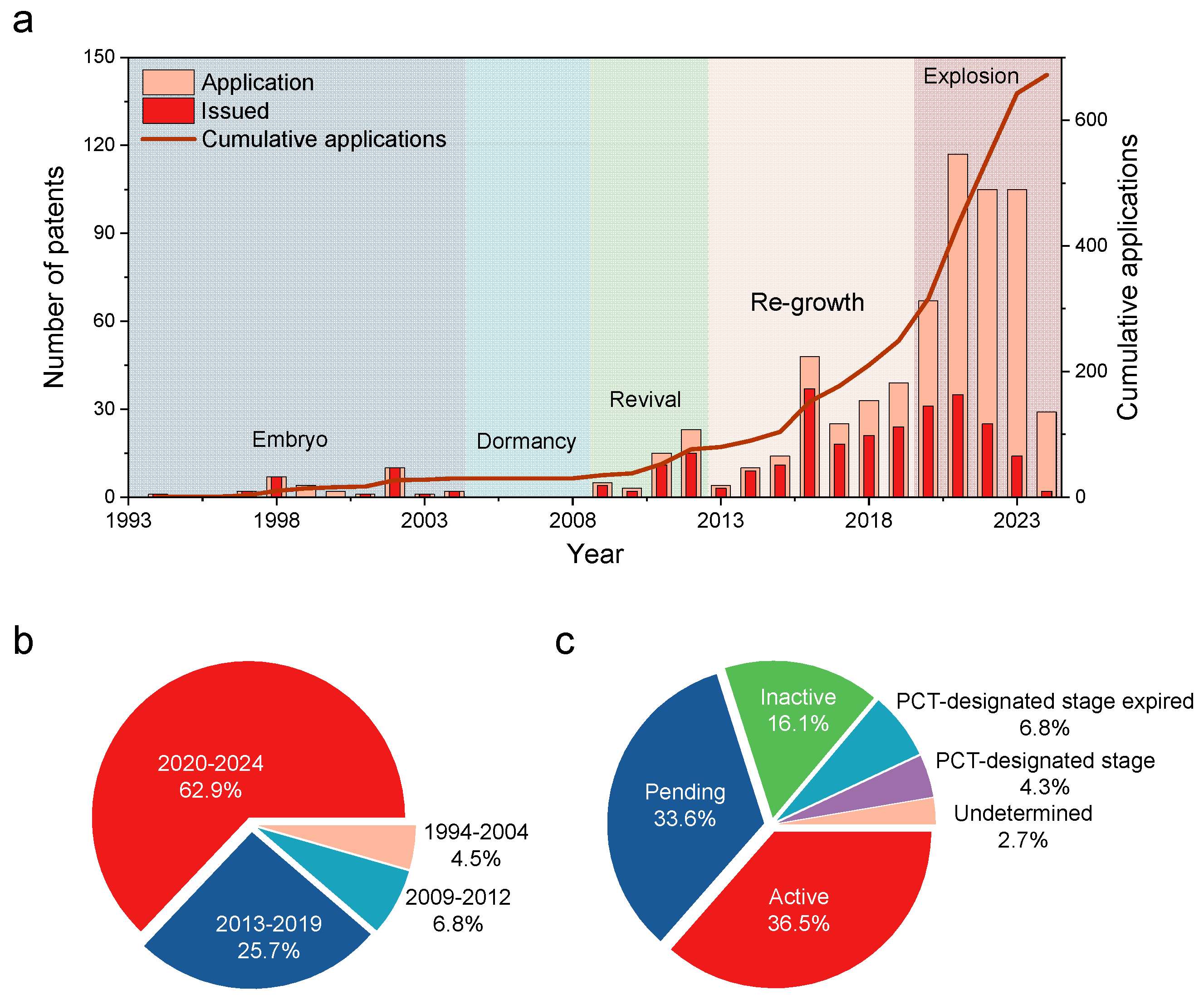
4. Temporal Evolution of Patenting Activities
5. Top Countries and Key Players in the Patent Filing
6. Important Patents (Patents with the Highest Forward Citations and the Largest Family Sizes)
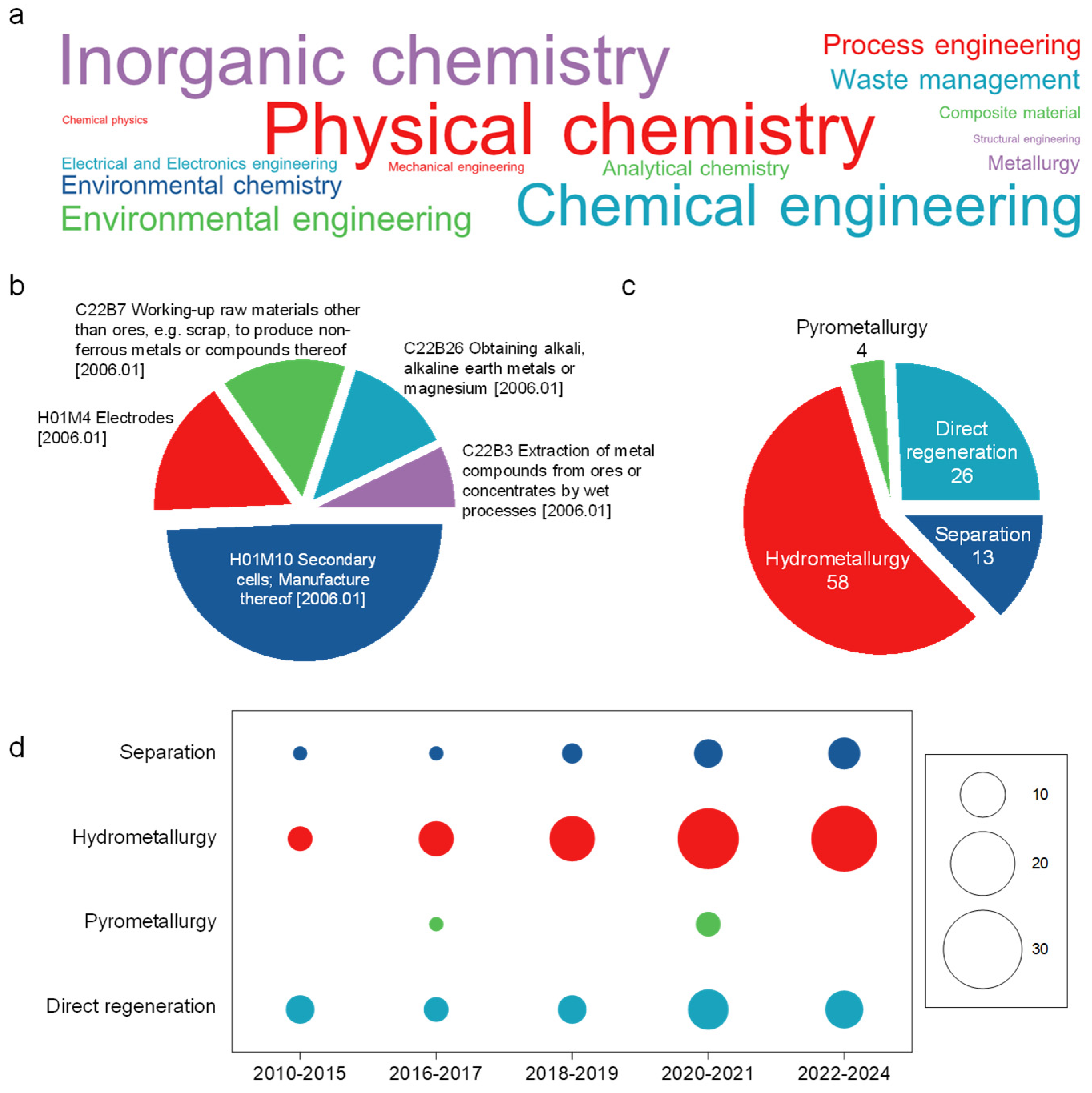
7. Technical Analysis of the Granted Patents
8. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion batteries |
| APEM | Active positive electrode materials |
| LFP | LiFePO4 |
| NCM | Li[NixCoyMn1−x−y]O2 |
| NCA | Li[NixCoyAl1−x−y]O2 |
| TAC | Topic/Abstract/Content |
| INPADOC | International Patent Documentation |
| EPO | European Patent Office |
| PCT | Patent Cooperation Treaty |
| LLC | Limited liability company |
| WIPO | World Intellectual Property Organization |
| PVDF | Polyvinylidene fluoride |
References
- Jannesar Niri, A.; Poelzer, G.A.; Zhang, S.E.; Rosenkranz, J.; Pettersson, M.; Ghorbani, Y. Sustainability challenges throughout the electric vehicle battery value chain. Renew. Sustain. Energy Rev. 2024, 191, 114176. [Google Scholar] [CrossRef]
- Degen, F.; Winter, M.; Bendig, D.; Tübke, J. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 2023, 8, 1284–1295. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, A.; Martens, I.; Vostrov, N.; Sun, Y.; Richard, M.-I.; Schülli, T.U.; Wang, L. High-Voltage Spinel Cathode Materials: Navigating the Structural Evolution for Lithium-Ion Batteries. Adv. Mater. 2024, 36, 2403482. [Google Scholar] [CrossRef]
- Knehr, K.W.; Kubal, J.J.; Nelson, P.A.; Ahmed, S. Battery Performance and Cost Modeling for Electric-Drive Vehicles (A Manual for BatPaC v5.0); Argonne National Lab. (ANL): Argonne, IL, USA, 2022. [Google Scholar]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2021, 42, 57–72. [Google Scholar] [CrossRef]
- Agency, I.E. Electric Vehicle Outlook 2024; Bloomberg: New York, NY, USA, 2024. [Google Scholar]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, K.; Lu, B.; Han, J.; Zhou, J. Hydrometallurgical Processes on Recycling of Spent Lithium-lon Battery Cathode: Advances and Applications in Sustainable Technologies. Acta Phys.-Chim. Sin. 2024, 40, 2309028. [Google Scholar] [CrossRef]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Ge, Z.; Zhang, S.; Song, B.; Tian, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Advances and perspectives towards spent LiFePO4 battery recycling. J. Clean. Prod. 2024, 434, 140077. [Google Scholar] [CrossRef]
