Plasticized Ionic Liquid Crystal Elastomer Emulsion-Based Polymer Electrolyte for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results
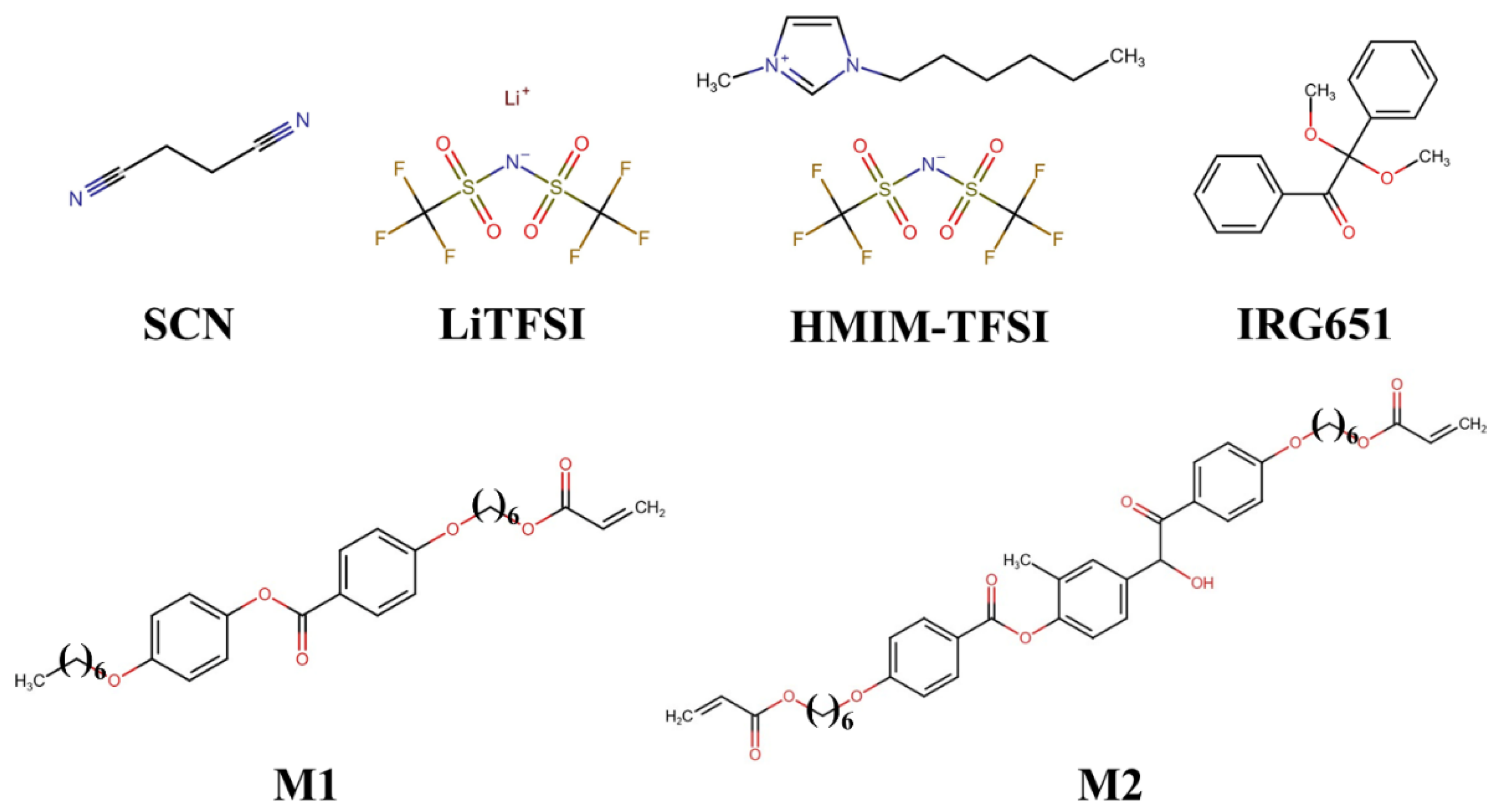
2.1. Material Composition
2.1.1. Synthesis of Ionic Liquid Crystal Elastomer
2.1.2. Fabrication of iLCE Electrolyte Membrane
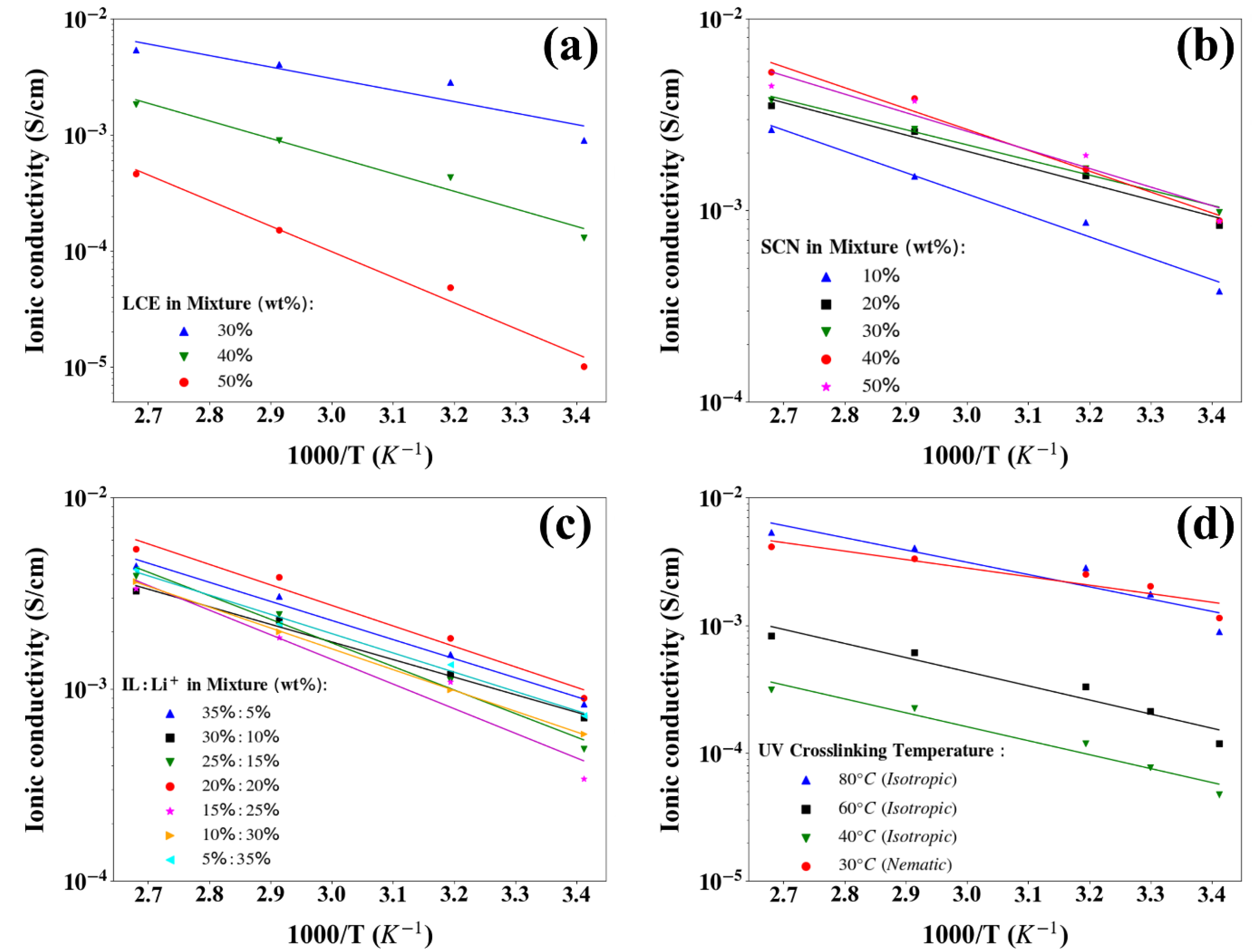
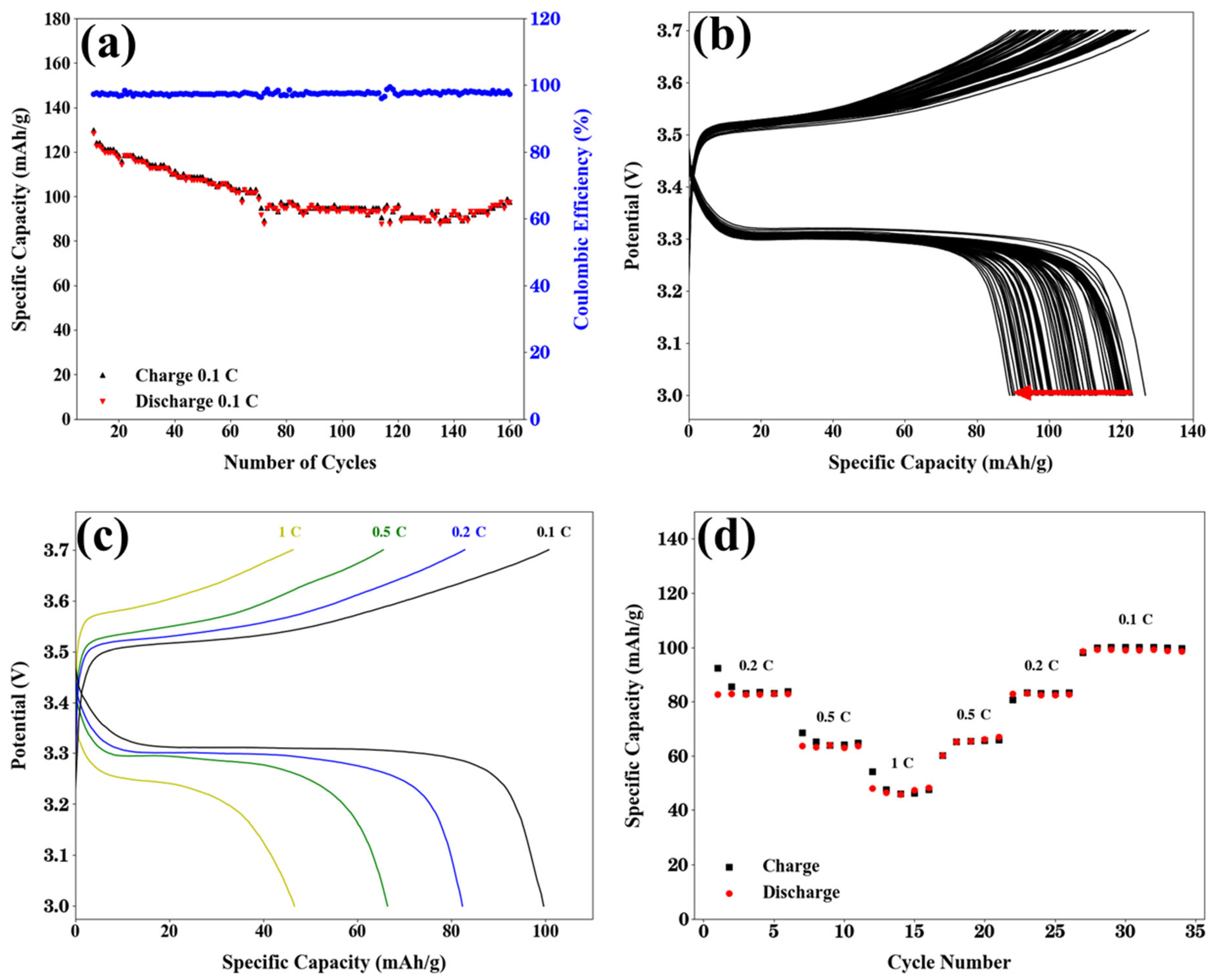
2.2. Electrochemical Performance
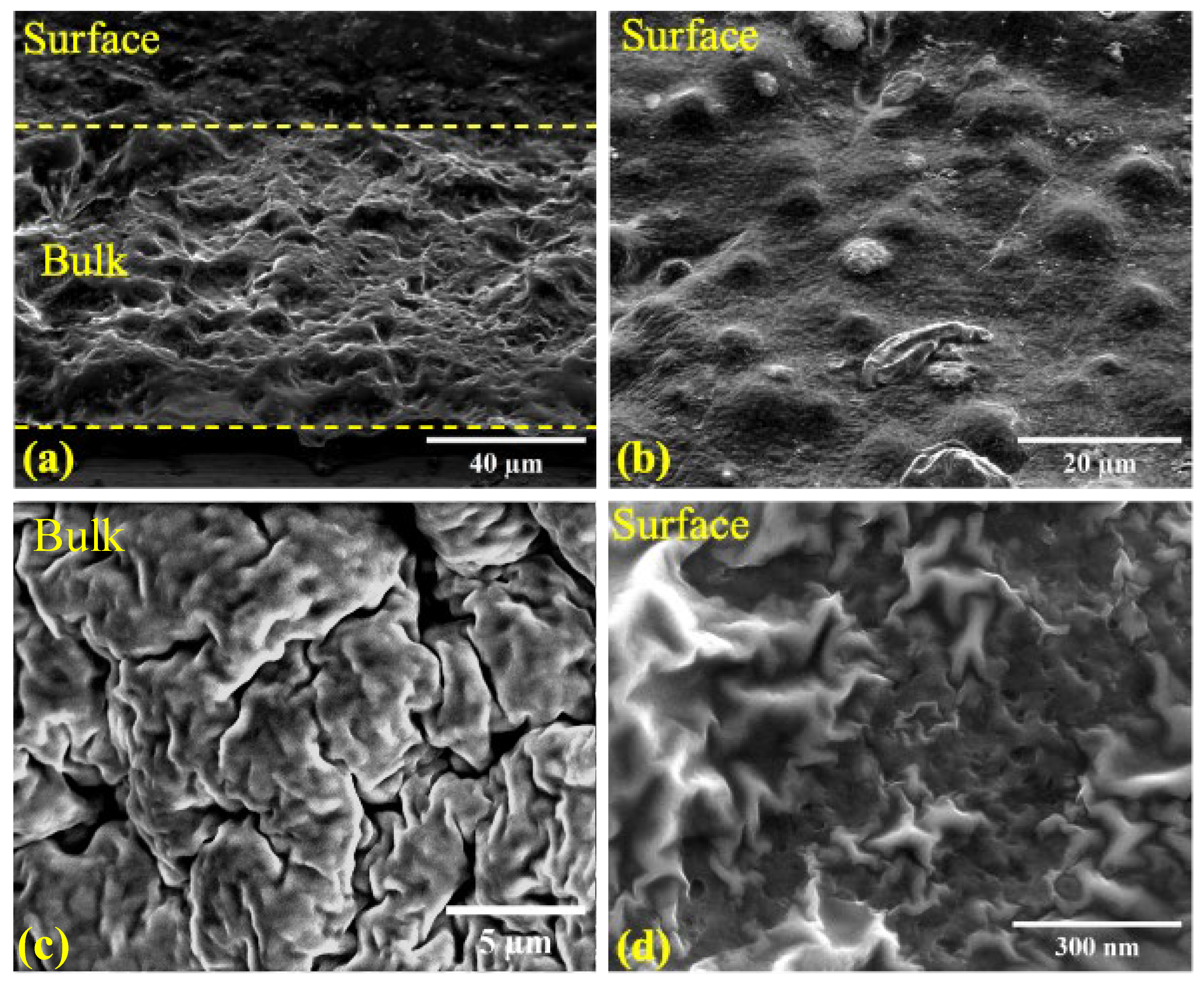
2.3. Structure of iLCE Electrolytes
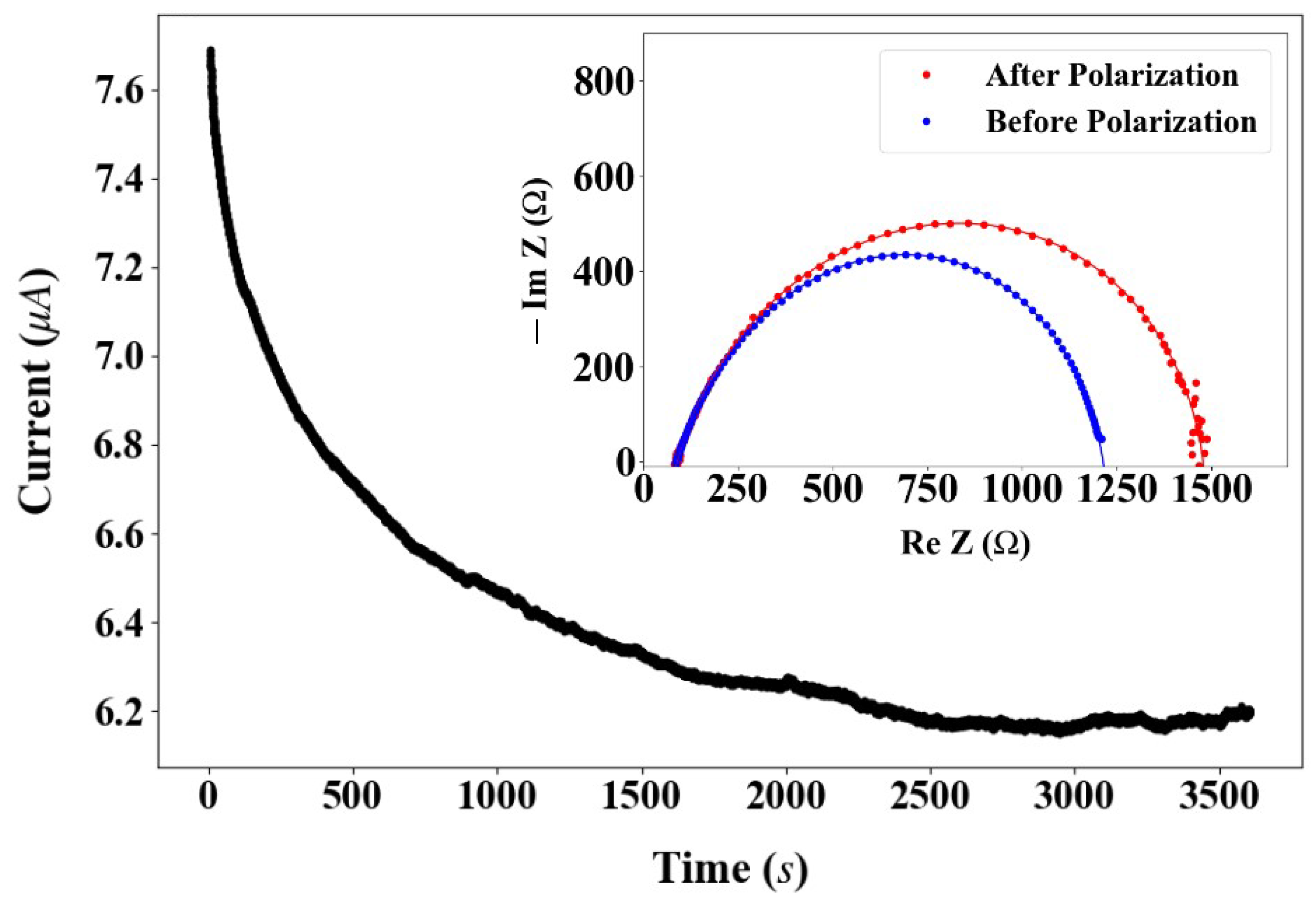
2.4. Cell Performance
3. Conclusions
4. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, C.W.; Cox, J.D.; Salomon, M. Conductivity and Electrochemical Stability of Electrolytes Containing Organic Solvent Mixtures with Lithium Tris(Trifluoromethanesulfonyl)Methide. J. Electrochem. Soc. 1996, 143, L80–L82. [Google Scholar] [CrossRef]
- Armand, M. The History of Polymer Electrolytes. Solid State Ion. 1994, 69, 309–319. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Nair, J.R.; Imholt, L.; Brunklaus, G.; Winter, M. Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges. Electrochem. Soc. Interface 2019, 28, 55–61. [Google Scholar] [CrossRef]
- Deng, K.; Qin, J.; Wang, S.; Ren, S.; Han, D.; Xiao, M.; Meng, Y. Effective Suppression of Lithium Dendrite Growth Using a Flexible Single-Ion Conducting Polymer Electrolyte. Small 2018, 14, 1801420. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single Lithium-Ion Conducting Solid Polymer Electrolytes: Advances and Perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef]
