Electrode Capacity Balancing for Accurate Battery State of Health Prediction and Degradation Analysis
Abstract
1. Introduction
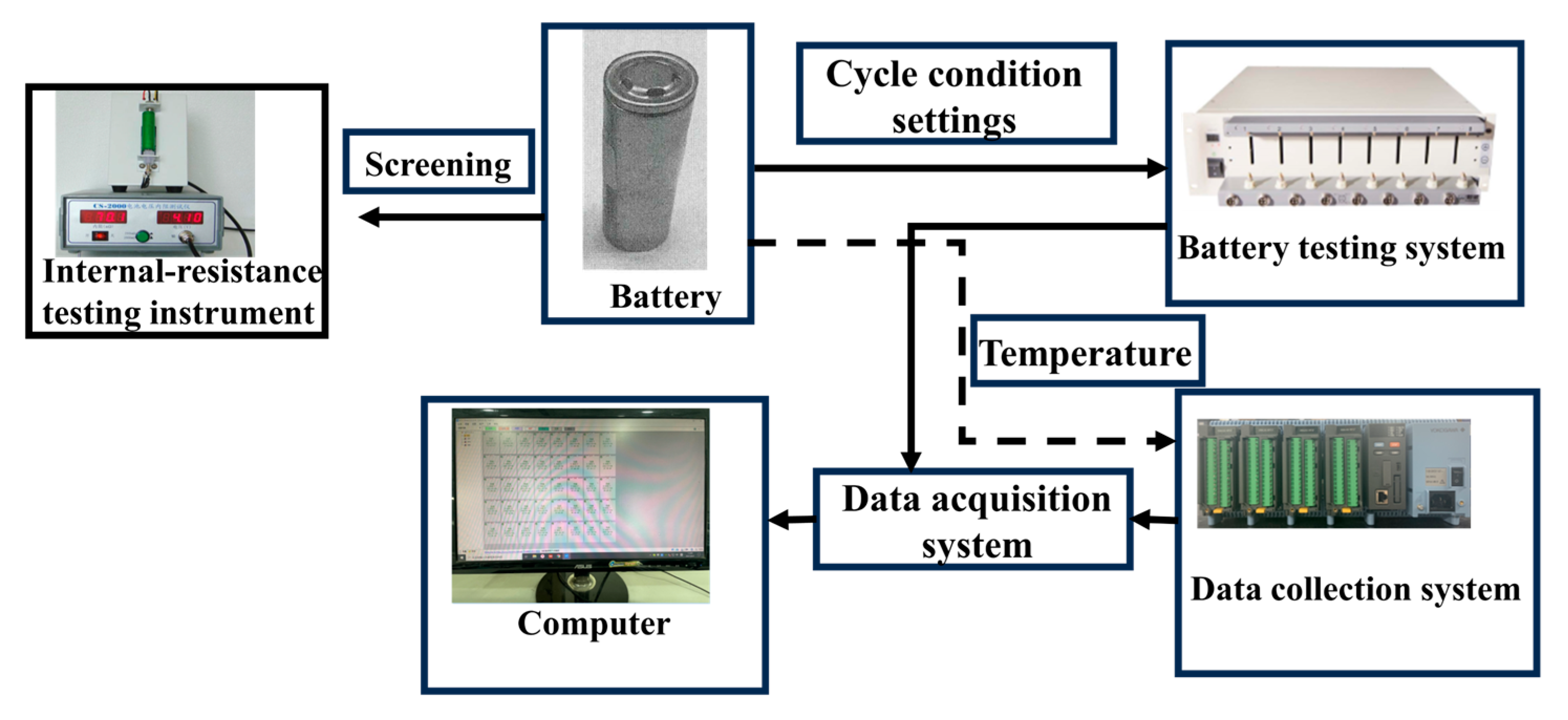
2. Experimental System and Conditions
3. Battery Health State Analysis
3.1. Internal Capacity Balance Model for