Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide
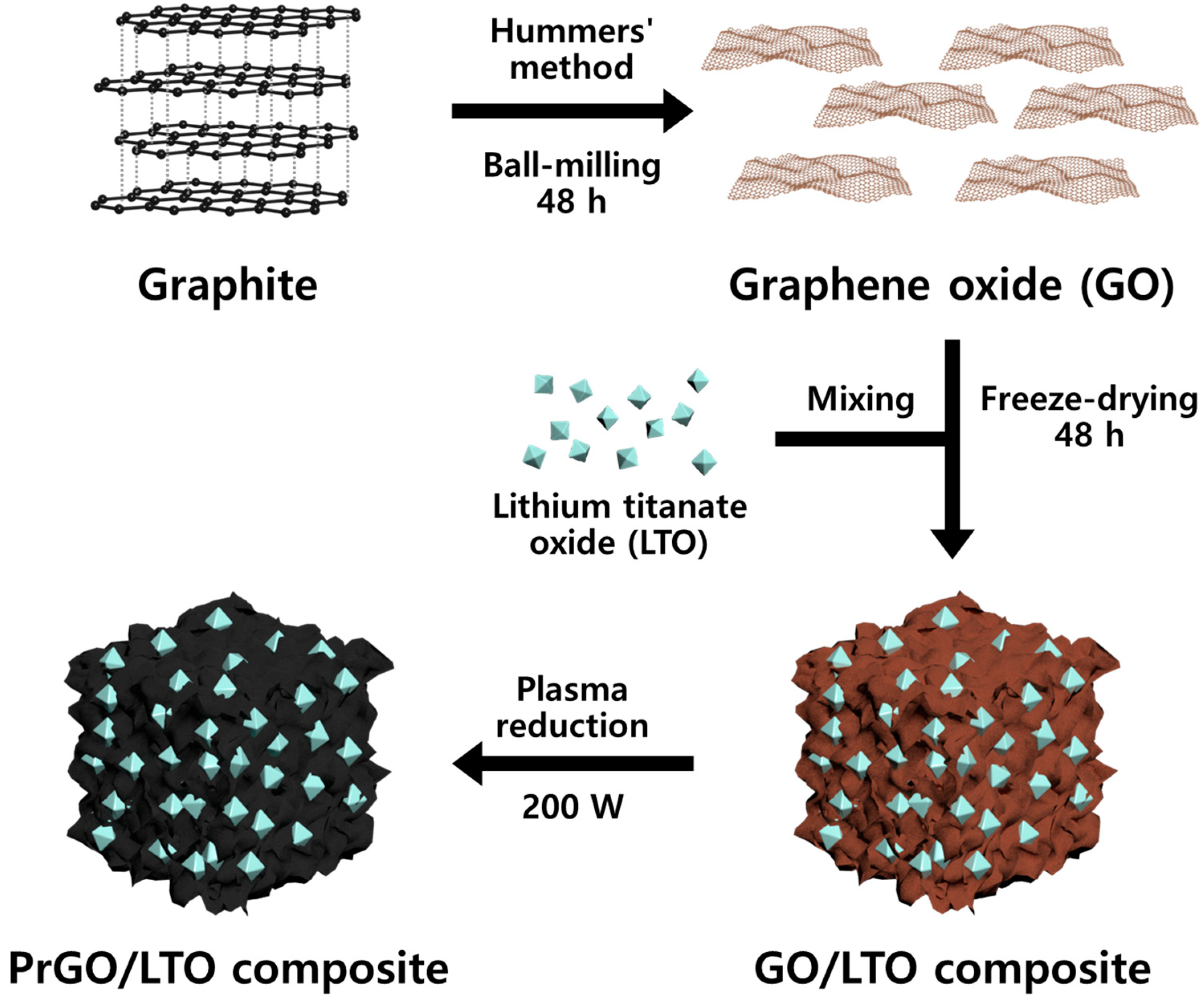
2.3. Preparation of Plasma-Reduced GO/LTO Composite (PrGO/LTO)
2.4. Characterization
2.5. Electrochemical Analysis
3. Results and Discussion
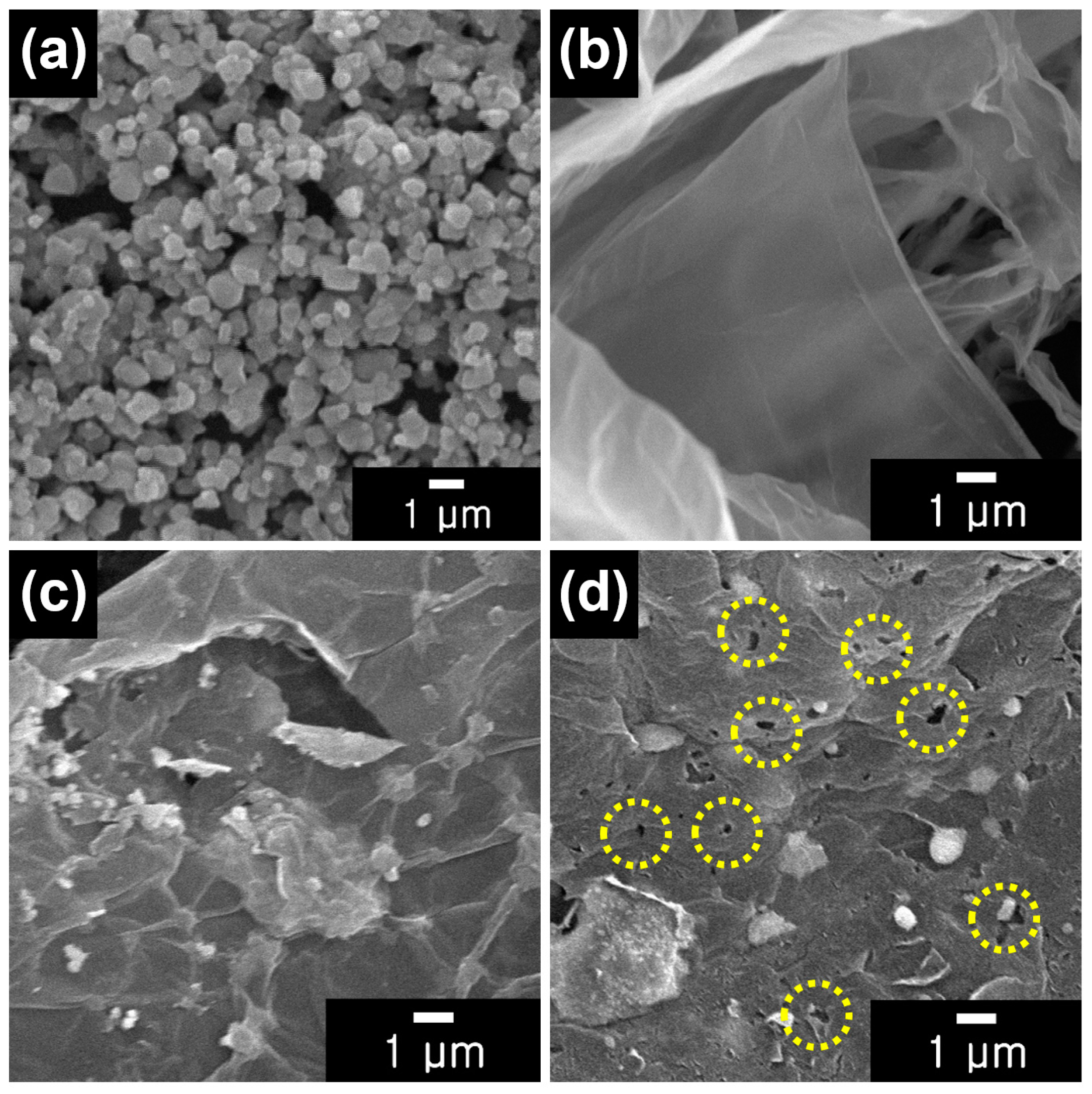
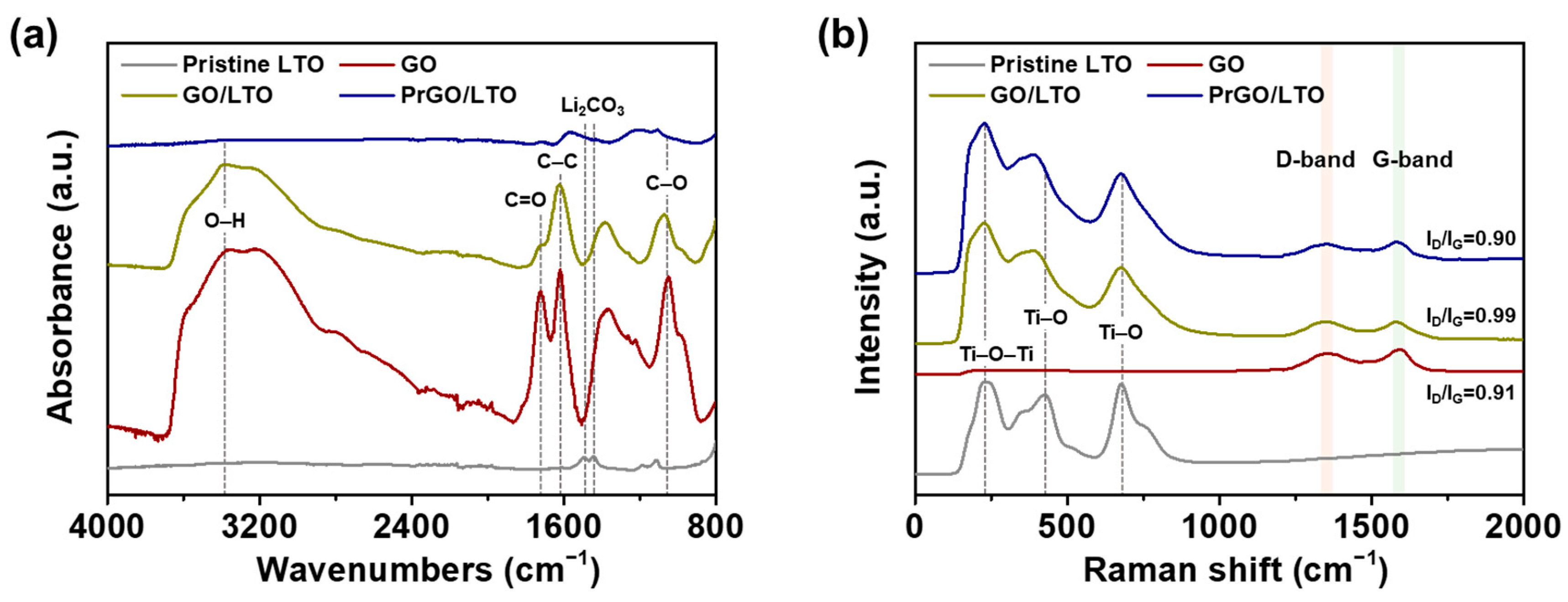
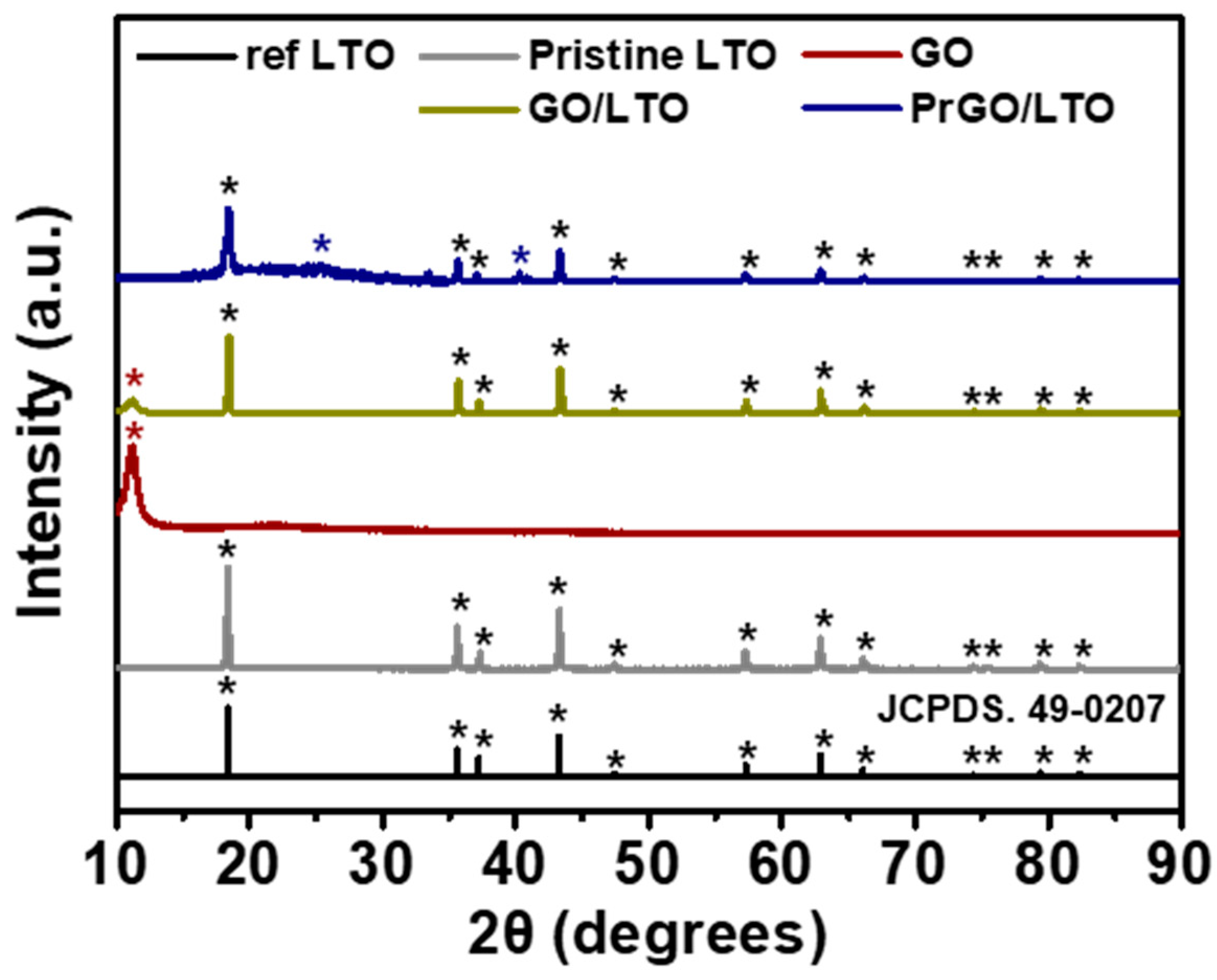
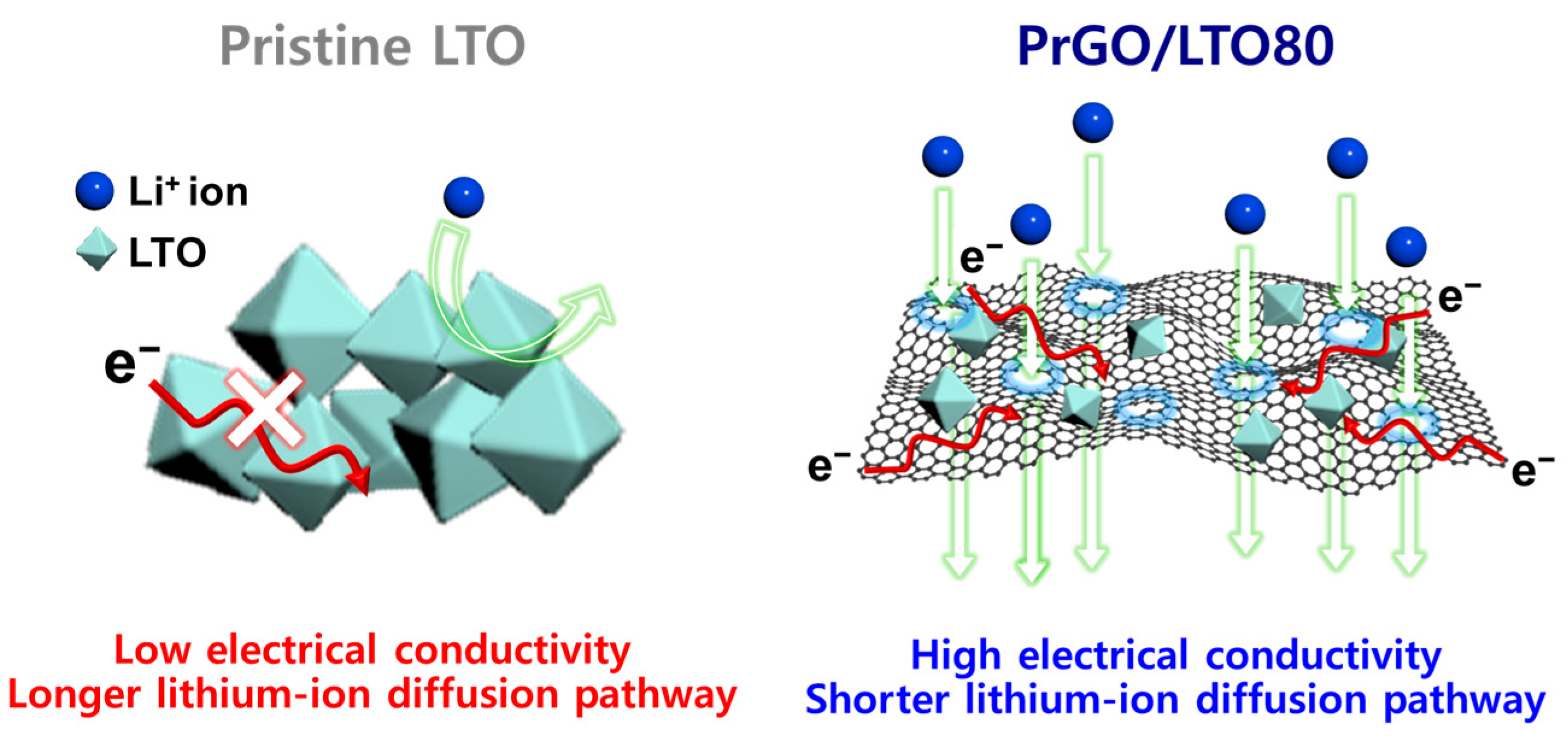
3.1. Fabrication of PrGO/LTO Composite
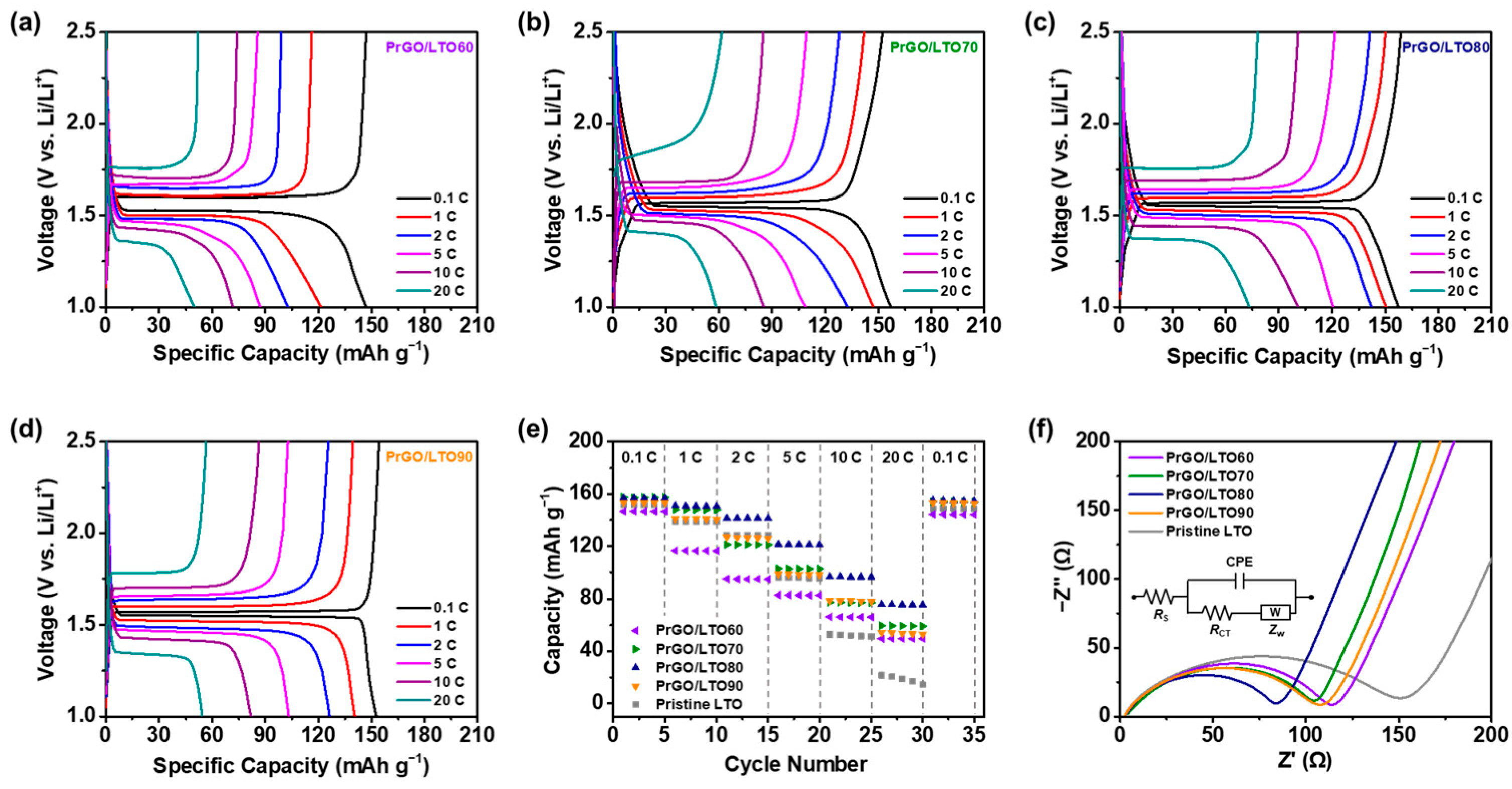
3.2. Electrochemical Performance of PrGO/LTO-Based Electrodes
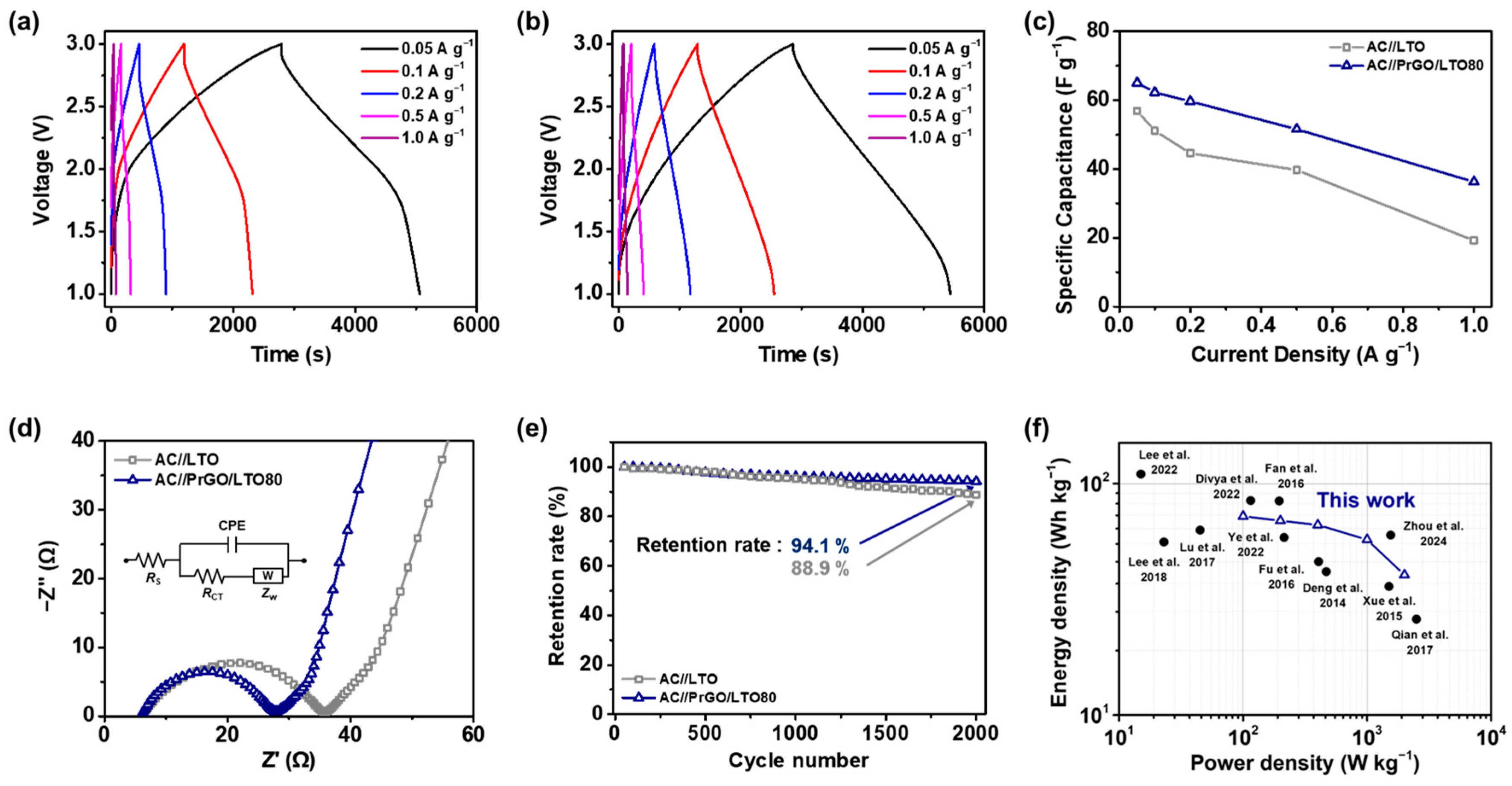
3.3. Electrochemical Performance of the AC//PrGO/LTO80 Full-Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Meng, J.; Chen, D.; Liu, H.; Hao, S.; Sui, X.; Du, X. A Review of Lithium-Ion Battery Capacity Estimation Methods for Onboard Battery Management Systems: Recent Progress and Perspectives. Batteries 2022, 8, 229. [Google Scholar] [CrossRef]
- Pameté, E.; Köps, L.; Kreth, F.A.; Pohlmann, S.; Varzi, A.; Brousse, T.; Balducci, A.; Presser, V. The Many Deaths of Supercapacitors: Degradation, Aging, and Performance Fading. Adv. Energy Mater. 2023, 13, 2301008. [Google Scholar] [CrossRef]
- Pandey, D.; Kumar, K.S.; Thomas, J. Supercapacitor electrode energetics and mechanism of operation: Uncovering the voltage window. Prog. Mater. Sci. 2024, 141, 101219. [Google Scholar] [CrossRef]
- Guo, L.; Hu, P.; Wei, H. Development of supercapacitor hybrid electric vehicle. J. Energy Storage 2023, 65, 107269. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A. Advances in Supercapacitor Development: Materials, Processes, and Applications. J. Electron. Mater. 2023, 52, 96–129. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Hung, P.Y.; Zhang, H.; Lin, H.; Guo, Q.; Lau, K.T.; Jia, B. Specializing liquid electrolytes and carbon-based materials in EDLCs for low-temperature applications. J. Energy Chem. 2022, 68, 580–602. [Google Scholar] [CrossRef]
- Zou, K.; Cai, P.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Carbon materials for high-performance lithium-ion capacitor. Curr. Opin. Electrochem. 2020, 21, 31–39. [Google Scholar] [CrossRef]
- Dong, S.; Lv, N.; Wu, Y.; Zhu, G.; Dong, X. Lithium-ion and sodium-ion hybrid capacitors: From insertion-type materials design to devices construction. Adv. Funct. Mater. 2021, 31, 2100455. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Li, C.; Wang, K.; Sun, X.; Ma, Y. High-efficiency sacrificial prelithiation of lithium-ion capacitors with superior energy-storage performance. Energy Storage Mater. 2020, 24, 160–166. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, S.Y.; Lee, J.S. Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles. Npj Comput. Mater. 2022, 8, 103. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Qin, N.; Zheng, J.; Wu, Q.; Zhang, C.; Zheng, J.P. A universal matching approach for high power-density and high cycling-stability lithium ion capacitor. J. Power Sources 2019, 441, 227211. [Google Scholar] [CrossRef]