- Nakayama, M.; Wada, S.; Kuroki, S.; Nogami, M. Factors Affecting Cyclic Durability of All-Solid-State Lithium Polymer Batteries Using Poly(Ethylene Oxide)-Based Solid Polymer Electrolytes. Energy Environ. Sci. 2010, 3, 1995–2002. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and Polymeric Solid Electrolytes for Lithium-Ion Batteries. J. Power Sources 2010, 195, 4554–5469. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Review of Composite Polymer-Ceramic Electrolytes for Lithium Batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.G.; Scrosati, B.; Plichta, E. Enhancement of Ion Transport in Polymer Electrolytes by Addition of Nanoscale Inorganic Oxides. J. Power Sources 2001, 97–98, 644–648. [Google Scholar] [CrossRef]
- Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium Dendrite Formation on a Lithium Metal Anode from Liquid, Polymer and Solid Electrolytes. Electrochemistry 2016, 84, 210–218. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zheng, W.; Cui, X.; Rojo, T.; Zhang, Q. Towards High-Safe Lithium Metal Anodes: Suppressing Lithium Dendrites via Tuning Surface Energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Bardhan, P.; Mandal, M.; Maji, T.K. Development of Wood Polymer Composites with Thermosetting Resin from Soybean Oil Cross-Linked with Rosin Derivative. Eur. J. Wood Wood Prod. 2020, 78, 1265–1278. [Google Scholar] [CrossRef]
- Goydaragh, M.G.; Taghizadeh-Mehrjardi, R.; Jafarzadeh, A.A.; Triantafilis, J.; Lado, M. Using Environmental Variables and Fourier Transform Infrared Spectroscopy to Predict Soil Organic Carbon. Catena 2021, 202, 105280. [Google Scholar] [CrossRef]
- Ehgartner, C.R.; Werner, V.; Selz, S.; Hüsing, N.; Feinle, A. Carboxylic Acid-Modified Polysilsesquioxane Aerogels for the Selective and Reversible Complexation of Heavy Metals and Organic Molecules. Microporous Mesoporous Mater. 2021, 312, 110759. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical Measurement of Transference Numbers in Polymer Electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent Development of Sulfide Solid Electrolytes and Interfacial Modification for All-Solid-State Rechargeable Lithium Batteries. J. Asian Ceram. Soc. 2013, 1, 17–25. [Google Scholar] [CrossRef]
