A Review of Pnictogenides for Next-Generation Anode Materials for Sodium-Ion Batteries
Abstract
1. Introduction
2. Physical and Chemical Properties of Pnictogenides
2.1. Metal Nitrides
| Materials | Crystal Structure | Density (g cm−3) | Electrical Conductivity (S m−1) | Young’s Modulus (GPa) | Bandgap (eV) | Ref. |
|---|---|---|---|---|---|---|
| Mo2N | Cubic | 9.46 | 2.97 | 365 | - | [35] |
| TiN | Cubic | 5.40 | 3.70 | 281 | 3.4 | [35] |
| VN | Cubic | 6.13 | 3.70 | 344 | Weak metallic | [40] |
| CoN | Cubic | 6.18 | - | 485 | Metallic | [41] |
| Ni3N | Hexagonal | 7.66 | - | 399 | Metallic | [42] |
2.2. Metal Phosphides
2.3. Metal Antimonides
3. Pnictogenides as Anode Materials for Sodium-Ion Batteries
3.1. Metal Nitrides as Anode Materials
3.1.1. Metal-Rich Nitrides (MaNb, a > b)
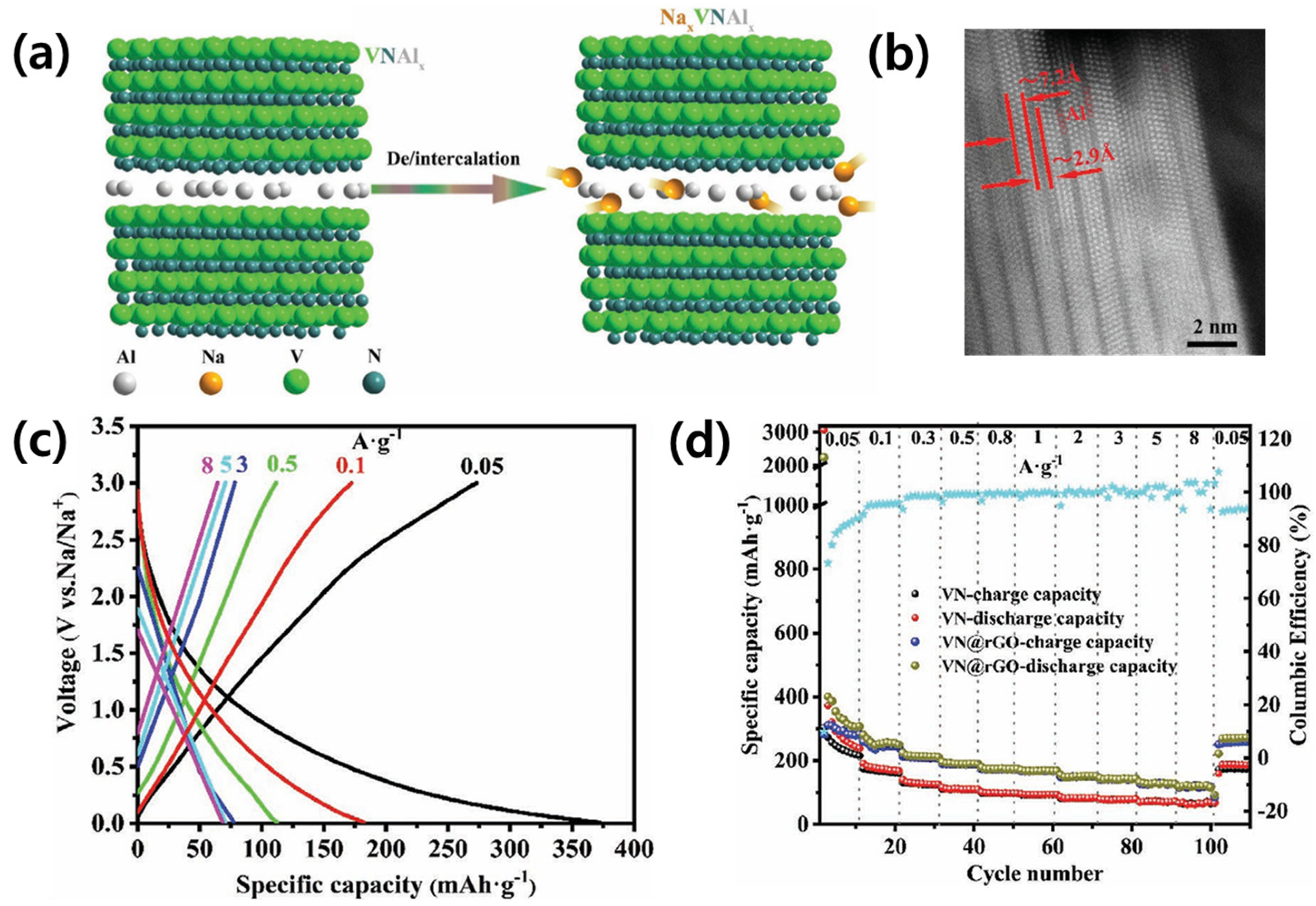
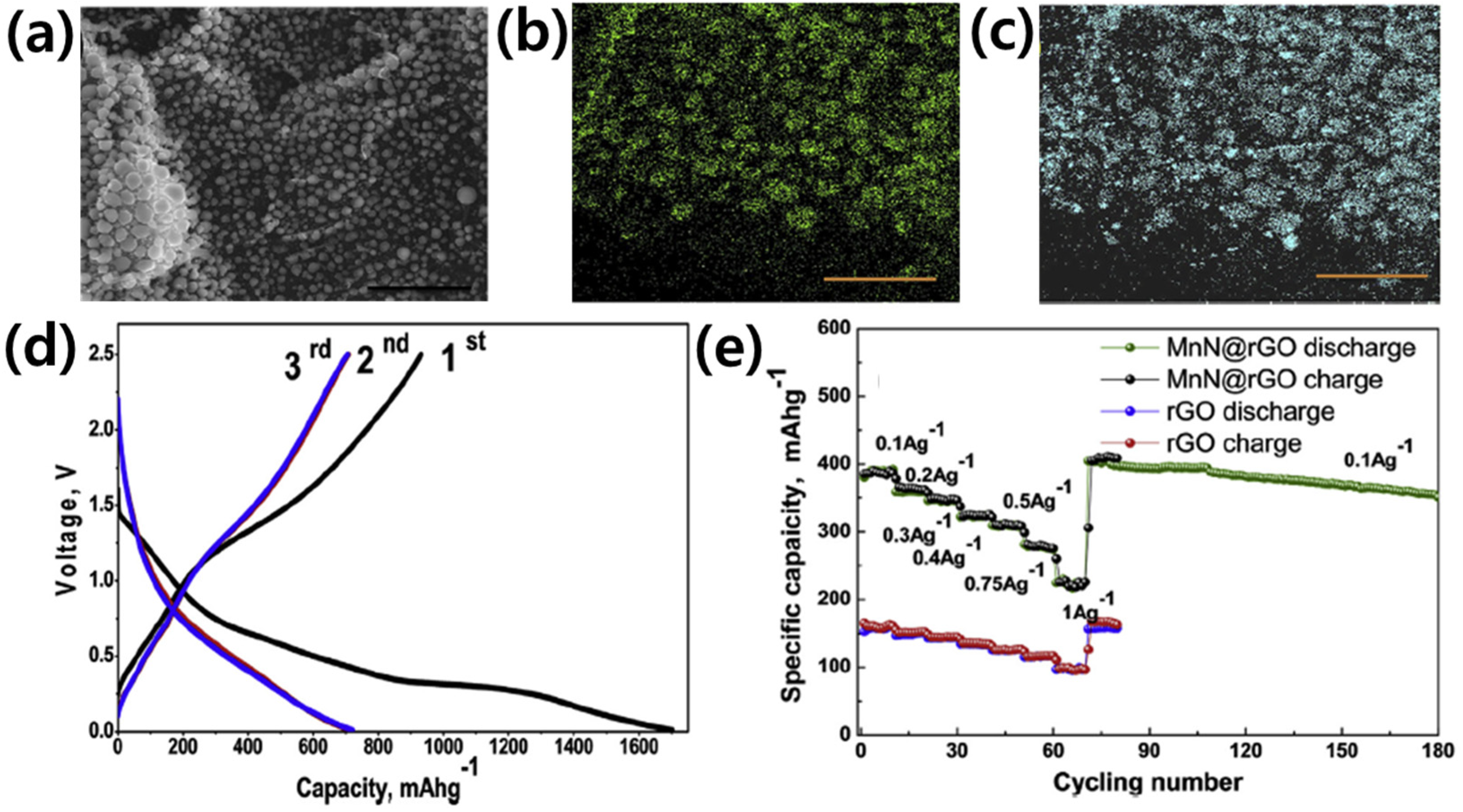
3.1.2. Metal Mono-Nitrides (MaNb, a = b)
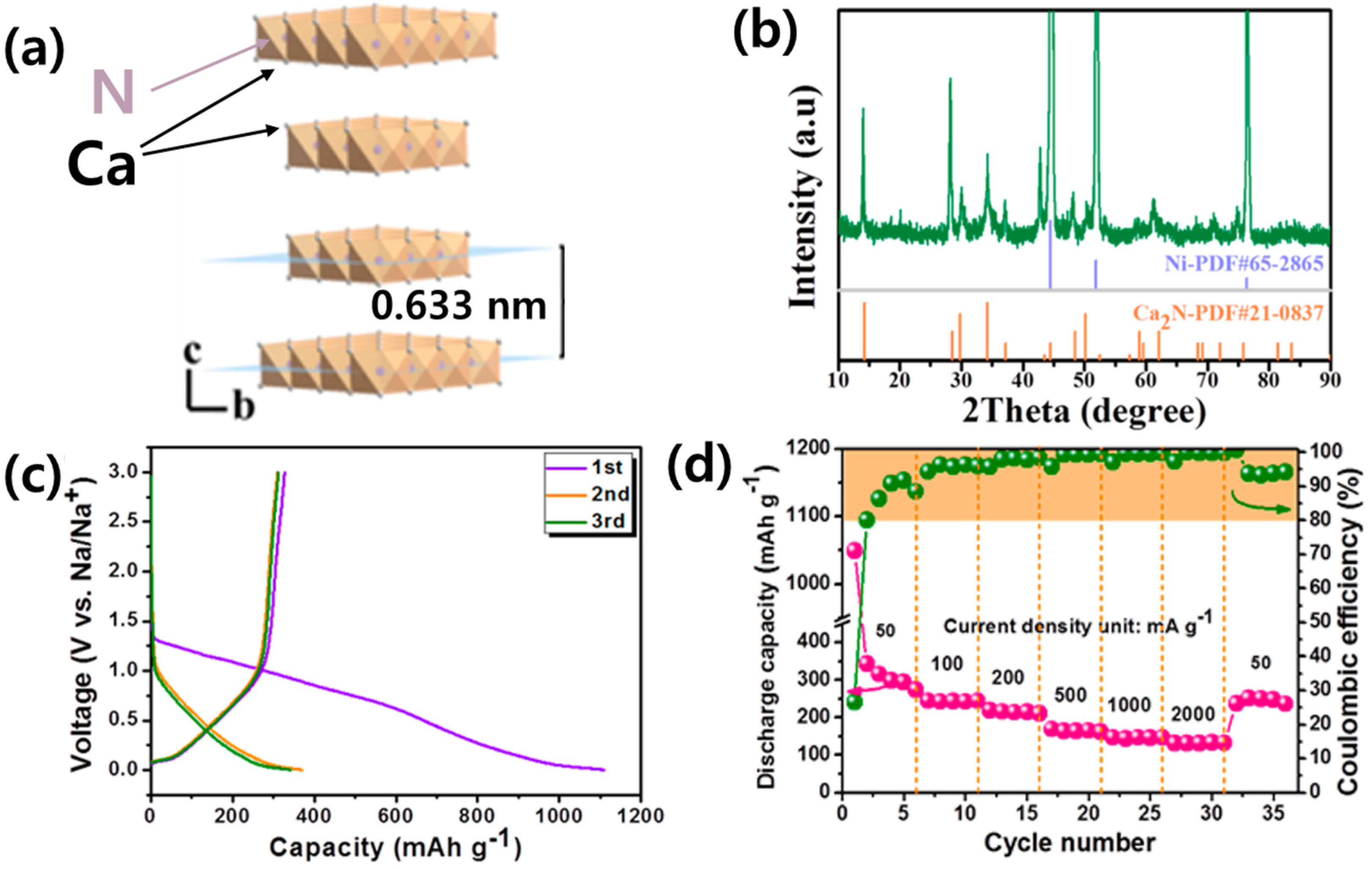
3.1.3. N-Rich Nitrides (MaNb, a < b)
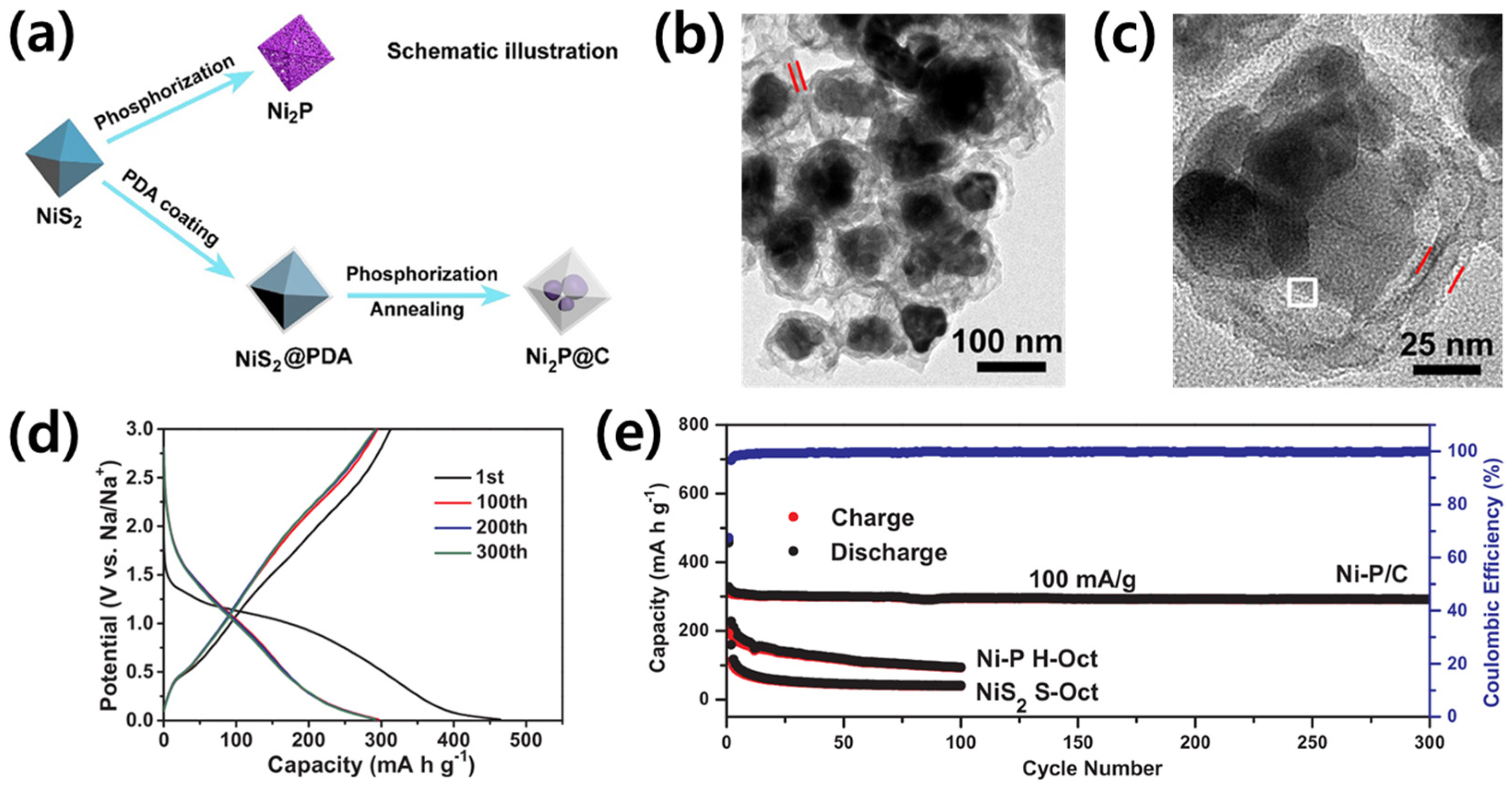
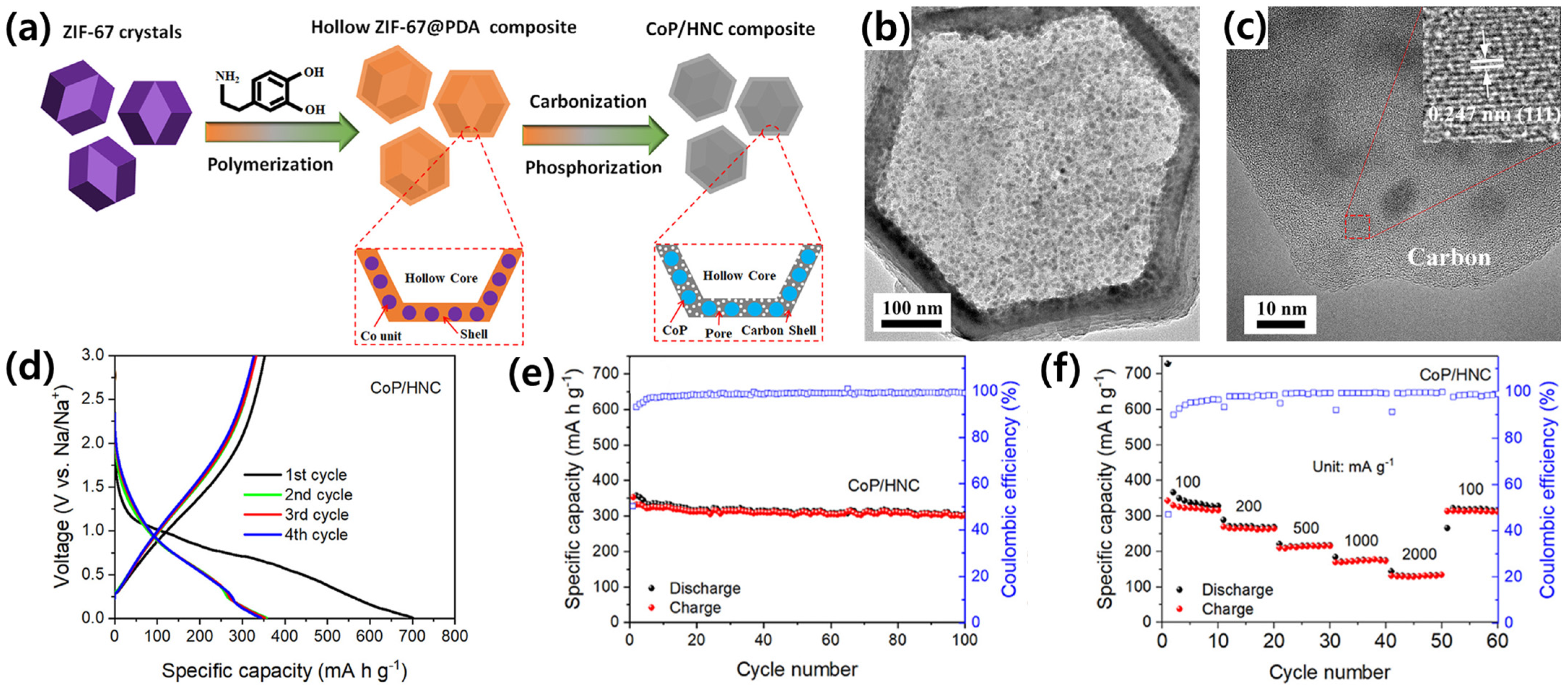
3.2. Metal Phosphides as Anode Materials
3.2.1. Metal-Rich Phosphides (MaPb, a > b)
3.2.2. Metal Mono-Phosphides (MaPb, a = b)
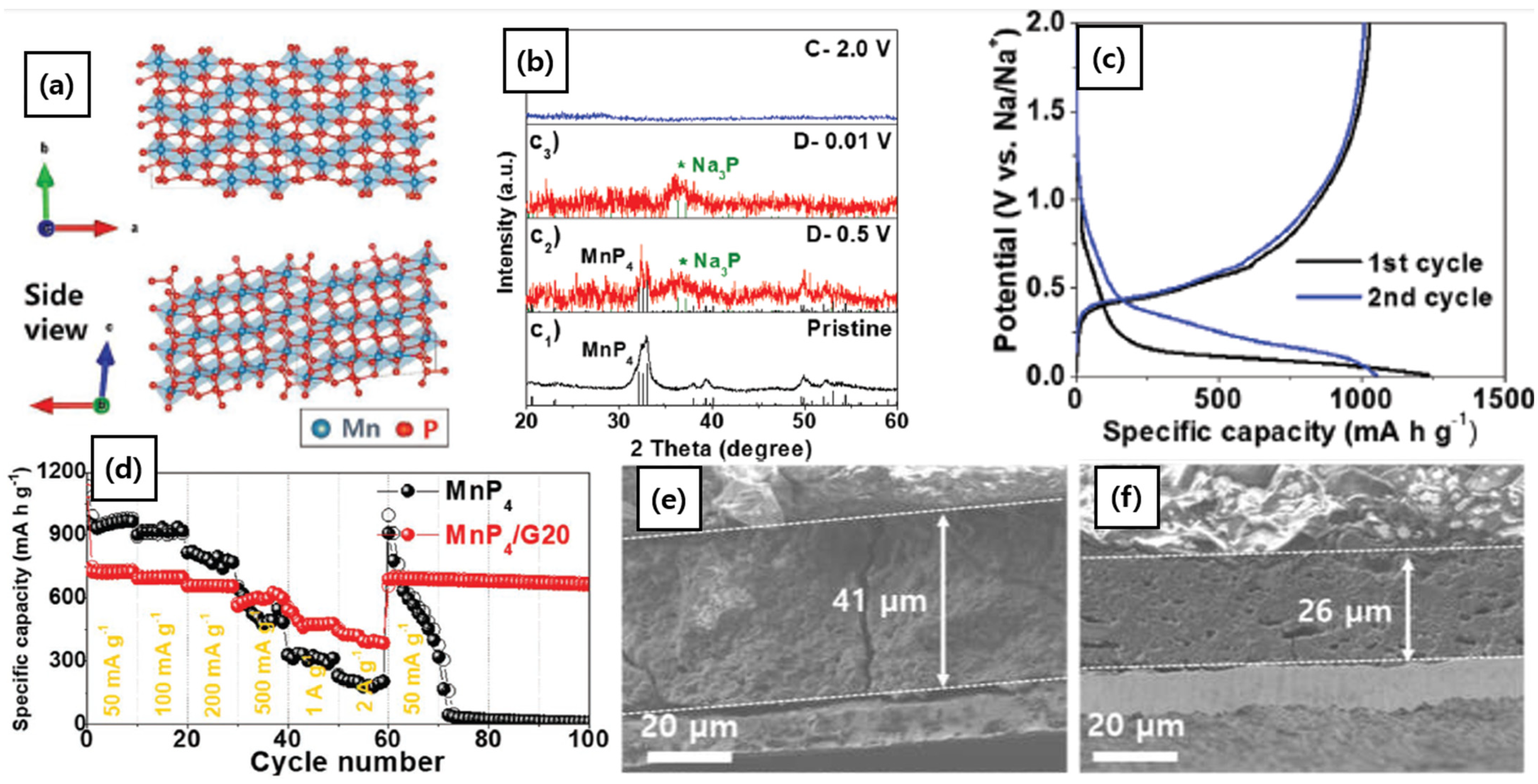
3.2.3. P-Rich Phosphides (MaPb, a < b)
3.3. Metal Antimonides as Anode Materials
3.3.1. Metal-Rich Antimonides (MaSbb, a > b)
3.3.2. Metal Mono-Antimonides (MaSbb, a = b)
3.3.3. Sb-Rich Antimonides (MaSbb, a < b)
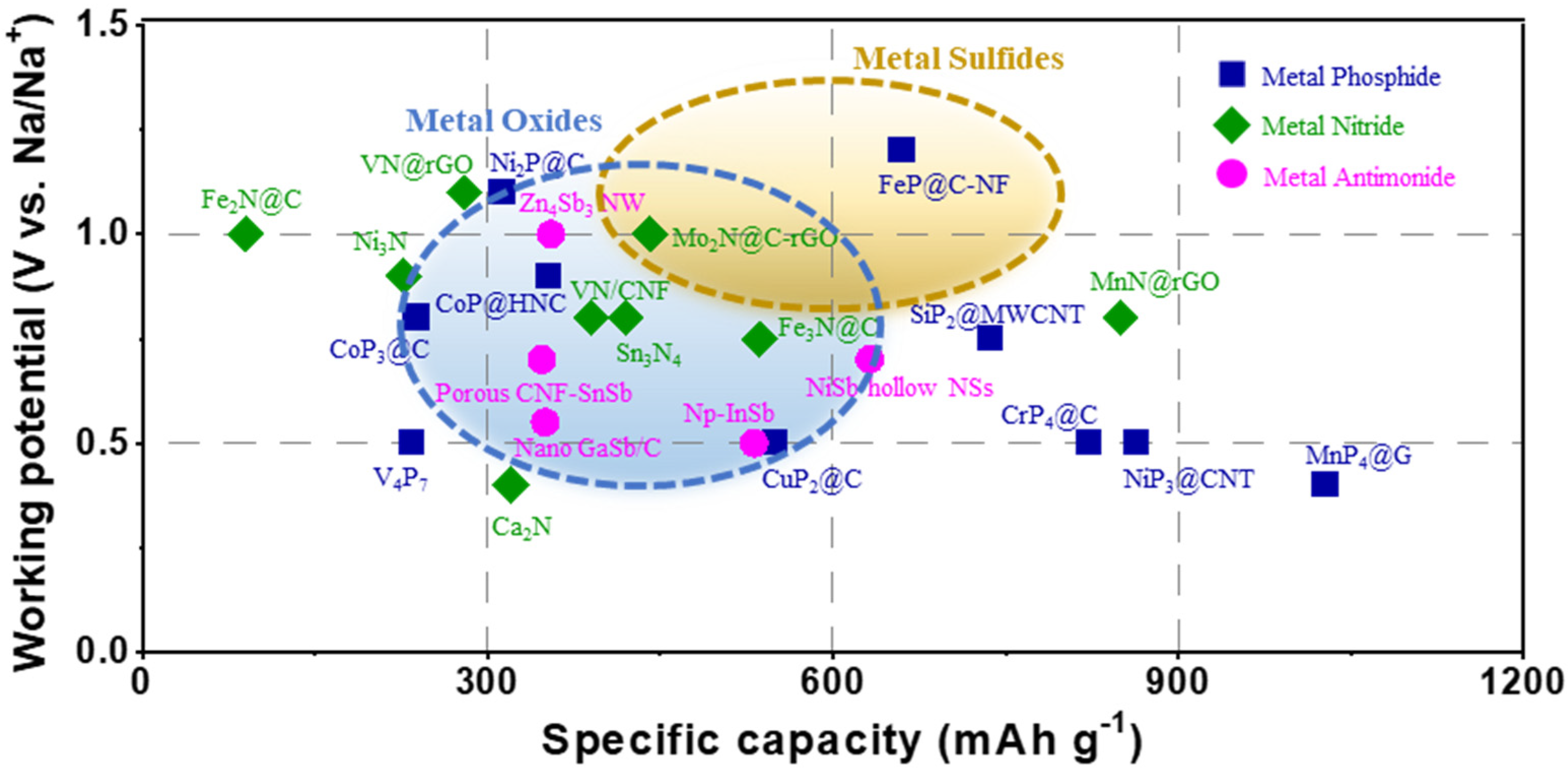
4. Addressing Challenges in Pnictogenides to be Applied for Commercial Electrodes
| System | Materials | Working Potential (V vs. Na/Na+) | Specific Capacity (mAh g−1) | Current Density (mA g−1) | I.C.E. (%) | Ref. |
|---|---|---|---|---|---|---|
| Metal nitrides (MNs) | Ni3N | 0.9 | 227 | 42 | 50 | [60] |
| Fe3N@C/3DNCF | 0.75 | 536 | 200 | 56.4 | [62] | |
| Fe2N@C | 1.0 | 93 | 500 | 60 | [103] | |
| Ca2N | 0.4 | 320 | 50 | 30 | [61] | |
| Mo2N@C-rGO | 1.0 | 441 | 200 | 42.3 | [63] | |
| VN@rGO | 1.1 | 280 | 50 | 12 | [65] | |
| VN/CNF | 0.8 | 390 | 100 | 49.2 | [64] | |
| MnN@rGO | 0.8 | 850 | 250 | 52.14 | [66] | |
| Sn3N4 | 0.8 | 420 | 200 | 32 | [68] | |
| Metal phosphides (MPs) | Ni2P@C | 1.1 | 313 | 100 | 67 | [77] |
| CoP/HNC | 0.9 | 353 | 100 | 50.35 | [79] | |
| FeP@C-NF | 1.2 | 660 | 100 | 69.8 | [78] | |
| V4P7 | 0.5 | 234 | 100 | 69.97 | [70] | |
| CuP2@C | 0.5 | 550 | 50 | 67.14 | [81] | |
| SiP2@MWCNT | 0.75 | 737 | 100 | 73.04 | [104] | |
| CoP3@C | 0.8 | 238 | 100 | 79 | [105] | |
| NiP3@CNT | 0.5 | 864 | 100 | 64.6 | [72] | |
| CrP4@C | 0.5 | 823 | 50 | 76.3 | [74] | |
| MnP4@G | 0.4 | 1028 | 50 | 83.3 | [73] | |
| Metal antimonides (MSbs) | CNF-SnSb | 0.6 | 347 | 100 | 53 | [86] |
| np-InSb | 0.5 | 531.5 | 200 | 74.7 | [87] | |
| nano GaSb/C | 0.6 | 350 | 50 | 76.8 | [106] | |
| NiSb NSs | 0.7 | 632 | 60 | 37.6 | [107] | |
| Zn4Sb3 NW | 0.25 | 355 | 41.4 | 45 | [85] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hasan, M.K.; Mahmud, M.; Ahasan Habib, A.K.M.; Motakabber, S.M.A.; Islam, S. Review of electric vehicle energy storage and management system: Standards, issues, and challenges. J. Energy Storage 2021, 41, 102940. [Google Scholar] [CrossRef]