Battery Degradation During Cycling
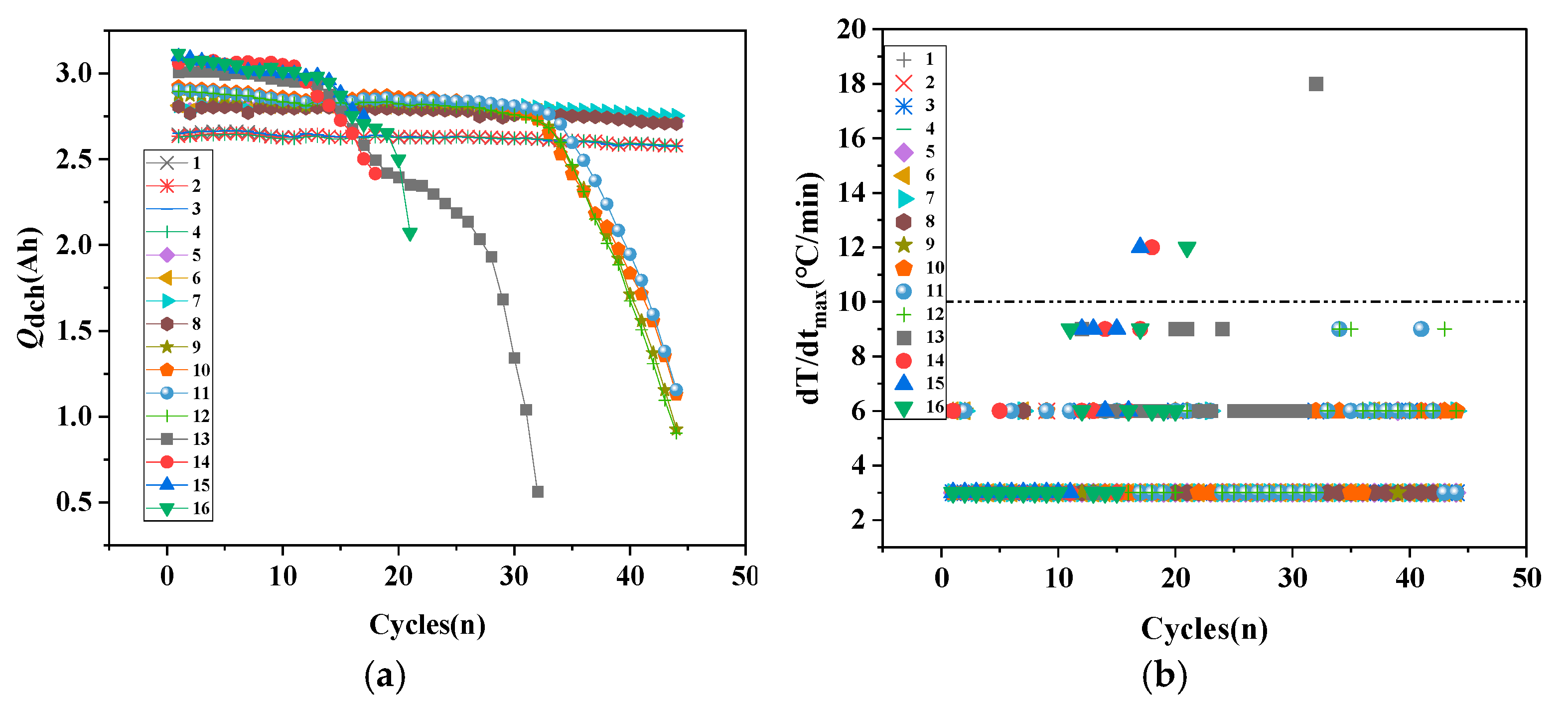
3.2. Analysis of Battery Health Performance Decline