- Xu, G.; Han, P.; Dong, S.; Liu, H.; Cui, G.; Chen, L. Li4Ti5O12-based energy conversion and storage systems: Status and prospects. Coord. Chem. Rev. 2017, 343, 139–184. [Google Scholar] [CrossRef]
- Shi, X.; Yu, S.; Deng, T.; Zhang, W.; Weitao, Z. Unlock the potential of Li4Ti5O12 for high-voltage/long-cycling-life and high-safety batteries: Dual-ion architecture superior to lithium-ion storage. J. Energy Chem. 2020, 44, 13–18. [Google Scholar] [CrossRef]
- Llaín-Jiménez, H.A.; Buchberger, D.A.; Winkowska-Struzik, M.; Ratyński, M.; Krajewski, M.; Boczar, M.; Hamankiewicz, B.; Czerwiński, A. Correlation between Lithium Titanium Oxide Powder Morphology and High Rate Performance in Lithium-Ion Batteries. Batteries 2022, 8, 168. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, F.; Zhao, W.; Liu, Z.; Wan, D. Synthesis of graphene-supported Li4Ti5O12 nanosheets for high rate battery application. J. Mater. Chem. 2012, 22, 11257–11260. [Google Scholar] [CrossRef]
- Pawlitzek, F.; Althues, H.; Schumm, B.; Kaskel, S. Nanostructured Networks for Energy Storage: Vertically Aligned Carbon Nanotubes (VACNT) as Current Collectors for High-Power Li4Ti5O12(LTO)//LiMn2O4(LMO) Lithium-Ion Batteries. Batteries 2017, 3, 37. [Google Scholar] [CrossRef]
- An, D.; Shen, L.; Lei, D.; Wang, L.; Ye, H.; Li, B.; Kang, F.; He, Y.-B. An ultrathin and continuous Li4Ti5O12 coated carbon nanofiber interlayer for high rate lithium sulfur battery. J. Energy Chem. 2019, 31, 19–26. [Google Scholar] [CrossRef]
- Lim, S.; Park, H.; Yamamoto, G.; Lee, C.; Suk, J.W. Measurements of the Electrical Conductivity of Monolayer Graphene Flakes Using Conductive Atomic Force Microscopy. Nanomaterials 2021, 11, 25785. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Doñoro, Á.; Muñoz-Mauricio, Á.; Etacheri, V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries 2021, 7, 26. [Google Scholar] [CrossRef]
- Albers, P.W.; Leich, V.; Ramirez-Cuesta, A.J.; Cheng, Y.; Hönig, J.; Parker, S.F. The characterisation of commercial 2D carbons: Graphene, graphene oxide and reduced graphene oxide. Mater. Adv. 2022, 3, 2810–2826. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Cho, S.; Yoon, C.-M.; Lee, C.; Ryu, J.; Jang, J. Multidimensional Polyaniline/Reduced Graphene Oxide/Silica Nanocomposite for Efficient Supercapacitor Electrodes. ChemNanoMat 2016, 2, 236–241. [Google Scholar] [CrossRef]