- Butt, F.S.; Lewis, A.; Chen, T.; Mazlan, N.A.; Wei, X.; Hayer, J.; Chen, S.; Han, J.; Yang, Y.; Yang, S.; et al. Lithium Harvesting from the Most Abundant Primary and Secondary Sources: A Comparative Study on Conventional and Membrane Technologies. Membranes 2022, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Rostami, H.; Valio, J.; Suominen, P.; Tynjälä, P.; Lassi, U. Advancements in cathode technology, recycling strategies, and market dynamics: A comprehensive review of sodium ion batteries. Chem. Eng. J. 2024, 495, 153471. [Google Scholar] [CrossRef]
- Mohan, I.; Raj, A.; Shubham, K.; Lata, D.B.; Mandal, S.; Kumar, S. Potential of potassium and sodium-ion batteries as the future of energy storage: Recent progress in anodic materials. J. Energy Storage 2022, 55, 105625. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Ciucci, F.; Huang, B.; Kim, J.-K. Rationally designed nanostructured metal chalcogenides for advanced sodium-ion batteries. Energy Storage Mater. 2021, 34, 582–628. [Google Scholar] [CrossRef]
- Ghahari, A.; Raissi, H. Architectural design of anode materials for superior alkali-ion (Li/Na/K) batteries storage. Sci. Rep. 2024, 14, 3959. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; He, X.-X.; Zhao, J.; Yang, Z.; Li, L.; Wu, X.; Li, L.; Chou, S.-L. Boosting the Development of Hard Carbon for Sodium-Ion Batteries: Strategies to Optimize the Initial Coulombic Efficiency. Adv. Funct. Mater. 2024, 34, 2302277. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q.-H. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Loaiza, L.C.; Monconduit, L.; Seznec, V. Si and Ge-Based Anode Materials for Li-, Na-, and K-Ion Batteries: A Perspective from Structure to Electrochemical Mechanism. Small 2020, 16, 1905260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, T.; Cheng, K.-w.E.; Lam, K.-h.; Boles, S.T. Insights into the Sodiation Kinetics of Si and Ge Anodes for Sodium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 100518. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent progress on sodium ion batteries: Potential high-performance anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Ali, Z.; Zhang, T.; Asif, M.; Zhao, L.; Yu, Y.; Hou, Y. Transition metal chalcogenide anodes for sodium storage. Mater. Today 2020, 35, 131–167. [Google Scholar] [CrossRef]
- Wu, C.; Dou, S.-X.; Yu, Y. The State and Challenges of Anode Materials Based on Conversion Reactions for Sodium Storage. Small 2018, 14, 1703671. [Google Scholar] [CrossRef]
- Kumar Prajapati, A.; Bhatnagar, A. A review on anode materials for lithium/sodium-ion batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Zhang, H.; Hasa, I.; Passerini, S. Beyond Insertion for Na-Ion Batteries: Nanostructured Alloying and Conversion Anode Materials. Adv. Energy Mater. 2018, 8, 1702582. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Wang, S.; Lv, Y.; Li, Y.; Chen, X.; Xia, A.; Li, Y.; Wu, J.; Ma, L. Phosphorus/Phosphide-Based Materials for Alkali Metal-Ion Batteries. Adv. Sci. 2022, 9, 2200740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Q.; Zhu, J.; Yan, Q.; Dou, S.X.; Sun, W. Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis. Adv. Funct. Mater. 2017, 27, 1702317. [Google Scholar] [CrossRef]