- Kaneko, F.; Wada, S.; Nakayama, M.; Wakihara, M.; Koki, J.; Kuroki, S. Capacity Fading Mechanism in All Solid-State Lithium Polymer Secondary Batteries Using PEG-Borate/Alumlnate Ester as Plasticizer for Polymer Electrolytes. Adv. Funct. Mater. 2009, 19, 918–925. [Google Scholar] [CrossRef]
- Bruce, P.G.; Evans, J.; Vincent, C.A. Conductivity and Transference Number Measurements on Polymer Electrolytes. Solid State Ion. 1988, 28–30, 918–922. [Google Scholar] [CrossRef]
- Strickberger, S.A.; Ravi, S.; Daoud, E.; Niebauer, M.; Man, K.C.; Morady, F. Relation between Impedance and Temperature during Radiofrequency Ablation of Accessory Pathways. Am. Heart J. 1995, 130, 1026–1030. [Google Scholar] [CrossRef]
- Feng, C.; Kyu, T. Role of Dinitrile Plasticizer Chain Lengths in Electrochemical Performance of Highly Conductive Polymer Electrolyte Membrane for Lithium Ion Battery. Electrochim. Acta 2020, 330, 135320. [Google Scholar] [CrossRef]
- Pal, U.; Chen, F.; Gyabang, D.; Pathirana, T.; Roy, B.; Kerr, R.; MacFarlane, D.R.; Armand, M.; Howlett, P.C.; Forsyth, M. Enhanced Ion Transport in an Ether Aided Super Concentrated Ionic Liquid Electrolyte for Long-Life Practical Lithium Metal Battery Applications. J. Mater. Chem. A Mater. 2020, 8, 18826–18839. [Google Scholar] [CrossRef]
