Abstract
In recent years, batteries have revolutionized electrification projects and accelerated the energy transition. Consequently, battery systems were hugely demanded based on large-scale electrification projects, leading to significant interest in low-cost and more abundant chemistries to meet these requirements in lithium-ion batteries (LIBs). As a result, lithium iron phosphate (LFP) share has increased considerably due to lower cost and higher safety compared to conventional nickel and cobalt-based chemistries. However, their fast-growing share is affected by updated chemistries, where cheaper systems like sodium-ion batteries (SIBs) are becoming more attractive. SIBs also benefited from the greener, more ethical, and evenly distributed elemental resources. SIBs are fast approaching market thanks to mature LIB’s technology and manufacturing scalability using existing Li-ion gigafactories. Additionally, SIBs can be adapted to other emerging technologies, including Li-ion batteries and silicon-based anodes, influencing projections for their broader use. However, despite the lower cost and abundance of sodium chemistries compared to lithium ones, limited manufacturing capacity discourages material suppliers from increasing production, which restricts the supply chain, raises costs, and diminishes Na battery manufacturing. Here, we aim to provide an overview of the progress of SIBs in gaining market share from LIBs. We first reviewed LIB and SIB histories, developments, and market share. Then, we analyzed the offered chemicals in battery components, their resources and supplies, material demand, and supply chain. The commercialization of each system was investigated in addition to the challenges related to energy density, environmental impact, sustainability, and safety. If all these concerns are addressed properly, LIBs and SIBs could potentially offer a more affordable, safer, and sustainable choice for the global energy storage outlook, particularly in short-range electric vehicles and stationary grid storage.
1. Introduction
Battery deployment must increase sevenfold by 2030 to achieve COP28 targets. To this end, based on net-zero emissions (NZE), battery demand will increase from 0.86 terawatt-hour (TWh) in 2023 to a total of 6 TWh in 2030, categorized in electric vehicles (EVs) (5.40 TWh), grid storage (0.52 TWh), and behind-the-meter (0.1 TWh) sectors (Figure 1a). Battery storage was the fastest growing energy technology in 2023, doubling in size compared to the previous year. The global addition of 42 GW in battery storage capacity came from strong growth in the power sector from 2010 to 2023. EV battery deployment also surged by 40%, with 14 million new electric cars dominating the energy sector’s battery usage. This considerable demand forced the manufacturer to find a solution for high battery costs, making them comparable with the internal combustion engines (ICEs). The technological development has substantially decreased the price from 1400 USD/kWh in 2010 to 140 USD/kWh in 2023 (90% decline). Further innovations in chemistries and manufacturing are expected to reduce lithium-ion battery (LIB) costs by over 40% up to 2030 (Figure 1b) [1]. Despite these cost reductions, many efforts have been made to offer new cost-effective alternatives. Na-ion systems are suggested as a solution with lower prices and more abundance thanks to the relevant sodium sources, making them more reliable for a sustainable battery future. This feature considerably attracted the manufacturer, with a 0.5 billion USD market value in 2023 and 1.2 billion USD in 2030 (21.5% compound annual growth rate (CAGR)) (Figure 1c) [2]. In contrast to the abundance of sodium-ion battery (SIB) precursors, LIB raw material reserves are unevenly distributed, and their large-scale applications are accompanied by the immense pressure of the value chain [3]. In fact, SIBs are growing fast owing to their cost and abundance advantages, making them a promising system in the sustainable battery prospect (Figure 1d).
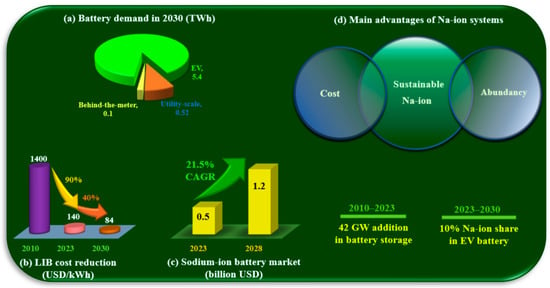
Figure 1.
(a) Battery demand in 2030 in alternative sectors based on the NZE scenario. (b) A considerable cost reduction in LIBs. (c) The growth in SIB’s market and their expected share of EV batteries. (d) The main advantages of SIBs over LIBs. Data was drawn from [1] for (a,b) and from [2] for (c).
1.1. Historical Evolution and Perspective
1.1.1. LIBs
Rechargeable LIBs commenced in 1913 with the origination of lithium metal batteries [4]. Significant advances occurred in 1965 when NASA employed lithium metal anodes in propylene carbonate-based electrolytes, with a low efficiency of approximately 50–70% [5]. By the mid-1970s, Argonne National Laboratory (ANL) had developed high-temperature lithium and lithium-aluminum/iron sulfide batteries for grids and EVs rather than for portable electronics [6,7]. The foundational cathode materials resembling those used in modern LIBs were developed by Whittingham using metal dichalcogenides like TiS2 and TaS2 for Li-ion storage through a highly reversible process known as “the intercalation mechanisms” and commercialized by Exxon in the late 1970s. Unfortunately, this product was limited to the coin cell level and only found to be applicable to watch batteries [8]. John Goodenough significantly advanced LIB technology with key developments. In the late 1970s, he introduced lithium cobalt oxide cathodes (LCO), essential in most personal electronics. Later, lithium manganese oxide cathodes were developed in the early 1980s, now crucial for EVs and some medical devices. In the 1990s, John Goodenough created lithium iron phosphate (LFP) cathodes, widely used in power tools [9]. A practical model of LIBs was patented in 1985 by Yoshino and colleagues, featuring a combined carbon anode and LCO cathode with an organic electrolyte [10]. In the early 1990s, Sony and A&T Battery (a joint venture of Asahi Kasei Co. and Toshiba) brought the LIB to market for use in consumer electronics with a 200 Wh/L and 80 Wh/kg energy density while being charged to 4.1 V [11]. The first commercial LIB-powered EV came with the release of the Nissan Altra in 1997. This model could achieve a range of up to 192 km and was equipped with a 364 kg battery pack (317 kg cells). Its battery pack consisted of 12 modules, each containing eight 100 Ah cells [12]. In 2000, the development of thin-film lithium and lithium-ion batteries at Oak Ridge National Laboratory advanced solid-state LIB technology with a promise for applications in consumer electronics and medical products [13]. Goodenough’s team in 2017 unveiled the solid-state glass batteries [14]. Karim Zaghib’s team also introduced the first two-electrode photo-rechargeable LIB (LFP/graphite) in 2017 [15]. They also received the Lionel-Boulet Award in 2019 for pioneering the use of LFP cathodes for lithium ion technology in Hydro-Québec [16].
Further improvement of LIBs depends significantly on market dynamics, technological advancements, and raw material considerations. As EV popularity is rising, cost reduction is pushing to make the price competitive with ICE vehicles. Although the price has decreased by approximately 97% since the early 1990s, precise price forecasting is challenging given the complexity of the battery landscape. Lithium, nickel, manganese, cobalt oxide, (NMC), and LFP will dominate the market until at least 2030 due to their technical feasibility and established infrastructure. However, technologies like SSBs and SIBs will be attractive after 2030. Recycling also plays a crucial role, whereas its impact on raw material demand and cost reduction remains uncertain [17]. LIB’s historical evolution, from the early stages to commercial applications, is demonstrated in a graphical history in Figure 2.
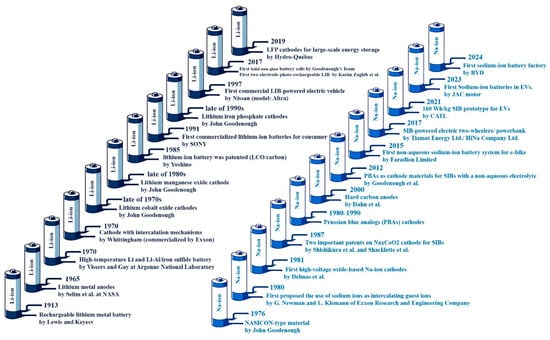
Figure 2.
LIBs and SIBs historical evolution, from the early stages to commercialization. Data was drawn from [4,5,6,7,8,9,10,11,12,13,14,15,16,17] for LIBs and from [18,19,20,21,22,23,24,25] for SIBs.
1.1.2. SIBs
The journey of SIB’s commercialization began in 1980 (Figure 2), when Exxon Research and Engineering Company first proposed using sodium ions as intercalating guest ions [23]. Polyanion cathode materials, including olivine and NASICON (Na-super ionic conductor)-like structures, are essential cathodes for SIBs. NASICON-type materials have been widely developed since their discovery in 1976 by Goodenough et al. [20]. Additionally, the discovery of LCO by Goodenough paved the way for the development of the first high-voltage oxide-based Na-ion cathodes: O3–NaCoO2 and P2-Na0.7CoO1.96 in 1981 [21]. Then, after the feasibility of an SIB was demonstrated in a patent filed in October 1987, employing a P2-NaxCoO2 cathode, 1 M NaPF6 in dimethoxyethane liquid electrolyte, and polymer-based anodes [21]. Shortly after, Showa Denko and Hitachi filed a patent in November 1987, detailing a system with an NaxCoO2 cathode, NaPF6 in ether-based electrolytes, and Na–Hg alloy-based anodes [22]. Prussian blue analogs (PBAs), a type of SIB cathode material, were developed between 1980 and 1990. Their use as cathode materials for SIBs with a nonaqueous electrolyte was demonstrated by Goodenough et al. in 2012 [20]. Graphite was initially considered for use as an anode in SIBs, but this attempt failed because of the instability and low potential of Na+ intercalation. Although solvated Na+ can be inserted, its capacity is insufficient for practical applications. As an alternative, hard carbon (HC), also known as nongraphitizable carbon, was introduced as anodes for SIBs in 2000 [19].