3.3. SOH Prediction Indicators
- (1)
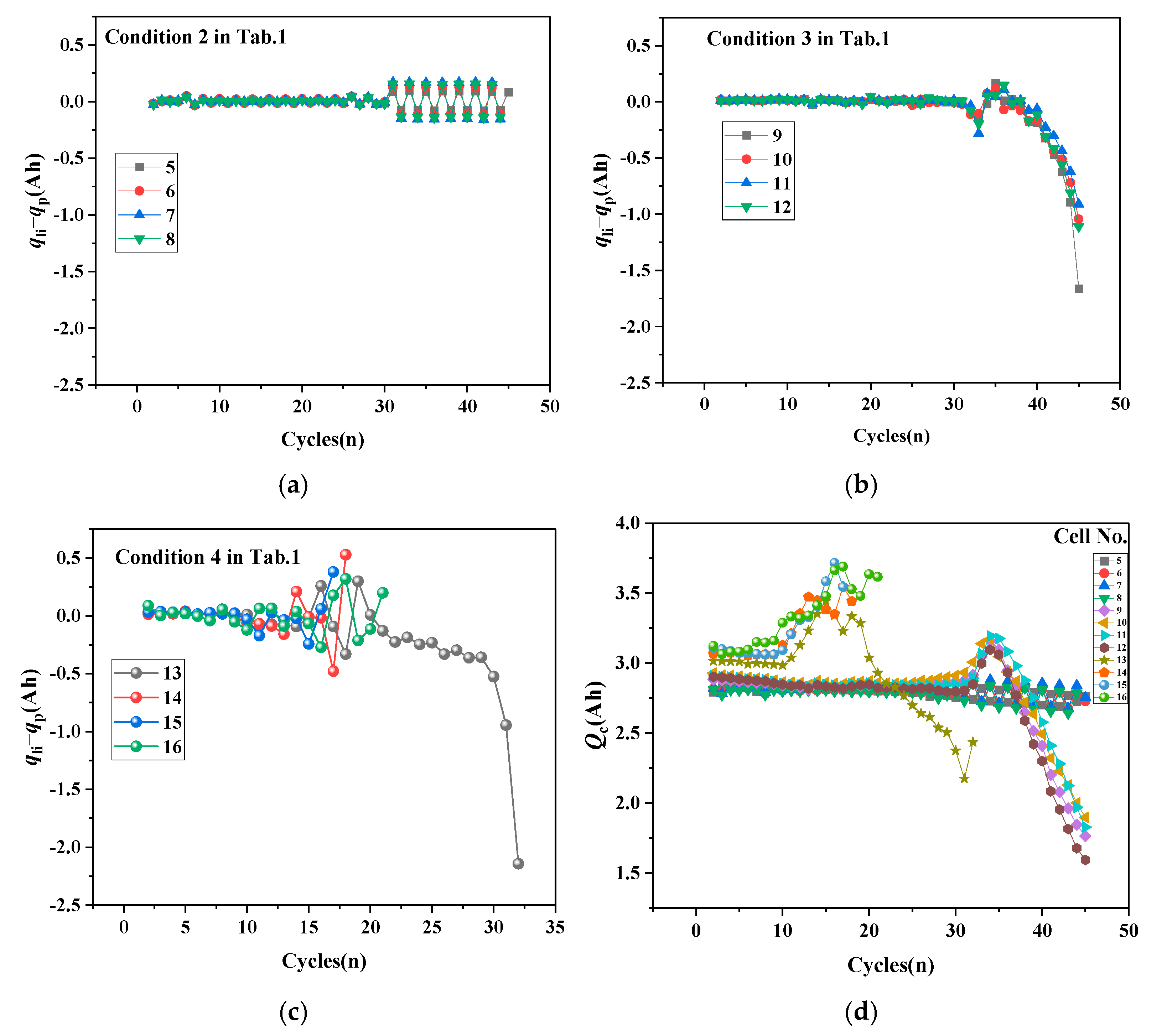
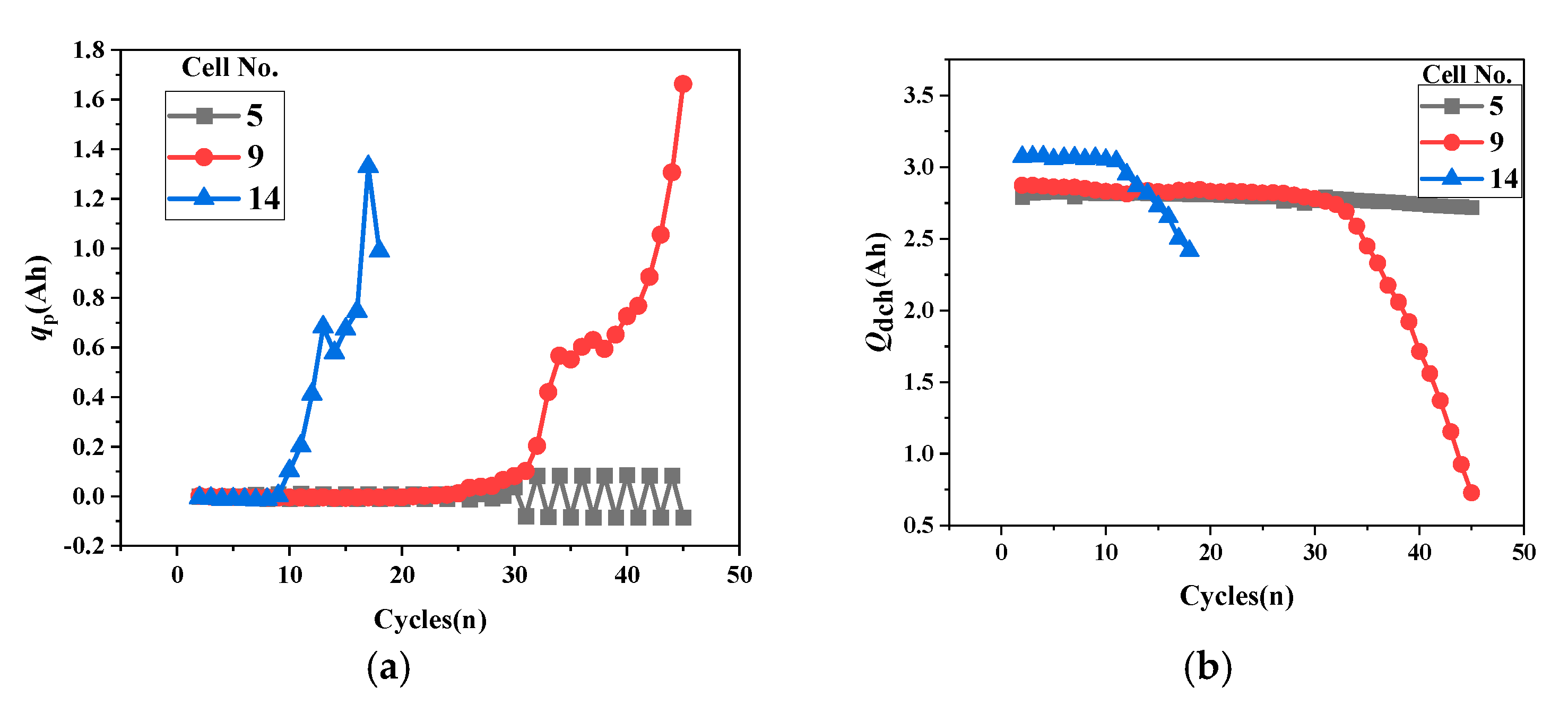
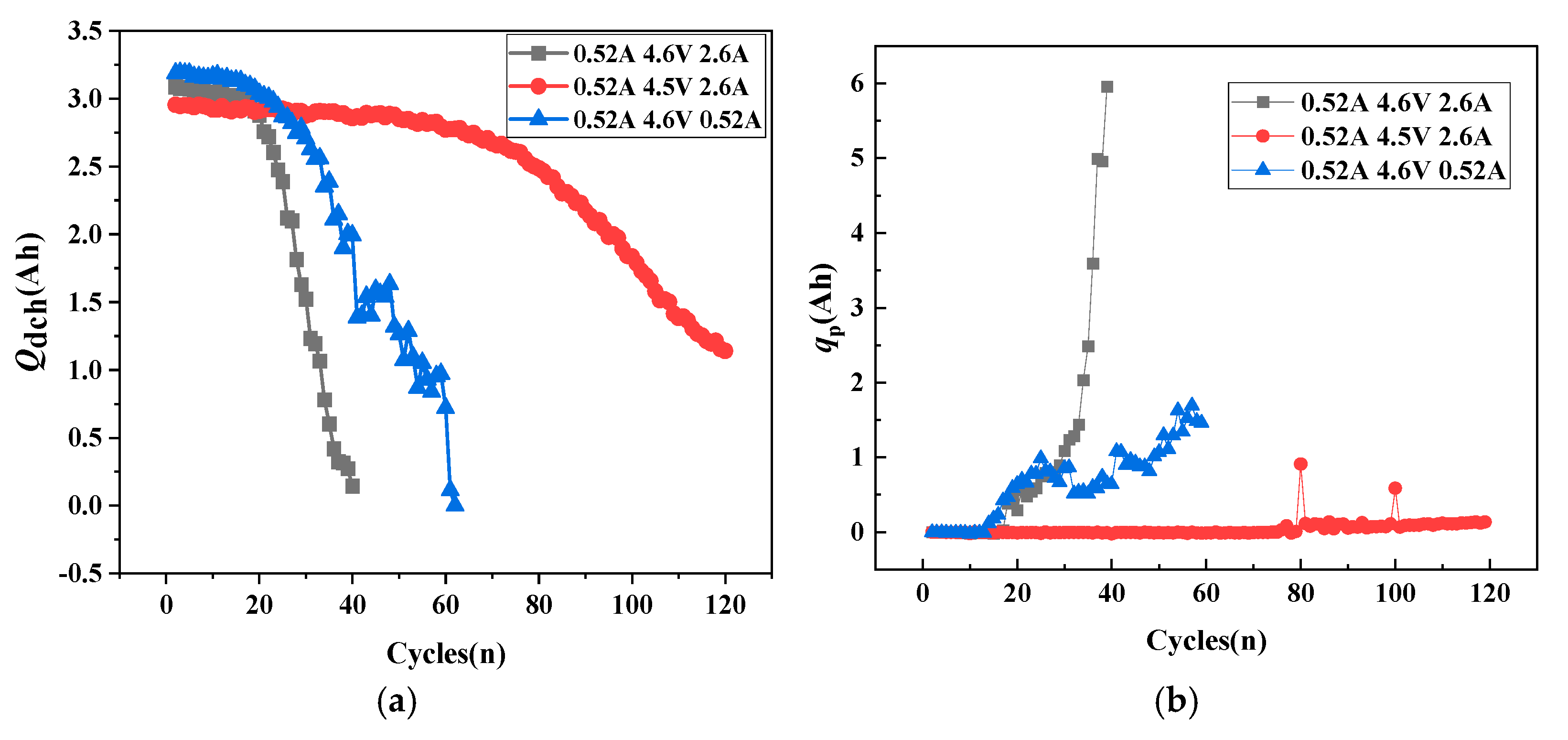
