Copper Wire Resistance Corrosion Test for Assessing Copper Compatibility of E-Thermal Fluids for Battery Electric Vehicles (BEVs)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
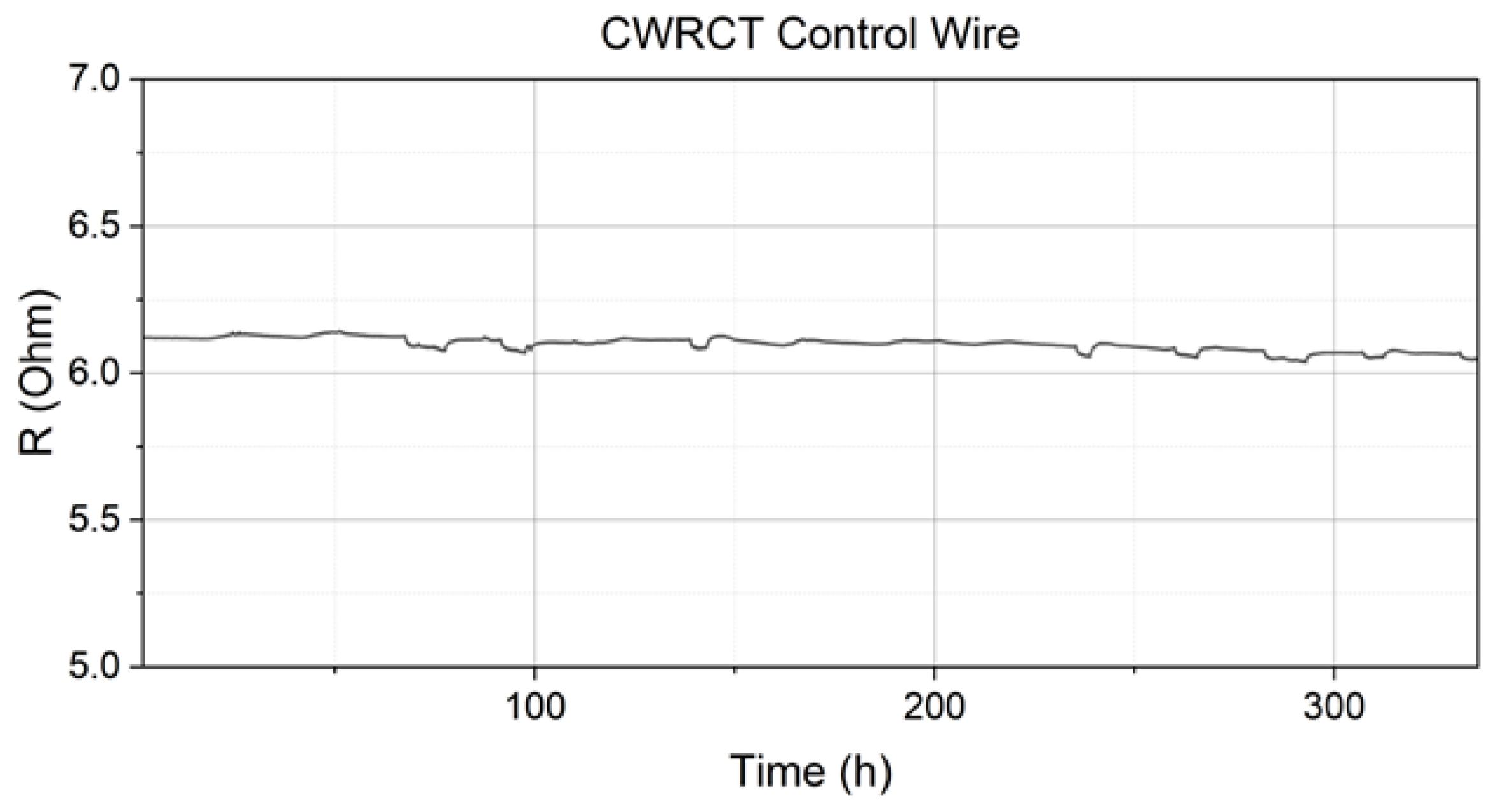
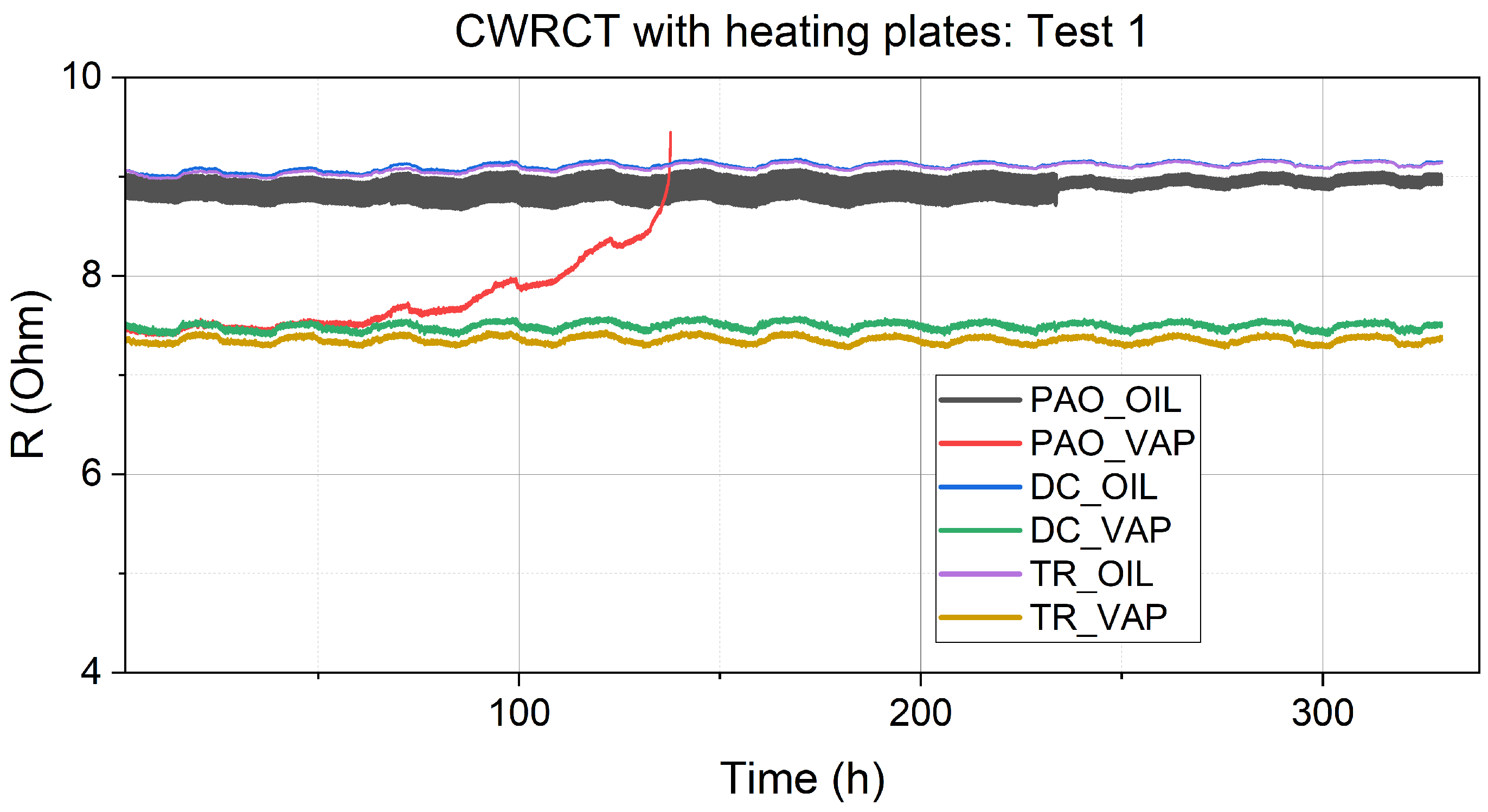
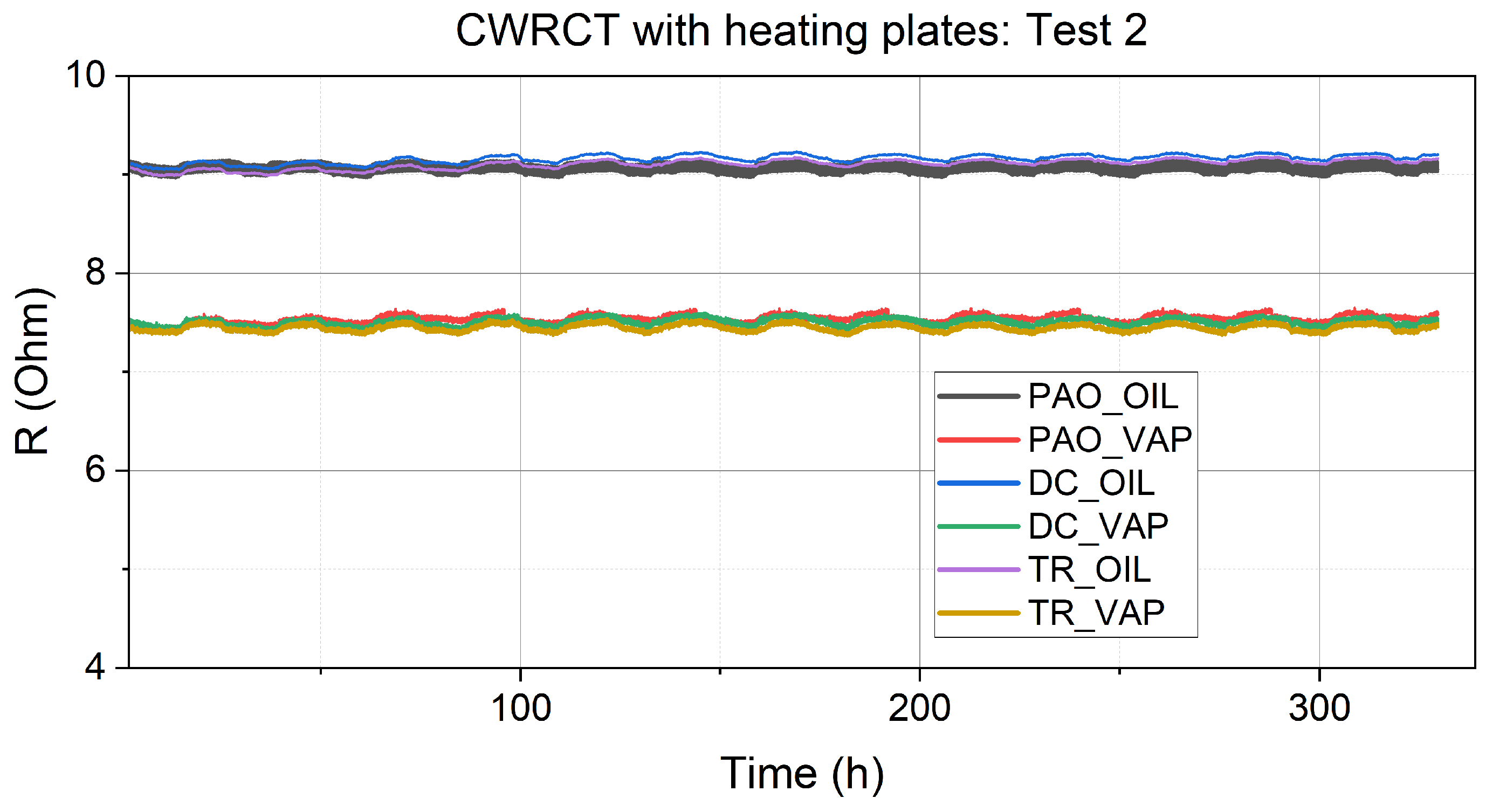
3.1. Resistance Measurements
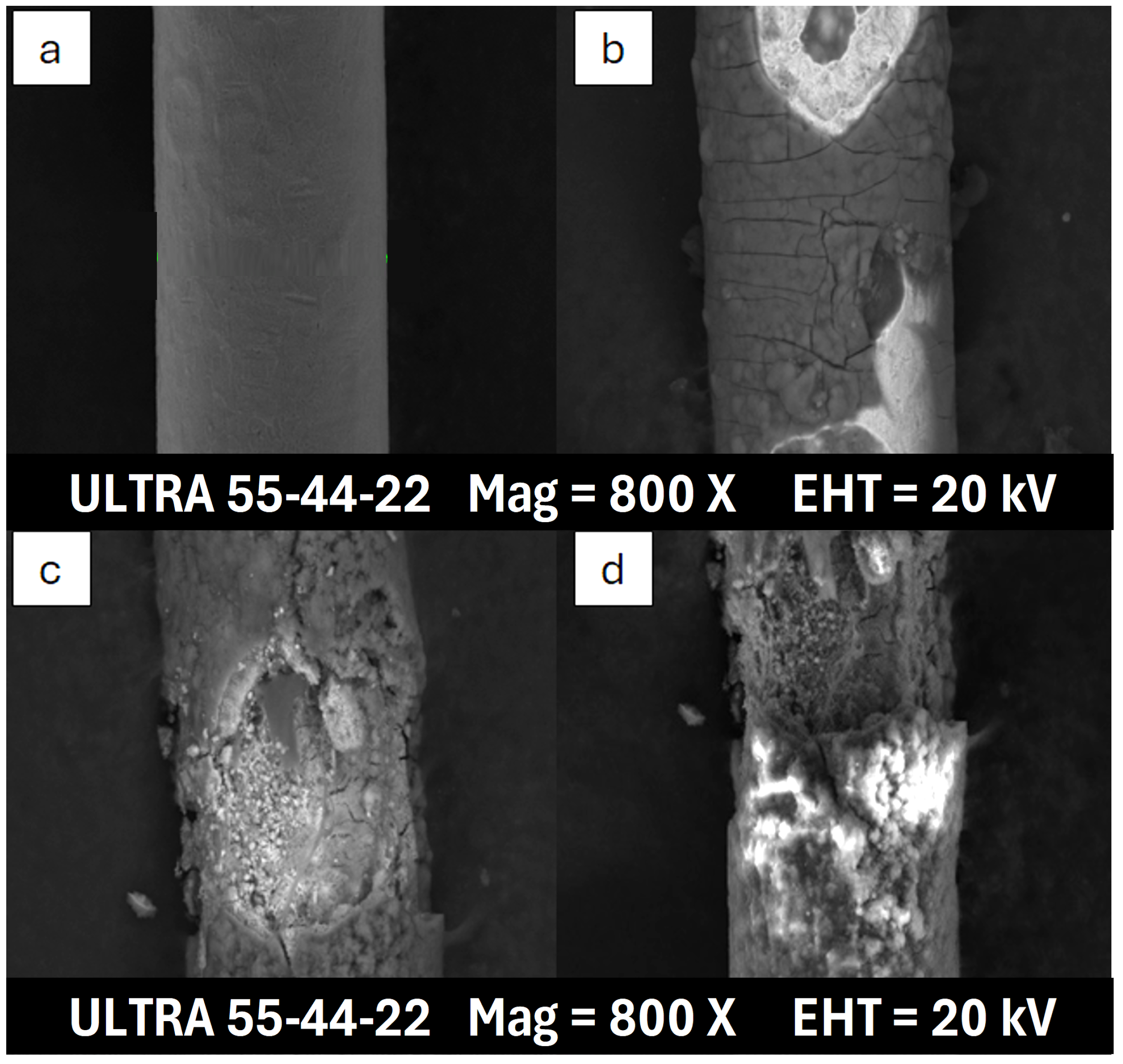
3.2. Scanning Electron Microscope Analysis
3.3. Copper Debris Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Electric Car Sales Break New Records with Momentum Expected to Continue through 2023. 2023. Available online: https://www.iea.org/reports/global-ev-outlook-2023/executive-summary (accessed on 5 February 2024).
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Pambudi, N.A.; Sarifudin, A.; Firdaus, R.A.; Ulfa, D.K.; Gandidi, I.M.; Romadhon, R. The immersion cooling technology: Current and future development in energy saving. Alex. Eng. J. 2022, 61, 9509–9527. [Google Scholar] [CrossRef]
- Sundin, D.W.; Sponholtz, S. Thermal management of Li-ion batteries with single-phase liquid immersion cooling. IEEE Open J. Veh. Technol. 2020, 1, 82–92. [Google Scholar] [CrossRef]
- Shell. Electric Vehicle Fluids. 2019. Available online: https://www.shell.com/content/dam/shell/assets/en/business-functions/shell-lubricants/documents/shell-white-paper.pdf (accessed on 6 February 2024).