- Bouzina, A.; Perrot, H.; Sel, O.; Debiemme-Chouvy, C. Preventing Graphene from Restacking via Bioinspired Chemical Inserts: Toward a Superior 2D Micro-supercapacitor Electrode. ACS Appl. Nano Mater. 2021, 4, 4964–4973. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Chen, Y.-H.; Yeh, Y.-S.; Hy, S.; Kuo, L.-Y.; Hwang, B.-J. Enhancement of Electrochemical Properties by Freeze-dried Graphene Oxide via Glucose-assisted Reduction. Electrochim. Acta 2016, 197, 146–151. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Wang, B.; Huang, M.; Wang, B.; Jiang, Y.; Suof, R. Freeze-Casting Produces a Graphene Oxide Aerogel with a Radial and Centrosymmetric Structure. ACS Nano 2018, 12, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiu, Y.; Liu, Y.; Xu, K.; Zhao, N.; Lao, C.; Shen, J.; Chen, Z. Novel 3D grid porous Li4Ti5O12 thick electrodes fabricated by 3D printing for high performance lithium-ion batteries. J. Adv. Ceram. 2022, 11, 295–307. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021, 55, 323–344. [Google Scholar] [CrossRef]
- Alazmi, A.; Tall, O.E.; Rasul, S.; Hedhili, M.N.; Patole, S.P.; Costa, P.M. A process to enhance the specific surface area and capacitance of hydrothermally reduced graphene oxide. Nanoscale 2016, 8, 17782–17787. [Google Scholar] [CrossRef]
- Zhao, J.; Rafat, M.N.; Yoon, C.-M.; Oh, W.-C. Novel Approach to Synthesis of AgZnS and TiO2 Decorated on Reduced Graphene Oxide Ternary Nanocomposite for Hydrogen Evolution Effect of Enhanced Synergetic Factors. Nanomaterials 2022, 12, 3639. [Google Scholar] [CrossRef] [PubMed]
- Akada, K.; Obata, S.; Saiki, K. Radio-frequency plasma assisted reduction and nitrogen doping of graphene oxide. Carbon 2022, 189, 571–578. [Google Scholar] [CrossRef]
- Wu, S.; Su, F.; Dong, X.; Ma, C.; Pang, L.; Peng, D.; Wang, M.; He, L.; Zhang, Z. Development of glucose biosensors based on plasma polymerization-assisted nanocomposites of polyaniline, tin oxide, and three-dimensional reduced graphene oxide. Appl. Surf. Sci. 2017, 401, 262–270. [Google Scholar] [CrossRef]
- Kondratowics, I.; Nadolska, M.; Şahin, S.; Łapiński, M.; Prześniak-Welenc, M.; Sawczak, M.; Yu, E.H.; Sadowski, W.; Żelechowska, K. Tailoring properties of reduced graphene oxide by oxygen plasma treatment. Appl. Surf. Sci. 2018, 440, 651–659. [Google Scholar] [CrossRef]
- Shabin, M.; Hanaa, M.H.; Ranwen, O.; Shasha, L.; Hongyu, M.; Xiaofang, C.; Tam, S.; Huanting, W. Effect of oxygen plasma treatment on the nanofiltration performance of reduced graphene oxide/cellulose nanofiber composite membranes. Green Chem. Eng. 2021, 2, 122–131. [Google Scholar]