- Wu, X.; Lan, X.; Hu, R.; Yao, Y.; Yu, Y.; Zhu, M. Tin-Based Anode Materials for Stable Sodium Storage: Progress and Perspective. Adv. Mater. 2022, 34, 2106895. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Wang, Y.; Liu, Y.; Nai, J.; Zhang, L.; Sheng, O.; Tao, X. Strategies to improve the performance of phosphide anodes in sodium-ion batteries. Nano Energy 2021, 90, 106475. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Liu, Q.; Wang, Y.; Wang, D.; Li, Z.; Zheng, P.; Liu, Z. Recent advances in nanostructured metal phosphides as promising anode materials for rechargeable batteries. J. Mater. Chem. A 2020, 8, 19113–19132. [Google Scholar] [CrossRef]
- Patel, P.C.; Awasthi, S.; Mishra, P.K.; Lakharwal, P.; Kashyap, J. Fe–As Intermetallic Alloys: A Way Out for Sodium-Ion Batteries. Energy Fuels 2023, 37, 16062–16071. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.; Chen, J.; Bruch, J. Exposures to silica mixed dust and cohort mortality study in tin mines: Exposure-response analysis and risk assessment of lung cancer. Am. J. Ind. Med. 2006, 49, 67–76. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A. Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol. Appl. Pharmacol. 2004, 198, 405–411. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Sb-based intermetallics and alloys for sodium-ion batteries: Trends, challenges and future prospects from material synthesis to battery performance. J. Mater. Chem. A 2021, 9, 5164–5196. [Google Scholar] [CrossRef]
- Chen, B.; Liang, M.; Wu, Q.; Zhu, S.; Zhao, N.; He, C. Recent Developments of Antimony-Based Anodes for Sodium- and Potassium-Ion Batteries. Trans. Tianjin Univ. 2022, 28, 6–32. [Google Scholar] [CrossRef]
- Hao, Y.; Shao, J.; Yuan, Y.; Li, X.; Xiao, W.; Sari, H.M.K.; Liu, T.; Lu, J. Design of Phosphide Anodes Harvesting Superior Sodium Storage: Progress, Challenges, and Perspectives. Adv. Funct. Mater. 2023, 33, 2212692. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Zhang, X.; Cui, G. Nanostructured transition metal nitrides for energy storage and fuel cells. Coord. Chem. Rev. 2013, 257, 1946–1956. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2015, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, W. Diffusional control of the near-surface microstructure in functional gradient hardmetals. Mater. Und Werkst. 2005, 36, 460–466. [Google Scholar] [CrossRef]
- Idrees, M.; Mukhtar, A.; Ata ur, R.; Abbas, S.M.; Zhang, Q.; Li, X. Transition metal nitride electrodes as future energy storage devices: A review. Mater. Today Commun. 2021, 27, 102363. [Google Scholar] [CrossRef]
