Hollow Carbon Nanorod-Encapsulated Eu2O3 for High-Energy Hybrid Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Eu2O3 Nanorods
2.2. Chemical Vapor Deposition (CVD)
2.3. Preparation of Electrodes and Supercapacitor Assembly
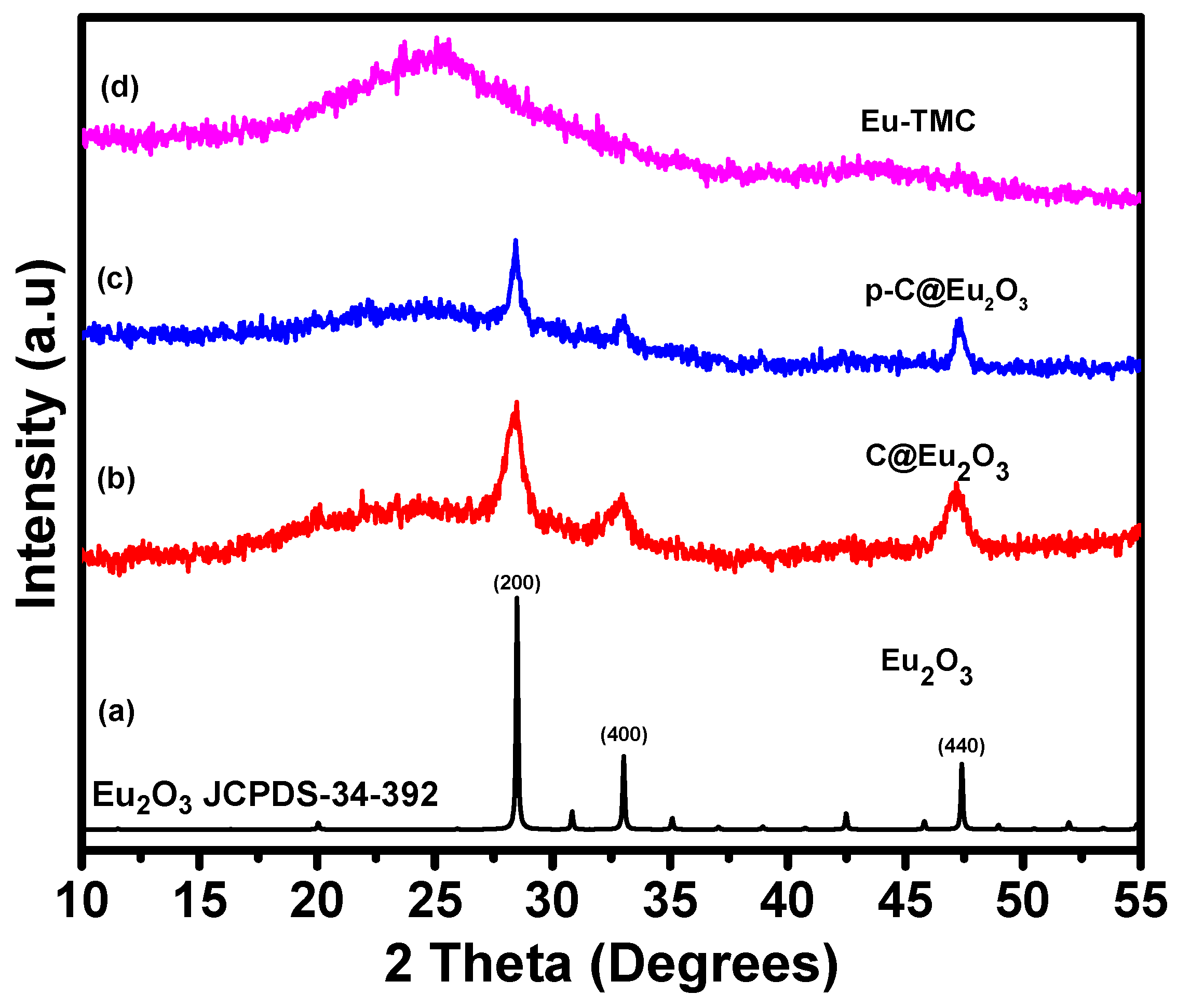
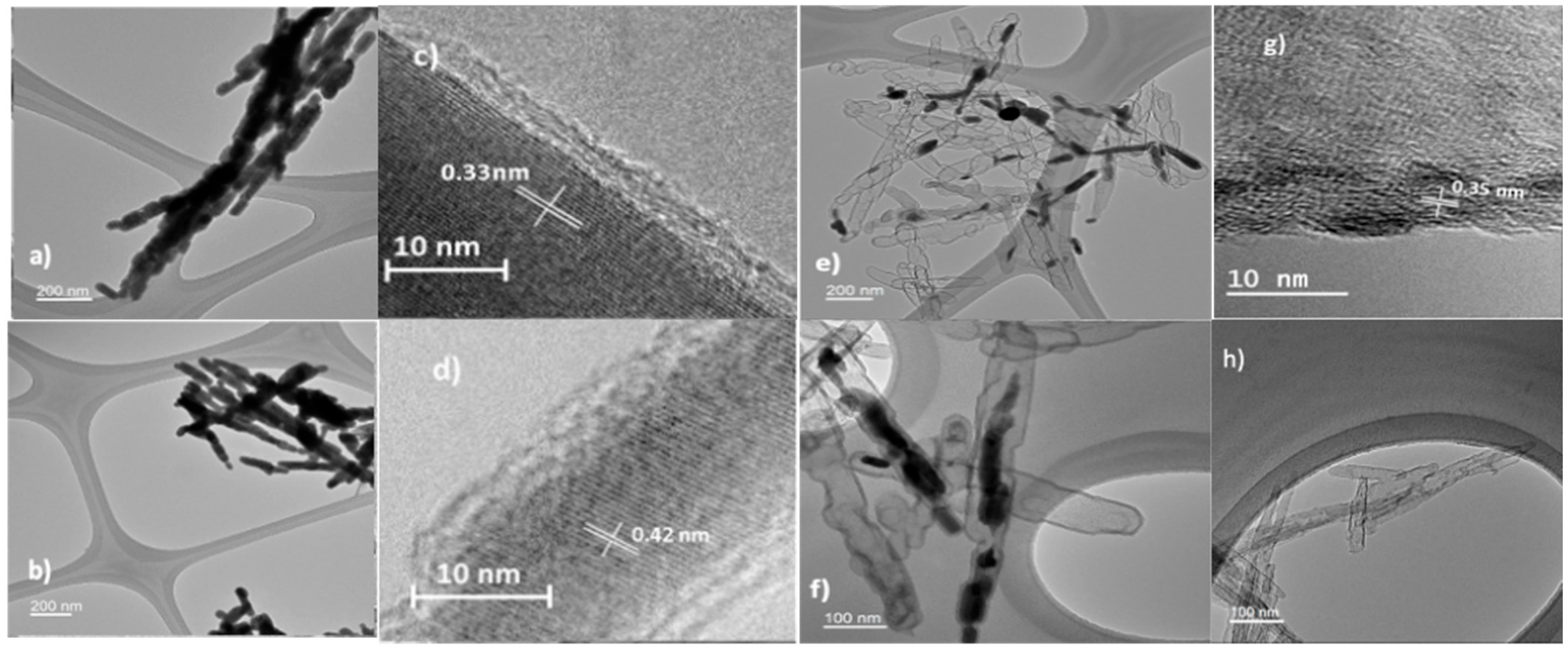
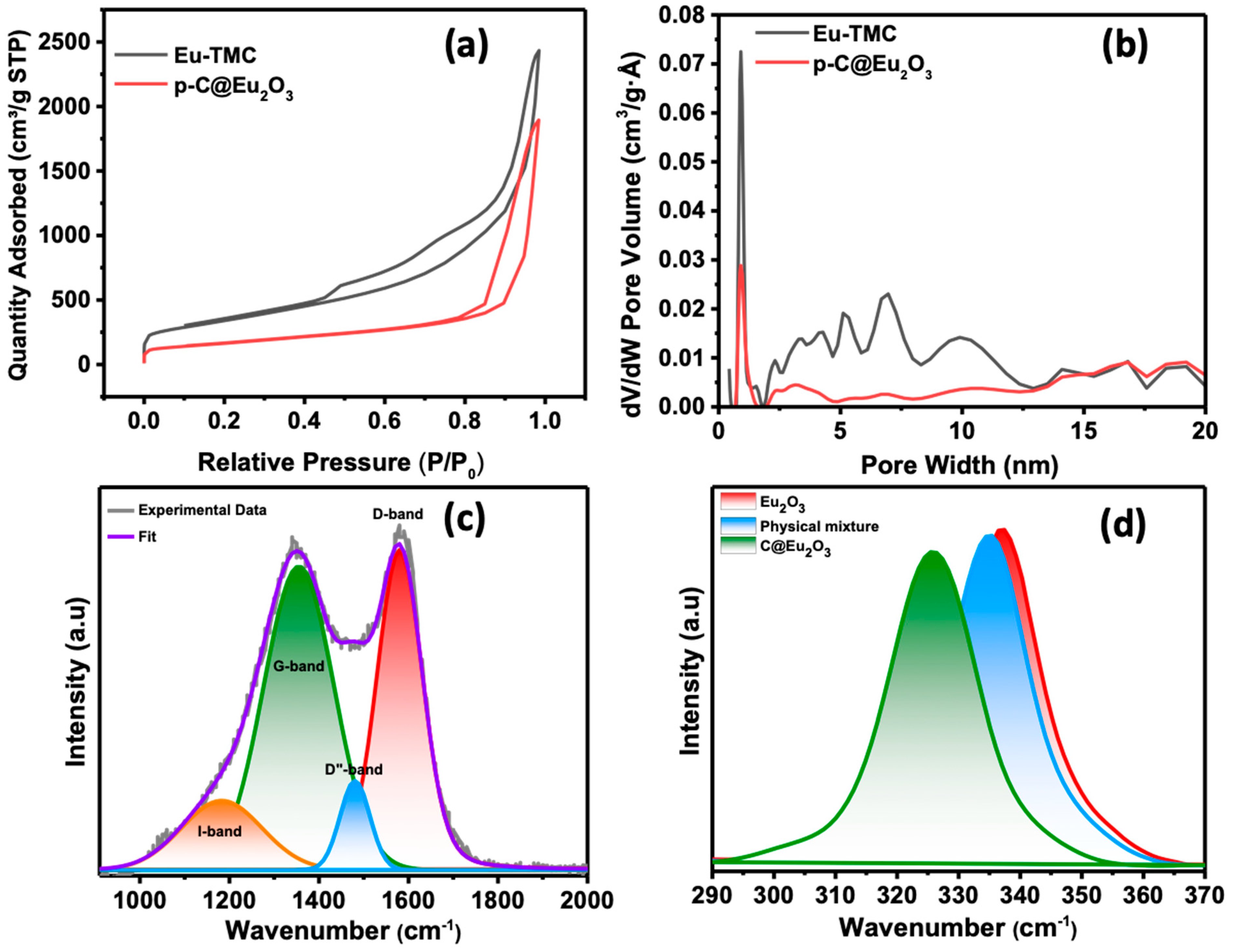
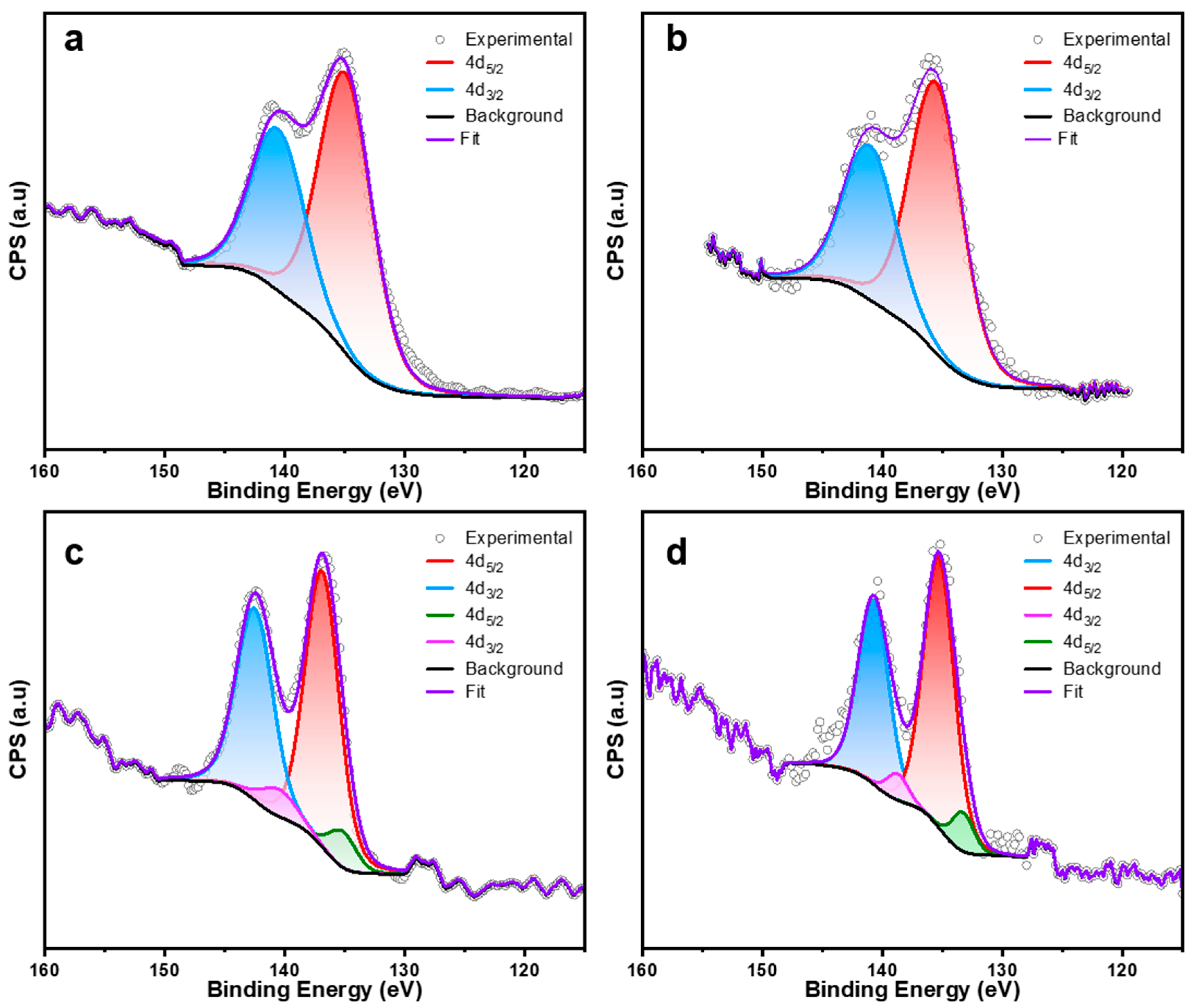
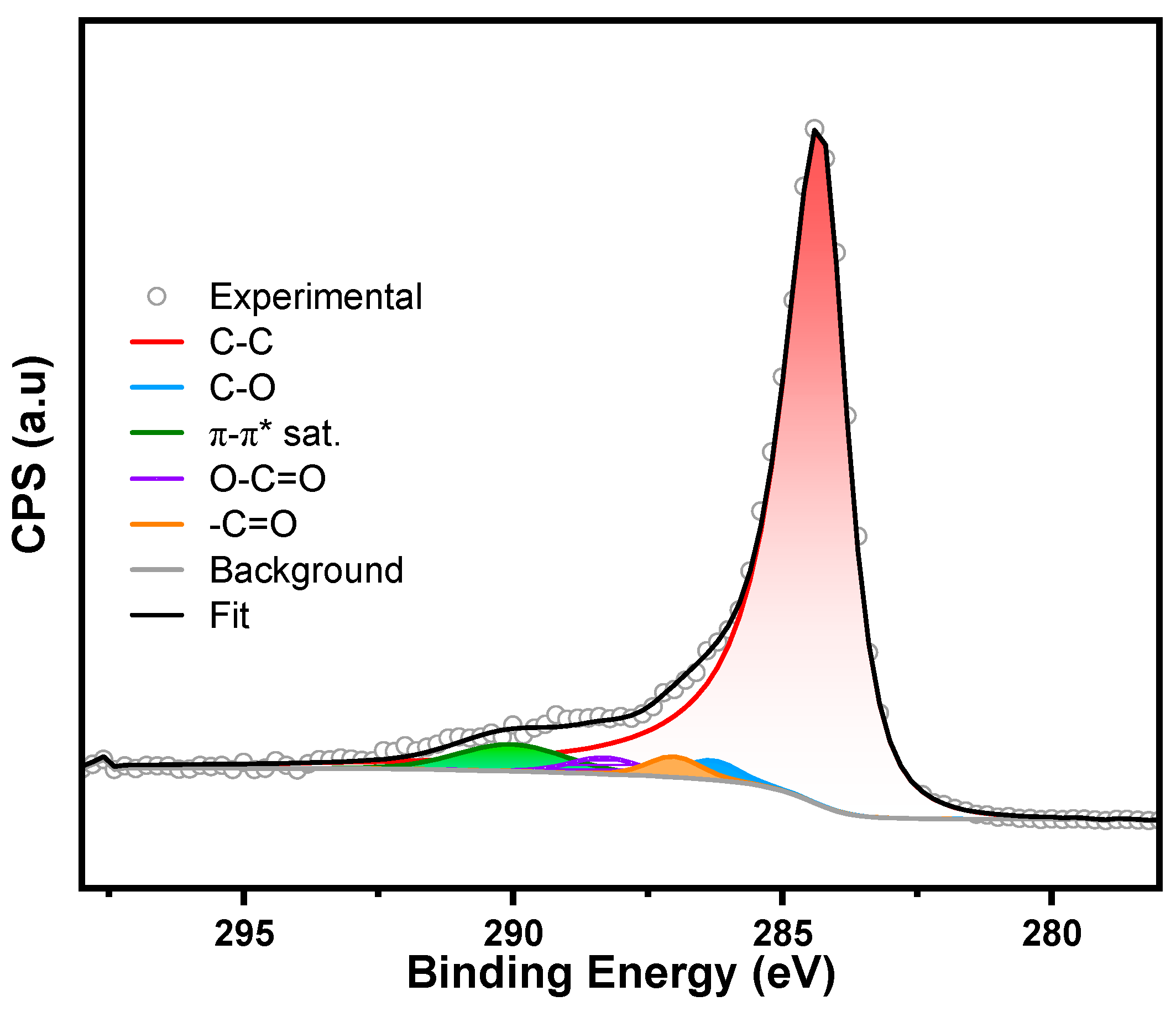
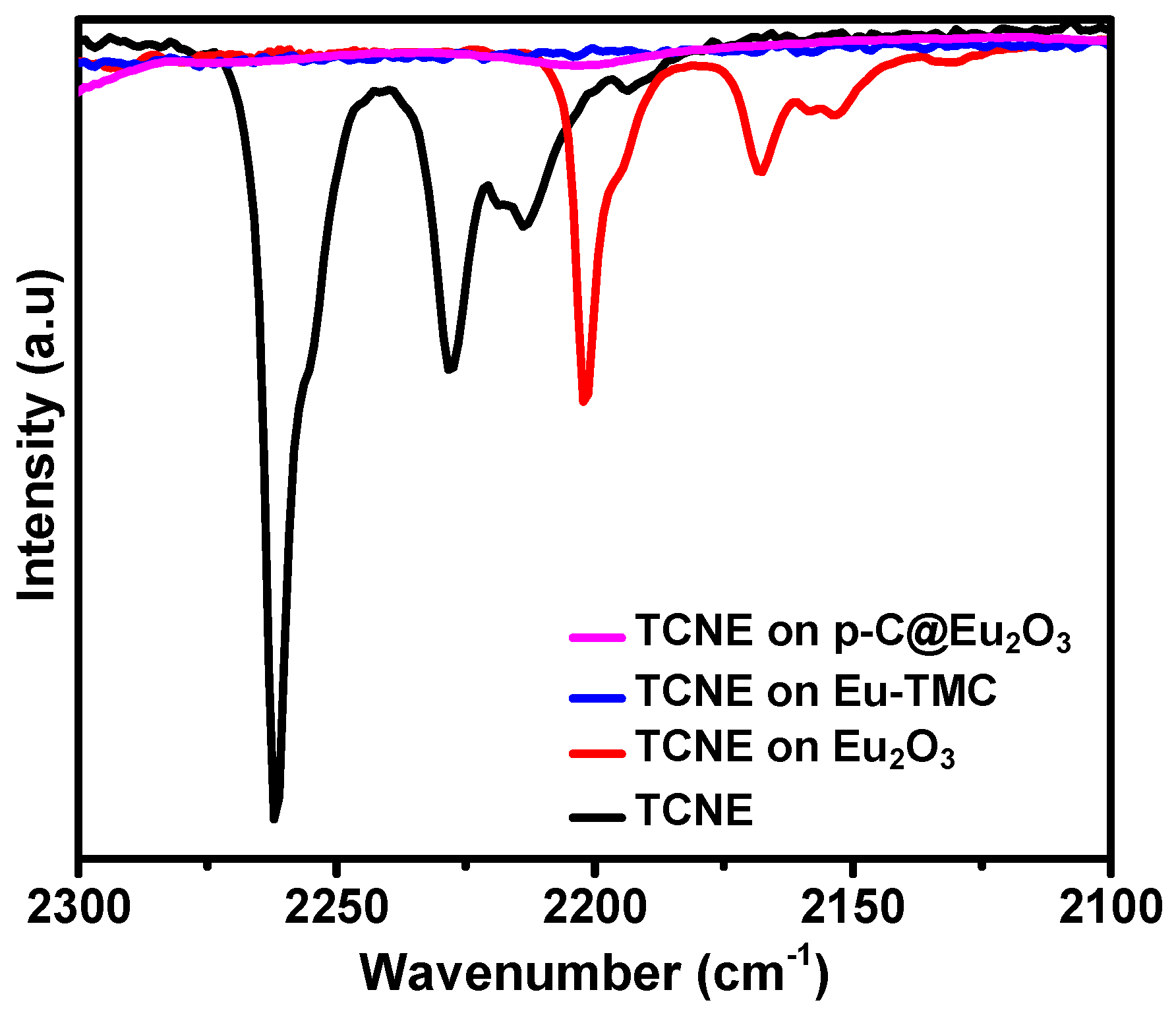
3. Characterization
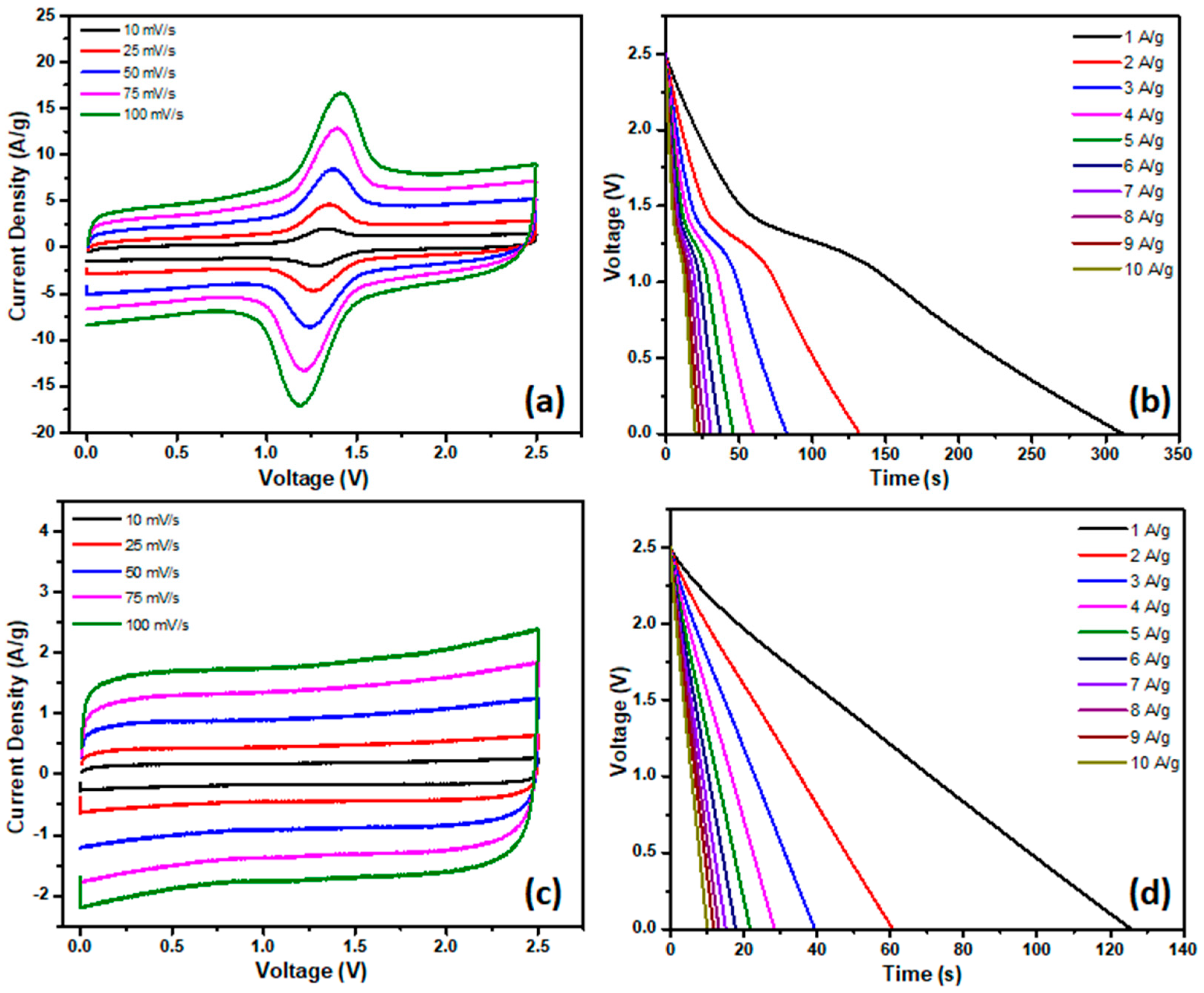
4. Electrochemical Measurements
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Sun, X.; Yan, X.; Gao, Y.; Zhang, X.; Wang, K.; Ma, Y. Review of Energy Storage Capacitor Technology. Batteries 2024, 10, 271. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Eskander, T.N.A.; Fathalla, M.; Zhukova, V.; Blanco, J.M.; Gonzalez, J.; Zhukov, A.; Abu-Dief, A.M. Empowering the Future: Cutting-Edge Developments in Supercapacitor Technology for Enhanced Energy Storage. Batteries 2025, 11, 232. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.K.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.U.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A Review of Supercapacitors: Materials Design, Modification, and Applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Obreja, V.V. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material—A review. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.-L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Banerjee, S.; Mordina, B.; Sinha, P.; Kar, K.K. Recent advancement of supercapacitors: A current era of supercapacitor devices through the development of electrical double layer, pseudo and their hybrid supercapacitor electrodes. J. Energy Storage 2025, 108, 115075. [Google Scholar] [CrossRef]