Besides the contributions of research groups, significant achievements were obtained by industry leaders. In 2015, Faradion Limited introduced the first nonaqueous SIB system for e-bikes by using O3/P2-type NaaNi1−x−y−zMnxMgyTizO2 layered oxides as the cathode and HC as the anode, with an operating potential from −20 to 60 °C. The French company Tiamat launched its first SIB in 2017 using cylindrical 18,650 cells with HC anodes and Na3V2(PO4)2F3 cathodes with 100–120 Wh/kg energy density. Simultaneously, the Chinese company HiNa developed power banks with 120 Wh/kg energy density by employing O3-type Nax[Cu,Fe,Mn]O2 layered oxide cathodes [20]. CATL (Contemporary Amperex Technology Co., Ltd.) has recently discovered a new SIB with a high energy density of 160 Wh/kg for EVs [24]. In 2023, Hina-JAC Battery in China integrated SIBs into EVs [25], and in 2024, BYD commenced the construction of its first SIB factory [18].
To progress, SIBs must build on successful prototypes and move towards commercialization to catch up with LIBs. Slight differences between the two could help SIBs eventually outperform LIBs [3]. For example, despite the numerous cathode active materials (CAM) proposed for SIBs, the evaluation should be performed compared to reference technologies such as LFP and NMC. Investigating potential CAMs, PBA, and NaFe2(SO4)3 seems more promising with low criticality, costs, and carbon footprint [26]. Since sodium ions (Na+) have a larger atomic radius than lithium ions (Li+), the volumetric energy density (Wh/L) of SIBs is inherently lower than that of LIBs. Thus, SIBs are suited for applications that do not require high energy density, including stationary storage and short- to medium-range EVs. However, with further technological advancements, SIBs could be comparable to LFP batteries in specific energy [27]. Na-ion technology has progressed remarkably in a short time compared to Li-ion technology. Continued improvements will come from optimizing cell component fabrication and assembly, similar to the developments seen in LIB technology over the past thirty years [28]. It seems that the SIB system can adopt the technological development and progress of LIBs, helping it toward a facile and more convenient transition. However, for a thorough commercialization of SIBs and their widespread application, the challenges must be addressed to make them industrially appealing.
1.2. Market
The demand for energy is growing exponentially because of rapid technological progress and the increasing population. As a result, transitioning from traditional fossil fuels to more sustainable and environmentally friendly alternatives is mandatory. Global electricity demand rise is expected from 25,000 TWh to 52,000–71,000 TWh by 2050 due to expanding markets and broader electrification (Figure 3). The transport sector will also observe a substantial increase in power needs, with passenger EVs reaching 1.3 billion by 2050. Industrial power demand is likely to double by 2050 with the electrification of specific processes and increased data center needs. Similarly, building power demand will double further electrification [29].
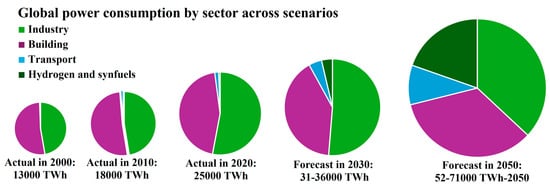
Figure 3.
Global power consumption and the demand to meet the global electrification target. Data was drawn from [29].
SIBs are expected to account for 7% of the worldwide energy storage market and 6% of the passenger EV market by 2033 [30]. The market share and material composition of different anode and cathode chemistries are displayed in Figure 4a,b. By 2035, LFP and LiMnFePO4 (LMFP) will emerge as the top cathode chemistries. LMFP, as a cobalt-free chemistry, is expected to show a high market share and replace LFP and NMC chemistries thanks to its higher energy density than LFP [30,31,32]. Systems with high cobalt and low nickel content, such as NMC333, may become obsolete by 2030. Due to their increased energy density compared to LFP, manganese-rich chemicals will play a smaller role in the mid-range market and a larger role in the long-range market. Long-range chemicals with high nickel content will dominate the market, but their EV market share will drop from 55% in 2023 to 40% in 2040, as manganese-rich and LFP/LMFP systems take their place. After 2030, SIBs are expected to grow their market share to approximately 10% by 2040. Silicon-doped graphite is expected to exhibit a growing share in the anode section, with a tendency toward greater silicon contents for entirely silicon anodes. After 2035, lithium metal will have a significant market share due to the growing use of solid-state batteries and sophisticated liquid electrolytes in long-range automobiles. A sizeable portion of the EV market is composed of HC anodes for SIBs [30].
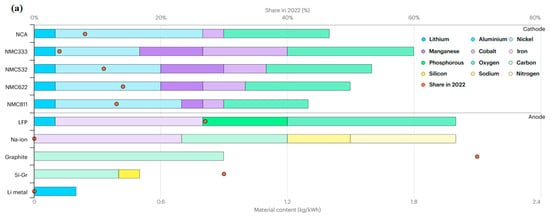
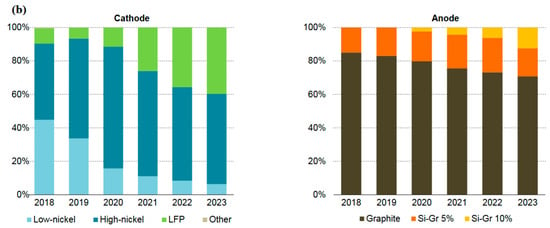
Figure 4.
(a) Material content in alternative anodes and cathodes in EV batteries in 2022. (b) Sale share in cathode and anode chemistries, 2018–2023. Low-nickel chemistries are comprised of NMC333 and NMC532, whereas high-nickel ones are NMC622, NMC721, NMC811, lithium nickel manganese cobalt aluminum oxide (NMCA), and lithium nickel cobalt aluminum oxide (NCA). Si-Gr means silicon-doped graphite with a percentage share of silicon. Sales share is measured based on capacity. With permission from [33] for (a) and [34] for (b).
The uptake of EVs is expected to be driven by the Chinese market, with local manufacturers like Chery, BYD, and JAC being the only ones to announce models featuring sodium-ion cells publicly [32]. IEA expected a 10% contribution of Na-ion cells in the EV battery sales [1]. Leading battery makers, including CATL and Northvolt, have already announced their Na-ion cells. However, the progress of this chemistry, mainly due to its price advantage over the Li-ion counterpart, was delayed in projected plans by the fall in lithium prices [34]. Several companies have commercialized SIBs, i.e., Faradion in the UK, CATL in China, Tiamat in France, and Natron in the United States [27]. CATL has developed SIBs with an estimated cost of 30% less than LFP batteries despite lower energy density (75–160 Wh/kg compared to 120–260 Wh/kg). Nearly 30 SIB plants are considered, mainly in China, to provide over 100 GWh capacity compared to the 1500 GWh capacity of LIB plants [33]. Tiamat plans to open an SIB gigafactory in Amiens in 2026 to produce SIBs mainly for power tools [35].
Several carmakers have announced Na-ion electric cars, such as BYD’s Seagull with a 300 km range priced at 11,600 USD and the Sehol EX10 from the VW-JAC joint venture with a 250 km range. While slightly more expensive than the cheapest small EVs like the Wuling Mini (5000–6500 USD, 170 km range), these models are more inexpensive than comparable options like BYD’s Dolphin, which costs over 15,000 USD for a similar range. BYD will utilize SIBs in all models below 29,000 USD. Hence, Na-ion EVs will hit the market by 2023–2024 with a technology readiness level (TRL) of 8–9. In 2022, Na-ion technology received TRL 6, with a jump from TRL 3–4 in 2021 [33]. Figure 5 summarizes a compilation of commercial battery cell manufacturers for LIBs and SIBs.

Figure 5.
Major cell manufacturers of sodium-ion and lithium-ion batteries [18,36,37].
2. Supply Chain and Material Demand
2.1. Chemistry Evolution
Figure 6 shows the final cell pricing derived for three distinct battery chemistries. The LFP battery has the greatest storage capacity price per kWh (229.3 €/kWh), followed by the SIB (223.4 €/kWh). The NMC-type LIB is the cheapest (168.5 €/kWh), owing to its high energy density. The material costs account for 37% to 42% of the finished cell price, with a further 18–19% representing investment expenses (depreciation). These figures are much lower than those reported by Vaalma et al. [38], who compared the material prices of an 11.5 kWh SIB and LIB. However, Vaalma et al. did not evaluate the battery at the cell level; instead, they evaluated it as a complete battery pack. The additional peripheral components and decreased energy density increased the cost per kWh of storage capacity. When material costs are calculated without the cell hardware, figures in the same order of magnitude are found, with the main cause for the variances being the varied energy densities of the modeled batteries and variable cathode compositions. The manufacturing plant (including R&D) provides 32–35%. Naturally, these (and thus the final cell price) depend highly on plant size and annual throughput. This is one of the reasons for the wide range of prices reported in the literature, with values ranging from 145 to 289 €/kWh for NMC and 225 to 303 €/kWh for LFP [39].
