Study on Blended Terpolymer Electrolyte Membrane for Enhanced Safety and Performance in Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials
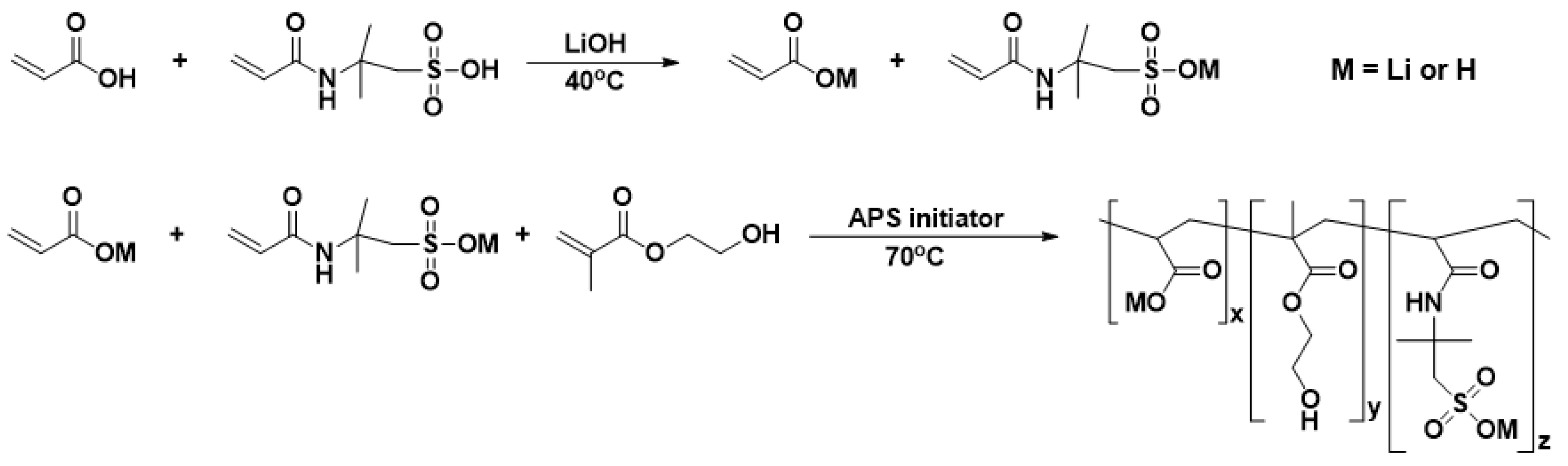
2.2. Synthesis of Poly (LiAA-HEMA-LiAMPS)
2.3. Preparation of Blended Terpolymer Electrolyte
2.4. Preparation of LFP Cathode
2.5. Cell Assemblies
2.6. The Structural Characterization of the Polymer Electrolytes
2.7. Thermal Performance Test
2.8. Electrochemical Measurements
3. Results and Discussions
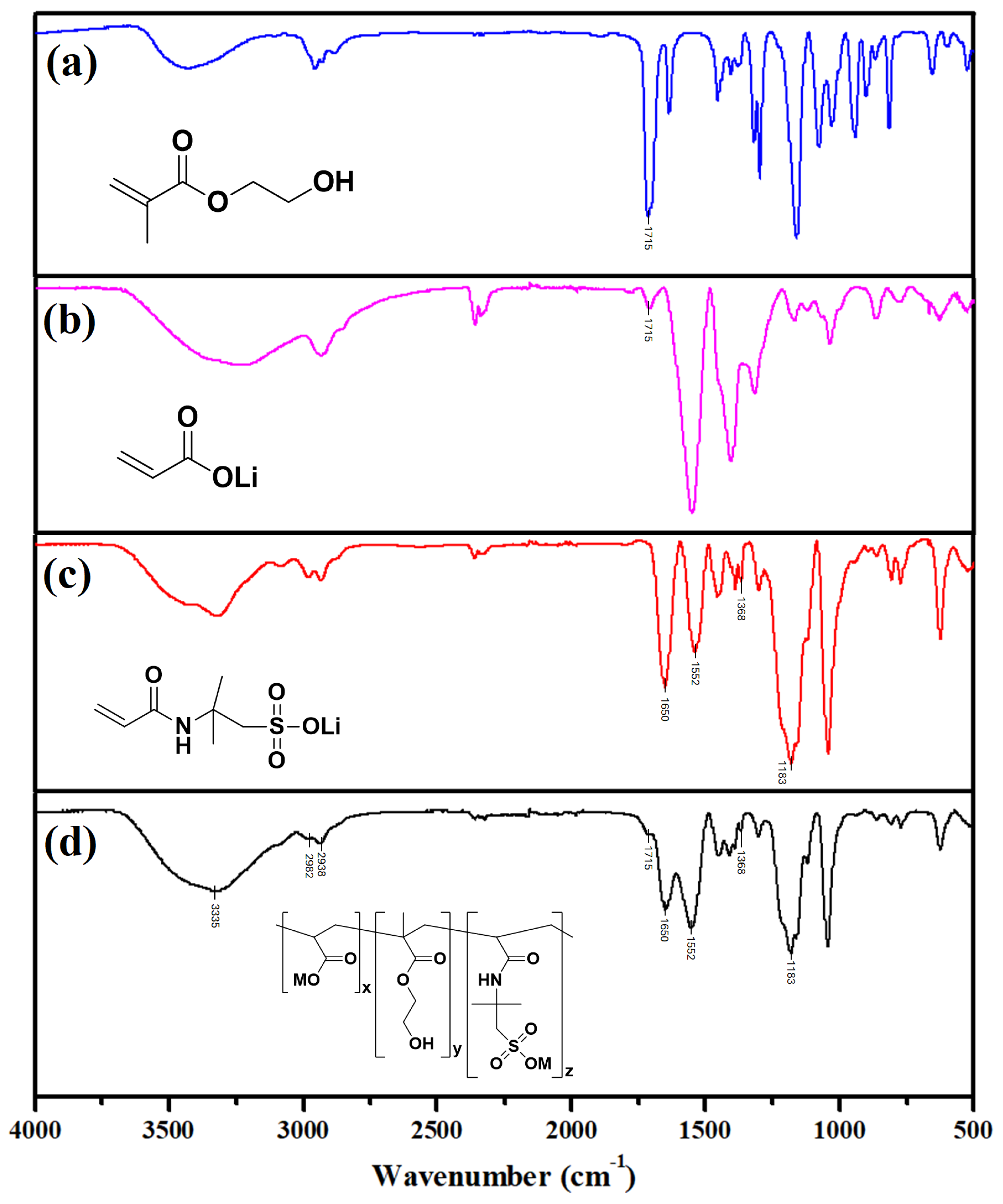
3.1. Characterization of Poly (LiAA-HEMA-LiAMPS) Terpolymer
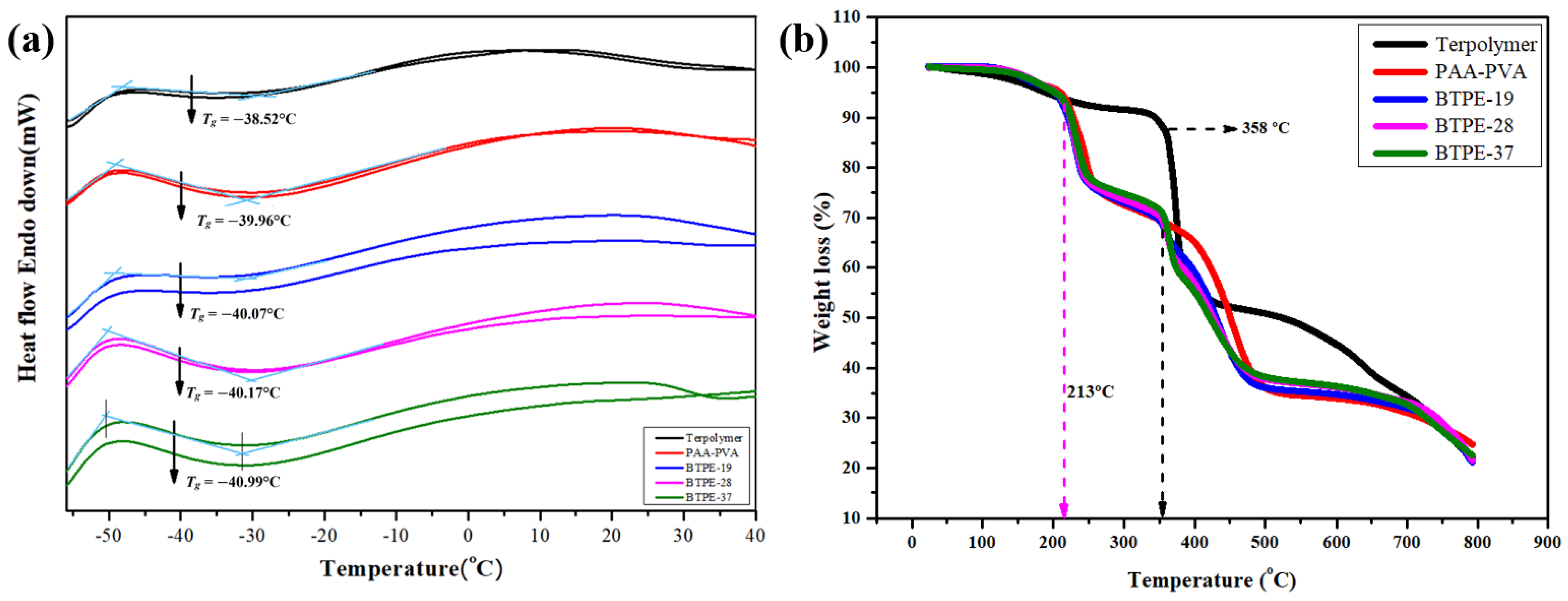
3.2. Thermal Properties of Blend Terpolymer Electrolyte Membrane
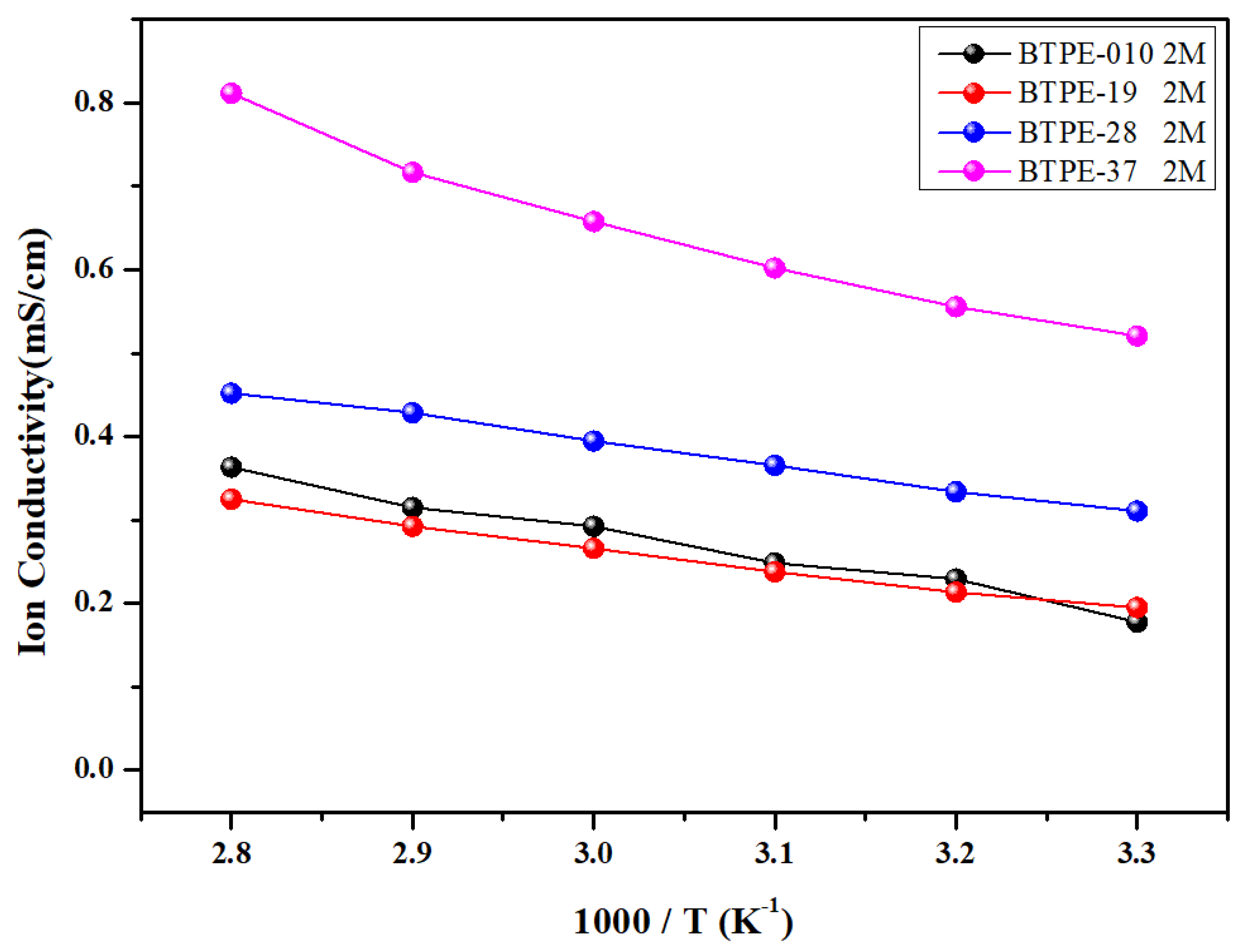
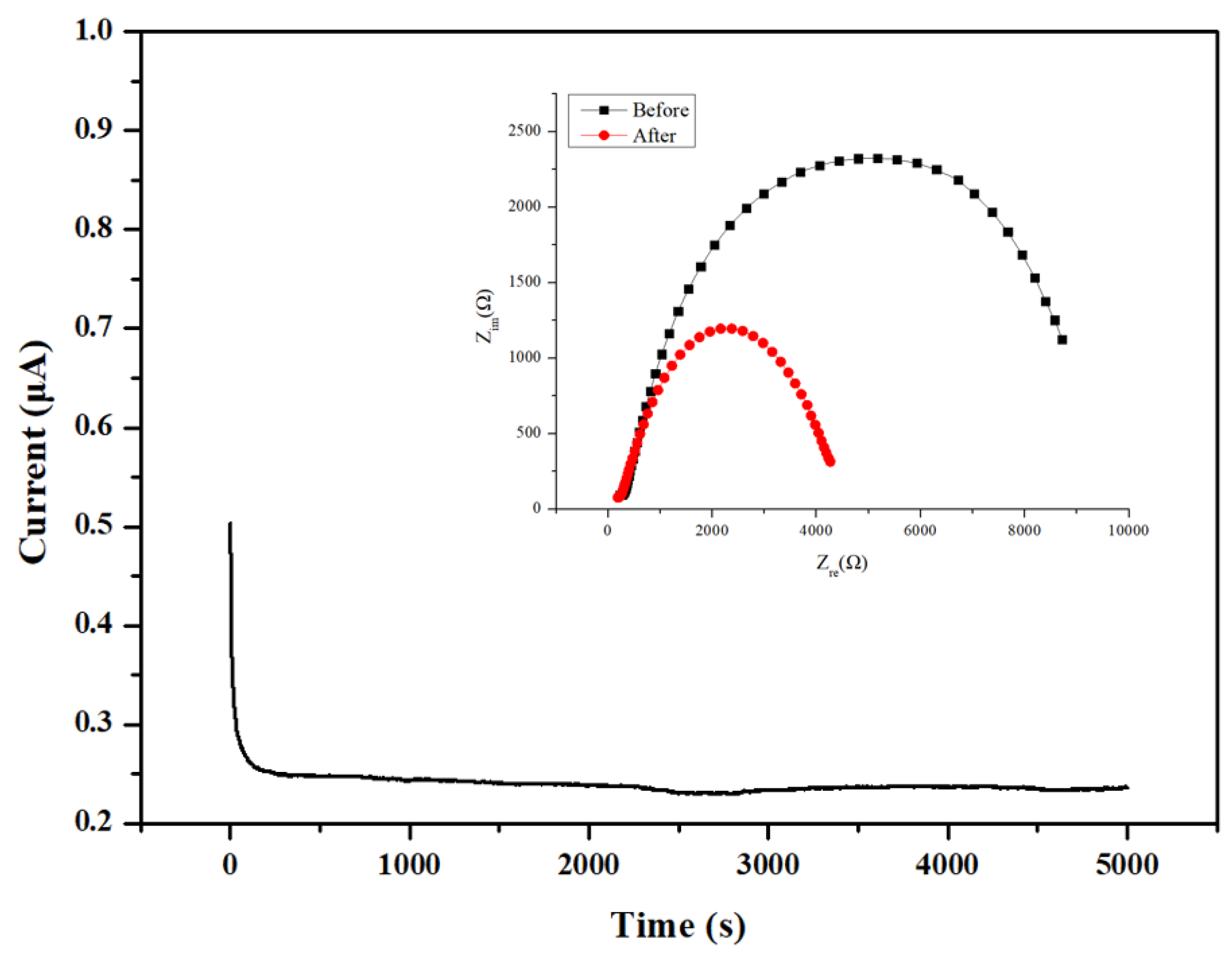
3.3. Electrochemical Properties of Blended Terpolymer Electrolyte Membrane
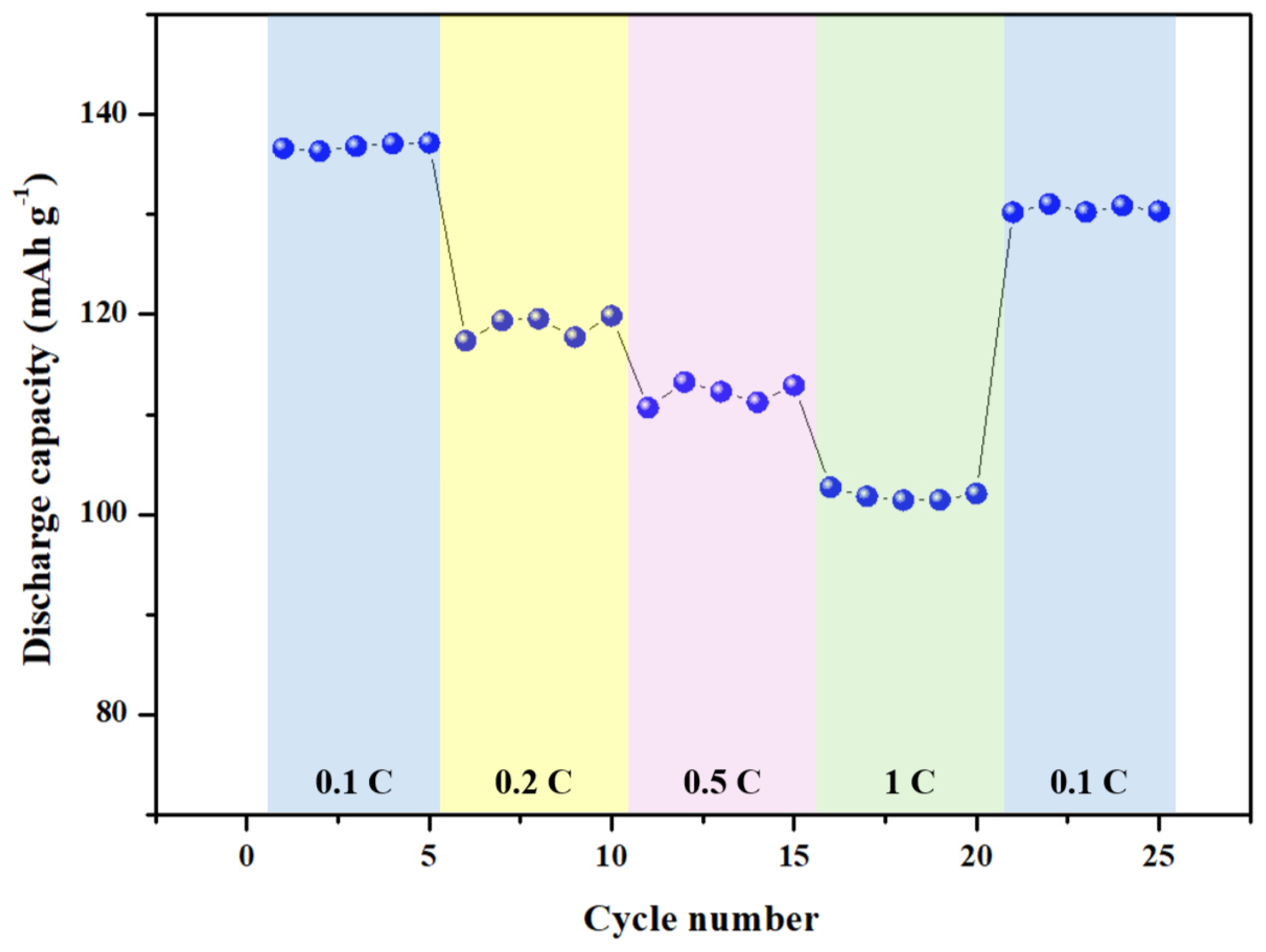
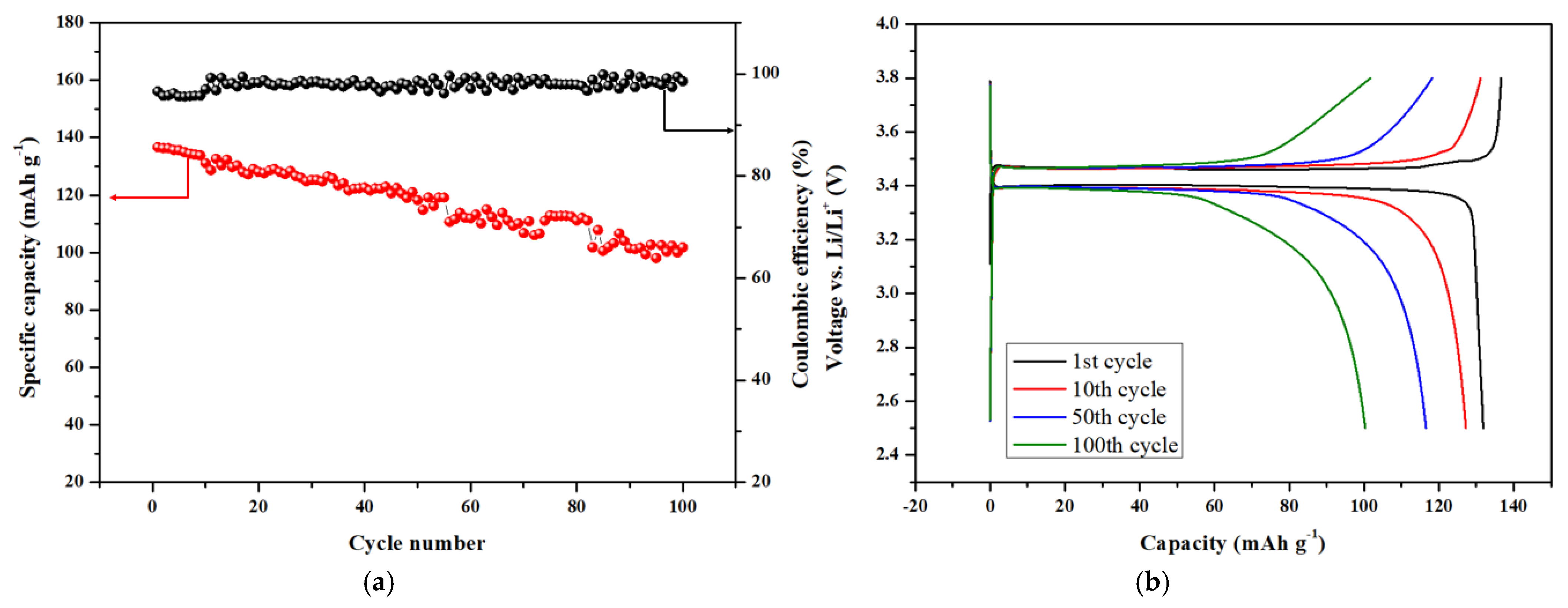
3.4. Electrochemical Performance of Blended Terpolymer Electrolyte-Based Li Metal Battery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.R.; Imholt, L.; Brunklaus, G.; Winter, M. Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges. Electrochem. Soc. Interface 2019, 28, 55. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C. High-Voltage Liquid Electrolytes for Li Batteries: Progress and Perspectives. Chem. Soc. Rev. 2021, 50, 10486–10566. [Google Scholar] [CrossRef] [PubMed]
- Pullanchiyodan, A.; Joy, R.; Sreeram, P.; Raphael, L.R.; Das, A.; Balakrishnan, N.T.M.; Ahn, J.-H.; Vlad, A.; Sreejith, S.; Raghavan, P. Recent Advances in Electrospun Fibers Based on Transition Metal Oxides for Supercapacitor Applications: A Review. Energy Adv. 2023, 2, 922–947. [Google Scholar] [CrossRef]
