Impact of Inert Materials on Commercial Lithium–Ion Cell Energy Density
Abstract
1. Introduction
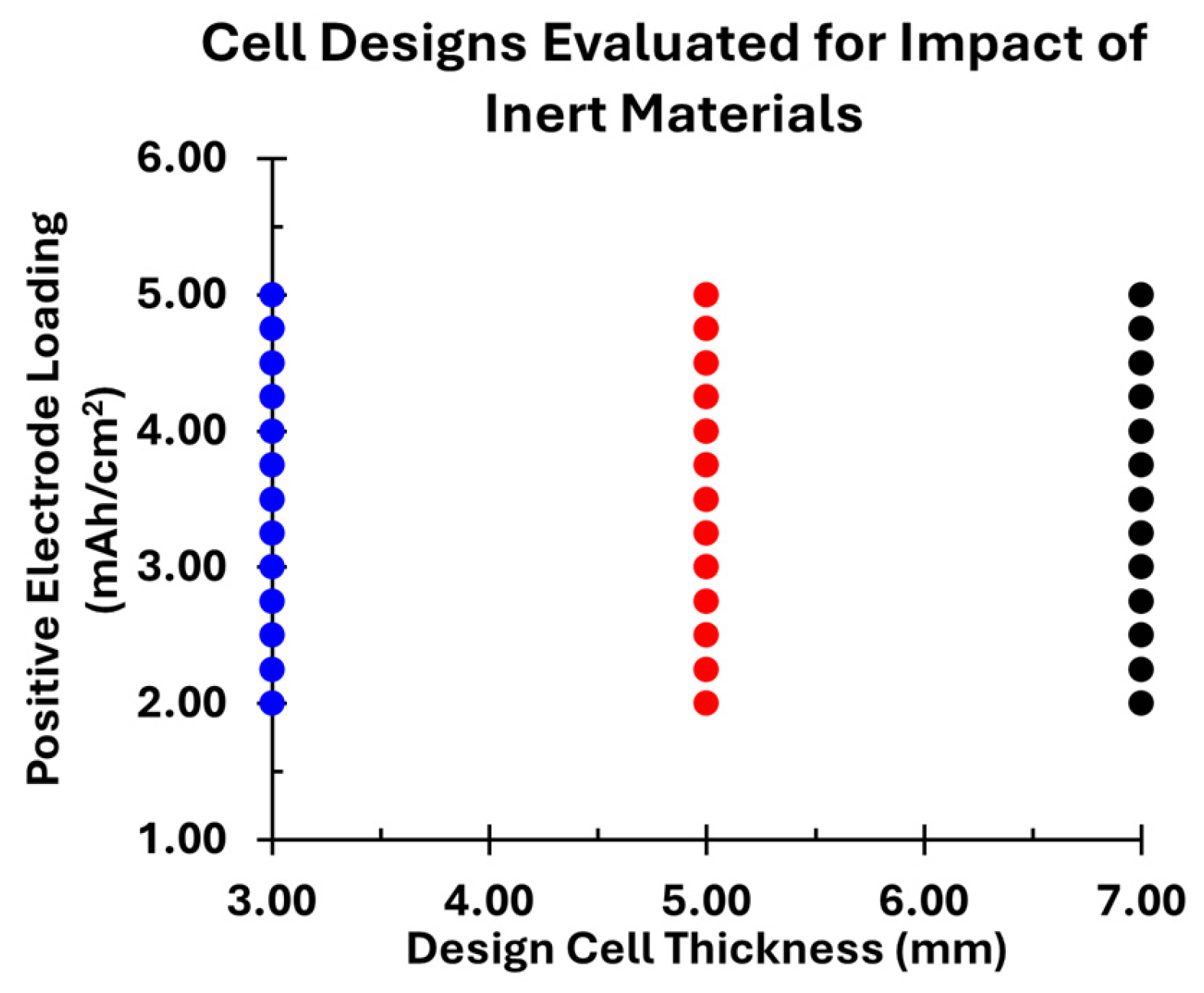
2. Materials and Methods
3. Results
3.1. Impact of Positive Current Collector Thickness
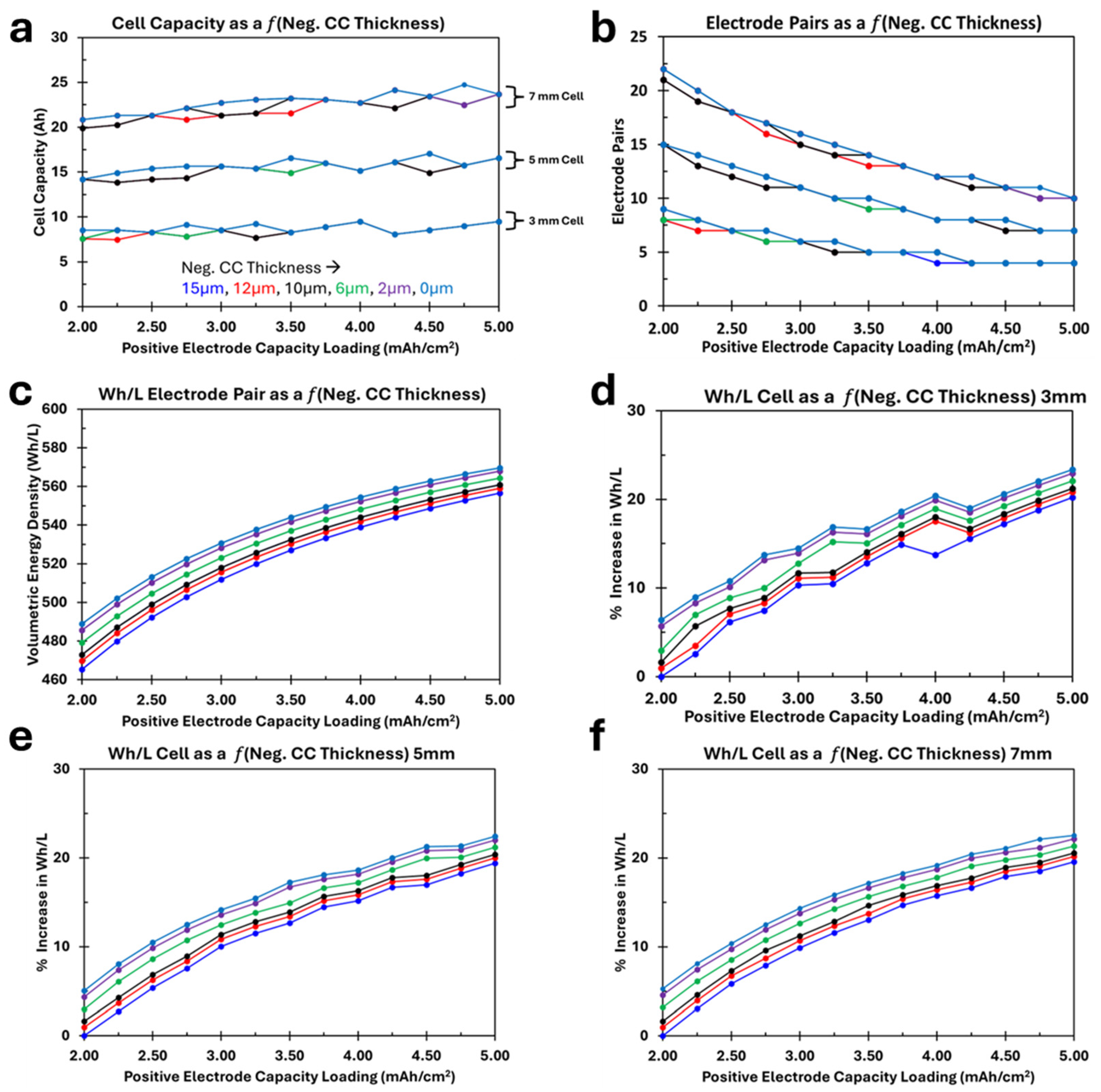
3.2. Impact of Negative Current Collector Thickness
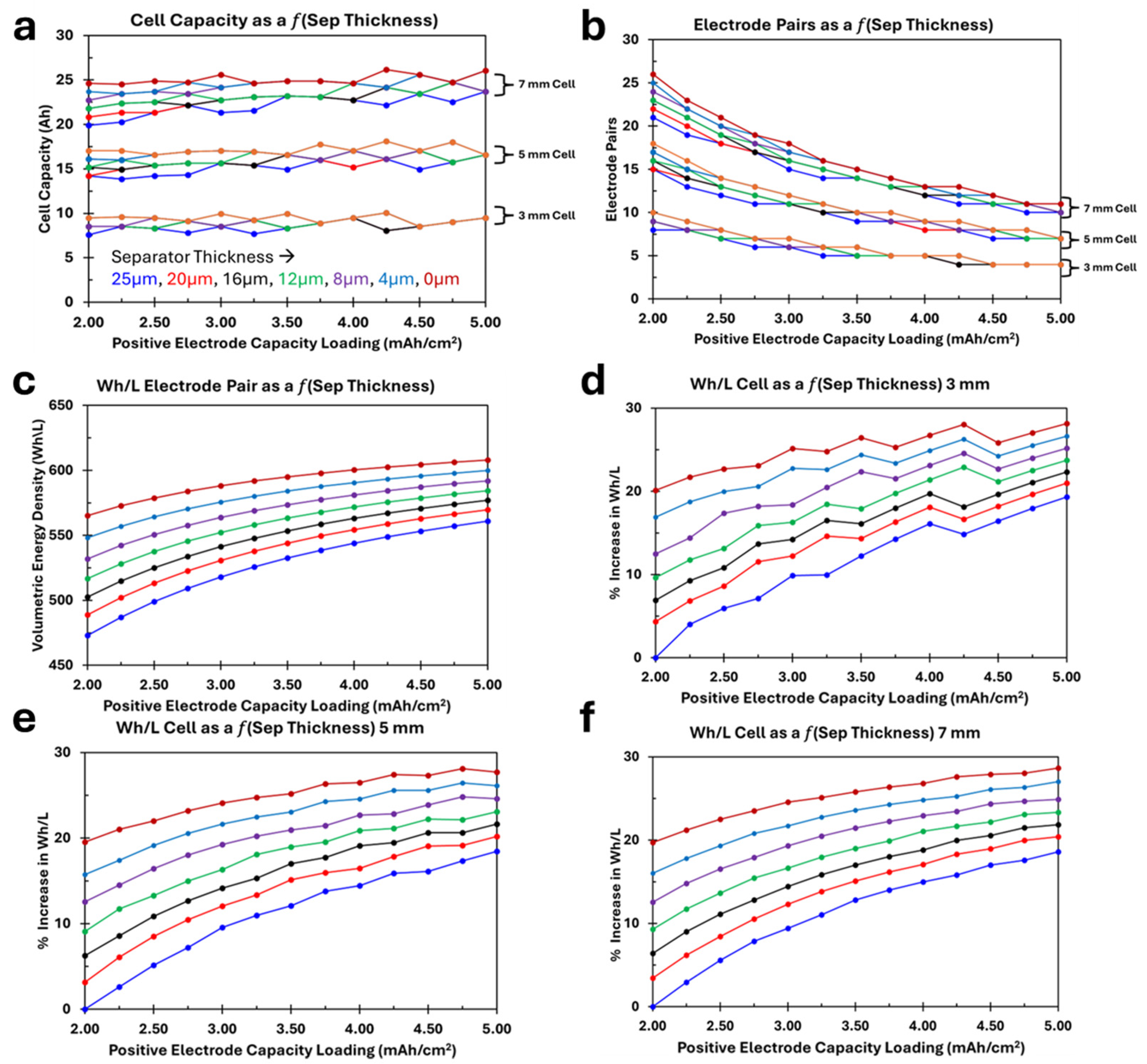
3.3. Impact of Separator Thickness
3.4. Impact of Aluminum Laminate Cell Package Thickness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, H.; Sharma, S.; Mishra, P.K. A Critical Review of Recent Progress on Lithium Ion Batteries: Challenges, Applications, and Future Prospects. Microchem. J. 2025, 212, 113494. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, N.; Lu, Y.; Zhang, Z.; Li, M.; Liu, J.; Song, W.; Zhao, Y.; Miao, Z. Strategies toward the Development of High-Energy-Density Lithium Batteries. J. Energy Storage 2024, 88, 111666. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A Retrospective on Lithium-Ion Batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhou, J.; Wang, Z.; Zhu, P.; Cao, Y.; Zheng, Y.; Zhou, X.; Yan, C.; Qian, T. Advances and Prospects in Improving the Utilization Efficiency of Lithium for High Energy Density Lithium Batteries. Adv. Funct. Mater. 2023, 33, 2302055. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, L.; Ma, H.; Wu, Z.; Zhao, Q.; Li, H.; Zhang, K.; Chen, J. Challenges and Advances in Wide-Temperature Rechargeable Lithium Batteries. Energy Environ. Sci. 2022, 15, 1711–1759. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational Design on Materials for Developing next Generation Lithium-Ion Secondary Battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Effect of Porosity on the Thick Electrodes for High Energy Density Lithium Ion Batteries for Stationary Applications. Batteries 2016, 2, 35. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Yourey, W. Cell Design Considerations and Impact on Energy Density—A Practical Approach to EV Cell Design. World Electr. Veh. J. 2023, 14, 279. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A Comprehensive Understanding of Electrode Thickness Effects on the Electrochemical Performances of Li-Ion Battery Cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Lee, T.; Kim, E.; An, M.; Park, J.; Cho, J.; Son, Y. Investigation of Mass Loading of Cathode Materials for High Energy Lithium-Ion Batteries. Electrochem. Commun. 2023, 147, 107437. [Google Scholar] [CrossRef]
- Choi, J.; Son, B.; Ryou, M.-H.; Kim, S.H.; Ko, J.M.; Lee, Y.M. Effect of LiCoO2 Cathode Density and Thickness on Electrochemical Performance of Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2013, 4, 27–33. [Google Scholar] [CrossRef]
- Malifarge, S.; Delobel, B.; Delacourt, C. Experimental and Modeling Analysis of Graphite Electrodes with Various Thicknesses and Porosities for High-Energy-Density Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1275–A1287. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A. The Dependence of Natural Graphite Anode Performance on Electrode Density. J. Power Sources 2004, 130, 247–253. [Google Scholar] [CrossRef]