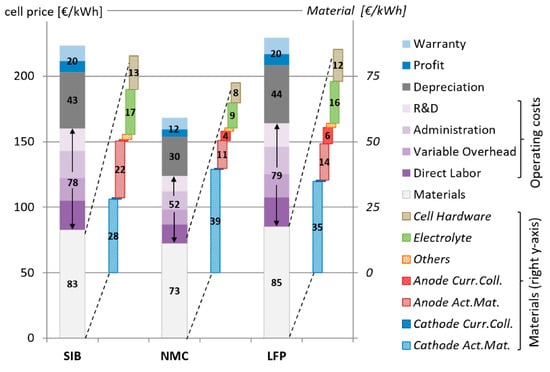
Figure 6.
Cell costs per kWh of storage capacity are broken down to battery components and materials compared with SIBs. With permission from [39].
Nevertheless, future research should aim to develop advanced anode and cathode materials with higher capacities and voltages to achieve specific energies near 200 Wh/kg [40,41,42]. The main concern in the anode part comes from the low effectiveness due to the Na ion’s large size and consequent sluggish kinetics. Carbon-based anodes are the most promising due to graphite’s commercial use in LIBs. However, graphite shows limited capacity in SIBs, forcing research toward alternatives like expanded graphite, nongraphitic carbon nanomaterials, and metal-organic frameworks (MOFs). These alternatives often rely on adsorption, intercalation, and pore-filling [43]. Innovative composites like Sn/C/Ni nanorods accommodate significant volume changes during sodium intercalation [44]. Engineering morphology can improve performance, doping to create more storage defects, and using ether instead of ester electrolytes [43]. Research into improved electrolytic systems is needed to enhance SIB’s performance at high charge-discharge rates across a wide temperature range, ensuring long cycle life and shelf life for energy storage applications. The electrolytes range from aqueous solutions, which lower operational voltages and stability, to ionic liquids and organic mixtures that offer wider electrochemical windows and enhanced stability [44]. Ester-derived electrolytes are widely studied, allowing for comparisons across numerous publications. Fluorinated salts like sodium hexafluorophosphate (NaPF6) or sodium bis(trifluoromethylsulfonyl)imide (NaTFSI) are recommended [45].
Like LIBs, the separators in SIBs are designed for efficient ion movement, whereas specific modifications are required to adopt the unique chemistry of sodium ions [44]. Polyether ether ketone (PEEK) and polyolefins are the best insulators, while fiberglass filters can serve as inexpensive separators. As a current collector, aluminum foil must be used for both electrodes, as it does not alloy with sodium. In binders, switching from polyvinylidene fluoride (PVDF)/N-methyl-2-pyrrolidone (NMP) to water-soluble binders (carboxymethyl cellulose (CMC), styrene butadiene rubber (SBR), poly(acrylic acid) (PAA), and sodium alginate (Na-Alg)) is advised for better performance, lower cost, and environmental benefits [45]. Possible chemistries for LIB and SIB components are summarized in Table 1.

Table 1.
Alternative materials in battery components and their related characteristics and properties. LMO: lithium manganese oxide, LNMO: lithium nickel manganese oxide, LTO: lithium titanium oxide, EC: ethylene carbonate, DEC: diethyl carbonate, DMC: dimethyl carbonate, and EMC: ethyl methyl carbonate, PE: polyethylene, PP: polypropylene, THF: tetrahydrofuran, HFP: hexafluoropropylene.
2.2. Resources and Supplies
Sodium is a promising substitute for lithium in battery systems due to its chemical and physical similarities to lithium. Sodium and lithium are present in varying concentrations in seawater and the Earth’s crust. Understanding lithium and sodium ore’s geological distribution and processing is paramount for sustainable and efficient resource utilization. Sodium ores present a significant contrast to lithium in abundance and diversity. Despite comprising only 0.002% of the Earth’s crust, lithium is a critical component in high-density batteries. Its ores, such as spodumene (LiAl(SiO3)2), petalite (LiAlSi4O10), and lepidolite (K(Li, Al)3(Al, Si)4O10(F, OH)2) (Figure 7), are extensively mined and processed to meet the escalating demand, primarily driven by the EV market and portable electronics industries [64,65,66,67,68]. Cobalt is among the least abundant metals (25 ppm), while nickel is relatively more abundant at 84 ppm in the earth’s crust [36].
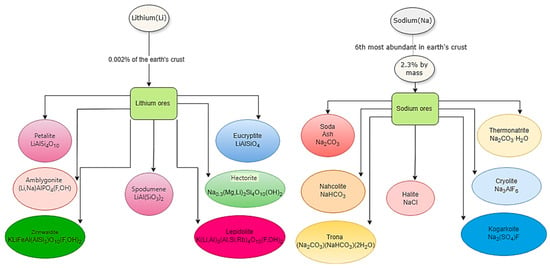
Figure 7.
Lithium and sodium abundancy and ores [69,70,71].
On the other hand, sodium is the sixth element abundant in the Earth’s crust, with a 2.3% mass contribution. Halite (NaCl), also known as rock salt, along with soda ash (Na2CO3), trona (Na3(HCO3)(CO3)·2H2O), and several others are the main. Soda ash, for instance, is utilized in glass, detergents, and chemical compound manufacturing [69,70,71]. Typical features of sodium and lithium are brought for comparison in Table 2. With a 3.0–3.6 V voltage and a 100–150 Wh/kg energy density, SIBs exhibit much lower performance metrics than LIBs [70,71]. Still, they are notably safer, reducing the risk of thermal runaway. They reveal 1000–3000 cycle lives, perform well across various temperatures, and utilize environmentally friendlier materials [72].

Table 2.
Comparative analysis of various features in sodium vs. lithium.
2.3. Comparison of Material Demand for LIBs and SIBs
The demand for LFP cathode chemistries is forecasted to be approximately 3.1 million ton (Mt) by 2035, over 13 times higher than in 2021 [83]. Global battery demand will reach approximately 1000 GWh annually by 2025 and exceed 2600 GWh by 2030. While expanding LIB production is an option, the limited minerals could hinder long-term development. Raw material demand is likely to grow by 2030, with an impact on four critical metals: lithium (6x), cobalt (2x), class 1 nickel (24x), and manganese (1.2x) [84]. The uneven distribution of resources makes the supply chain more vulnerable. Currently, most lithium is mined in Australia and China, but by 2030, Africa and North America will supply 30% of mined lithium. Nickel demand will also surge while the supply remains concentrated in Asia. Even new technological developments in recycling relying on urban mining could only propose 157,000 tons of lithium carbonate equivalent in 2030 to meet just 7% of overall demand. However, nearly 2 Mt will be recycled annually by 2050 [85].
Layered sodium nickel-manganese-iron (NMF) oxide was invented from NMC concepts in ANL for SIBs with efficient sodium insertion and extraction. This cobalt-free formula alleviates the demand for this scarce, expensive metal. Sodium, with more abundance than lithium and easy mining, affects the cost per kilogram of battery material. The suggested formula is neither susceptible to price fluctuations nor disrupted in the supply chain. Thus, the price will be one-third less than LIBs, making it a more sustainable energy storage solution [86]. Cobalt scarcity is more problematic given the required expansion scale of LIBs to meet the projected demand in the transportation and electricity sectors. A published work in 2017 mentioned doubling the price of elemental cobalt in only a year to 50 USD/kg due to the expansion in battery demand [87].
In contrast, SIBs have less susceptibility to the supply chain and element price because of their nonexpensive abundance of elements. However, SIBs are currently estimated to be 1.33 times more expensive for grid-level storage than LFP batteries for a 250 kW, 2-h system. Based on current data, this estimate could change if LFP battery costs are affected by their increased use in EVs. Assuming the 1.33 scaling factor remains constant for a 100 MW, 10-h system, SIBs would cost 215.88 USD/kWh compared to 162.32 USD/kWh for LFP batteries. Developing a solid-electrolyte industry to supply materials like β”-alumina and NaSICON could streamline Na battery production. However, the immature and small-scale manufacturing and supply chain ecosystems are challenging. Limited manufacturing volumes discourage material suppliers from increasing production, which raises costs and restricts Na battery manufacturing [88].
3. Commercialization for Practical Applications
The field of battery technology is changing in response to increasing costs and supply chain challenges facing LIBs, which have been the primary choice for portable energy storage devices and EVs. There is a growing need to consider alternative battery technologies, like SIBs. This section thoroughly examines the aspects of commercialization and the future outlook of both SIBs and LIBs. We compared critical factors affecting their adoption and the impact on global energy systems, specifically applications in transportation, portable electronic devices, and stationary storage. Through this comparison, we demonstrated the SIB’s potential for sustainable energy storage solutions.