- Jianlang, F.; Yunqing, Y.; Meng, X.; Ganbg, W.; Yu, K. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar]
- Wang, S.; Wang, R.; Zhao, Q.; Ren, L.; Wen, J.; Chang, J.; Fang, X.; Hu, N.; Xu, C. Freeze-drying induced self-assembly approach for scalable constructing MoS2/graphene hybrid aerogels for lithium-ion batteries. J. Colloid Interface Sci. 2019, 544, 37–45. [Google Scholar] [CrossRef]
- Noh, J.; Jekal, S.; Yoon, C.-M. Polyaniline-Coated Mesoporous Carbon Nanosheets with Fast Capacitive Energy Storage in Symmetric Supercapacitors. Adv. Sci. 2023, 10, 2301923. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jekal, S.; Kim, D.-H.; Noh, J.; Kim, J.; Kim, H.-Y.; Kim, C.-G.; Chu, Y.-R.; Oh, W.-C. 3D Hierarchically Structured Tin Oxide and Iron Oxide-Embedded Carbon Nanofiber with Outermost Polypyrrole Layer for High-Performance Asymmetric Supercapacitor. Nanomaterials 2023, 13, 1614. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ma, G.; Du, X.; Du, L.; Jing, P.; Wang, Y.; Wu, K.; Wu, H.; Wang, Q.; et al. Investigation on process mechanism of a novel energy-saving synthesis for high performance Li4Ti5O12 anode material. J. Energy Chem. 2022, 70, 266–275. [Google Scholar] [CrossRef]
- Liyuan, Z.; Gang, H.; Dali, Z.; Jiabei, Z.; Qianqian, Y. Study on transformation mechanism of lithium titanate modified with hydrochloric acid. Ionics 2016, 22, 2007–2014. [Google Scholar]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Lee, K.; Jang, J. Enhanced Electrorheological Performance of Mixed Silica Nanomaterial Geometry. ACS Appl. Mater. Interface 2017, 9, 36358–36367. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual external field-responsive polyaniline-coated magnetite/silica nanoparticles for smart fluid applications. Chem. Commun. 2017, 53, 6645–6648. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, C.; Yoon, C.-M.; Kim, M.; Jang, J. High-Performance Three-Dimensional Mesoporous Graphene Electrode for Supercapacitors using Lyophilization and Plasma Reduction. ACS Appl. Mater. Interfaces 2017, 9, 5222–5230. [Google Scholar] [CrossRef]
- Hong, S.-A.; Lee, S.B.; Joo, O.-S.; Kang, J.W.; Cho, B.-W.; Lim, J.-S. Synthesis of lithium titanium oxide (Li4Ti5O12) with ultrathin carbon layer using supercritical fluids for anode materials in lithium batteries. J. Mater. Sci. 2016, 51, 6220–6534. [Google Scholar] [CrossRef]
- Gul, W.; Alrobei, H. Effect of Graphene Oxide Nanoparticles on the Physical and Mechanical Properties of Medium Density Fiberboard. Polymers 2021, 13, 1818. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Peng, H.-T.; Michalska, M.; Xiao, Y.; Lin, H.-Y.; Hsieh, C.-K. Investigation of carbon coating approach on electrochemical performance of Li4Ti5O12/C composite anodes for high-rate lithium-ion batteries. J. Solid State Electrochem. 2018, 22, 1851–1861. [Google Scholar] [CrossRef]