- Davies, A.; Yu, A. Material advancements in supercapacitors: From activated carbon to carbon nanotube and graphene. Can. J. Chem. Eng. 2011, 89, 1342–1357. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Yamani, Z.H. Recent Progress in Carbonaceous and Redox-Active Nanoarchitectures for Hybrid Supercapacitors: Performance Evaluation, Challenges, and Future Prospects. Chem. Rec. 2022, 22, e202200018. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Zhang, H.; Jin, L.; Chu, X.; Gu, B.; Huang, H.; Yang, W. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin. Carbon 2019, 149, 105–116. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, X. Pseudocapacitive Storage in High-Performance Flexible Batteries and Supercapacitors. Batteries 2025, 11, 63. [Google Scholar] [CrossRef]
- Liu, F.; Feng, X.; Wu, Z.-S. The key challenges and future opportunities of electrochemical capacitors. J. Energy Chem. 2023, 76, 459–461. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K. Conducting polymer electrolytes for flexible supercapacitors. Flex. Supercapacitor Nanoarchitectonics 2021, 233–262. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, W.; Yushin, G. Carbons from biomass for electrochemical capacitors. Prod. Mater. Sustain. Biomass Resour. 2019, 153–184. [Google Scholar] [CrossRef]
- Li, J.; Tang, J.; Yuan, J.; Zhang, K.; Shao, Q.; Sun, Y.; Qin, L.-C. Interactions between graphene and ionic liquid electrolyte in supercapacitors. Electrochimica Acta 2016, 197, 84–91. [Google Scholar] [CrossRef]