- Zu, C.; Yu, H.; Li, H. Enabling the Thermal Stability of Solid Electrolyte Interphase in Li-ion Battery. InfoMat 2021, 3, 648–661. [Google Scholar] [CrossRef]
- Balakrishnan, N.T.M.; Das, A.; Joyner, J.D.; Jabeen Fatima, M.J.; Raphael, L.R.; Pullanchiyodan, A.; Raghavan, P. Quest for High-Performance Gel Polymer Electrolyte by Enhancing the Miscibility of the Bi-Polymer Blend for Lithium-Ion Batteries: Performance Evaluation in Extreme Temperatures. Mater. Today Chem. 2023, 29, 101407. [Google Scholar] [CrossRef]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid Garnet Batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhang, X.D.; Wang, T.S.; Yin, Y.X.; Shi, J.L.; Wang, C.R.; Guo, Y.G. Ameliorating the Interfacial Problems of Cathode and Solid-State Electrolytes by Interface Modification of Functional Polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Frechero, M.A. Strategies for Rational Design of Polymer-Based Solid Electrolytes for Advanced Lithium Energy Storage Applications. Energy Storage Mater. 2022, 52, 430–464. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, D.; Wang, Q.; Yang, C.; Wang, X.; Wu, K.; Mu, Y.; Wu, J.; Liu, Z.; Liu, W. Accelerating Li+/Li Redox through the Regulation of the Electric Double Layer for Efficient Lithium Metal Anodes. Chem. Eng. J. 2023, 468, 143676. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Du, Z.; He, C.; Bi, J.; Li, S.; Guan, W.; Du, H.; Ai, W. Integrated Gradient Cu Current Collector Enables Bottom-Up Li Growth for Li Metal Anodes: Role of Interfacial Structure. Adv. Sci. 2023, 10, 2301288. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Dandan, Y.; Chengwei, K.; Liwei, C.; Wang, L.; Xilan, F.; Zheng, Z.; Xinbo, Z.; Yu, Z. Alkali Metal Anodes for Rechargeable Batteries. Chem 2019, 5, 313–338. [Google Scholar]