- Nikiforov, A.A.; Kuznetsov, D.K.; Nasara, R.N.; Govindarajan, K.; Lin, S.-K.; Pelegov, D.V. Fast and Slow Laser-Stimulated Degradation of Mn-Doped Li4Ti5O12. Batteries 2022, 8, 251. [Google Scholar] [CrossRef]
- Wunderlich, P.; Küpper, J.; Simon, U. Optimizing Discharge Capacity of Graphite Nanosheet Electrodes for Lithium–Oxygen Batteries. Batteries 2020, 6, 36. [Google Scholar] [CrossRef]
- Shen, Y.; Jing, T.; Ren, W.; Zhang, J.; Jiang, Z.-G.; Yu, Z.-Z.; Dasari, A. Chemical and thermal reduction of graphene oxide and its electrically conductive polylactic acid nanocomposites. Compos. Sci. Technol. 2012, 72, 1430–1435. [Google Scholar] [CrossRef]
- Babu, B.V.; Babu, K.V.; Aregai, G.T.; Devi, L.S.; Latha, B.M.; Reddi, M.S.; Samatha, K.; Veeraiah, V. Structural and electrical properties of Li4Ti5O12 anode material for lithium-ion batteries. Results Phys. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Storm, M.M.; Overgaard, M.; Younesi, R.; Reeler, N.E.A.; Vosch, T.; Nielsen, U.G.; Edström, K.; Norby, P. Reduced graphene oxide for Li–air batteries: The effect of oxidation time and reduction conditions for graphene oxide. Carbon 2015, 85, 233–244. [Google Scholar] [CrossRef]
- Qiao, X.; Liao, S.; You, C.; Chen, R. Phosphorus and Nitrogen Dual Doped and Simultaneously Reduced Graphene Oxide with High Surface Area as Efficient Metal-Free Electrocatalyst for Oxygen Reduction. Catalysts 2015, 5, 981–991. [Google Scholar] [CrossRef]
- Su, H.; Zhang, C.; Li, X.; Wu, L.; Chen, Y. Aggregation prevention: Reduction of graphene oxide in mixed medium of alkylphenol polyoxyethylene (7) ether and 2-methoxyethanol. RSC Adv. 2018, 8, 39140–39148. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, Z.; Mao, J. Mechanisms of the decrease in low-temperature electrochemical performance of Li4Ti5O12-based anode materials. Sci. Rep. 2017, 7, 15292. [Google Scholar] [CrossRef]
- Patat, S.; Rahman, S.; Dokan, F.K. The effect of sodium and niobium co-doping on electrochemical performance of Li4Ti5O12 as anode material for lithium-ion batteries. Ionics 2022, 28, 3177–3185. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. Micromachines 2024, 15, 310. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Li, Y.; Guo, X.; Shen, Y.; Wang, D.; Zhang, Y.; Wang, Z. Fabrication of Li4Ti5O12-TiO2 Nanosheets with Structural Defects as High-Rate and Long-Life Anodes for Lithium-Ion Batteries. Sci. Rep. 2017, 7, 2960. [Google Scholar] [CrossRef]