- Zhuo, J.; Zheng, Y.; Li, S.; Sha, J. A supercapacitor with high specific volumetric capacitance produced by 12-phosphomolybdate anchored on graphene balls. ACS Appl. Energy Mater. 2022, 5, 13627–13634. [Google Scholar] [CrossRef]
- Liu, P.; Ge, Y.; Li, H.; Wen, Y.; Chen, T.; Zeng, X. New insights into the performance of biomass carbon-based supercapacitors based on interpretable machine learning approach. J. Energy Storage 2025, 118, 116300. [Google Scholar] [CrossRef]
- Umasankar, Y.; Brooks, D.B.; Brown, B.; Zhou, Z.; Ramasamy, R.P. Three dimensional carbon nanosheets as a novel catalyst support for enzymatic bioelectrodes. Adv. Energy Mater. 2014, 4, 1301306. [Google Scholar] [CrossRef]
- Aziz, A.; Shah, S.S.; Kashem, A. Preparation and Utilization of Jute-Derived Carbon: A Short Review. Chem. Rec. 2020, 20, 1074–1098. [Google Scholar] [CrossRef]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-derived carbons–from porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Li, C.; Sun, X.-Z.; Wang, K.; Su, F.-Y.; Liu, F.-Y.; Ma, Y.-W. Recent advances in transition metal chalcogenides for lithium-ion capacitors. Rare Met. 2022, 41, 2971–2984. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-based supercapacitor electrodes: Advances and challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Perera, S.D.; Rudolph, M.; Mariano, R.G.; Nijem, N.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J., Jr. Manganese oxide nanorod–graphene/vanadium oxide nanowire–graphene binder-free paper electrodes for metal oxide hybrid supercapacitors. Nano Energy 2013, 2, 966–975. [Google Scholar] [CrossRef]