- ASTMD130; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM International: West Conshohocken, PA, USA, 2019.
- Hunt, G.J.; Gahagan, M.P.; Peplow, M.A. Wire resistance method for measuring the corrosion of copper by lubricating fluids. Lubr. Sci. 2017, 29, 279–290. [Google Scholar] [CrossRef]
- Hunt, G.; Prengaman, C. Understanding Vapor and Solution Phase Corrosion of Lubricants Used in Electrified Transmissions; Technical Report, SAE Technical Paper; SAE International: Warrendale, PA, USA, 2020. [Google Scholar]
- Hunt, G. New Perspectives on Lubricant Additive Corrosion: Comparison of Methods and Metallurgy; Technical Report, SAE Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Wang, A.; Yin, X.; Xin, Z.; Cao, F.; Wu, Z.; Sundén, B.; Xiao, D. Performance optimization of electric vehicle battery thermal management based on the transcritical CO2 system. Energy 2023, 266, 126455. [Google Scholar] [CrossRef]
- Shrestha, S.; Baral, B.; Shah, M.; Chitrakar, S.; Shrestha, B.P. Measures to resolve range anxiety in electric vehicle users. Int. J. Low-Carbon Technol. 2022, 17, 1186–1206. [Google Scholar] [CrossRef]
- Lacroix-Andrivet, O.; Hubert-Roux, M.; Loutelier Bourhis, C.; Moualdi, S.; Mendes Siqueira, A.L.; Afonso, C. Characterization of Base Oil and Additive Oxidation Products from Formulated Lubricant by Ultra-High Resolution Mass Spectrometry. Lubricants 2023, 11, 345. [Google Scholar] [CrossRef]
- Soleimani, M.; Dehabadi, L.; Wilson, L.D.; Tabil, L.G. Antioxidants Classification and Applications in Lubricants. In Tribology, Lubricants and Additives; Johnson, D.W., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 2. [Google Scholar] [CrossRef]
- Aromaa, J.; Kekkonen, M.; Mousapour, M.; Jokilaakso, A.; Lundström, M. The Oxidation of Copper in Air at Temperatures up to 100 °C. Corros. Mater. Degrad. 2021, 2, 625–640. [Google Scholar] [CrossRef]