- Chen, K.; Pathak, R.; Gurung, A.; Adhamash, E.A.; Bahrami, B.; He, Q.; Qiao, H.; Smirnova, A.L.; Wu, J.J.; Qiao, Q.; et al. Flower-shaped lithium nitride as a protective layer via facile plasma activation for stable lithium metal anodes. Energy Storage Mater. 2019, 18, 389–396. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Xue, X.; Wang, J.; Wang, Y.; Bresser, D.; Liu, X.; Chen, M.; Passerini, S. Achieving stable lithium metal anode via constructing lithiophilicity gradient and regulating Li3N-rich SEI. Nano Energy 2025, 133, 110439. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Huang, Y.; Qiu, W.; Yang, H.; Ji, H.; Tong, Y. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today 2017, 20, 425–451. [Google Scholar] [CrossRef]
- Reddy, M.V.; Prithvi, G.; Loh, K.P.; Chowdari, B.V.R. Li Storage and Impedance Spectroscopy Studies on Co3O4, CoO, and CoN for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, P.; Li, X.; Tong, Y.; Ding, H.; Wu, X.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Metallic Nickel Nitride Nanosheets Realizing Enhanced Electrochemical Water Oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sankapal, B.R. Metal phosphides: Topical advances in the design of supercapacitors. J. Mater. Chem. A 2021, 9, 20241–20276. [Google Scholar] [CrossRef]
- Tahri, W.; Zhou, X.; Khan, R.; Sajid, M. Recent Trends in Transition Metal Phosphide (TMP)-Based Seawater Electrolysis for Hydrogen Evolution. Sustainability 2023, 15, 14389. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kim, H.-H.; Hong, S.-H. NiP2/C nanocomposite as a high performance anode for sodium ion batteries. Electrochim. Acta 2022, 403, 139686. [Google Scholar] [CrossRef]
- Chang, G.; Zhao, Y.; Dong, L.; Wilkinson, D.P.; Zhang, L.; Shao, Q.; Yan, W.; Sun, X.; Zhang, J. A review of phosphorus and phosphides as anode materials for advanced sodium-ion batteries. J. Mater. Chem. A 2020, 8, 4996–5048. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Chen, H.; Lv, X.; Jiang, Y.; Feng, Y.; Rui, X.; Yu, Y. Advances in metal phosphides for sodium-ion batteries. SusMat 2021, 1, 359–392. [Google Scholar] [CrossRef]
- Liu, Y.; McCue, A.J.; Li, D. Metal Phosphides and Sulfides in Heterogeneous Catalysis: Electronic and Geometric Effects. ACS Catal. 2021, 11, 9102–9127. [Google Scholar] [CrossRef]
- Pöhls, J.-H.; Faghaninia, A.; Petretto, G.; Aydemir, U.; Ricci, F.; Li, G.; Wood, M.; Ohno, S.; Hautier, G.; Snyder, G.J.; et al. Metal phosphides as potential thermoelectric materials. J. Mater. Chem. C 2017, 5, 12441–12456. [Google Scholar] [CrossRef]
- Bhunia, K.; Chandra, M.; Kumar Sharma, S.; Pradhan, D.; Kim, S.-J. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord. Chem. Rev. 2023, 478, 214956. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Gong, N.; Deng, C.; Wu, L.; Wan, B.; Wang, Z.; Li, Z.; Gou, H.; Gao, F. Structural Diversity and Electronic Properties of 3d Transition Metal Tetraphosphides, TMP4 (TM = V, Cr, Mn, and Fe). Inorg. Chem. 2018, 57, 9385–9392. [Google Scholar] [CrossRef]
- Dwivedi, A.; Pandey, A.; Bajpai, A. Stability and electronic properties of transition metal antimonides in ionic and neutral case-TMmSbn (m, n=1-2). J. Comput. Methods Mol. Des. 2014, 4, 38–45. [Google Scholar]
- Jing, W.T.; Yang, C.C.; Jiang, Q. Recent progress on metallic Sn- and Sb-based anodes for sodium-ion batteries. J. Mater. Chem. A 2020, 8, 2913–2933. [Google Scholar] [CrossRef]