LIB or SIB’s employment and share in EVs, portable electronic devices, and stationary storage depends on several critical parameters: infrastructure, cost, energy density, cycle life, charging rate, and safety. Infrastructure becomes crucial as it enables the rapid and cost-effective industrialization of new battery technologies. The compatibility of SIB manufacturing with existing LIB infrastructure enhances feasibility [89] and reduces costs with minimal investment. Cost-effectiveness is crucial to encouraging broader adoption and scaling up manufacturing. Energy density affects how much power can be stored in a given volume and defines the portability of devices and the range of EVs. Cycle life is attributed to the lifespan and replacement frequency of batteries. The charging rate is essential for user convenience. Lastly, safety is critical to preventing accidents due to battery failures, which significantly impact consumer trust and regulatory approval. Accordingly, we will investigate SIBs and LIBs in detail to evaluate these critical metrics and their suitability for commercialization.
3.1. Scale-Up and Infrastructure
The manufacturing processes for SIBs are strongly compatible with existing LIB production lines compared to lithium–sulfur batteries (LSBs) and sulfuric and oxide-based solid-state batteries (SSBs). Figure 8 illustrates the battery cell production for different battery technologies. While SIB and LIB’s production steps are identical, anode production for lithium-sulfur, solid-state, and lithium-air batteries requires an inert gas or dry room atmosphere due to the metallic lithium and laser application for cutting, slitting, and contacting steps. This compatibility comes from similar fundamental electrochemistry and cell designs, which allow using the same production facilities and equipment. Specifically, the electrode production, cell assembly, and conditioning are similar. This similarity enables the utilization of existing manufacturing capacities and reduces investments. Thus, the transition to SIB production is becoming economically possible for manufacturers already producing LIBs [89]. Additionally, less expensive and more abundant raw materials influence the scale and location of manufacturing facilities due to variations in material supply chains and costs [38]. Nevertheless, more cells (assuming identical geometry) must be produced to store the same energy in kWh. This results in higher processing costs due to the need for additional machines to be acquired, installed, and operated. As a result, there has been a reported increase in processing costs of approximately 15% [89].
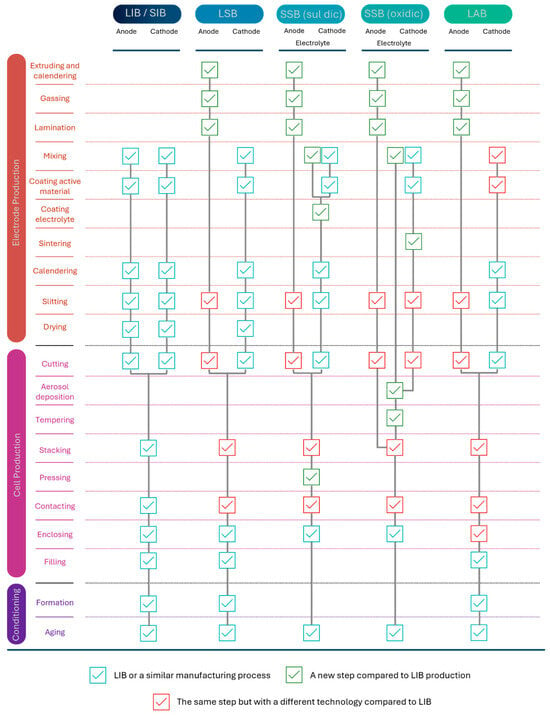
Figure 8.
Battery cell production process for battery technologies. Data was drawn from [89].
3.2. Cost
Battery pack’s cost is high, mostly in the case of EVs. The cost structure of battery electric vehicles (BEVs) is different from that of internal combustion engine vehicles (ICEVs). Battery expenses can contribute to up to one-third of overall vehicle prices (Figure 9). The cost of a battery in a compact ICEV is comparable to a BEV with a 50 kWh battery. In 2020, an ICEV is still much cheaper than a BEV, but by 2030, declining battery prices will reduce the price difference to just 9%. The EV traction battery is given special attention because it is the critical component of the BEV’s powertrain. The total vehicle cost structure is presented in the figure. We consider other vital components besides electrification because driving function automation is a potential development in the automotive industry for driver assistance systems or autonomous driving [90].
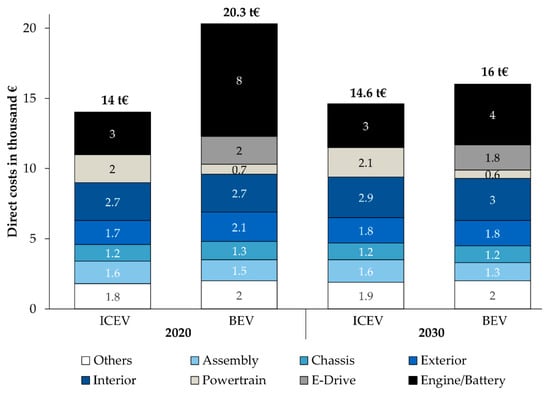
Figure 9.
Cost structure of current and future BEVs compared to ICEVs. With permission from [90].
3.2.1. Materials Costs
In the near future, the expenses associated with Na-based batteries are anticipated to be lower than those for LIBs. This expectation stems from the fact that sodium is the Earth’s fourth most abundant element, and sodium salts are more stable, making their preparation easier when compared to lithium salts [91]. The sodium concentration in the earth’s crust and water is 28,400 mg/kg and 11,000 mg/L, respectively, in contrast with lithium, with 20 mg/kg in the earth’s crust and 0.18 mg/L in water [92]. Due to the utilization of widely available sodium (5000 USD/ton compared to 150 USD/ton for Li sources, Table 1), cobalt-free electrode materials, cost-effective electrolytes, and readily accessible anode materials, SIBs have become appealing to battery manufacturers, reducing expenses. Lithium carbonate prices will rise due to higher demand than the supply capacity [36]. During 2023–2024, sodium carbonate (soda ash) and lithium carbonate experienced frequent price changes. The average US sodium carbonate cost was 180 USD/ton in 2023 [93]. The annual average US price decreased by 32% from 2022 to 46,000 USD/ton. Lithium carbonate prices in China dropped from approximately 76,000 USD/ton in January 2023 to approximately 23,000 USD/ton in November 2023 [93]. However, lithium carbonate prices increased by 16,590 USD/ton in 2024 due to high demand from the EV sector [94].
The cathode materials account for approximately 32% of Li-ion batteries’ total cell construction cost [95]. The rise in expenses for advanced technologies has exceeded the growth in performance based on market prices. For instance, upgrading from NMC532 to NMC811 resulted in an approximately 18% increase in specific capacity at a material level but also led to a 38% increase in total cost per kilogram [96]. This upgrade underscores the importance of either eliminating or reducing these cathode elements. Several SIB companies have opted not to use expensive materials such as Co (and naturally Li). Instead, they are basing their technologies on more abundant elements like Na, Mn, Fe, Ti, and Mg while reducing the amount of elements such as Cu or Ni [97]. An analysis additionally examines the impact of cathode materials on the cost. The SIB achieves a cell price of 223 EUR/kWh, while the LFP and NMC batteries reach 229 EUR/kWh and 168 EUR/kWh, respectively [39].
In the anode part, although HC is relatively more costly than traditional graphite anodes, this challenge can be overcome by producing HC from renewable, ecofriendly, biowaste materials. Organic waste and fossil coke precursor materials have the potential to yield cost-effective HC [39]. Other active materials of biomethods in low-cost and environmentally benign strategies, e.g., natural protein-derived active materials, are also promising for the sustainable energy storage devices. These renewable and low-cost natural resources in versatile bio-macromolecules rich in amino acids, functional groups, and heteroatoms, greatly enhance the performance of sustainable energy storage devices [98]. Based on the reports regarding the percentage of anode cost in the total battery cost, anode cost in SIBs will account for 36% in the short term (2023–2024), decreasing to 31% in the medium term (2025–2028) and further to 19% in the long term (2029+). In contrast, in LFP-based LIBs, anode costs will constitute 13% in the short term, increase slightly to 15% in the medium term, and rise to 17% in the long term [99].
3.2.2. Battery Components
The primary expense in LIBs comprises the cost of the cathode, anode, collectors, separator, and electrolyte [100]. In contrast, a range of essential supplementary components, including separator, conductive agent, binder, and case, are included in the cost [101,102] (Figure 10). Substituting copper foil with aluminum as the anode current collector in SIBs significantly influences the cost structure. For example, for cells composed of LMO-synthetic graphite materials, the cost contributions of aluminum and copper as current collectors are 2.7% and 11.6%, respectively. This substitution results in a 55% considerable mass decrease. Consequently, BatPaC calculations show that this material exchange is projected to lower the cost by 8.7% relative to the reference cell materials [38]. Using lighter and less expensive aluminum instead of copper reduces inactive materials in SIB full cells. This leads to a higher energy density, which prepares the SIBs for use in passenger and commercial EVs. Substituting lithium with sodium only results in a slight cost reduction from 16.1 to 15.8 EUR/L, which has minimal impact on the final cell price [39].