- Lee, S.K.; Hsu, H.C.; Tuan, W.H. Oxidation Behavior of Copper at a Temperature below 300 °C and the Methodology for Passivation. Mater. Res. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- De Carlo, I.; Baudino, L.; Klapetek, P.; Serrapede, M.; Michieletti, F.; De Leo, N.; Pirri, F.; Boarino, L.; Lamberti, A.; Milano, G. Electrical and Thermal Conductivities of Single CuxO Nanowires. Nanomaterials 2023, 13, 2822. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, B.; Wang, Z.; Guo, J.; Wu, B.; Hao, S.; Zheng, N. Surface coordination layer passivates oxidation of copper. Nature 2020, 586, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Young, H.D.; Freedman, R.A.; Ford, A.L.; Sears, F.W. Sears and Zemansky’s University Physics: With Modern Physics, 13th ed.; Pearson Addison-Wesley: San Francisco, CA, USA, 2012. [Google Scholar]
- Areny, R.P. Tetrapolar bioimpedance measurements compared to four-wire resistance measurements. J. Electr. Bioimpedance 2018, 9, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ladino, L.; Rondón, S. Resistance of copper wire as a function of temperature. Phys. Educ. 2014, 50, 42. [Google Scholar] [CrossRef]
- Sancarlos-González, A.; Pineda-Sanchez, M.; Puche-Panadero, R.; Sapena-Bano, A.; Riera-Guasp, M.; Martinez-Roman, J.; Perez-Cruz, J.; Roger-Folch, J. Application of the parametric proper generalized decomposition to the frequency-dependent calculation of the impedance of an AC line with rectangular conductors. Open Phys. 2017, 15, 929–935. [Google Scholar] [CrossRef]
- Młot, A.; Korkosz, M.; Grodzki, P.; Łukaniszyn, M. Analysis of the proximity and skin effects on copper loss in a stator core. Arch. Electr. Eng. 2014, 63, 211–225. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, H.; Huo, D.; Wu, F.; Shao, L.; Zheng, P.; Jiang, Y.; Zheng, X.; Qiu, X.; Liu, Y.; et al. N-doped graphene-based copper nanocomposite with ultralow electrical resistivity and high thermal conductivity. Sci. Rep. 2018, 8, 9248. [Google Scholar] [CrossRef] [PubMed]