- Dai, H.; Xi, K.; Liu, X.; Lai, C.; Zhang, S. Cationic Surfactant-Based Electrolyte Additives for Uniform Lithium Deposition via Lithiophobic Repulsion Mechanisms. J. Am. Chem. Soc. 2018, 140, 17515–17521. [Google Scholar] [CrossRef]
- Zhang, H.; Eshetu, G.G.; Judez, X.; Li, C.; Rodriguez-Martínez, L.M.; Armand, M. Electrolyte Additives for Lithium Metal Anodes and Rechargeable Lithium Metal Batteries: Progress and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 15002–15027. [Google Scholar] [CrossRef]
- Zhou, T.; Mu, Y.; Chen, L.; Li, D.; Liu, W.; Yang, C.; Zhang, S.; Wang, Q.; Jiang, P.; Ge, G.; et al. Toward Stable Zinc Aqueous Rechargeable Batteries by Anode Morphology Modulation via Polyaspartic Acid Additive. Energy Storage Mater. 2022, 45, 777–785. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, Z.; Xu, K.; Lu, J. New Concepts in Electrolytes. Chem. Rev. 2020, 120, 6783–6819. [Google Scholar] [CrossRef] [PubMed]
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Wu, J.; Zhou, M.; Liu, W.; Zhou, H. Advanced Electrolyte Design for Stable Lithium Metal Anode: From Liquid to Solid. Nano Energy 2021, 80, 105516. [Google Scholar] [CrossRef]
- Li, Z.; Lu, W.; Zhang, N.; Pan, Q.; Chen, Y.; Xu, G.; Zeng, D.; Zhang, Y.; Cai, W.; Yang, M. Single Ion Conducting Lithium Sulfur Polymer Batteries with Improved Safety and Stability. J. Mater. Chem. A Mater. 2018, 6, 14330–14338. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P.; Magistris, A. PEO-Based Composite Polymer Electrolytes. Solid State Ion. 1998, 110, 1–14. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Cui, X.; Zhang, R.; Guo, J.; Li, R.; Chao, Y.; Xu, G.; He, C.; Chen, F.; Li, L.; et al. Utilizing an Oxygen-Rich Interface by Hydroxyapatite to Regulate the Linear Diffusion for the Stable Solid-State Electrolytes. ACS Appl. Mater. Interfaces 2022, 14, 33392–33399. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Ke, B.; Xie, Y.; Cheng, S.; Zhang, C.; Zhang, H.; Lu, B.; Wang, X. All-Solid-State Thin-Film Lithium-Sulfur Batteries. Nanomicro Lett. 2023, 15, 73. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer Electrolytes for Lithium Polymer Batteries. J. Mater. Chem. A Mater. 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Jin, L.; Lim, H.; Bae, W.; Song, S.; Joo, K.; Jang, H.; Kim, W. Crosslinked Gel Polymer Electrolyte from Trimethylolpropane Triglycidyl Ether by In Situ Polymerization for Lithium-Ion Batteries. Gels 2024, 10, 40. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, L.; Bae, W.; Park, S.; Jeon, M.; Lee, S.; Lee, S.; Jang, H.; Kim, W. In-Situ Generated Solid-State Electrolytes with Intimate Interface Affinity Enable Conductivity and High Performances for Lithium-Ion Batteries. Electrochim. Acta 2023, 465, 142932. [Google Scholar] [CrossRef]
- Grewal, M.S.; Tanaka, M.; Kawakami, H. Bifunctional Poly(Ethylene Glycol) Based Crosslinked Network Polymers as Electrolytes for All-Solid-State Lithium Ion Batteries. Polym. Int. 2019, 68, 684–693. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Wang, S.; Zhou, Y.; Song, D.; Zhang, H.; Shi, X.; Zhang, L. Li Salt Initiated In-Situ Polymerized Solid Polymer Electrolyte: New Insights via in-Situ Electrochemical Impedance Spectroscopy. Chem. Eng. J. 2022, 429, 132483. [Google Scholar] [CrossRef]