- Wang, Y.; Goikolea, E.; de Larramendi, I.R.; Lanceros-Méndez, S.; Zhang, Q. Recycling methods for different cathode chemistries—A critical review. J. Energy Storage 2022, 56, 106053. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Azimi, G.; Chan, K.H. A review of contemporary and emerging recycling methods for lithium-ion batteries with a focus on NMC cathodes. Resour. Conserv. Recycl. 2024, 209, 107825. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2021, 406, 124332. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Silvester, D.S.; Banks, C.E.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Direct regeneration of cathode materials in spent lithium-ion batteries toward closed-loop recycling and sustainability. J. Power Sources 2024, 589, 233728. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Zhuang, Z.; Liang, Z.; Jia, K.; Ji, G.; Zhou, G.; Cheng, H.-M. Toward Direct Regeneration of Spent Lithium-Ion Batteries: A Next-Generation Recycling Method. Chem. Rev. 2024, 124, 2839–2887. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, X.; Fang, R.; Lu, C.; Wang, K.; Gan, Y.; He, X.; Zhang, J.; Huang, H.; Zhang, W.; et al. Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials. Carbon Neutralization 2023, 2, 169–185. [Google Scholar] [CrossRef]
- Tong, Z.; Zhu, X. A patent landscape analysis on the high-voltage spinel LiNi0.5Mn1.5O4 for next-generation lithium-ion batteries. Next Energy 2024, 5, 100158. [Google Scholar] [CrossRef]
- Mejia, C.; Kajikawa, Y. Emerging topics in energy storage based on a large-scale analysis of academic articles and patents. Appl. Energy 2020, 263, 114625. [Google Scholar] [CrossRef]
- Yang, C.; Mu, X.-Y. Mapping the trends and prospects of battery cathode materials based on patent landscape. Front. Energy 2023, 17, 822–832. [Google Scholar] [CrossRef]
- Aaldering, L.J.; Song, C.H. Tracing the technological development trajectory in post-lithium-ion battery technologies: A patent-based approach. J. Clean. Prod. 2019, 241, 118343. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Wilke, C.; Kaas, A.; Peuker, U.A. Influence of the Cell Type on the Physical Processes of the Mechanical Recycling of Automotive Lithium-Ion Batteries. Metals 2023, 13, 1901. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184. [Google Scholar] [CrossRef]