- Chang, Z.; Qiao, Y.; Deng, H.; Yang, H.; He, P.; Zhou, H. A Liquid Electrolyte with De-Solvated Lithium Ions for Lithium-Metal Battery. Joule 2020, 4, 1776–1789. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.; Zhu, Y.; Lin, M.C.; Chen, H.; Li, Y.Y.; Hung, W.H.; Zhou, B.; Wang, X.; Bai, Y.; et al. High-Safety and High-Energy-Density Lithium Metal Batteries in a Novel Ionic-Liquid Electrolyte. Adv. Mater. 2020, 32, 2001741. [Google Scholar] [CrossRef]
- Kobayashi, K.; Pagot, G.; Vezzù, K.; Bertasi, F.; Di Noto, V.; Tominaga, Y. Effect of Plasticizer on the Ion-Conductive and Dielectric Behavior of Poly(Ethylene Carbonate)-Based Li Electrolytes. Polym. J. 2021, 53, 149–155. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Y.; Zhuo, S.; Zhu, S.; Chen, Y.; Jiang, C.; Wang, C. Synergistic Effect of Organic Plasticizer and Lepidolite Filler on Polymer Electrolytes for All-Solid High-Voltage Li-Metal Batteries. J. Mater. Chem. A Mater. 2020, 8, 5968–5974. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Kajiura, R.; Miura, A.; Tadanaga, K. Organic−inorganic Hybrid Materials for Interface Design in All-Solid-State Batteries with a Garnet-Type Solid Electrolyte. ACS Appl. Energy Mater. 2020, 3, 11260–11268. [Google Scholar] [CrossRef]
- Ibrahim, S.; Johan, M.R. Conductivity, Thermal and Neural Network Model Nanocomposite Solid Polymer Electrolyte System (PEO-LiPF 6-EC-CNT). Int. J. Electrochem. Sci. 2011, 6, 5565–5587. [Google Scholar] [CrossRef]
- Klongkan, S.; Pumchusak, J. Effects of Nano Alumina and Plasticizers on Morphology, Ionic Conductivity, Thermal and Mechanical Properties of PEO-LiCF3SO3 Solid Polymer Electrolyte. Electrochim. Acta 2015, 161, 171–176. [Google Scholar] [CrossRef]
- Christie, A.M.; Lilley, S.J.; Staunton, E.; Andreev, Y.G.; Bruce, P.G. Increasing the Conductivity of Crystalline Polymer Electrolytes. Nature 2005, 433, 50–53. [Google Scholar] [CrossRef]
- Phan, T.N.T.; Issa, S.; Gigmes, D. Poly(Ethylene Oxide)-Based Block Copolymer Electrolytes for Lithium Metal Batteries. Polym. Int. 2019, 68, 7–13. [Google Scholar] [CrossRef]