- Abosede, O.O.; Obaleye, J.A. New One-Pot Synthetic Route and Spectroscopic Characterization of Hydroxo-Bridged Stepped-Cubane Copper(II) Complexes. Asian J. Appl. Sci. Technol. 2022, 6, 21–31. [Google Scholar] [CrossRef]
- Li, Y.; Fan, W.; Zhang, Z.; Xie, X.; Xiang, S.; Huang, D. Copper(II)-hydroxide facilitated C-C bond formation: The carboxamido pyridine system versus the methylimino pyridine system. Dalton Trans. 2020, 49, 12189–12196. [Google Scholar] [CrossRef] [PubMed]
- Duvanova, E.; Mariichak, A.Y.; Baumer, V.; Rozantsev, G.M.; Radio, S.V. Crystal Structure of Double Sodium–Copper(II) Paratungstate B, Na2Cu4[W12O40(OH)2]·22H2O, and Mixed Copper (II) Paratungstate B–Hydroxide, Cu5[W12O40(OH)2]·2Cu(OH)2·30H2O. J. Struct. Chem. 2021, 62, 379–389. [Google Scholar] [CrossRef]
- Saikova, S.; Vorob’ev, S.A.; Nikolaeva, R.; Mikhlin, Y. Conditions for the formation of copper nanoparticles by reduction of copper(II) ions with hydrazine hydrate solutions. Russ. J. Gen. Chem. 2010, 80, 1122–1127. [Google Scholar] [CrossRef]
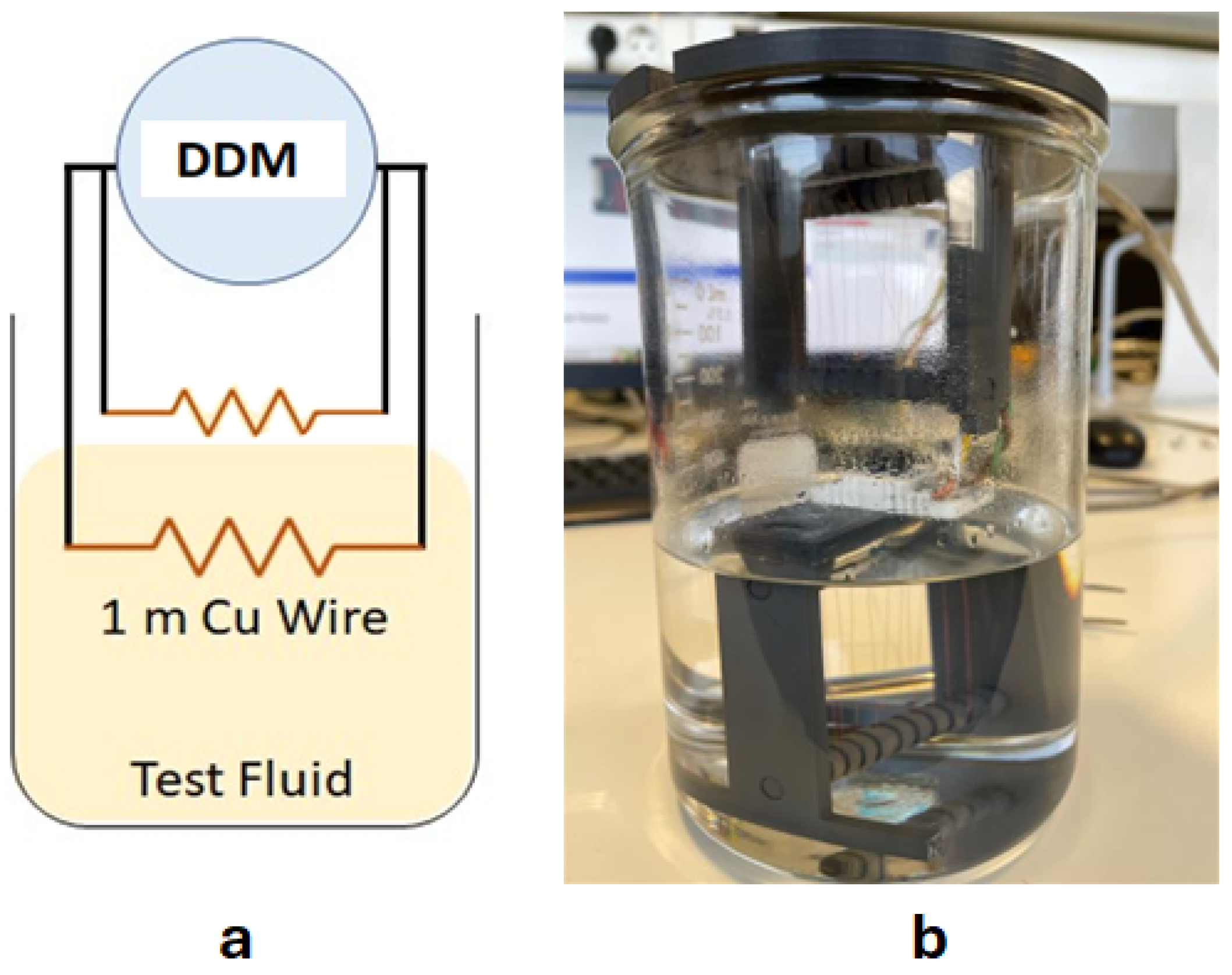



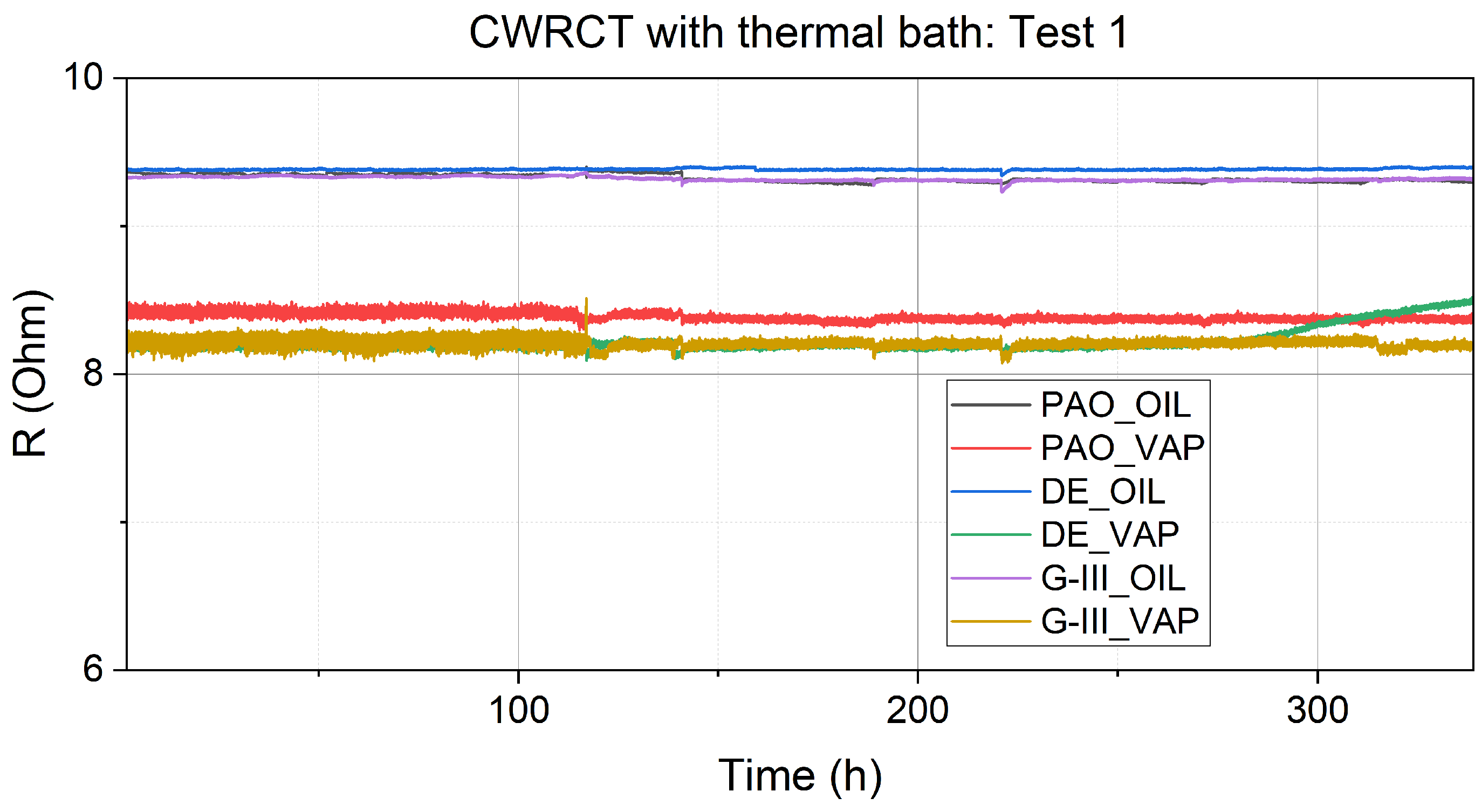
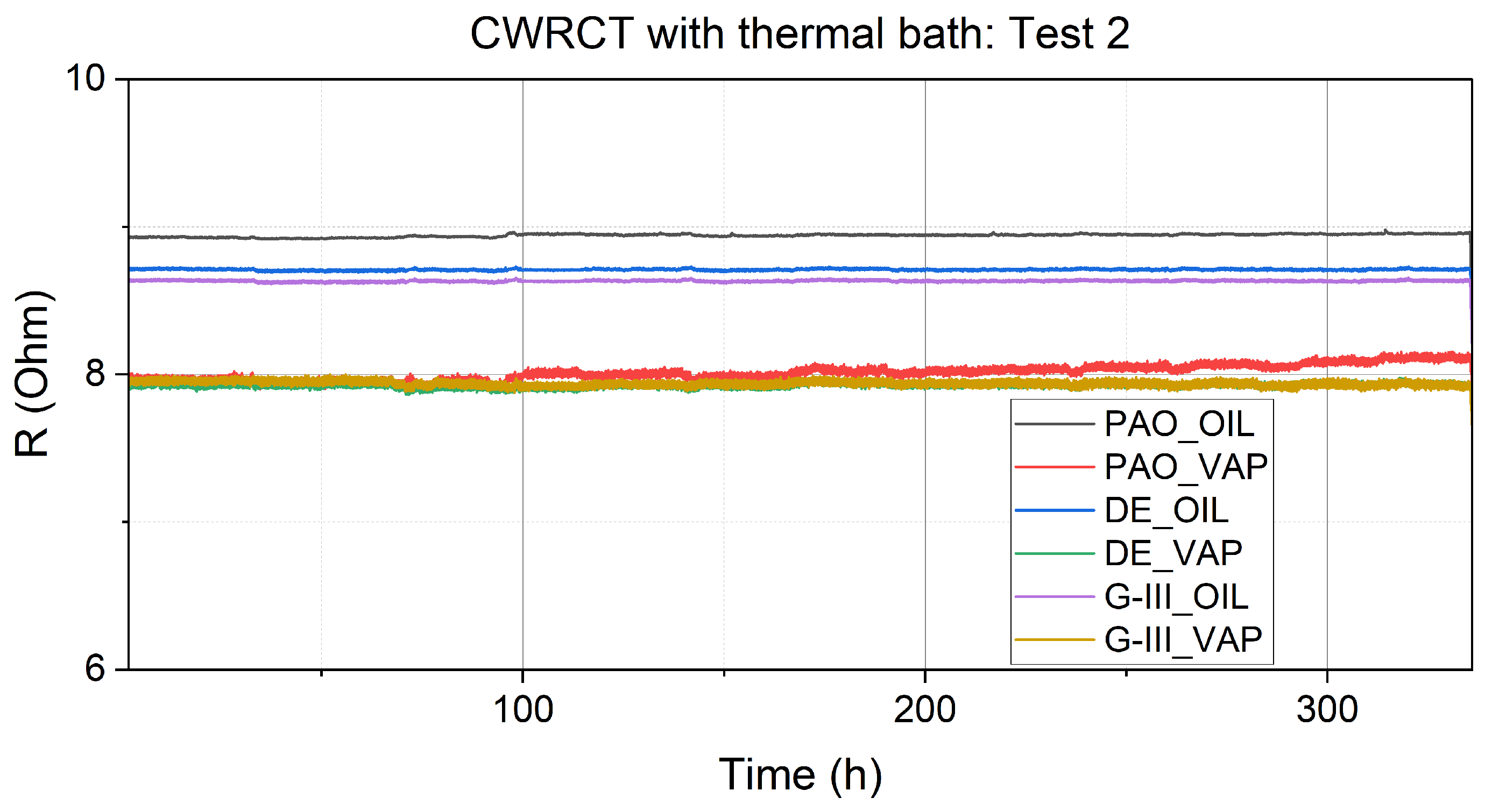
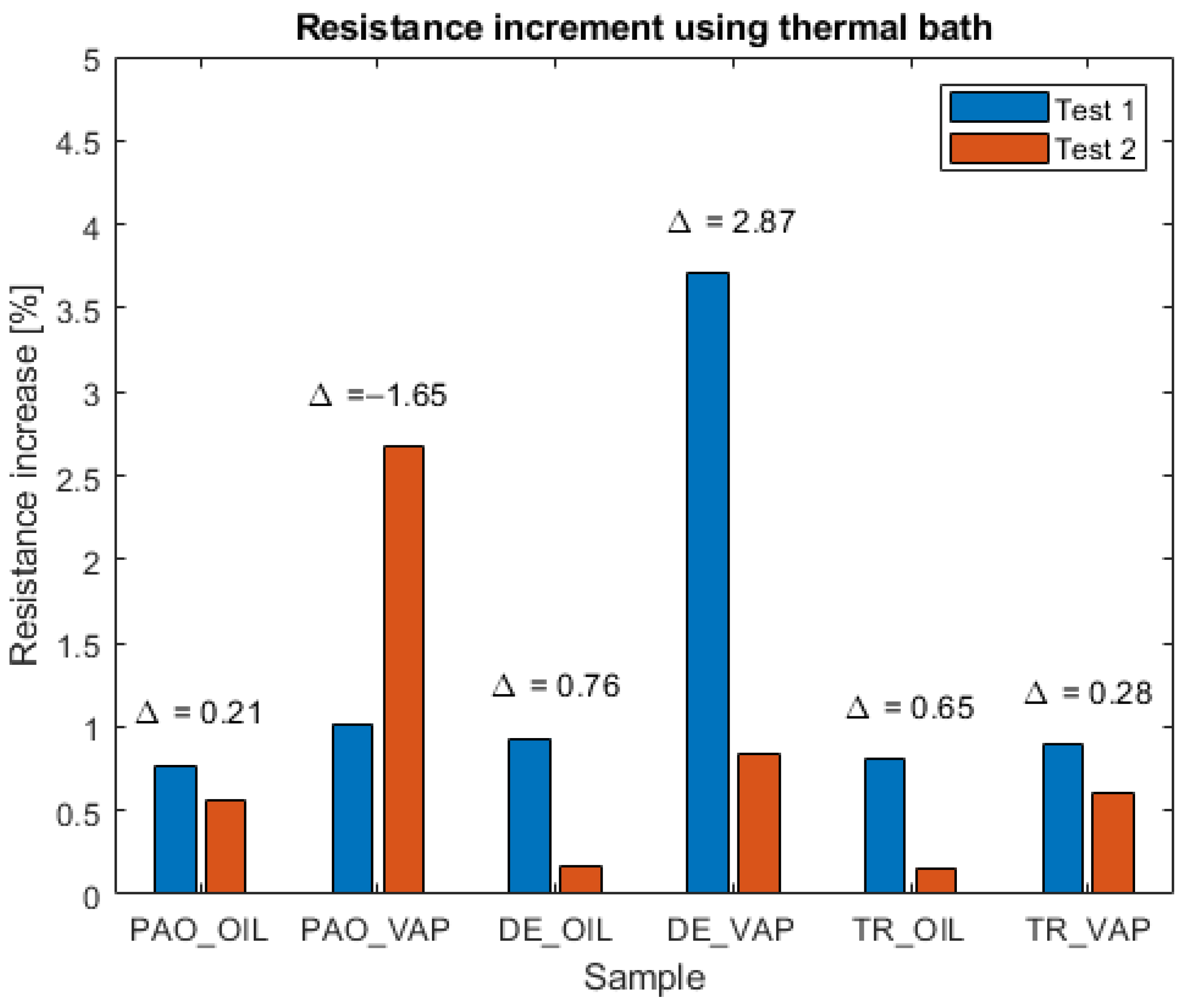

| Equipment | Variable Measured/Controlled | Accuracy |
|---|---|---|
| Thermal bath | Temperature | ±0.25 °C |
| Heating plates | Temperature | ±0.5 °C |
| DDM | Resistance | ±57.8 m |
| ICP | Concentration | ±1 ppm |
| Heating Method | Fluid | Notation | Type |
|---|---|---|---|
| Heating plates (HP), Thermal bath (TB) | PAO4 | PAO | Base stock Polyalphaolefin (API G-IV) |
| Thermal bath (TB) | DIESTER | DE | Base stock synthetic Ester (API G-V) |