- Mu, G.; Agrawal, S.; Sittisomwong, P.; Bai, P. Impacts of Negative to Positive Capacities Ratios on the Performance of Next-Generation Lithium-Ion Batteries. Electrochim. Acta 2022, 406, 139878. [Google Scholar] [CrossRef]
- Kim, C.S.; Jeong, K.M.; Kim, K.; Yi, C.W. Effects of Capacity Ratios between Anode and Cathode on Electrochemical Properties for Lithium Polymer Batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A Review of Current Collectors for Lithium-Ion Batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Recovery of Cathode Materials and Al from Spent Lithium-Ion Batteries by Ultrasonic Cleaning. Waste Manag. 2015, 46, 523–528. [Google Scholar] [CrossRef]
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent Advances in Lithium-Ion Battery Materials for Improved Electrochemical Performance: A Review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Matsumoto, F.; Fukunishi, M. Review of Current Collector-, Binder-, Conductive Additive-Free, and Freestanding Electrodes in Lithium and Related Batteries. Batteries 2024, 10, 330. [Google Scholar] [CrossRef]
- Johnson, B.A.; White, R.E. Characterization of Commercially Available Lithium-Ion Batteries. J. Power Sources 1998, 70, 48–54. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Hao, H.; Tan, R.; Ye, C.; Low, C.T.J. Carbon-Coated Current Collectors in Lithium-Ion Batteries and Supercapacitors: Materials, Manufacture and Applications. Carbon. Energy 2024, 6, e604. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A Review of Recent Developments in Membrane Separators for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Rajan, D.; Kannan, R.; Terala, K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the Separator Thickness and Porosity on the Performance of Lithium-Ion Batteries. Int. J. Electrochem. 2018, 2018, 1925708. [Google Scholar] [CrossRef]
- Horváth, D.V.; Tian, R.; Gabbett, C.; Nicolosi, V.; Coleman, J.N. Quantifying the Effect of Separator Thickness on Rate Performance in Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 030503. [Google Scholar] [CrossRef]
- Choi, S.; Kim, U.; Roh, Y.; Dzakpasu, C.B.; Lim, J.; Song, M.; Rhee, J.; Lee, Y.S.; Lee, Y.M. Roll-to-Roll Fabrication and Characterization of Ultra-Thin Ceramic-Coated Separator for High-Energy-Density Lithium-Ion Batteries. J. Power Sources 2024, 623, 235427. [Google Scholar] [CrossRef]
- Yoo, S.; Hong, C.; Chong, K.T.; Seul, N. Analysis of Pouch Performance to Ensure Impact Safety of Lithium-Ion Battery. Energies 2019, 12, 2865. [Google Scholar] [CrossRef]
- Yourey, W. Theoretical Impact of Manufacturing Tolerance on Lithium-Ion Electrode and Cell Physical Properties. Batteries 2020, 6, 23. [Google Scholar] [CrossRef]
- Yourey, W. Silicon Negative Electrodes—What Can Be Achieved for Commercial Cell Energy Densities. Batteries 2023, 9, 576. [Google Scholar] [CrossRef]


| Positive Electrode (LCO) | ||
| Material | Weight Percent | Density (g/cm3) |
| LiCoO2 | 90 | 5.00 |