- Raut, B.; Ahmed, M.S.; Kim, H.-Y.; Rahman Khan, M.M.; Bari, G.A.K.M.R.; Islam, M.; Nam, K.-W. Battery-Type Transition Metal Oxides in Hybrid Supercapacitors: Synthesis and Applications. Batteries 2025, 11, 60. [Google Scholar] [CrossRef]
- Aryanrad, P.; Naderi, H.R.; Kohan, E.; Ganjali, M.R.; Baghernejad, M.; Dezfuli, A.S. Europium oxide nanorod-reduced graphene oxide nanocomposites towards supercapacitors. RSC Adv. 2020, 10, 17543–17551. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Choudhary, R.B.; Thakur, A.K.; Kumar, U. Augmented gravimetric and volumetric capacitive performance of rare earth metal oxide (Eu2O3) incorporated polypyrrole for supercapacitor applications. J. Electroanal. Chem. 2017, 804, 42–52. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, X.; Abbas, M.; Tague, D.W.; Wunch, M.A.; Ferraris, J.P.; Balkus, K.J. Magnesium hydroxide templated hierarchical porous carbon nanosheets as electrodes for high-energy-density supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6805–6813. [Google Scholar] [CrossRef]
- Brown, A.T.; Lin, J.; Vizuet, J.P.; Thomas, M.C.; Balkus, K.J. Graphene-like carbon from calcium hydroxide. ACS Omega 2021, 6, 31066–31076. [Google Scholar] [CrossRef]
- Kim, K.; Lee, T.; Kwon, Y.; Seo, Y.; Song, J.; Park, J.K.; Lee, H.; Park, J.Y.; Ihee, H.; Cho, S.J. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template. Nature 2016, 535, 131–135. [Google Scholar] [CrossRef]
- Brown, A.T.; Agrawal, V.S.; Wunch, M.A.; Lin, J.; Thomas, M.C.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J., Jr. Yttrium oxide-catalyzed formation of electrically conductive carbon for supercapacitors. ACS Appl. Energy Mater. 2021, 4, 12499–12507. [Google Scholar] [CrossRef]