| Thermal bath (TB) | G-III | G-III | Base stock mineral oil (API G-III) |
| Heating plates (HP) | TRANSFORMER OIL | TR | Fully formulated mineral transformer oil |
| Heating plates (HP) | DIELECTRIC COOLANT | DC | Fully formulated biodegradable synthetic hydrocarbon |
| Fluid | Density @20 °C (kg/m3) | K. Viscosity @100 °C (cSt) | Thermal Conductivity @20 °C (W/m K) | S. Heat Capacity @20 °C (kJ/kg K) | Flash Point (°C) |
|---|---|---|---|---|---|
| PAO | 816 | 4.03 | 0.145 | 2.314 | 204 |
| DE | 913 | 3.25 | 0.146 | 2.076 | 220 |
| G-III | 828 | 4.29 | 0.138 | 2.210 | 198 |
| TR | 852 | 5.91 | 0.135 | 2.184 | 187 |
| DC | 823 | 2.20 | 0.137 | 2.127 | 190 |
| Method | R () |
|---|---|
| Theoretical (calculated using Equation (1)) | 6.0974 |
| Experimental (measured) | 6.1012 |
| Heating Method | Fluid | State | Cu (%) | O (%) | C (%) | Other (%) |
|---|---|---|---|---|---|---|
| - | - | Clean wire | 92.33 | 0.06 | 7.61 | - |
| Heating plates 1 | PAO | OIL | 65.45 | 6.71 | 27.69 | 0.15 |
| VAP | 30.65 | 25.84 | 43.19 | 0.32 | ||
| DC | OIL | 67.49 | 6.38 | 25.95 | 0.18 | |
| VAP | 55.92 | 7.61 | 36.38 | 0.09 | ||
| TR | OIL | 61.96 | 10.86 | 26.54 | 0.64 | |
| VAP | 66.98 | 4.18 | 28.84 | - | ||
| Heating plates 2 | PAO | OIL | 67.74 | 6.94 | 25.21 | 0.09 |
| VAP | 56.25 | 7.91 | 35.64 | 0.19 | ||
| DC | OIL | 69.18 | 6.55 | 23.99 | 0.27 | |
| VAP | 57.60 | 7.84 | 34.56 | 0.00 | ||
| TR | OIL | 64.97 | 8.49 | 26.25 | 0.29 | |
| VAP | 68.22 | 4.25 | 27.43 | 0.10 | ||
| Thermal bath 1 | PAO | OIL | 72.91 | 4.09 | 22.83 | 0.17 |
| VAP | 65.56 | 3.49 | 30.86 | 0.09 | ||
| DE | OIL | 73.32 | 4.74 | 21.70 | 0.24 | |
| VAP | 34.86 | 20.16 | 44.68 | 0.30 | ||
| G-III | OIL | 72.66 | 4.27 | 23.07 | - | |
| VAP | 60.13 | 6.40 | 33.39 | 0.08 | ||
| Thermal bath 2 | PAO | OIL | 73.43 | 5.67 | 20.80 | 0.10 |