- Jekal, S.; Kim, J.; Kim, D.-H.; Noh, J.; Kim, M.-J.; Kim, H.-Y.; Kim, M.-S.; Oh, W.-C.; Yoon, C.-M. Synthesis of LiDAR-Detectable True Black Core/Shell Nanomaterial and Its Practical Use in LiDAR Applications. Nanomaterials 2022, 12, 3689. [Google Scholar] [CrossRef] [PubMed]
- Jekal, S.; Otgonbayar, Z.; Noh, J.; Sa, M.; Kim, J.; Kim, C.-G.; Chu, Y.-R.; Kim, H.-Y.; Song, S.; Choi, H.; et al. Designing Novel LiDAR-Detectable Plate-Type Materials: Synthesis, Chemistry, and Practical Application for Autonomous Working Environment. ACS Appl. Mater. Interfaces 2024, 16, 19121–19136. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Jung, W.-G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Coelho, J.; Pokel, A.; Park, S.-H.; McEvoy, N.; Berner, N.C.; Duesberg, G.S.; Nicolosi, V. Lithium Titanate/Carbon Nanotubes Composites Processed by Ultrasound Irradiation as Anodes for Lithium Ion Batteries. Nature 2017, 7, 7614. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, L.; Yang, L.; Zhou, X.; Zhang, Y. Structural Properties of Graphene Oxide Prepared from Graphite by Three Different Methods and the Effect on Removal of Cr(VI) from Aqueous Solution. Nanomaterials 2023, 13, 279. [Google Scholar] [CrossRef]
- Anthony, G.; Genwei, C.; Mark, R.; Kun, W.; Yizhi, X.; Zhe, Q. Accelerated Synthesis of Ordered Mesoporous Carbons Using Plasma. ACS Omega 2023, 8, 15781–15789. [Google Scholar]
- Mihiri, Y.G.E.; Sahrooz, R.; Rusen, Z.; Renwu, Z.; Patrick, J.C.; Anthony, P.M.; Jennifer, M.; Kostya, K.O. Power-to-decarbonization: Mesoporous carbon-MgO nanohybrid derived from plasma-activated seawater salt-loaded biomass for efficient CO2 capture. J. CO2 Util. 2021, 53, 101711. [Google Scholar]
- Alireza, B.S.; Eslam, G.S.; Mohammad, H.; Maryam, S. Synergistic catalytic degradation of ciprofloxacin using magnetic carbon nanomaterial/NiFe2O4 promoted cold atmospheric pressure plasma jet: Influence of charcoal, multi walled carbon nanotubes and walnut shell. J. Taiwan Inst. Chem. Eng. 2022, 132, 104131. [Google Scholar]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Thermal Characterizations of a Lithium Titanate Oxide-Based Lithium-Ion Battery Focused on Random and Periodic Charge-Discharge Pulses. Appl. Syst. Innov. 2021, 4, 24. [Google Scholar] [CrossRef]
- Truptimayee, A.; Anil, D.P.; Soobhankar, P. High-Temperature Electrochemical Performance of Lithium Titanate (Li4Ti5O12) Anode Material in Secondary Lithium-ion Batteries. J. Energy Storage 2023, 67, 107529. [Google Scholar]
- Shilei, D.; Zelong, J.; Jing, G.; Hongliang, Z.; Jiajia, C.; Dongdong, W. Carbon-coated lithium titanate: Effect of carbon precursor addition processes on the electrochemical performance. Front. Chem. Sci. Eng. 2021, 15, 148–155. [Google Scholar]