- The relationship between cathode active particle lithiation loss qp and discharge capacity.
- (2)
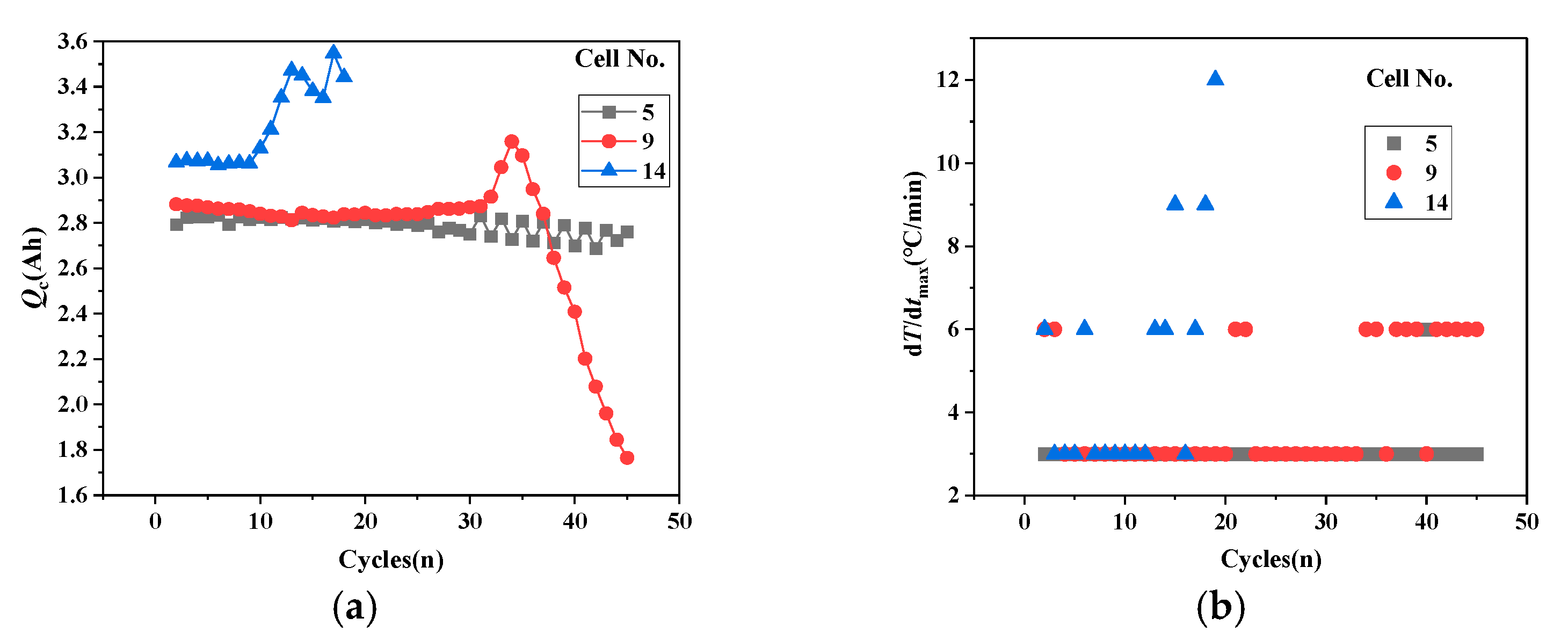