- Bennett, B.; Magno, R.; Boos, J.; Kruppa, W.; Ancona, M. Antimonide-based compound semiconductors for electronic devices: A review. Solid-State Electron. 2005, 49, 1875–1895. [Google Scholar] [CrossRef]
- Usui, H.; Domi, Y.; Takada, N.; Sakaguchi, H. Reaction Mechanism of Indium Antimonide as a Sodium Storage Material. Cryst. Growth Des. 2021, 21, 218–226. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Wriedt, H.A. The N-Ni (Nitrogen-Nickel) system. Bull. Alloy Phase Diagr. 1985, 6, 558–563. [Google Scholar] [CrossRef]
- Li, X.; Hasan, M.M.; Hector, A.L.; Owen, J.R. Performance of nanocrystalline Ni3N as a negative electrode for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 6441–6445. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Li, H.; Li, Y.; Wang, Z.; Ni, Q.; Liu, L.; Wu, F.; Yao, Y.; Wu, C. Multilayered Electride Ca2N Electrode via Compression Molding Fabrication for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 6666–6669. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xiong, X.; Zeng, J.; Zou, P.; Lin, Z.; Liu, M. Melamine-assisted synthesis of Fe3N featuring highly reversible crystalline-phase transformation for ultrastable sodium ion storage. J. Mater. Chem. A 2020, 8, 6768–6775. [Google Scholar] [CrossRef]
- Vadahanambi, S.; Park, H. Carbon sheathed molybdenum nitride nanoparticles anchored on reduced graphene oxide as high-capacity sodium-ion battery anodes and supercapacitors. New J. Chem. 2018, 42, 5668–5673. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, P.; Zeng, L.; Liu, R.; Liu, J.; Luo, F.; Li, X.; Chen, Q.; Wei, M.; Qian, Q. Facile fabrication of a vanadium nitride/carbon fiber composite for half/full sodium-ion and potassium-ion batteries with long-term cycling performance. Nanoscale 2020, 12, 10693–10702. [Google Scholar] [CrossRef]
- Wei, S.; Wang, C.; Chen, S.; Zhang, P.; Zhu, K.; Wu, C.; Song, P.; Wen, W.; Song, L. Dial the Mechanism Switch of VN from Conversion to Intercalation toward Long Cycling Sodium-Ion Battery. Adv. Energy Mater. 2020, 10, 1903712. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Manganese nitride stabilized on reduced graphene oxide substrate for high performance sodium ion batteries, super-capacitors and EMI shielding. J. Alloys Compd. 2019, 808, 151748. [Google Scholar] [CrossRef]
- Li, Z.; Ding, J.; Mitlin, D. Tin and Tin Compounds for Sodium Ion Battery Anodes: Phase Transformations and Performance. Acc. Chem. Res. 2015, 48, 1657–1665. [Google Scholar] [CrossRef]
- Fitch, S.D.S.; Cibin, G.; Hepplestone, S.P.; Garcia-Araez, N.; Hector, A.L. Solvothermal synthesis of Sn3N4 as a high capacity sodium-ion anode: Theoretical and experimental study of its storage mechanism. J. Mater. Chem. A 2020, 8, 16437–16450. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Zhang, J.; Lou, X.W. Necklace-Like Structures Composed of Fe3N@C Yolk–Shell Particles as an Advanced Anode for Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1800525. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Choi, J.; Hong, S.-H. Superior electrochemical sodium storage of V4P7 nanoparticles as an anode for rechargeable sodium-ion batteries. Chem. Commun. 2019, 55, 3207–3210. [Google Scholar] [CrossRef]