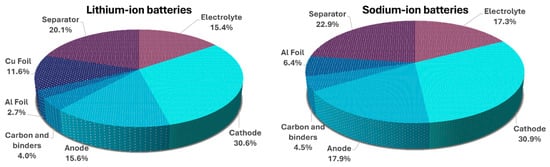
Figure 10.
Cost comparison of model sodium-ion and lithium-ion batteries. The model systems are 7 kW, 11.5 kWh batteries with calculated cell material costs for the reference LMO–sG battery and a theoretical LMO–sG battery in which the copper foil is replaced with aluminum foil and lithium is replaced with sodium. Data was drawn from [38].
3.2.3. Indirect Parameters
SIBs could be more cost-effective for transporting battery cells than LIBs [103]. SIBs can be safely discharged to 0 V or 0% state of charge (SOC) and even stored and transported in a shorted state, enhancing safety and reducing manufacturing and transportation costs. This is possible because SIBs use aluminum current collectors, which do not oxidize like the copper collectors in LIBs. In contrast, Li-ion cells cannot safely be discharged near 0 V without risk of oxidation and potential damage. Consequently, LIBs must be transported at approximately 30% SOC according to international regulations, adding to their cost and reducing safety during transportation [104].
Sodium is larger than lithium and has a higher molecular weight (Mw). This larger size and weight could potentially reduce theoretical energy density and increase cell-level costs [104]. Another approach that can lead to cost reduction involves the addition of electrolyte additives to the electrolyte. With any improvement in the SEI layer, this method can influence the cycle life, thereby potentially reducing the costs associated with battery production [105]. For example, ZSM-5 nanozeolite, a low-cost additive for SIBs, improves cycle life and capacity retention from 40% to 62% after 480 cycles [106]. In LIBs, a study on dioxolone derivatives revealed a 30–40% improvement after 400 cycles at a 1C charging rate [107].
As SIBs have a lower energy density than LIBs [108], assessing the cost per kWh is crucial for commercialization. A cost analysis performed on 18650-type SIB cells calculated a final price for the NMO-HC setup at 252 USD/kWh in comparison to 258 USD/kWh for LFP-type LIB and 190 USD/kWh for NMC-type LIB [39]. In another study [109], the BatPac model was developed by ANL to determine the capital cost of a 53 kWh SIB battery pack (Na0.67Fe0.5Mn0.5O2/HC) at 5925 USD, which is closely comparable to the 5875 USD cost of a similar LIB battery pack (Li1.05(Ni0.6Mn0.2Co0.2)0.95O2 (NMC622)/graphite). Figure 11 schematically represents the comparison of cost factors. If successfully developed on a large scale, SIBs could be priced up to 20% lower than existing technologies and fit into compact EVs and stationary storage systems. The development and economic advantages of SIBs are intricately associated with the price of lithium. Presently, its low cost is deterring investments in sodium-ion technology and postponing expansion efforts. Additionally, supply chain challenges for high-quality cathode and anode materials tailored to SIBs could hinder short-term expansion efforts [110]. Another competitive edge of SIBs compared to LIBs is the supply chain for HC, which results in higher costs for anodes.
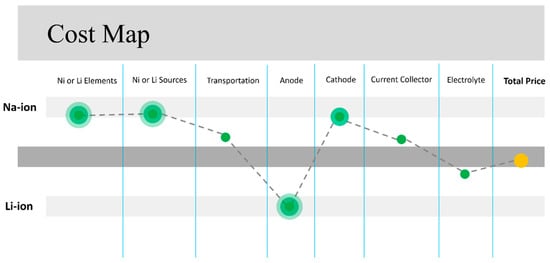
Figure 11.
Competitive advantages in commercialization cost factor of SIBs and LIBs. The circle’s radius highlights the intensity of the advantages of each parameter. The figure is schematically produced based on the [90,93,102,107,110,111].
3.3. Low-Temperature Performance
The use of batteries in cold conditions, such as in EVs, space applications, or stationary storage in some countries, is hindered by their poor performance at low temperatures. SIBs offer a wider temperature range than LIBs due to their low melting point and high-temperature stability as electrolytes, such as propylene carbonate versus ethylene carbonate in LIBs [112]. Thus, high-capacity utilization is possible at extremely low temperatures, down to −40 °C. The wider temperature range of SIBs offers use in environments with extreme temperatures [113,114]. LFP-based LIBs demonstrate a significant reduction in capacity at temperatures as low as −20 °C, with capacity falling to less than 50% due to the sluggish electrochemical reactions and high internal resistances at low temperatures [115]. A study comparing spirally wound full-cells composed of Li0.1Na0.7Co0.5Mn0.5O2 (or Li0.8Co0.5Mn0.5O2) and HC revealed that the power output of SIB at −30 °C is approximately 21% greater than that of LIB. SIB also exhibited capacity retention in cycle testing at 0 °C of approximately ~53%, compared to only ~29% for LIB [116]. In addition, using inorganic antifreeze additives has effectively lowered the electrolyte’s freezing point and improved ionic conductivity and stability over numerous cycles. When tested at −30 °C, cycling stability of 6000 cycles with no obvious capacity decay was achieved [117].
3.4. Fast Charging
The Advanced Battery Alliance of America determines the benchmark for fast charging of an EV as achieving 80% battery capacity in just 15 min [118]. The current LIBs used in EVs face challenges, such as lithium plating on the anodes and cathode instability. These issues affect fast charging, especially in cold temperatures, reducing range, and slower charging. As a result, it is crucial to focus on designing electrodes, electrolytes, and interfaces to achieve reliable fast-charging capability [118]. For example, activated graphite demonstrated improved rate capability for anode improvement, reaching a capacity of 209 mAh/g at 10 C compared to the pristine graphite’s 15 mAh/g. This enhancement is linked to a pseudocapacitive lithium storage behavior. Additionally, activated graphite can achieve 82% and 96% SOC within 6 and 15 min when paired with LFP in a full-cell configuration [119]. Expanded graphite displayed a high specific capacity of 185 mAh/g at a fast charging rate of 1000 mA/g after 500 cycles [120]. As mentioned, the graphite anode is the primary reason for the limited fast-charging capability of LIBs.
On the other hand, SIBs utilize a different Na ion storage mechanism within HC anodes. The functional surface and porous structure of graphitic nanodomains in HC anodes improve charge transfer properties when compared to Li-ion in graphite. As a result, it is essential to identify effective cathode and electrolyte materials for SIBs to enable practical fast charging. Tiamat has demonstrated impressive power performance with its 18,650 SIBs featuring NVPF/HC chemistry by maintaining over 90% capacity at a discharge rate of 20 C [121,122]. SIBs inherently offer higher fast charging abilities than LIBs, thanks to the specific combination of an HC anode utilized in all high-capacity Na-ion cells and the improved kinetics of Na-ion electrolytes overall [123]. Thus, in recent advancements, the significance of fast charging for battery applications in EVs, portable electronics, and stationary storage systems has been emphasized. Rapid charging enhances convenience with an extended driving range for EVs. For portable devices, fast charging is crucial for user convenience, reducing the time spent connected to charging devices. In stationary storage, it enables quicker energy storage during low-demand periods to manage peak loads properly.
3.5. Recyclability
The pivotal issue concerns the cost-effectiveness of lithium recycling in the foreseeable future. As the production of large battery modules escalates, uncertainties in lithium supply may inflate the costs of LIBs. Currently, the LIB industry consumes one-third of the globally produced lithium. Additionally, the availability of other critical raw materials for LIBs, particularly cobalt, is threatened by market fluctuations driven by political and environmental factors. Manufacturers will not need alternatives if LIBs can be fully recycled and a circular economy is established. As for sodium, there will be no pressure for “low-cost recycling” because Na is easily obtained by evaporating seawater (11,000 mg/L in seawater) [92]. Therefore, sodium-based batteries present a viable solution for the mass production and assembly of large modules [103]. However, the recyclability must be considered due to the environmental issues.
4. Challenges and Issues
4.1. Energy Density and Capacity
The replacement of lithium with sodium in a battery seems straightforward at first. Still, some parameters are considered, such as reactions in the electrode and the redox behavior of lithium or sodium electrodes. The aim is to decrease cost while maintaining or improving energy density, safety, cycle and calendar life, and low-temperature tolerance. Overall, the energy density of LIBs has continuously increased by approximately 7–8 Wh/Kg per year. The values slightly exceed 300 Wh/kg [124]. The Na ion (r = 1.02 c, CN = 6) is larger than the Li ion (r = 0.59 c, CN = 4) [125], and the less polarizing size and polarizing nature of the Na ion affect the phase behavior and diffusion properties compared to the Li ion. Na ion also has a lower desolvation energy, approximately 30% less than lithium, which affects the reactions at the electrode-electrolyte interface, and the charge transfer resistance is small for SIBs [126]. LiCrO2 and NaCrO2 both possess quite similar crystal structures, but the former is inactive in Li cells while the latter is active in Na cells [127]. In both positive and negative electrodes, the intercalation potentials of Li+ and Na+ are highly structure-dependent, but for given intercalation hosts, they are lower for sodium than for lithium.