- Abbas, M.; Haque, S.F.B.; Tian, Y.; Ferraris, J.P.; Balkus, K.J., Jr. Organic–Inorganic Nanohybrids in Supercapacitors. In Hybrid Nanomaterials: Biomedical, Environmental and Energy Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 359–383. [Google Scholar]
- Yan, T.; Zhang, D.; Shi, L.; Li, H. Facile synthesis, characterization, formation mechanism and photoluminescence property of Eu2O3 nanorods. J. Alloys Compd. 2009, 487, 483–488. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Swain, B.; Hiltwine, J.; Brooks, D.B.; Zhou, Z. Carbon nanosheet buckypaper: A graphene-carbon nanotube hybrid material for enhanced supercapacitor performance. J. Power Sources 2014, 272, 979–986. [Google Scholar] [CrossRef]
- Tran, C.; Lawrence, D.; Richey, F.W.; Dillard, C.; Elabd, Y.A.; Kalra, V. Binder-free three-dimensional high energy density electrodes for ionic-liquid supercapacitors. Chem. Commun. 2015, 51, 13760–13763. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Sobiesiak, M. Comparison of heterogeneous pore models QSDFT and 2D-NLDFT and computer programs ASiQwin and SAIEUS for calculation of pore size distribution. Adsorption 2016, 22, 459–464. [Google Scholar] [CrossRef]
- Kang, J.-G.; Jung, Y.; Min, B.-K.; Sohn, Y. Full characterization of Eu(OH)3 and Eu2O3 nanorods. Appl. Surf. Sci. 2014, 314, 158–165. [Google Scholar] [CrossRef]