- Baik, J.H.; Kim, S.; Hong, D.G.; Lee, J.C. Gel Polymer Electrolytes Based on Polymerizable Lithium Salt and Poly(Ethylene Glycol) for Lithium Battery Applications. ACS Appl. Mater. Interfaces 2019, 11, 29718–29724. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Xiao, Y.; Xi, C.; Xu, G.; Li, B.; Yang, C.; Yu, Y. Flame-Retardant Composite Gel Polymer Electrolyte with a Dual Acceleration Conduction Mechanism for Lithium Ion Batteries. Chem. Eng. J. 2021, 422, 130526. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Fan, L.; Nan, C.; Zhang, Q. Intercalated Electrolyte with High Transference Number for Dendrite-free Solid-state Lithium Batteries. Adv. Funct. Mater. 2019, 29, 1901047. [Google Scholar] [CrossRef]
- Maia, B.A.; Magalhães, N.; Cunha, E.; Braga, M.H.; Santos, R.M.; Correia, N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, Y.; Zhang, Q.; Zhao, Y.; Lian, F. A Single-ion Conducting Network as Rationally Coordinating Polymer Electrolyte for Solid-state Li Metal Batteries. Adv. Energy Mater. 2022, 12, 2103530. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ng, H.M.; Numan, A.; Ramesh, S. Poly(Acrylic Acid)–Based Hybrid Inorganic–Organic Electrolytes Membrane for Electrical Double Layer Capacitors Application. Polymers 2016, 8, 179. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Shen, Y.; Ma, M.; Ruan, W.; Zhang, M. ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes. Polymers 2024, 16, 1128. [Google Scholar] [CrossRef]