- Kumar, N.; Setshedi, K.; Masukume, M.; Suprakas, S.R. Facile scalable synthesis of graphene oxide and reduced graphene oxide: Comparative investigation of different reduction methods. Carbon Lett. 2022, 32, 1031–1046. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Seop, K.H. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Sun, X.; An, Y.; Song, S.; Li, C.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; et al. Electrochemical impedance spectroscopy study of lithium-ion capacitors: Modeling and capacity fading mechanism. J. Power Sources 2021, 488, 229454. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Kallioinen, J.; Kallio, T. Comparative study of carbon free and carbon containing Li4Ti5O12 electrodes. J. Power Sources 2015, 279, 481–486. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, F.; Chen, J. Investigation of effects of carbon coating on the electrochemical performance of Li4Ti5O12/C nanocomposites. J. Mater. Chem. A 2013, 1, 9484–9490. [Google Scholar] [CrossRef]
- Lee, B.-G.; Lee, S.-H. Application of hybrid supercapacitor using granule Li4Ti5O12/activated carbon with variation of current density. J. Power Sources 2017, 343, 545–549. [Google Scholar] [CrossRef]
- Dsoke, S.; Fuchs, B.; Gucciardi, E.; Wohlfahrt-Mehrens, M. The importance of the electrode mass ratio in a Li-ion capacitor based on activated carbon and Li4Ti5O12. J. Power Sources 2015, 282, 385–393. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Zhang, X.; Peng, H.; Zhang, Y.; Wang, G.; Wang, Z.; Cao, G.; Umirov, N.; Bakenov, Z. Effect of graphene nanosheets on electrochemical performance of Li4Ti5O12 in lithium-ion capacitors. Ceram. Int. 2017, 46, 6554–6562. [Google Scholar] [CrossRef]
- Fu, C.C.; Zhang, L.J.; Peng, J.H.; Wang, H.; Yan, H. Synthesis of Li4Ti5O12-reduced graphene oxide composite and its application for hybrid supercapacitors. Ionics 2016, 22, 1829–1836. [Google Scholar] [CrossRef]
- Qian, Y.; Cai, X.; Zhang, C.; Jiang, H.; Zhou, L.; Li, B. A free-standing Li4Ti5O12/graphene foam composite as anode material for Li-ion hybrid supercapacitor. Electrochim. Acta 2017, 258, 1311–1319. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Sun, S.; Wang, H.; Liu, J.; Yan, H. Synthesis and electrochemical properties of Li4Ti5O12 spheres and its application for hybrid supercapacitors. Electrochim. Acta 2014, 146, 37–43. [Google Scholar] [CrossRef]
- Xue, R.; Yan, J.; Jiang, L.; Yi, B. Fabrication of lithium titanate/graphene composites with high rate capability as electrode materials for hybrid electrochemical supercapacitors. Mater. Chem. Phys. 2015, 160, 375–382. [Google Scholar] [CrossRef]