- Pol, V.G.; Palchik, O.; Gedanken, A.; Felner, I. Synthesis of europium oxide nanorods by ultrasound irradiation. J. Phys. Chem. B 2002, 106, 9737–9743. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, X.; Abbas, M.; Tague, D.W.; Ferraris, J.P.; Balkus, K.J., Jr. Two-dimensional hexagonal-shaped mesoporous carbon sheets for supercapacitors. ACS Omega 2022, 7, 27896–27902. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Cheng, H.-M. Carbon nanotubes: Surface, porosity, and related applications. In Carbon Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 323–359. [Google Scholar]
- Wang, Z.; Perera, W.A.; Perananthan, S.; Ferraris, J.P.; Balkus, K.J., Jr. Lanthanum hydroxide nanorod-templated graphitic hollow carbon nanorods for supercapacitors. ACS Omega 2018, 3, 13913–13918. [Google Scholar] [CrossRef] [PubMed]
- Hausbrand, R.; Jaegermann, W. Reaction Layer Formation and Charge Transfer at Li-Ion Cathode—Electrolyte Interfaces: Concepts and Results Obtained by a Surface Science Approach; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Dilawar, N.; Mehrotra, S.; Varandani, D.; Kumaraswamy, B.V.; Haldar, S.K.; Bandyopadhyay, A.K. A Raman spectroscopic study of C-type rare earth sesquioxides. Mater. Charact. 2008, 59, 462–467. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and magnetic studies of pulsed laser deposited Fe doped Eu2O3 thin film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Everitt, G.F. Transition-Metal Complexes of Tetracyanoethylene. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, Louisiana, 1971. [Google Scholar]
- Miller, J.S. Tetracyanoethylene (TCNE): The characteristic geometries and vibrational absorptions of its numerous structures. Angew. Chem. Int. Ed. 2006, 45, 2508–2525. [Google Scholar] [CrossRef]