- Hu, T.; Shen, X.; Peng, L.; Liu, Y.; Wang, X.; Ma, H.; Zhang, P.; Zhao, J. Preparation of Single-Ion Conductor Solid Polymer Electrolyte by Multi-Nozzle Electrospinning Process for Lithium-Ion Batteries. J. Phys. Chem. Solids 2021, 158, 110229. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Han, D.W.; Shin, D.M. Single-Ion Conducting Polymer Electrolytes as a Key Jigsaw Piece for next-Generation Battery Applications. Chem. Sci. 2021, 12, 13248–13272. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; El Desouky, E.A.; Rafea, M.A.; Abusaif, M.S. Toward Highly Ionic Polyelectrolyte Membrane: Synthesis, Characterization, Membrane Performance and Theoretical Studies of PVDF-g-PAN/PAMPS Hybrid Membranes. Mater. Today Commun. 2024, 40, 110232. [Google Scholar] [CrossRef]
- Lee, A.-R.; Kim, Y.-D.; Lee, S.-K.; Jo, N.-J. Poly (Hydroxyethyl Methacrylate) Based Networked Solid Polymer Electrolyte. J. Nanosci. Nanotechnol. 2013, 13, 7208–7211. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Preparation and Characterization of PVA/PAA Membranes for Solid Polymer Electrolytes. J. Memb. Sci. 2006, 275, 127–133. [Google Scholar] [CrossRef]
- Thayumanasundaram, S.; Rangasamy, V.S.; Seo, J.W.; Locquet, J.-P. Electrochemical Performance of Polymer Electrolytes Based on Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Blend and Pyrrolidinium Ionic Liquid for Lithium Rechargeable Batteries. Electrochim. Acta 2017, 240, 371–378. [Google Scholar] [CrossRef]