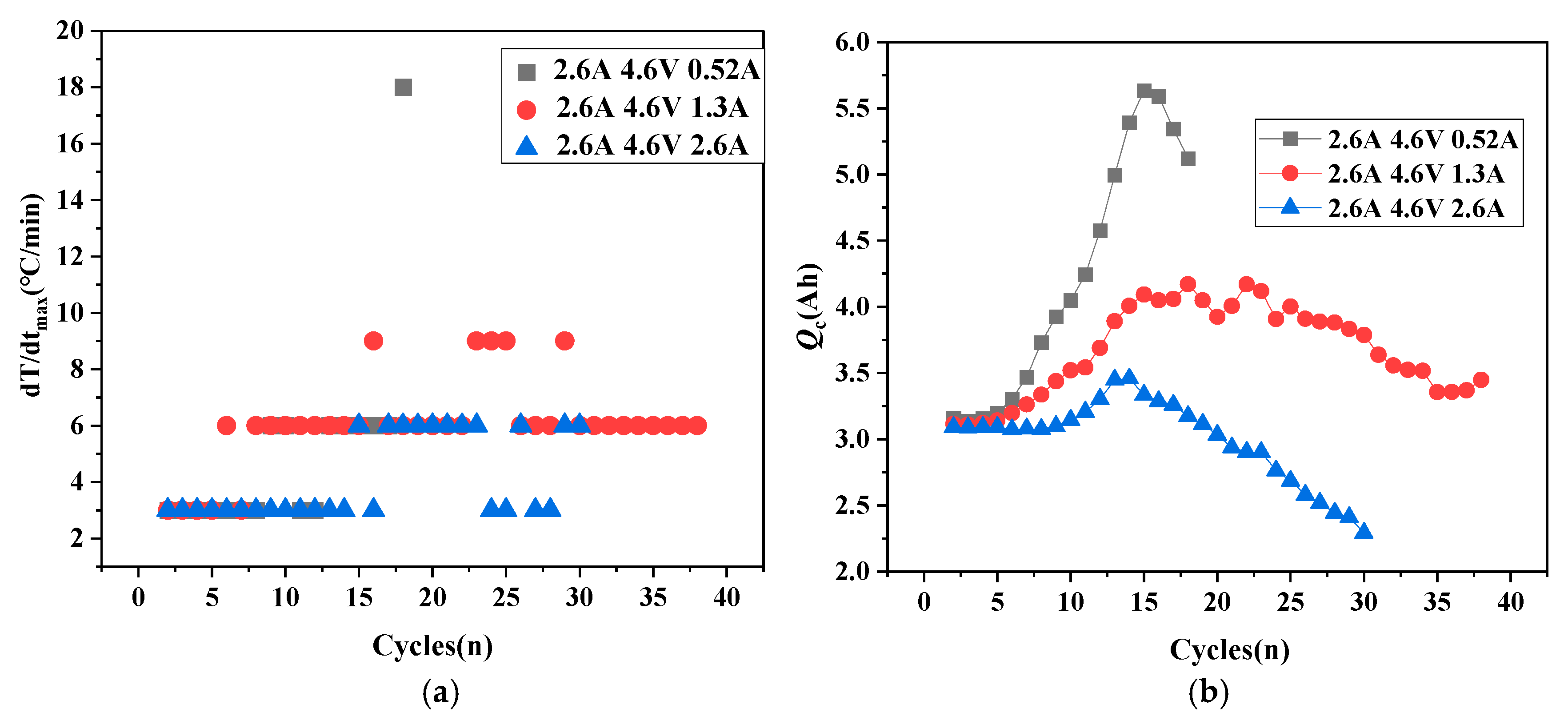
- The relationship between the cathode cyclic lithium Qc and the surface temperature rise rate.