- Han, L.; Xu, Z.; Wu, J.; Guo, X.; Zhu, H.; Cui, H. Controllable preparation of graphene/MnO2/Co3O4 for supercapacitors. J. Alloys Compd. 2017, 729, 1183–1189. [Google Scholar] [CrossRef]
- R, R.; Thejas Prasannakumar, A.; V, M.; Varma, S.J. V2O5/MnO2 Nanostructured Electrodes for High-Energy-Density Supercapacitors. ACS Appl. Nano Mater. 2025, 8, 13861–13875. [Google Scholar] [CrossRef]
- Vigneshwaran, J.; Abraham, S.; Muniyandi, B.; Prasankumar, T.; Li, J.-T.; Jose, S. Fe2O3 decorated graphene oxide/polypyrrole matrix for high energy density flexible supercapacitor. Surf. Interfaces 2021, 27, 101572. [Google Scholar] [CrossRef]
- Moghaddam, M.R.; Ganjali, M.R.; Hosseini, M.; Faridbod, F. A novel electrochemiluminescnece sensor based on an Ru(bpy)32+-Eu2O3-nafion nanocomposite and its application in the detection of diphenhydramine. Int. J. Electrochem. Sci. 2017, 12, 5220–5232. [Google Scholar] [CrossRef]
- Regueiro-Figueroa, M.; Barriada, J.L.; Pallier, A.; Esteban-Gomez, D.; de Blas, A.; Rodríguez-Blas, T.; Toth, E.; Platas-Iglesias, C. Stabilizing divalent europium in aqueous solution using size-discrimination and electrostatic effects. Inorg. Chem. 2015, 54, 4940–4952. [Google Scholar] [CrossRef]
- Burnett, M.E.; Adebesin, B.; Funk, A.M.; Kovacs, Z.; Sherry, A.D.; Ekanger, L.A.; Allen, M.J.; Green, K.N.; Ratnakar, S.J. Electrochemical investigation of the Eu3+/2+ redox couple in complexes with variable numbers of glycinamide and acetate pendant arms. Eur. J. Inorg. Chem. 2017, 2017, 5001–5005. [Google Scholar] [CrossRef]
- Mei, B.-A.; Lau, J.; Lin, T.; Tolbert, S.H.; Dunn, B.S.; Pilon, L. Physical interpretations of electrochemical impedance spectroscopy of redox active electrodes for electrical energy storage. J. Phys. Chem. C 2018, 122, 24499–24511. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef]