Sodium has a smaller cohesive energy than lithium, which should enable higher cell voltages by 0.53 V. However, Na+ intercalation into a fixed positive electrode is less energetically favorable than Li+ intercalation, which is one of the drawbacks of SIBs [128]. Developing new cathode materials with abundant active sites and ionic channels is crucial for high-energy-dense SIBs. Transition metal oxides, polyanionic compounds, and ferrocyanides are promising options with favorable electrochemical properties. SIBs have a lower energy density than LIBs due to the atomic weight of Na+ ions and the structural requirements for sodium accommodation. Certain materials exhibit high-rate capability and a stable cycle life of up to ten thousand cycles. NaVOPO4, Na2MnFe(CN)6, and Na2CoFe(CN)6 achieve energy densities of approximately 500 Wh/kg, comparable to some LIB materials. Layered oxide cathode materials offer unique sodium storage characteristics with a theoretical capacity of 230–250 mAh/g. However, their multiphase transition reactions and complex metal doping/substitution make them more complicated than other materials [129].
SIB cathodes include Prussian blue and its analogs, which show reversible capacities of 80–120 mAh/g in Na half-cells at 3–3.5 V voltages. However, they have the drawback of low density, providing lower volumetric energy densities for SIBs compared to transition metal oxide cathodes with the same specific capacity [41]. PBAs face issues of moisture sensitivity and limited reversibility when cycling more than one atom of sodium per formula unit, leading to capacity fading. This is often due to the limited electronic conductivity, water presence, and phase transitions of highly sodiated compounds [97]. Layered materials such as P2 and O3 have potential as Na-ion cathodes. There are variations in electrochemical performance due to cation permutation. The notations P2, O3, and P3 describe the crystal structures adopted by Na-ion cathode materials, denoting the coordination of Na-ion sites between metal-oxide sheets. Due to their unique sodium-ion environment, P2-based sodium batteries offer better power performance than LIBs. O3-based systems provide the best battery capacity and performance. Over-sedation may lead to further improvement in performance [124,129].
The layered oxides utilized in SIBs have a distinctive P-type characteristic. In this type, the sodium ions in the alkali metal layer occupy prismatic sites, resulting in exceptional specific capacity and high-rate capabilities [130]. Layered NaxMO2 has a higher energy density and is suitable for high-energy devices. Due to sodium deficiency, O3-type materials are more practical, but phase transformation and air sensitivity hinder their application. Layered NaxMO2 compounds with sufficient cycle life, energy density, and air stability are required. High-performance O3-type or mixed P-/O-type materials are preferable with low-cost and nontoxic elements such as Fe, Mn, and Cu [124,130].
Na3V2(PO4)2F3 (NVPF) compound is a promising battery cathode material due to its crystal structure that allows Na+ ions fast diffusion. It can release two Na+ per formula unit at an average potential of 3.9 V with a specific energy of 507 Wh/kg, comparable to LFP. This report suggests a way to improve the energy density of Na3V2(PO4)2F3 batteries by 14% while maintaining good cycle life and rate capabilities. Moreover, it indicates that the low insertion plateau is a practical measure for designing SIBs that do not suffer from performance degradation even when discharged to zero volts [131]. Tiamat’s prototype cells using NVPF/HC chemistry achieved 122 Wh/kg at 1C rate with high power rate capability and long cycling life. However, toxic vanadium is a drawback, and scientists are exploring alternatives like less toxic and abundant 3d metals (Mn). To resolve a volume change and consequent structural degradation during long-term cycling in sustainable Fe-/Mn-based P2-type cathode, researchers introducing vacancies into the transition metal layer of P2-Na0.7Fe0.1Mn0.75□0.15O2 (‘□’ represents a vacancy). The result shows this cathode design enables pouch cells with energy densities of 170 Wh/kg and 120 Wh/kg to operate for over 600 and 1000 cycles, respectively [130].
4.2. Environmental Impact
SIBs are known for their eco-friendliness and avoidance of using rare or critical materials. It is currently challenging for SIB to match LIB in terms of global warming potential (GWP). However, ongoing efforts to reduce greenhouse gas emissions associated with SIB’s production are expected to help them become more competitive with LIB’s. Raw materials and energy sources for processing will play a significant role in the overall GWP of future battery cells, potentially outweighing considerations of cell chemistry. SIBs using abundant materials like NaMMO (Na2/3(Mn0.95Mg0.05)O2), NaPBA (Na2Fe[Fe(CN)6]), and NaNMMT (Na1.1(Ni0.3Mn0.5Mg0.05Ti0.05)O2) are a better choice, as they do not rely on critical materials, and have a positive life cycle that outperforms LIBs. NaMVP (Na4MnV(PO4)3) cells have some unfavorable results, which can be partially attributed to specific vanadium production processes. However, alternative sourcing methods can help mitigate this issue. SIBs generally cause higher acidification potential impacts because of the anode active material, HC, which usually comes from petroleum coke [132]. While this material has good environmental performance, the lack of specific sulfur dioxide (SO2) abatement technologies in the process needs further attention. Figure 12 provides information on the potential environmental impacts of producing 1 kWh of battery cell capacity [132]. SIB coin cells have much lower energy densities (0.1–0.4 Wh/kg) and consequently, a much higher environmental impact, with approximately 56,000 kg CO2eq/kWh for lab-scale scenarios and approximately 5200 kg CO2eq/kWh for industrial-scale scenarios [133].
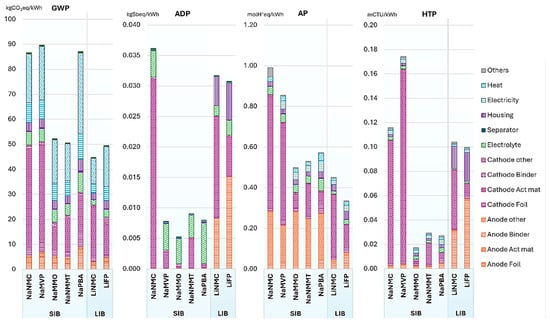
Figure 12.
Environmental impacts per kWh of a battery cell in manufacturing. With permission from [132].
In assessing SIBs employing Ti1Al1TiC1.85 MXene as anode material (1 kg of produced battery), SIBs exhibit the same CO2eq emission as LIBs. However, regarding resource utilization and the impact of minerals and metals, SIBs demonstrated a 20% reduction. Nonetheless, until SIBs attain comparable energy density and efficiency during electrode production, LIBs will persist as the primary environmentally friendly choice. Although SIBs present potential as a sustainable substitute with diminished geopolitical supply risk, further investigation is imperative to mitigate natural resource consumption and production expenses [134]. Another study revealed that the production of reduced graphene oxide can generate emissions ranging from 1060 to 2360 kg CO2eq/kg. In comparison, the synthesis of graphene oxide may yield emissions between 217 and 501 kg CO2eq/kg [135]. In the evaluation of a Na3V2(PO4)3/Na half-cell setup, global warming potential ranges from 423.9 to 1380 kg CO2eq/kg (539.8 to 1622.1 kg CO2eq/kWh) of the cathode. Introducing essential carbon additives to Na3V2(PO4)3 can strike a favorable equilibrium between CO2 footprint and storage capacity [136]. Cathode materials constitute over 50% of carbon emissions in making LIBs, but not so much for most SIBs, especially Na-S and Na-PBA batteries, where cathode production emits very little carbon. The Na-PBA battery has the highest carbon emissions at 130.05 kg CO2eq/kWh due to its low energy density. NaMMO battery has the lowest carbon emissions at 8.47 kg CO2eq/kg and 63.45 kg CO2eq/kWh due to its low input of heavy metal materials and energy in manufacturing. SIBs have a lower environmental impact than LIBs regarding human toxicity potential, land use, and mineral and metal values [137]. Analyzing the climate impact shows that from 2020 to 2050, the climate impact of SIBs decreased by 43–57%. The contribution of the battery manufacturing process will decrease from 18–32% to 2–4% due to the increasing share of clean energy in the electricity grid [27].
4.3. Sustainability
The significant advantage of SIBs is sustainability, which is essential for a world striving to be free of carbon-based energy sources. We can foresee SIBs with hard-carbon anodes and cobalt-free cathodes as sustainable lower-cost alternatives to LIBs for applications, such as short-range EVs and large-scale energy storage in a world that is increasingly being transformed into wind, solar, and hydroelectric power, which depend on battery energy storage for uninterrupted performance. Future research should focus on discovering advanced anode and cathode materials for SIBs with higher specific capacities and voltages to produce practical SIBs with specific energies up to 200 Wh/kg. Efforts should also be made to develop advanced electrolytes that enable SIB performance at high charge–discharge rates over a wide temperature range [41]. As the rising costs, increasing material demand, and sustainability concerns drive, it is crucial to address how to direct these efforts towards sustainability before the technology becomes adaptable [132].
Chile (8.6 Mt) and Australia (2.8 Mt) hold the largest reserves of lithium and are the top exporters, followed by Argentina and China (Figure 13a) [138]. The US has the world’s largest natural soda ash reserves at 23 Mt. This gives the country a significant advantage in production and supply chain [139]. The US Department of Energy (DOE) has identified critical and near-critical elements for the medium term (2025–2035) due to their importance in energy and supply risk, as shown in Figure 13b [140]. The appealing qualities of sodium increased the interest in using sodium-containing precursors for rechargeable batteries, leading to the development of new projects.