- (1)
- First, qLi-qp determines battery development trends. When cathode lithiation compensates for anode lithium loss, the battery shows aging trends. When cathode lithiation cannot compensate for continuous anode lithium loss, the battery shows thermal runaway trends. When qLi-qp approaches zero, the battery maintains capacity balance and stability.
- (2)
- Using qLi-qp to distinguish battery stability, aging, and thermal runaway trends, qp assesses subsequent aging development, while Qc evaluates thermal runaway progression, enabling appropriate adjustment strategies to modify battery development trends.
3.4. Development Trends in Battery Cycling Condition Regulation
- (1)
- Mitigation of battery aging through charging cut-off voltage and discharge rate control.
- (2)
- Enhanced battery safety through a reduction in the charging cut-off voltage.
4. Conclusions
- (1)
- Based on the relationship between electrode capacity and battery stability, aging and thermal runaway processes, an electrode capacity balance, a model is proposed. The correlation between inherent cathode degradation-induced lithium loss and anode lithium inventory loss determines cathode structural lithium deposition, which, in turn, governs cathode structural stability.
- (2)
- The battery State of Health (SOH) prediction indicators based on the capacity balance model include the following: (a) The battery development trend: qLi − qp determines the battery development trend. When qLi − qp > 0 continues to increase, thermal runaway occurs; when qLi − qp < 0, battery aging occurs; and when qLi − qp = 0, battery performance remains stable. (b) Development degree assessment: qp indicates battery aging and Qc indicates thermal runaway; the operating conditions can be adjusted to improve these trends.
- (3)
- Battery thermal runaway and aging trends depend on whether active particle loss and lithium inventory loss can maintain electrode capacity balance. When cathode inherent degradation cannot compensate for anode lithium loss, increased cathode structural lithium deposition leads to active particle loss compensating for anode lithium inventory loss. When active particle loss exceeds anode lithium inventory loss, cathode saturation triggers rapid capacity decay. When active particle loss is lower than lithium inventory loss, anode lithium loss causes increased anode potential. At a constant charging cut-off voltage, more structural lithium from the cathode is deposited, raising the cathode potential and destabilizing the cathode structure. Deteriorating conditions cause delithiated cathode materials to decompose and release oxygen, generating heat through reactions with electrolytes. These exothermic reactions, combined with the reduction reactions from anode lithium loss, ultimately trigger thermal runaway.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berecibar, M.; Gandiaga, I.; Villarreal, I.; Omar, N.; Van Mierlo, J.; Van den Bossche, P. Critical Review of State of health estimation methods of Li-ion batteries for real applications. Renew. Sustain. Energy Rev. 2016, 56, 572–587. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Xu, M.; Reichman, B.; Wang, X. Modeling the effect of electrode thickness on the performance of lithium-ion batteries with experimental validation. Energy 2019, 186, 115864. [Google Scholar] [CrossRef]
- Chang, C.-C.; Huang, S.-Y.; Chen, W.-H. Thermal and solid electrolyte interphase characterization of lithium-ion battery. Energy 2019, 174, 999–1011. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Spotnitz, R. Simulation of capacity fade in lithium-ion batteries. J. Power Sources 2003, 113, 72–80. [Google Scholar] [CrossRef]
- Chu, Y.; Shen, Y.; Guo, F.; Zhao, X.; Dong, Q.; Zhang, Q.; Li, W.; Chen, H.; Luo, Z.; Chen, L. Advanced characterizations of solid electrolyte Interphases in lithium-ion batteries. Electrochem. Energy Rev. 2019, 3, 187–219. [Google Scholar] [CrossRef]
- SAE J2464; Electric and Hybrid Electric Vehicle Rechargeable Energy Storage System (RESS) Safety and Abuse Testing. Society of Automotive Engineers: Warrendale, PA, USA, 2009.