- Ni, H.; Yang, Y.; Chen, Y.; Liu, J.; Zhang, L.; Wu, M. Preparation of a Poly(DMAEMA-Co-HEMA) Self-Supporting Microfiltration Membrane with High Anionic Permselectivity by Electrospinning. e-Polymers 2017, 17, 149–157. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.Y.; Toktarbay, Z.; Zhursumbaeva, M.B.; Seitkaliyeva, N.Z. Flocculating Properties of 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid-Co-Allylamine Polyampholytic Copolymers. Polym. Bull. 2022, 79, 10741–10756. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Ghaffarlou, M.; Sütekin, S.D.; Pasanphan, W.; Güven, O. Preparation of Multifunctional Poly (Acrylic Acid)-Poly (Ethylene Oxide) Nanogels from Their Interpolymer Complexes by Radiation-Induced Intramolecular Crosslinking. Colloid. Polym. Sci. 2018, 296, 1599–1608. [Google Scholar] [CrossRef]
- Mehto, A.; Mehto, V.R.; Chauhan, J. Preparation and Characterization of Polyvinyl Alcohol (PVA)/ZrO2 Composite Membranes. Phys. Status Solidi (B) 2023, 260, 2300164. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Z.; Yuan, S.; Han, A.; Shen, Y.; Cheng, X.; Liang, Y.; Shen, S.; Zhang, J. Structural and Transport Properties of Ultrathin Perfluorosulfonic Acid Ionomer Film in Proton Exchange Membrane Fuel Cell Catalyst Layer: Ass Review. J. Power Sources 2022, 536, 231523. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development—A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Daojun, Y.; Xiaojie, L.; Ningning, W.; Wenhuai, T. Effect of moisture content on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2/graphite battery. Electrochim. Acta 2016, 188, 611–618. [Google Scholar]
- Guochun, Y.; Xinhai, L.; Zhixing, W.; Huajun, G.; Wenjie, P.; Qiyang, H. Lithium difluoro(oxalato)borate as an additive to suppress the aluminum corrosion in lithium bis(fluorosulfonyl)imide-based nonaqueous carbonate electrolyte. J. Solid. State Electrochem. 2016, 20, 507–516. [Google Scholar]