- He, L.; Guo, J.; Liu, S.; Wang, F.; Li, X.; Su, Z. Progress of metal phosphides as the anode materials for sodium ion batteries. J. Alloys Compd. 2024, 997, 174924. [Google Scholar] [CrossRef]
- Ihsan-Ul-Haq, M.; Huang, H.; Cui, J.; Yao, S.; Wu, J.; Chong, W.G.; Huang, B.; Kim, J.-K. Chemical interactions between red P and functional groups in NiP3/CNT composite anodes for enhanced sodium storage. J. Mater. Chem. A 2018, 6, 20184–20194. [Google Scholar] [CrossRef]
- Kim, K.-H.; Hong, S.-H. Manganese Tetraphosphide (MnP4) as a High Capacity Anode for Lithium-Ion and Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003609. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.; Kim, K.-H.; Hong, S.-H. Chromium tetraphosphide (CrP4) as a high-performance anode for Li ion and Na ion batteries. J. Mater. Chem. A 2024, 12, 11463–11472. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Li, C.-L.; Wang, W.-H.; Yu, D.Y.W. Facile synthesis of hollow Cu3P for sodium-ion batteries anode. Rare Met. 2021, 40, 3460–3465. [Google Scholar] [CrossRef]
- Hino, M.; Iikubo, S.; Ohtani, H. Thermodynamic Analysis of the Cu-Sn-P Ternary System. High Temp. Mater. Process. 2011, 30, 387–404. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, X.; Pan, X.; Teng, C.; Wang, N. Yolk-shelled Ni2P@carbon nanocomposite as high-performance anode material for lithium and sodium ion batteries. Appl. Surf. Sci. 2019, 473, 699–705. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.; Lee, D.C.; Bell, C.; Drexler, M.; Ma, Z.F.; Magasinski, A.; Yushin, G.; Alamgir, F.M. Porous FeP/C composite nanofibers as high-performance anodes for Li-ion/Na-ion batteries. Mater. Today Energy 2020, 16, 100410. [Google Scholar] [CrossRef]
- Huang, W.; Shangguan, H.; Zheng, X.; Engelbrekt, C.; Yang, Y.; Li, S.; Mølhave, K.; Xiao, X.; Lin, X.; Ci, L.; et al. Three-dimensional hollow nitrogen-doped carbon shells enclosed monodisperse CoP nanoparticles for long cycle-life sodium storage. Electrochim. Acta 2021, 395, 139112. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Chen, Z.; Wang, Y.; Li, Y.; Chai, J.; Han, N.; Tang, B.; Rui, Y.; Jiang, L. Metal Phosphide Anodes in Sodium-Ion Batteries: Latest Applications and Progress. Small 2024, 20, 2310426. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Wang, L.; Tang, L.; Zhu, M.; Wu, C.; Dou, S.-X.; Wu, M. Graphene-Encapsulated CuP2: A Promising Anode Material with High Reversible Capacity and Superior Rate-Performance for Sodium-Ion Batteries. Nano Lett. 2019, 19, 2575–2582. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.-J.; Zhu, J.; Xia, Y.; Müller-Buschbaum, P. A Chronicle Review of Nonsilicon (Sn, Sb, Ge)-Based Lithium/Sodium-Ion Battery Alloying Anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Baggetto, L.; Ganesh, P.; Sun, C.-N.; Meisner, R.A.; Zawodzinski, T.A.; Veith, G.M. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: Experiment and theory. J. Mater. Chem. A 2013, 1, 7985–7994. [Google Scholar] [CrossRef]