- Young, W.S.; Epps, T.H. Ionic Conductivities of Block Copolymer Electrolytes with Various Conducting Pathways: Sample Preparation and Processing Considerations. Macromolecules 2012, 45, 4689–4697. [Google Scholar] [CrossRef]
- Ben youcef, H.; Garcia-Calvo, O.; Lago, N.; Devaraj, S.; Armand, M. Cross-Linked Solid Polymer Electrolyte for All-Solid-State Rechargeable Lithium Batteries. Electrochim. Acta 2016, 220, 587–594. [Google Scholar] [CrossRef]
- Stalin, S.; Choudhury, S.; Zhang, K.; Archer, L.A. Multifunctional Cross-Linked Polymeric Membranes for Safe, High-Performance Lithium Batteries. Chem. Mater. 2018, 30, 2058–2066. [Google Scholar] [CrossRef]
- Schulze, M.W.; McIntosh, L.D.; Hillmyer, M.A.; Lodge, T.P. High-Modulus, High-Conductivity Nanostructured Polymer Electrolyte Membranes via Polymerization-Induced Phase Separation. Nano Lett. 2014, 14, 122–126. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Feng, Y.; Tan, R.; Zuo, Y.; Gao, R.; Zhao, Y.; Han, L.; Wang, Z.; Pan, F. Flexible Composite Solid Electrolyte Facilitating Highly Stable “Soft Contacting” Li–Electrolyte Interface for Solid State Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701437. [Google Scholar] [CrossRef]
- Bhattacharyya, A.J.; Fleig, J.; Guo, Y.G.; Maier, J. Local Conductivity Effects in Polymer Electrolytes. Adv. Mater. 2005, 17, 2630–2634. [Google Scholar] [CrossRef]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion Transport in Self-Organized Columnar Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Ionic Liquid Crystal as a Hole Transport Layer of Dye-Sensitized Solar Cells. Chem. Commun. 2005, 6, 740–742. [Google Scholar] [CrossRef]