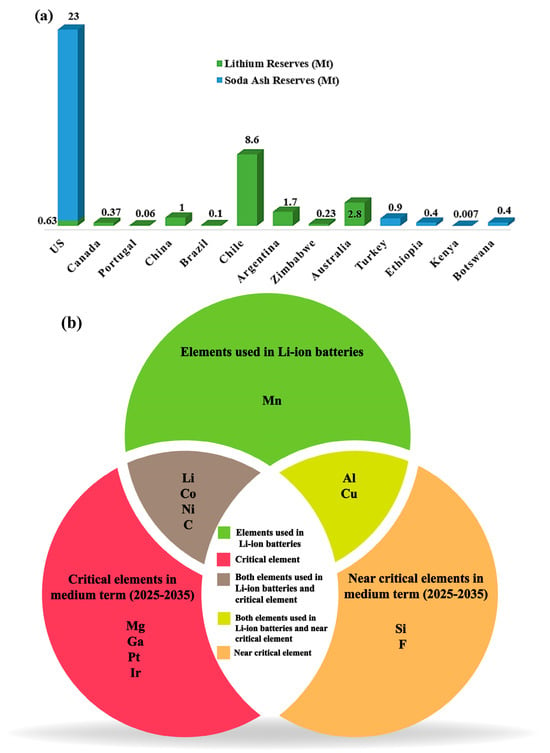
Figure 13.
(a) Li and Na reserves (Soda Ash) in the world reported in 2020. Data was drawn from [138]. (b) Critical and near-critical elements for the medium term (2025–2035). Data was drawn from [123].
4.4. Safety
Although SIBs are often considered a safer alternative to LIBs, limited information is available regarding their safety risks. Like LIBs, using flammable organic electrolytes brings similar safety concerns. Analyzing thermal abuse on SIBs revealed that the electrolyte emits flammable gases. Exploring different cathode and anode materials may allow for safer electrolyte solvents, e.g., overall safety could improve utilizing solid-state electrolytes instead of flammable organic liquid electrolytes [139]. SSBs are stable and can operate over a wider temperature range, allowing them to function in extreme weather conditions without requiring extreme thermal management [141]. SIB electrolytes can be classified into five types: organic, ionic liquids, aqueous, solid polymer, and inorganic. Among them, organic electrolytes based on esters and ethers are most used due to their high ionic conductivity and superior wettability (Figure 14). Synergistic cosolvent systems have improved SIB’s ion conductivity, viscosity, electrochemical stability window, and safety [142]. Research is focused on developing film-forming additives to improve the cycling performance and safety of SIBs. Fluoroethylene carbonate (FEC) is a successful additive for SIBs [143]. To enhance the safety of the current mature electrolyte system, one could modify its components instead of changing its skeleton composition. This can be performed by adding conventional flame-retardant additives or a small dose of ionic liquids as an electrolyte additive for stability and safety issues of SIBs [143,144]. Sodium bis(fluorosulfonyl)imide (NaFSI) is a substance that improves the interaction between positively charged ions and solvents. This leads to better performance, and it also has fire-extinguishing properties. By using organic electrolytes that are inspired by the concept of salt-concentrated electrolytes, fires can be extinguished. A model using NaN(SO2F)2 (NaFSA)/trimethyl phosphate (TMP) for SIBs has been shown to achieve stable cycling for over 1000 cycles, outperforming conventional flammable electrolytes. This concentrated electrolyte, which uses TMP without additives, ensures long-lasting battery safety and paves the way for higher energy density [145]. Another nonflammable electrolyte for SIBs was developed by adding a flame retardant called ethoxy(pentafluoro)cyclotriphosphazene (EFPN). EFPN not only prevents the electrolyte from catching fire, but it also improves the performance of both the anode and cathode. Adding 5% EFPN to a standard 1 M NaPF6 in EC:DEC (1:1, volume ratio) electrolyte decreased the self-extinguish time from 58 s to 0 s [139].
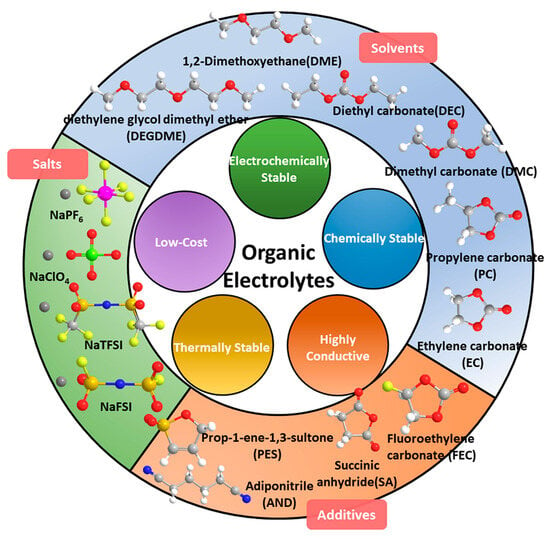
Figure 14.
Typical components of organic electrolytes based on ethers and esters in SIBs. With permission from [142].
Phosphorus, a fire-retardant element, reduces self-heating and shows the potential use of organic phosphate additives/electrolytes in SIBs. Traditional phosphates have linear or cyclic chemical structures [146]. A nonflammable electrolyte consisting of polyanionic compounds, PBAs, and commercial HC, 1.2 M NaTFSI-TMP/bis(2,2,2-trifluoroethyl) ether (BTFE)/vinylene carbonate (VC) (0.53:1:1.5:0.15 molar ratio) is compatible with various electrodes. This electrolyte exhibits high cycling Coulombic efficiency with cycling stability. Its wide availability and compatibility make it desirable for multiple combinations of nonflammable or flame-retardant solvents and alkaline salts to develop safe and high-performance rechargeable batteries [147]. LFP cathode is taking the share from NMC in the EV market due to its high level of safety. In LFP, the P–O covalent bond is strong and inhibits oxygen release compared to the layered oxide NMC cathodes. This results in improved thermal stability. Similarly, using cathode materials with high thermal stability can lead to high specific energy (Wh/kg) and energy density (Wh/L) of SIBs at the pack level [140].
5. Conclusions and Perspective
With the fast-growing market, sodium chemistries might compete for the dominance of lithium counterparts for a long time. However, with a 10% prediction for the EV battery share up to 2030, this dominance will be a far destiny. As sodium is heavier than lithium, the weight of the battery system and lower energy density are significant issues to consider. This causes sodium systems to be more favorable for short-range urban transportation, which needs lower energy density and stationary energy storage systems, such as grid storage or industrial applications. For widespread commercialization, SIBs must adopt LIB’s technological advancements to proceed faster. Additionally, challenges related to lower energy density must be addressed to attract the manufacturer’s attention. Large employment will resolve other issues like a limited supply chain, which causes higher costs for SIBs despite the lower price and more abundance of their precursors. As of today, the driving factors are not cost-related; instead, they include the safety benefits, the natural abundance of sodium-based precursor salts, and their less toxic nature. Over time, continued innovative research in this field is expected to advance SIB technology, paving the way for a brighter future in energy storage. The energy storage market will be segmented between low-cost LIBs based on olivine cathodes such as LFP or LMFP and SIBs with hard carbon as an anode. In parallel, the green stable chain supply for SIBs will be built to meet the high demand for energy storage and power electronic applications.
Author Contributions
A.N., M.D., M.R., M.D.B. and A.K.M.R.R. worked equally on conceptualization, investigation, writing, and reviewing. X.L., S.D. and K.Z. contributed in conceptualization, editing and reviewing, K.Z. in supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This review paper was made possible through the generous financial support of Concordia University, AI–Mogul, Innovéé (Quebec Government), and Natural Sciences and Engineering Research Council of Canada (NSERC).
Data Availability Statement
Figure’s data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Batteries and Secure Energy Transitions; International Energy Agency (IEA): Paris, France, 2024. [Google Scholar]
- Sodium-Ion Battery Market. Available online: https://www.marketsandmarkets.com/Market-Reports/sodium-ion-battery-market-207269067.html (accessed on 3 April 2024).