| VAP | 39.58 | 18.38 | 41.92 | 0.12 | ||
| DE | OIL | 70.80 | 4.89 | 24.31 | - | |
| VAP | 59.17 | 6.93 | 33.81 | 0.09 | ||
| G-III | OIL | 73.24 | 4.08 | 22.67 | 0.01 | |
| VAP | 61.99 | 6.18 | 31.72 | 0.11 |
| Method | Condition | TR | DC | PAO | G-III | DE |
|---|---|---|---|---|---|---|
| Heating plates 1 | Fresh | n.d. 1 | n.d. | <1 | - | - |
| 336 h | 3.79 | n.d. | 4.92 | - | - | |
| Heating plates 2 | Fresh | n.d. | n.d. | n.d. | - | - |
| 336 h | 3.01 | n.d. | 1.51 | - | - | |
| Thermal bath 1 | Fresh | - | - | n.d. | n.d. | n.d. |
| 336 h | - | - | 1.03 | 1.83 | 3.67 | |
| Thermal bath 2 | Fresh | - | - | n.d. | n.d. | n.d. |
| 336 h | - | - | 1.37 | 1.52 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tormos, B.; Ruiz, S.; Alvis-Sanchez, J.; Farfan-Cabrera, L.I. Copper Wire Resistance Corrosion Test for Assessing Copper Compatibility of E-Thermal Fluids for Battery Electric Vehicles (BEVs). Batteries 2024, 10, 285. https://doi.org/10.3390/batteries10080285
Tormos B, Ruiz S, Alvis-Sanchez J, Farfan-Cabrera LI. Copper Wire Resistance Corrosion Test for Assessing Copper Compatibility of E-Thermal Fluids for Battery Electric Vehicles (BEVs). Batteries. 2024; 10(8):285. https://doi.org/10.3390/batteries10080285
Chicago/Turabian StyleTormos, Bernardo, Santiago Ruiz, Jorge Alvis-Sanchez, and Leonardo Israel Farfan-Cabrera. 2024. "Copper Wire Resistance Corrosion Test for Assessing Copper Compatibility of E-Thermal Fluids for Battery Electric Vehicles (BEVs)" Batteries 10, no. 8: 285. https://doi.org/10.3390/batteries10080285
APA StyleTormos, B., Ruiz, S., Alvis-Sanchez, J., & Farfan-Cabrera, L. I. (2024). Copper Wire Resistance Corrosion Test for Assessing Copper Compatibility of E-Thermal Fluids for Battery Electric Vehicles (BEVs). Batteries, 10(8), 285. https://doi.org/10.3390/batteries10080285