- Lee, S.; Becht, G.A.; Lee, B.; Burns, C.T.; Firestone, M.A. Electropolymerization of a Bifunctional Ionic Liquid Monomer Yields an Electroactive Liquid-Crystalline Polymer. Adv. Funct. Mater. 2010, 20, 2063–2070. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Wang, A.; Wang, Z.; Chen, J.; Zeng, Q.; Wang, X.; Zhang, L. An Ionic Liquid Crystal-Based Solid Polymer Electrolyte with Desirable Ion-Conducting Channels for Superior Performance Ambient-Temperature Lithium Batteries. Polym. Chem. 2018, 9, 4674–4682. [Google Scholar] [CrossRef]
- Mukai, T.; Yoshio, M.; Kato, T.; Yoshizawa, M.; Ohno, H. Anisotropic Ion Conduction in a Unique Smectic Phase of Self-Assembled Amphiphilic Ionic Liquids. Chem. Commun. 2005, 10, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Matsumoto, T.; Akita, T.; Nishiyama, N. Ion Conductive Properties in Ionic Liquid Crystalline Phases Confined in a Porous Membrane. J. Mater. Chem. C Mater. 2015, 3, 6144–6147. [Google Scholar] [CrossRef]
- Kuo, D.; Soberats, B.; Kumar, K.R.S.; Yoshio, M.; Ichikawa, T.; Ohno, H.; Zeng, X.; Ungar, G.; Kato, T. Switching of Ionic Conductivities in Columnar Liquid-Crystalline Anilinium Salts: Effects of Alkyl Chains, Ammonium Cations and Counter Anions on Thermal Properties and Switching Temperatures. Mol. Syst. Des. Eng. 2019, 4, 342–347. [Google Scholar] [CrossRef]
- Alyami, A.; Rajapaksha, C.P.H.; Feng, C.; Paudel, P.R.; Paul, A.; Adaka, A.; Dharmarathna, R.; Lüssem, B.; Jákli, A. Ionic Liquid Crystal Elastomers for Actuators, Sensors, and Organic Transistors. Liq. Cryst. 2023, 50, 1151–1161. [Google Scholar] [CrossRef]
- Luo, S.C.; Sun, S.; Deorukhkar, A.R.; Lu, J.T.; Bhattacharyya, A.; Lin, I.J.B. Ionic Liquids and Ionic Liquid Crystals of Vinyl Functionalized Imidazolium Salts. J. Mater. Chem. 2011, 21, 1866–1873. [Google Scholar] [CrossRef]