| Conductive Additive | 5 | 2.00 |
| PVDF Electrode Binder | 5 | 1.78 |
| Positive Mixture | - | 4.29 |
| Negative Electrode | ||
| Material | Weight Percent | Density (g/cm3) |
| Active Carbon | 90 | 2.20 |
| Conductive Additive | 2 | 2.00 |
| PVDF Electrode Binder | 8 | 1.78 |
| Negative Mixture | - | 2.15 |
| Material | Thicknesses Evaluated (µm) |
|---|---|
| Positive Current Collector | 20, 16, 12, 8, 4, 0 |
| Negative Current Collector | 15, 12, 10, 6, 2, 0 |
| Separator | 25, 20, 16, 12, 8, 4, 0 |
| Package Material | 156, 150, 125, 100, 75, 50, 25, 0 |
| Component | Dimensions |
|---|---|
| Negative Electrode | 200 mm × 120 mm |
| Positive Electrode | 199 mm × 119 mm |
| Separator 1 | 201.75 mm × 120 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yourey, W.; Nong, K.; Babaiahgari, B. Impact of Inert Materials on Commercial Lithium–Ion Cell Energy Density. Batteries 2025, 11, 353. https://doi.org/10.3390/batteries11100353
Yourey W, Nong K, Babaiahgari B. Impact of Inert Materials on Commercial Lithium–Ion Cell Energy Density. Batteries. 2025; 11(10):353. https://doi.org/10.3390/batteries11100353
Chicago/Turabian StyleYourey, William, Kayla Nong, and Bhanu Babaiahgari. 2025. "Impact of Inert Materials on Commercial Lithium–Ion Cell Energy Density" Batteries 11, no. 10: 353. https://doi.org/10.3390/batteries11100353
APA StyleYourey, W., Nong, K., & Babaiahgari, B. (2025). Impact of Inert Materials on Commercial Lithium–Ion Cell Energy Density. Batteries, 11(10), 353. https://doi.org/10.3390/batteries11100353