- Lee, B.-G.; Lee, S.-H.; Ahn, H.-J.; Yoon, J.-R. High performance hybrid supercapacitors using granule Li4Ti5O12/Carbon nanotube anode. J. Alloy. Compd. 2018, 748, 882–888. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, M.; Meng, Q.; Cao, B.; Yu, Y. Activated-Nitrogen-Doped Graphene-Based Aerogel Composites as Cathode Materials for High Energy Density Lithium-Ion Supercapacitor. J. Electrochem. Soc. 2016, 163, A1736–A1742. [Google Scholar] [CrossRef]
- Ye, Z.; Zhong, F.; Chen, Y.; Zou, Z.; Jiang, C. Unique CNTs-chained Li4Ti5O12 nanoparticles as excellent high rate anode materials for Li-ion capacitors. Ceram. Int. 2022, 48, 20237–20244. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, Y.; Zhang, T.A. Cost-Effective Production Route of Li4Ti5O12 Resisting Unsettled Market and Subsequent Application in the Li-Ion Capacitor. Small Struct. 2024, 5, 2300377. [Google Scholar] [CrossRef]
- Divya, M.L.; Lü, H.Y.; Lee, Y.S.; Aravindan, V. Pre-lithiated Li4+xTi5O12 (0 ≤ x ≤ 3) anodes towards building high-performance Li-ion capacitors. Sustain. Energy Fuels 2022, 6, 4884–4892. [Google Scholar] [CrossRef]
- Lee, S.H. Smart Multi-Layer Architecture Electrodes for High Energy Density Lithium-Ion Capacitors. Batter. Supercaps. 2022, 6, e202200380. [Google Scholar] [CrossRef]
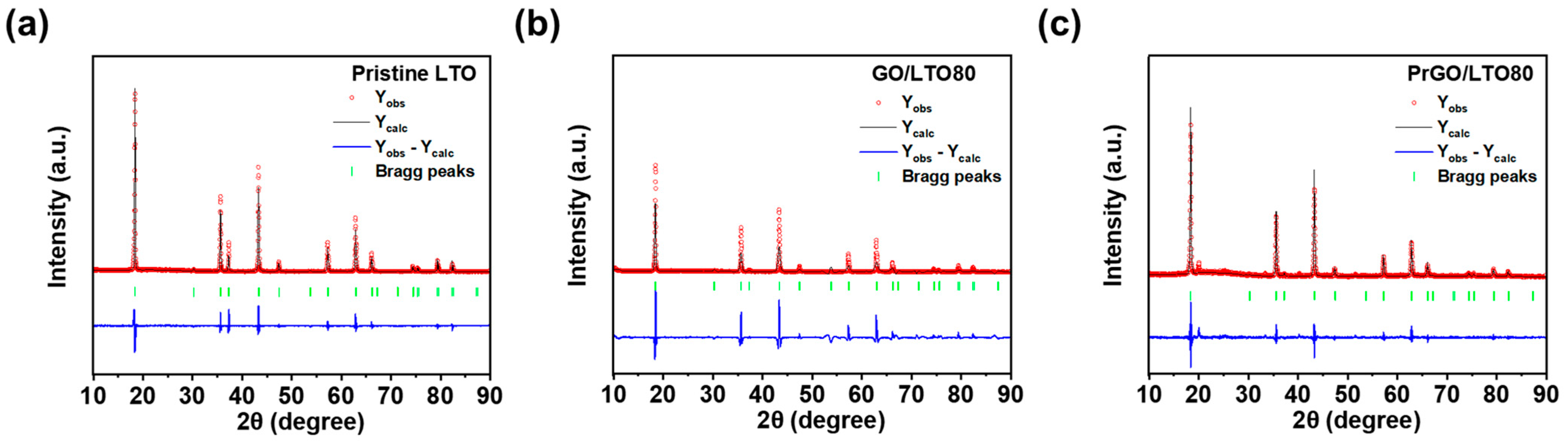
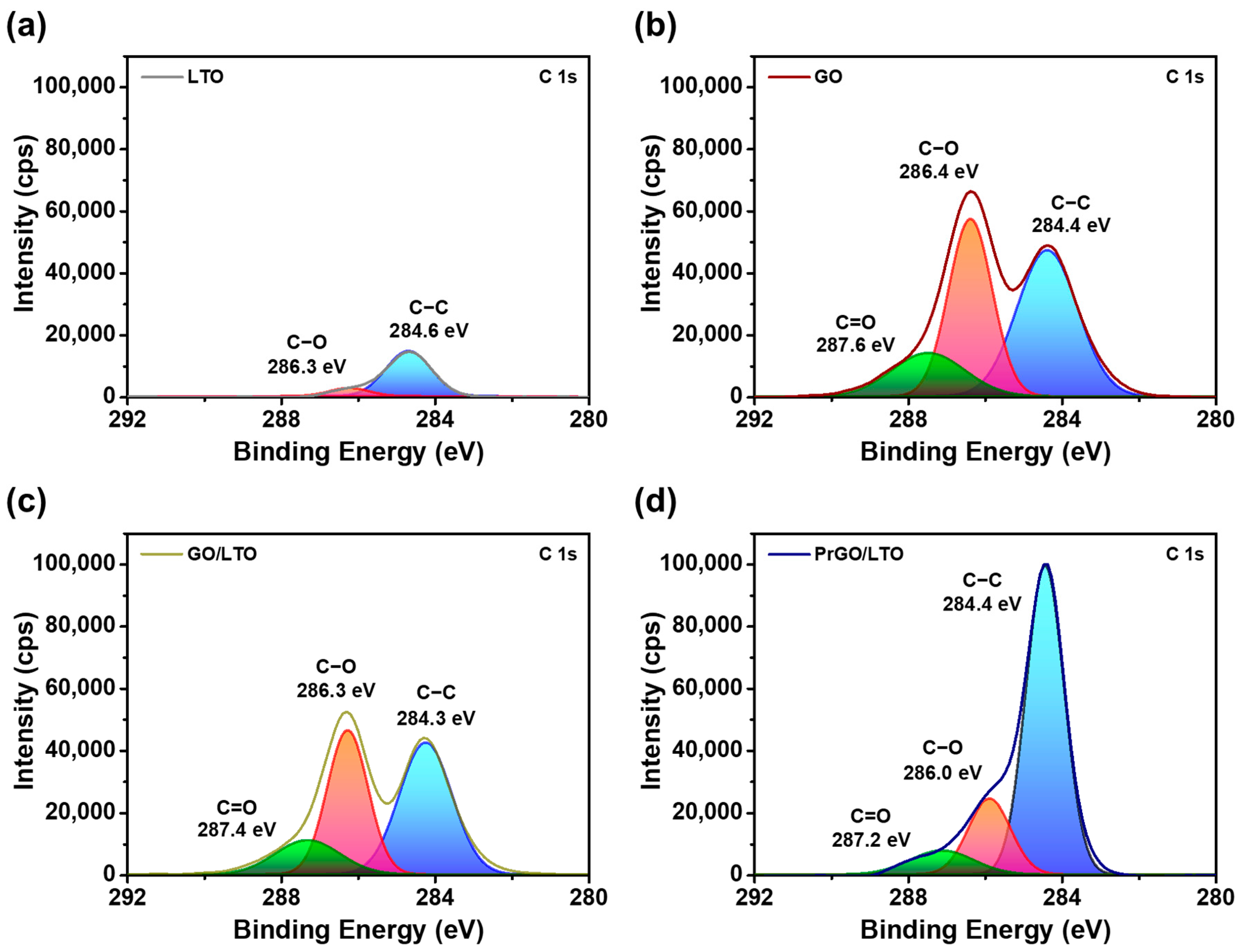
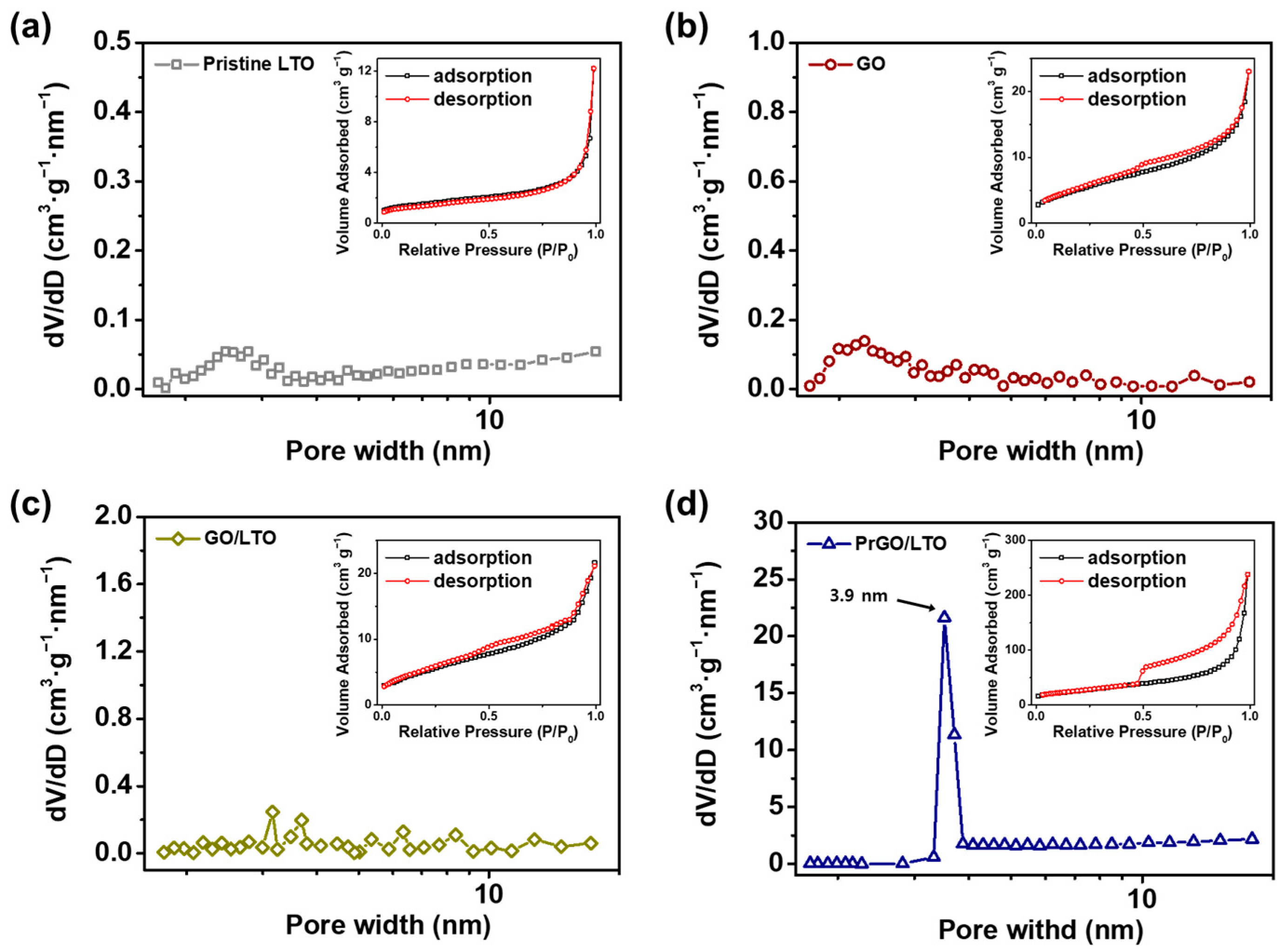
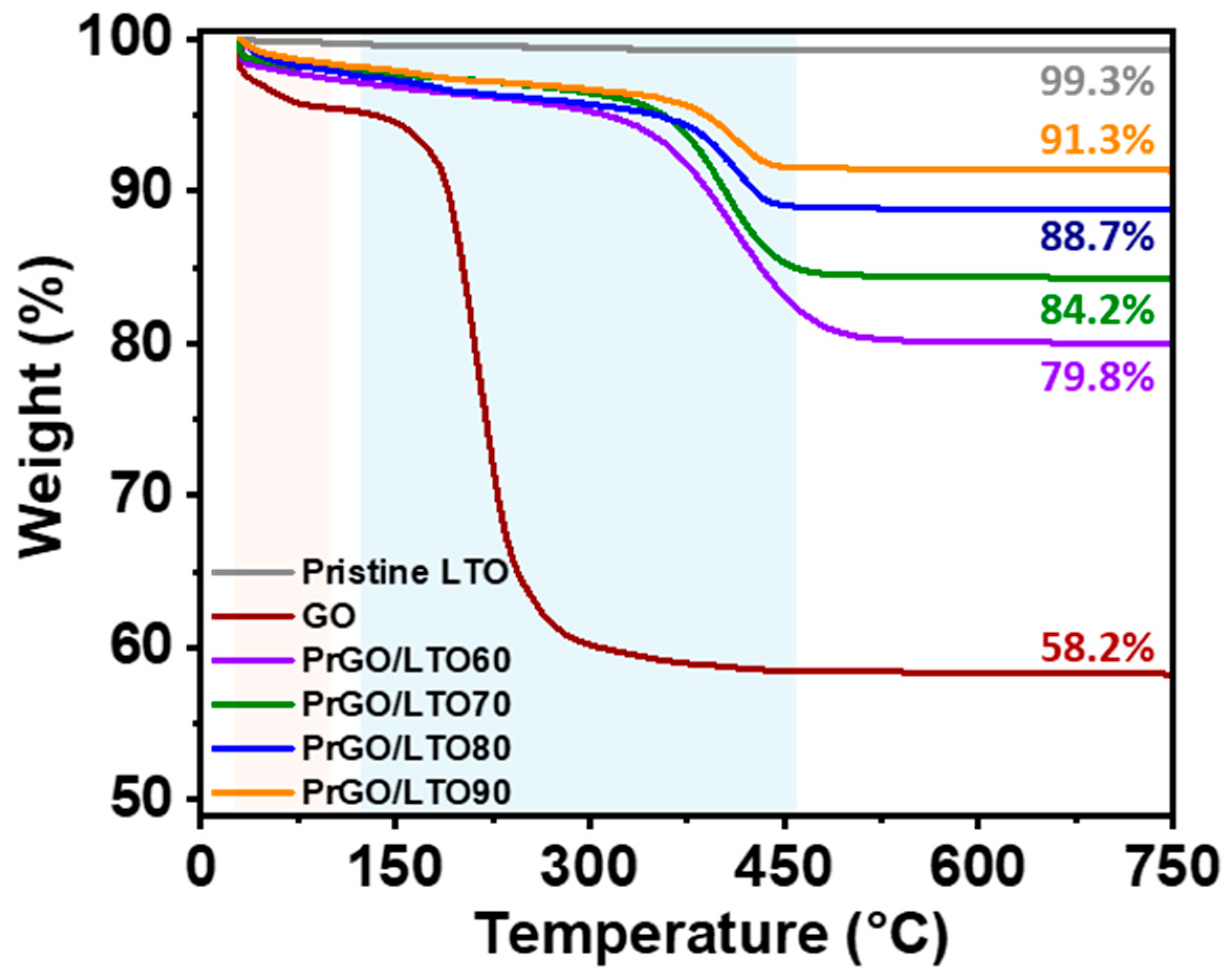
| Sample | Yobs | Ycal | Bragg Rfactor | Vol (nm3) | Rp (%) | Rexp (%) | Rwp (%) |
|---|---|---|---|---|---|---|---|
| LTO | 3312.7 | 3312.5 | 2.6 | 583.6 | 21.7 | 31.2 | 17.7 |
| GO/LTO80 | 30,127 | 2912.5 | 3.4 | 582.4 | 59.5 | 63.1 | 6.76 |
| PrGO/LTO80 | 128.5 | 118.3 | 8.1 | 584.7 | 19.2 | 8.3 | 21.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-G.; Jekal, S.; Otgonbayar, Z.; Kim, J.; Ra, Y.-H.; Noh, J.; Oh, W.-C.; Yoon, C.-M. Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material. Batteries 2024, 10, 311. https://doi.org/10.3390/batteries10090311
Kim C-G, Jekal S, Otgonbayar Z, Kim J, Ra Y-H, Noh J, Oh W-C, Yoon C-M. Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material. Batteries. 2024; 10(9):311. https://doi.org/10.3390/batteries10090311
Chicago/Turabian StyleKim, Chan-Gyo, Suk Jekal, Zambaga Otgonbayar, Jiwon Kim, Yoon-Ho Ra, Jungchul Noh, Won-Chun Oh, and Chang-Min Yoon. 2024. "Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material" Batteries 10, no. 9: 311. https://doi.org/10.3390/batteries10090311
APA StyleKim, C.-G., Jekal, S., Otgonbayar, Z., Kim, J., Ra, Y.-H., Noh, J., Oh, W.-C., & Yoon, C.-M. (2024). Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material. Batteries, 10(9), 311. https://doi.org/10.3390/batteries10090311