- Drzaic, P.S. Liquid Crystal Dispersions; World Scientific: River Edge, NJ, USA, 1995. [Google Scholar]
- Scharf, T. Polarized Light in Liquid Crystals and Polymers; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Méry, A.; Rousselot, S.; Lepage, D.; Dollé, M. A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes. Materials 2021, 14, 3840. [Google Scholar] [CrossRef] [PubMed]
- Seidl, L.; Grissa, R.; Zhang, L.; Trabesinger, S.; Battaglia, C. Unraveling the Voltage-Dependent Oxidation Mechanisms of Poly(Ethylene Oxide)-Based Solid Electrolytes for Solid-State Batteries. Adv. Mater. Interfaces 2022, 9, 2100704. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, J.; Parrondo, J.; Zamani, S.; Atienza, D.; Kyu, T. Enhanced Energy Storage in Lithium-Metal Batteries via Polymer Electrolyte Polysulfide-Polyoxide Conetworks. ACS Appl. Mater. Interfaces 2023, 15, 27173–27182. [Google Scholar] [CrossRef] [PubMed]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The Plastic-Crystalline Phase of Succinonitrile as a Universal Matrix for Solid-State Ionic Conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Yan, R.; Niu, H.; He, W.; Miao, Z. Liquid Crystal Elastomer-Based Solid Electrolyte with Intelligently Regulated Rigidity–Flexibility toward High-Energy Lithium Batteries. Chem. Eng. J. 2025, 503, 158552. [Google Scholar] [CrossRef]
- Fu, J.; Li, Z.; Zhou, X.; Guo, X. Ion Transport in Composite Polymer Electrolytes. Mater. Adv. 2022, 3, 3809–3819. [Google Scholar] [CrossRef]