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Qiu, X.; Wang, S. Low-temperature reversible capacity loss and aging mechanism in lithium-ion batteries for different discharge profiles. Int. J. Energy Res. 2018, 43, 243–253. [Google Scholar] [CrossRef]
- Attia, P.M.; Bills, A.; Brosa Planella, F.; Dechent, P.; dos Reis, G.; Dubarry, M.; Gasper, P.; Gilchrist, R.; Greenbank, S.; Howey, D.; et al. Review—“Knees” in lithium-ion battery aging trajectories. J. Electrochem. Soc. 2022, 169, 060517. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium-ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Gauthier, R.; Luscombe, A.; Bond, T.; Bauer, M.; Johnson, M.; Harlow, J.; Louli, A.; Dahn, J.R. How do depth of discharge, C-rate and calendar age affect capacity retention, impedance growth, the electrodes, and the electrolyte in li-ion cells? J. Electrochem. Soc. 2022, 169, 020518. [Google Scholar] [CrossRef]
- Wen, J.; Zhu, Y.; Wang, S. Detection of the chain reaction pathway and early warning method of thermal runaway by battery management system for lithium cobalt manganese oxide (NCM) batteries. J. Energy Storage 2025. submitted for publication. [Google Scholar]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium batteries and the solid electrolyte interphase (SEI)—Progress and outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium-ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Smith, A.J.; Burns, J.C.; Xiong, D.; Dahn, J.R. Interpreting high precision coulometry results on Li-Ion Cells. J. Electrochem. Soc. 2011, 158, A1136. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Smith, K.; Saxon, A.; Keyser, M.; Lundstrom, B.; Ziwei, C.; Roc, A. Life prediction model for grid-connected Li-Ion Battery Energy Storage System. In Proceedings of the 2017 American Control Conference (ACC), Seattle, WA, USA, 24–26 May 2017. [Google Scholar] [CrossRef]

| Cell No. | Condition | Charging Current/A | Charging Voltage/V | Discharge Current/A | Discharge Voltage/V |
|---|---|---|---|---|---|
| 1 | 1 | 2.6 | 4.2 | 2.6 | 2.75 |
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 | 2 | 2.6 | 4.4 | 2.6 | 2.75 |
| 6 | |||||
| 7 | |||||
| 8 | |||||
| 9 | 3 | 2.6 | 4.5 | 2.6 | 2.75 |
| 10 | |||||
| 11 | |||||
| 12 | |||||
| 13 | 4 | 2.6 | 4.6 | 2.6 | 2.75 |
| 14 | |||||
| 15 | |||||
| 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Zhu, Y.; Wang, S. Electrode Capacity Balancing for Accurate Battery State of Health Prediction and Degradation Analysis. Batteries 2025, 11, 367. https://doi.org/10.3390/batteries11100367
Wen J, Zhu Y, Wang S. Electrode Capacity Balancing for Accurate Battery State of Health Prediction and Degradation Analysis. Batteries. 2025; 11(10):367. https://doi.org/10.3390/batteries11100367
Chicago/Turabian StyleWen, Jianghui, Yu Zhu, and Shixue Wang. 2025. "Electrode Capacity Balancing for Accurate Battery State of Health Prediction and Degradation Analysis" Batteries 11, no. 10: 367. https://doi.org/10.3390/batteries11100367
APA StyleWen, J., Zhu, Y., & Wang, S. (2025). Electrode Capacity Balancing for Accurate Battery State of Health Prediction and Degradation Analysis. Batteries, 11(10), 367. https://doi.org/10.3390/batteries11100367