- Yun, L.; Linh, D.; Shui, L.; Peng, X.; Garg, A.; Le, M.L.P.; Asghari, S.; Sandoval, J. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles. Resour. Conserv. Recycl. 2018, 136, 198–208. [Google Scholar] [CrossRef]
- Kaas, A.; Wilke, C.; Vanderbruggen, A.; Peuker, U.A. Influence of different discharge levels on the mechanical recycling efficiency of lithium-ion batteries. Waste Manag. 2023, 172, 1–10. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Gantz, P.C.; Panjiyar, L.; Neumann, A.; Neumann, M.; Roggendorf, H.; Wehrspohn, R.; Stöber, S.; Stephan-Scherb, C. Lithium-Phase Identification in an Industrial Lithium-Ion-Battery Recycling Slag: Implications for the Recovery of Lithium. Adv. Energy Sustain. Res. 2024, 2400338. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.D.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy. Processes 2024, 12, 1485. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Zhou, G.; Yao, W.; Cheng, H.-M.; Tang, Y. Direct Regenerating Cathode Materials from Spent Lithium-Ion Batteries. Adv. Sci. 2024, 11, 2304425. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, T.; Zhang, M. Advances in Intelligent Regeneration of Cathode Materials for Sustainable Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201526. [Google Scholar] [CrossRef]
- Yang, T.; Luo, D.; Yu, A.; Chen, Z. Enabling Future Closed-Loop Recycling of Spent Lithium-Ion Batteries: Direct Cathode Regeneration. Adv. Mater. 2023, 35, 2203218. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Kim, S.; Hatton, T.A.; Mo, H.; Waite, T.D. Lithium recovery using electrochemical technologies: Advances and challenges. Water Res. 2022, 221, 118822. [Google Scholar] [CrossRef]
- Jose, S.A.; Stoll, J.L.; Smith, T.; Jackson, C.; Dieleman, T.; Leath, E.; Eastwood, N.; Menezes, P.L. Critical Review of Lithium Recovery Methods: Advancements, Challenges, and Future Directions. Processes 2024, 12, 2203. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.; Park, S.; Lee, J.; Bae, J.; Kim, D.; Joo, H.; Ban, S.; Lee, H.; Kim, Y.; et al. A comprehensive review on the recovery of cathode active materials via direct recycling from spent Li-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113693. [Google Scholar] [CrossRef]


| Publication Number | Title | Legal Status | Current Assignee | Publication Date | INPADOC Family Cited by Count |
|---|---|---|---|---|---|
| US20050100793A1 | Active metal electrolyzer | Active | PolyPlus Battery Co., Inc. | 12 May 2005 | 1427 |