- Gerdroodbar, A.E.; Alihemmati, H.; Safavi-Mirmahaleh, S.A.; Golshan, M.; Damircheli, R.; Eliseeva, S.N.; Salami-Kalajahi, M. A Review on Ion Transport Pathways and Coordination Chemistry between Ions and Electrolytes in Energy Storage Devices. J. Energy Storage 2023, 74, 109311. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.W.; Kyu, T. A Comparative Study on Electrochemical Performance of Single versus Dual Networks in Lithium Metal/Polysulfide-Polyoxide Co-Network/Lithium Titanium Oxide Cathode. Batteries 2024, 10, 163. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(Ethylene Oxide)-Based Electrolytes for Lithium-Ion Batteries. J. Mater. Chem. A Mater. 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Derollez, P.; Lefebvre, J.; Descamps, M.; Press, W.; Fontaine, H. Structure of Succinonitrile in Its Plastic Phase. J. Phys. Condens. Matter 1990, 2, 6893. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic Conductivity and Ion Transport Mechanisms of Solid-State Lithium-Ion Battery Electrolytes: A Review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiquee, Z.; Lee, H.; Xu, W.; Kyu, T.; Jákli, A. Plasticized Ionic Liquid Crystal Elastomer Emulsion-Based Polymer Electrolyte for Lithium-Ion Batteries. Batteries 2025, 11, 106. https://doi.org/10.3390/batteries11030106
Siddiquee Z, Lee H, Xu W, Kyu T, Jákli A. Plasticized Ionic Liquid Crystal Elastomer Emulsion-Based Polymer Electrolyte for Lithium-Ion Batteries. Batteries. 2025; 11(3):106. https://doi.org/10.3390/batteries11030106
Chicago/Turabian StyleSiddiquee, Zakaria, Hyunsang Lee, Weinan Xu, Thein Kyu, and Antal Jákli. 2025. "Plasticized Ionic Liquid Crystal Elastomer Emulsion-Based Polymer Electrolyte for Lithium-Ion Batteries" Batteries 11, no. 3: 106. https://doi.org/10.3390/batteries11030106
APA StyleSiddiquee, Z., Lee, H., Xu, W., Kyu, T., & Jákli, A. (2025). Plasticized Ionic Liquid Crystal Elastomer Emulsion-Based Polymer Electrolyte for Lithium-Ion Batteries. Batteries, 11(3), 106. https://doi.org/10.3390/batteries11030106