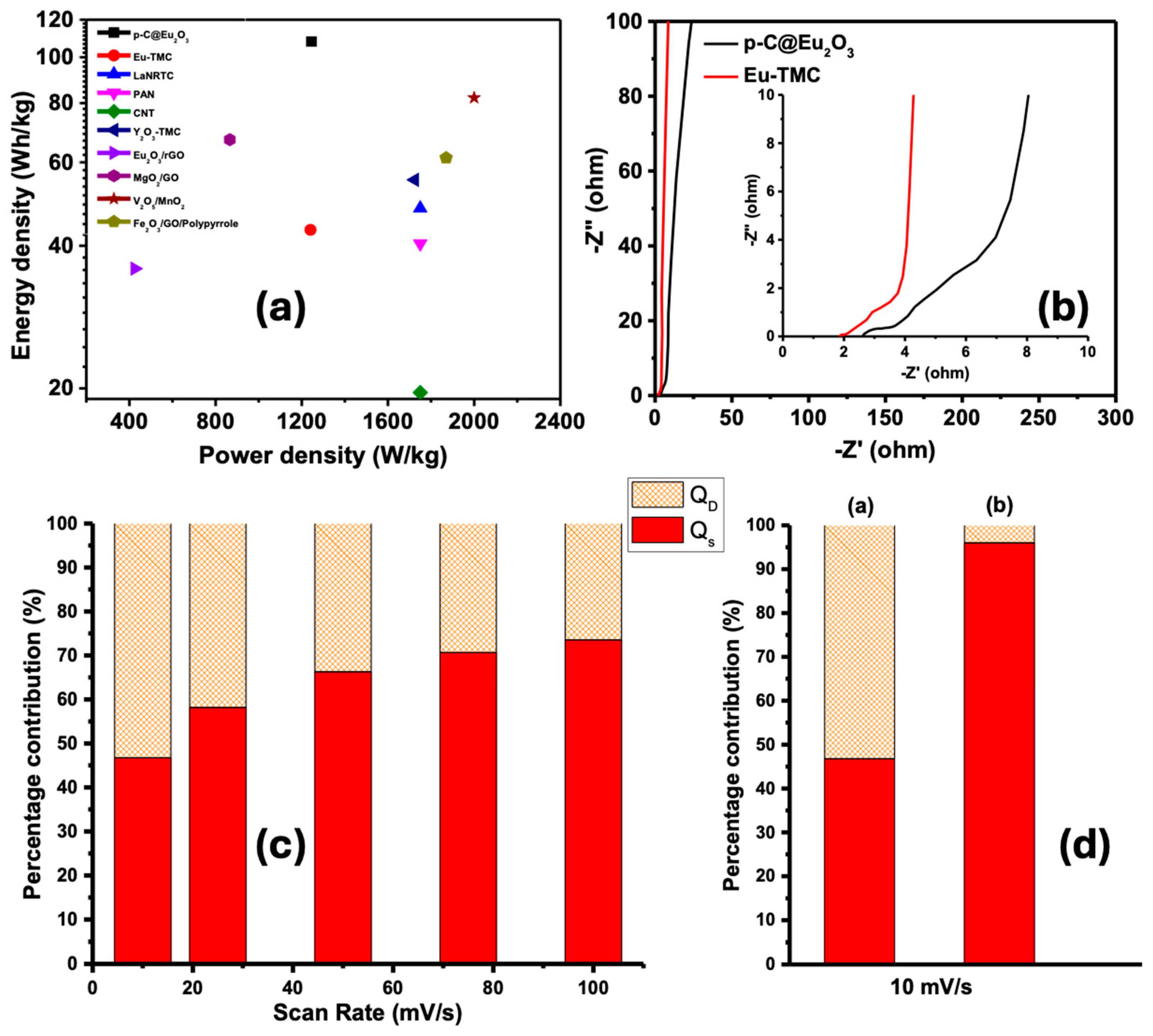
| Electrode Material | Capacitance | Energy Density | Reference |
|---|---|---|---|
| Eu2O3@rGO composite | 403 F/g | 35.8 Wh/kg | [25] |
| MgO-templated carbon nanosheets | ~280 F/g | 70 Wh/kg | [27] |
| G/Co3O4/MnO2 | 502.3 F/g | - | [51] |
| V2O5/MnO2 | 394.5 F/g | 82 Wh/Kg | [52] |
| Fe2O3-GO/polypyrrole | 442 F/g | 61.3 Wh/kg | [53] |
| La2O3-templated porous carbon | ~220 F/g | ~50 Wh/kg | [29] |
| Y2O3-templated graphitic carbon | ~240 F/g | 55 Wh/kg | [30] |
| This work: p-C@Eu2O3 | 501.2 F/g | 108 Wh/kg | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer, A.; Tague, D.W.; Abbas, M.; Ferraris, J.P.; Balkus, K.J., Jr. Hollow Carbon Nanorod-Encapsulated Eu2O3 for High-Energy Hybrid Supercapacitors. Batteries 2025, 11, 355. https://doi.org/10.3390/batteries11100355
Umer A, Tague DW, Abbas M, Ferraris JP, Balkus KJ Jr. Hollow Carbon Nanorod-Encapsulated Eu2O3 for High-Energy Hybrid Supercapacitors. Batteries. 2025; 11(10):355. https://doi.org/10.3390/batteries11100355
Chicago/Turabian StyleUmer, Arslan, Daniel W. Tague, Muhammad Abbas, John P. Ferraris, and Kenneth J. Balkus, Jr. 2025. "Hollow Carbon Nanorod-Encapsulated Eu2O3 for High-Energy Hybrid Supercapacitors" Batteries 11, no. 10: 355. https://doi.org/10.3390/batteries11100355
APA StyleUmer, A., Tague, D. W., Abbas, M., Ferraris, J. P., & Balkus, K. J., Jr. (2025). Hollow Carbon Nanorod-Encapsulated Eu2O3 for High-Energy Hybrid Supercapacitors. Batteries, 11(10), 355. https://doi.org/10.3390/batteries11100355