- Baggetto, L.; Carroll, K.J.; Hah, H.-Y.; Johnson, C.E.; Mullins, D.R.; Unocic, R.R.; Johnson, J.A.; Meng, Y.S.; Veith, G.M. Probing the Mechanism of Sodium Ion Insertion into Copper Antimony Cu2Sb Anodes. J. Phys. Chem. C 2014, 118, 7856–7864. [Google Scholar] [CrossRef][Green Version]
- Nie, A.; Gan, L.-Y.; Cheng, Y.; Tao, X.; Yuan, Y.; Sharifi-Asl, S.; He, K.; Asayesh-Ardakani, H.; Vasiraju, V.; Lu, J.; et al. Ultrafast and Highly Reversible Sodium Storage in Zinc-Antimony Intermetallic Nanomaterials. Adv. Funct. Mater. 2016, 26, 543–552. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofibers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Guo, Z.; Yu, B.; Cheng, G.; Yang, W.; Zhang, Z. Dealloying-induced dual-scale nanoporous indium-antimony anode for sodium/potassium ion batteries. J. Energy Chem. 2022, 75, 154–163. [Google Scholar] [CrossRef]
- Edison, E.; Sreejith, S.; Madhavi, S. Melt-Spun Fe–Sb Intermetallic Alloy Anode for Performance Enhanced Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 39399–39406. [Google Scholar] [CrossRef]
- Baggetto, L.; Allcorn, E.; Unocic, R.R.; Manthiram, A.; Veith, G.M. Mo3Sb7 as a very fast anode material for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2013, 1, 11163–11169. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. Boost sodium-ion batteries to commercialization: Strategies to enhance initial Coulombic efficiency of hard carbon anode. Nano Energy 2021, 82, 105738. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, B.; Bai, J.; Tong, P.; Zhu, X.; Sun, Y. Transition Metal Nitrides in Lithium- and Sodium-Ion Batteries: Recent Progress and Perspectives. Adv. Mater. Interfaces 2022, 9, 2200606. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From spent graphite to recycle graphite anode for high-performance lithium ion batteries and sodium ion batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.-T.; Sun, Y.-K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Niu, J.; Mai, W.; Liu, L.; Xu, Z. Self-healing Sn4P3@Hard carbon Co-storage anode for sodium-ion batteries. J. Alloys Compd. 2021, 851, 156746. [Google Scholar] [CrossRef]
- Cheng, H.; Garcia-Araez, N.; Hector, A.L. Synthesis of Vanadium Nitride–Hard Carbon Composites from Cellulose and Their Performance for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4286–4294. [Google Scholar] [CrossRef]
- Capone, I.; Hurlbutt, K.; Naylor, A.J.; Xiao, A.W.; Pasta, M. Effect of the Particle-Size Distribution on the Electrochemical Performance of a Red Phosphorus–Carbon Composite Anode for Sodium-Ion Batteries. Energy Fuels 2019, 33, 4651–4658. [Google Scholar] [CrossRef]
- Mogensen, R.; Colbin, S.; Younesi, R. An Attempt to Formulate Non-Carbonate Electrolytes for Sodium-Ion Batteries. Batter. Supercaps 2021, 4, 791–814. [Google Scholar] [CrossRef]
- Patra, J.; Huang, H.-T.; Xue, W.; Wang, C.; Helal, A.S.; Li, J.; Chang, J.-K. Moderately concentrated electrolyte improves solid–electrolyte interphase and sodium storage performance of hard carbon. Energy Storage Mater. 2019, 16, 146–154. [Google Scholar] [CrossRef]