| Electrolyte Name | Weight of Pure Terpolymer (g) | Wight of Pure PAA–PVA (g) | Ratio | Weight of LiFSI (g) |
|---|---|---|---|---|
| BTPE–010 2M | 0 | 0.5 | 0:10 | 0.187 |
| BTPE–19 2M | 0.05 | 0.45 | 1:9 | 0.187 |
| BTPE–28 2M | 0.1 | 0.4 | 2:8 | 0.187 |
| BTPE–37 2M | 0.15 | 0.35 | 3:7 | 0.187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, W.; Sutradhar, S.C.; Song, S.; Joo, K.; Lee, D.; Kang, D.; Na, H.; Lee, J.; Kim, W.; Jang, H. Study on Blended Terpolymer Electrolyte Membrane for Enhanced Safety and Performance in Lithium-Ion Batteries. Batteries 2025, 11, 103. https://doi.org/10.3390/batteries11030103
Bae W, Sutradhar SC, Song S, Joo K, Lee D, Kang D, Na H, Lee J, Kim W, Jang H. Study on Blended Terpolymer Electrolyte Membrane for Enhanced Safety and Performance in Lithium-Ion Batteries. Batteries. 2025; 11(3):103. https://doi.org/10.3390/batteries11030103
Chicago/Turabian StyleBae, Wansu, Sabuj Chandra Sutradhar, Subeen Song, Kijong Joo, Doyul Lee, Donghoon Kang, Hyewon Na, Jiye Lee, Whangi Kim, and Hohyoun Jang. 2025. "Study on Blended Terpolymer Electrolyte Membrane for Enhanced Safety and Performance in Lithium-Ion Batteries" Batteries 11, no. 3: 103. https://doi.org/10.3390/batteries11030103
APA StyleBae, W., Sutradhar, S. C., Song, S., Joo, K., Lee, D., Kang, D., Na, H., Lee, J., Kim, W., & Jang, H. (2025). Study on Blended Terpolymer Electrolyte Membrane for Enhanced Safety and Performance in Lithium-Ion Batteries. Batteries, 11(3), 103. https://doi.org/10.3390/batteries11030103