| US20130302226A1 | Method and apparatus for recycling lithium-ion batteries | Active | Worcester Polytechnic Institute | 14 November 2013 | 273 |
| AU2003205087C1 | System and method for removing electrolyte from energy storage and/or conversion device using supercritical fluid | Active | Eco-Bat Indiana LLC, Indianapolis, IN, USA | 6 November 2008 | 228 |
| KR1019980063266A | Process and apparatus for recovering components of sealed-type battery | Inactive | Canon, Inc., Tokyo, Japan | 7 October 1998 | 225 |
| CA2319285A1 | Method for neutralizing and recycling spent lithium metal polymer rechargeable batteries | Inactive | Avestor, Portland, OR, USA | 13 March 2002 | 200 |
| Publication Number | Title | Legal Status | Current Assignee | Publication Date | INPADOC Family Size |
|---|---|---|---|---|---|
| US20050100793A1 | Active metal electrolyzer | Active | PolyPlus Battery Co., Inc. | 12 May 2005 | 54 |
| CN114174545A | Process for recovery of lithium from waste lithium-ion batteries | Pending | BASF AB, Ludwigshafen, Germany | 11 March 2022 | 34 |
| US20170005374A1 | Process for recycling Li-ion batteries | Active | Umicore SA, Brussels, Belgium | 5 January 2017 | 26 |
| US20190024212A1 | Recovery of lithium from acid solution | Granted | Larry Lien (US) | 24 January 2019 | 25 |
| MYPI2018000006A0 | Method of recovering metals from spent Li-ion batteries | Active | Attero Recycling Pvt Ltd., Noida, India | 6 January 2017 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Zhu, X. A Patent Landscape Analysis on the Recycling of Lithium-Ion Battery Positive Electrode Materials: Trends, Technologies, and the Future. Batteries 2025, 11, 110. https://doi.org/10.3390/batteries11030110
Tong Z, Zhu X. A Patent Landscape Analysis on the Recycling of Lithium-Ion Battery Positive Electrode Materials: Trends, Technologies, and the Future. Batteries. 2025; 11(3):110. https://doi.org/10.3390/batteries11030110
Chicago/Turabian StyleTong, Zhuoya, and Xiaobo Zhu. 2025. "A Patent Landscape Analysis on the Recycling of Lithium-Ion Battery Positive Electrode Materials: Trends, Technologies, and the Future" Batteries 11, no. 3: 110. https://doi.org/10.3390/batteries11030110
APA StyleTong, Z., & Zhu, X. (2025). A Patent Landscape Analysis on the Recycling of Lithium-Ion Battery Positive Electrode Materials: Trends, Technologies, and the Future. Batteries, 11(3), 110. https://doi.org/10.3390/batteries11030110