- Rakov, D.A.; Chen, F.; Ferdousi, S.A.; Li, H.; Pathirana, T.; Simonov, A.N.; Howlett, P.C.; Atkin, R.; Forsyth, M. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat. Mater. 2020, 19, 1096–1101. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Mu, P.; Wang, G.; Luo, C.; Zhang, X.; Gao, C.; Zhou, X.; Cui, G. Covalently Cross-Linked Chemistry of a Three-Dimensional Network Binder at Limited Dosage Enables Practical Si/C Composite Electrode Applications. ACS Nano 2024, 18, 2475–2484. [Google Scholar] [CrossRef]
- Xu, M.; Wei, X.; Yan, Z.; Huang, J.; Wu, S.; Ye, K.-H.; Lin, Z. A Fast Self-Healing Binder for Highly Stable SiOx Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 55353–55361. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Yang, Z.; He, X.-X.; Liu, X.-H.; Zhang, H.; Gao, Y.; Qiao, Y.; Li, L.; Chou, S.-L. Binders for sodium-ion batteries: Progress, challenges and strategies. Chem. Commun. 2021, 57, 12406–12416. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Wang, W.; Yang, L.; Zhu, K.; Wang, H. Synthesis of coral-like Fe2N@C nanoparticles and application in sodium ion batteries as a novel anode electrode material. RSC Adv. 2016, 6, 86131–86136. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Yi, Y.-H.; Chang, W.-C.; Kao, T.-L.; Tuan, H.-Y. Multi-walled carbon nanotube-wrapped SiP2 as a superior anode material for lithium-ion and sodium-ion batteries. J. Power Sources 2018, 399, 49–58. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Wang, G.; Long, X.; Li, Y.; Zhang, W.; Zhang, P. Carbon-coated CoP3 nanocomposites as anode materials for high-performance sodium-ion batteries. Appl. Surf. Sci. 2018, 445, 167–174. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, Y.-H.; Yoon, J.-M.; Hwa, Y.; Park, C.-M. GaSb nanocomposite: New high-performance anode material for Na- and K-ion batteries. Compos. Part B Eng. 2022, 243, 110142. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Wang, J.; Gu, L.; Maier, J.; Yu, Y. Three-dimensionally interconnected nickel–antimony intermetallic hollow nanospheres as anode material for high-rate sodium-ion batteries. Nano Energy 2015, 16, 389–398. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.; Kim, J.; Kim, D.W.; Suh, J.M.; Kim, K.-H. A Review of Pnictogenides for Next-Generation Anode Materials for Sodium-Ion Batteries. Batteries 2025, 11, 54. https://doi.org/10.3390/batteries11020054
Ha S, Kim J, Kim DW, Suh JM, Kim K-H. A Review of Pnictogenides for Next-Generation Anode Materials for Sodium-Ion Batteries. Batteries. 2025; 11(2):54. https://doi.org/10.3390/batteries11020054
Chicago/Turabian StyleHa, Sion, Junhee Kim, Dong Won Kim, Jun Min Suh, and Kyeong-Ho Kim. 2025. "A Review of Pnictogenides for Next-Generation Anode Materials for Sodium-Ion Batteries" Batteries 11, no. 2: 54. https://doi.org/10.3390/batteries11020054
APA StyleHa, S., Kim, J., Kim, D. W., Suh, J. M., & Kim, K.-H. (2025). A Review of Pnictogenides for Next-Generation Anode Materials for Sodium-Ion Batteries. Batteries, 11(2), 54. https://doi.org/10.3390/batteries11020054