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-ion batteries toward Na-ion chemistries: Challenges and opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Lewis, G.N.; Keyes, F.G. The potential of the lithium electrode. J. Am. Chem. Soc. 1913, 35, 340–344. [Google Scholar] [CrossRef]
- Selim, R.G.; Hill, K.R.; Rao, M.L.B. Research and Development of a High Capacity, Nonaqueous Secondary Battery. PR Mallory & Company, Laboratory for Physical Science: Indianapolis, IN, USA, 1966. [Google Scholar]
- Vissers, D.R.; Tomczuk, Z.; Steunenberg, R.K. A Preliminary Investigation of High Temperature Lithium/Iron Sulfide Secondary Cells. J. Electrochem. Soc. 1974, 121, 665. [Google Scholar] [CrossRef]
- Gay, E.C.; Vissers, D.R.; Martino, F.J.; Anderson, K.E. Performance characteristics of solid lithium-aluminum alloy electrodes. J. Electrochem. Soc. 1976, 123, 1591–1596. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Evarts, E.C. Lithium batteries: To the limits of lithium. Nature 2015, 526, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Wei, S.; Taylor, M.; Chen, J.; Reports, J.K.-E. Hybrid Anode Materials for Rechargeable Batteries—A Review of Sn/TiO2 Based Nanocomposites. Energy Rep. 2021, 7, 2836. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Gaines, L.; Cuenca, R. Costs of Lithiumion Batteries for Vehicles. Technical report by Argonne National Laboratory; The US Department of Energy (DOE): Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Bates, J. Thin-film lithium and lithium-ion batteries. Solid. State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Lithium-Ion Battery Inventor Introduces New Technology for Fast-Charging, Noncombustible Batteries. Available online: https://news.utexas.edu/2017/02/28/goodenough-introduces-new-battery-technology/ (accessed on 3 February 2017).
- Paolella, A.; Faure, C.; Bertoni, G.; Marras, S.; Guerfi, A.; Darwiche, A.; Hovington, P.; Commarieu, B.; Wang, Z.; Prato, M.; et al. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 2017, 8, 14643. [Google Scholar] [CrossRef]
- Hydro-Québec Researcher Karim Zaghib Wins the Lionel-Boulet Award. Available online: https://news.hydroquebec.com/en/press-releases/1549/hydro-quebec-researcher-karim-zaghib-wins-the-lionel-boulet-award/ (accessed on 3 October 2019).
- Bajolle, H.; Lagadic, M.; Louvet, N. The future of lithium-ion batteries: Exploring expert conceptions, market trends, and price scenarios. Energy Res. Soc. Sci. 2022, 93, 102850. [Google Scholar] [CrossRef]
- Newman, G.H.; Klemann, L.P. Ambient temperature cycling of an Na-TiS2 cell. J. Electrochem. Soc. 1980, 127, 2097–2099. [Google Scholar] [CrossRef]
- Sayahpour, B.; Hirsh, H.; Parab, S.; Nguyen, L.H.B.; Zhang, M.; Meng, Y.S. Perspective: Design of cathode materials for sustainable sodium-ion batteries. MRS Energy Sustain. 2022, 9, 183–197. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of high energy density sodium-ion batteries: Faradion’s journey and outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High capacity anode materials for rechargeable sodium-ion batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- CATL: CATL Unveils Its Latest Breakthrough Technology by Releasing Its First Generation of Sodium-Ion Batteries. Available online: https://www.catl.com/en/news/665.html (accessed on 14 May 2024).
- IEA. Global EV Outlook 2023; International Energy Agency (IEA): Paris, France, 2023. [Google Scholar]
- Jones, N. The new car batteries that could power the electric vehicle revolution. Nature 2024, 626, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Häringer, M.; Schmidt, M.; Schneider, L.; Peters, J.F.; Bauer, W.; Binder, J.R.; Weil, M. Prospective sustainability screening of sodium-ion battery cathode materials. Adv. Energy Mater. 2022, 12, 2202636. [Google Scholar] [CrossRef]
- Zhang, S.; Steubing, B.; Karlsson Potter, H.; Hansson, P.A.; Nordberg, Å. Future climate impacts of sodium-ion batteries. Resour. Conserv. Recycl. 2024, 202, 107362. [Google Scholar] [CrossRef]
- ScienceDaily. Sodium-Ion Batteries Are a Valid Alternative to Lithium-Ion Batteries; ScienceDaily: Rockville, MD, USA, 2020. [Google Scholar]
- Patrick Chen, Tamara Grünewald, Jesse Noffsinger, Eivind Samseth: Global Energy Perspective 2023: Power Outlook. Available online: https://www.mckinsey.com/industries/oil-and-gas/our-insights/global-energy-perspective-2023-power-outlook#/ (accessed on 10 May 2024).
- IEA. Global Critical Minerals Outlook 2024; International Energy Agency (IEA): Paris, France, 2024. [Google Scholar]
- Nekahi, A.; Kumar, M.R.A.; Li, X.; Deng, S.; Zaghib, K. Sustainable LiFePO4 and LiMnxFe1-xPO4 (x=0.1–1) cathode materials for lithium-ion batteries: A systematic review from mine to chassis. Mater. Sci. Eng. R. Rep. 2024, 159, 100797. [Google Scholar] [CrossRef]
- Sun, Y.-K. A Rising Tide of Co-Free Chemistries for Li-Ion Batteries. ACS Energy Lett. 2022, 7, 1774–1775. [Google Scholar] [CrossRef]
- Yang, L.; Deng, W.; Xu, W.; Tian, Y.; Wang, A.; Wang, B.; Zou, G.; Hou, H.; Deng, W.; Ji, X. Olivine LiMnxFe1−xPO4 cathode materials for lithium ion batteries: Restricted factors of rate performances. J. Mater. Chem. A 2021, 9, 14214–14232. [Google Scholar] [CrossRef]
- Trends in Batteries, Battery Demand for EVs Continues to Rise. 2023. Available online: https://www.iea.org/reports/global-ev-outlook-2023/trends-in-batteries (accessed on 3 April 2024).
- King, A.; Pass the Salt Please. Power Lies within. 2024. Available online: https://projects.research-and-innovation.ec.europa.eu/en/horizon-magazine/pass-salt-please-power-lies-within (accessed on 3 April 2024).
- Nagmani; Pahari, D.; Verma, P.; Puravankara, S. Are Na-ion batteries nearing the energy storage tipping point?–Current status of non-aqueous, aqueous, and solid-sate Na-ion battery technologies for sustainable energy storage. J. Energy Storage 2022, 56, 105961. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering 2023, 24, 172–183. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Peters, J.; Peña Cruz, A.; Weil, M. Exploring the economic potential of sodium-ion batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- Reid, M. Sodium-Ion Batteries: Disrupt and Conquer? Wood Mackenzie: Edinburgh, UK, 2023. [Google Scholar]
- Abraham, K.M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of today for Na-based batteries of the future: From materials to cell metrics. J. Power Sources 2021, 482, 228872. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in anode materials for sodium ion batteries-A review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Sawicki, M.; Shaw, L.L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129–53154. [Google Scholar] [CrossRef]
- Alvira, D.; Antorán, D.; Manyà, J.J. Assembly and electrochemical testing of renewable carbon-based anodes in SIBs: A practical guide. J. Energy Chem. 2022, 75, 457–477. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zhou, S.; Mei, T.; Wang, X.; Qian, Y. Crystal structural design of exposed planes: Express channels, high-rate capability cathodes for lithium-ion batteries. Nanoscale 2018, 10, 17435–17455. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium titanate as anode material for lithium-ion cells: A review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- Huang, S.; Cheong, L.-Z.; Wang, D.; Shen, C. Nanostructured phosphorus doped silicon/graphite composite as anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 23672–23678. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhou, Q.; Li, X.; Xiong, X. Fast-charging anodes for lithium ion batteries: Progress and challenges. Chem. Commun. 2024, 60, 2472–2488. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kim, H.W.; Lee, S.S.; Kim, H.J.; Kim, J.; Jung, Y.; Kim, Y. Ceramic-based composite solid electrolyte for lithium-ion batteries. ChemPlusChem 2015, 80, 1100–1103. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, S.; Luo, L.; Ding, M.S.; Zheng, J.; Cartmell, S.S.; Wang, C.-M.; Xu, K.; Zhang, J.-G.; Xu, W. Wide-temperature electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent development of polyolefin-based microporous separators for Li−ion batteries: A review. Chem. Rec. 2020, 20, 570–595. [Google Scholar] [CrossRef]
- Wang, Y.; Travas-Sejdic, J.; Steiner, R. Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery. Solid. State Ion. 2002, 148, 443–449. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884. [Google Scholar] [CrossRef]
- Xie, M.; Xu, M.; Huang, Y.; Chen, R.; Zhang, X.; Li, L.; Wu, F. Na2NixCo1−xFe(CN)6: A class of Prussian blue analogs with transition metal elements as cathode materials for sodium ion batteries. Electrochem. Commun. 2015, 59, 91–94. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, A.; Peng, L.; Zhao, F.; Tang, Y.; Zhou, Y.; Yu, G. Cyanogel-enabled homogeneous Sb–Ni–C ternary framework electrodes for enhanced sodium storage. ACS Nano 2018, 12, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Zhu, Y.; Culver, J.N.; Lundgren, C.A.; Xu, K.; Wang, C. Tin-Coated Viral Nanoforests as Sodium-Ion Battery Anodes. ACS Nano 2013, 7, 3627–3634. [Google Scholar] [CrossRef]
- Monti, D.; Jónsson, E.; Boschin, A.; Palacín, M.R.; Ponrouch, A.; Johansson, P. Towards standard electrolytes for sodium-ion batteries: Physical properties, ion solvation and ion-pairing in alkyl carbonate solvents. Phys. Chem. Chem. Phys. 2020, 22, 22768–22777. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572. [Google Scholar] [CrossRef]
- Norton, J.J.; Schlegel, D.M. Lithium resources of North America; US Government Printing Office: Washington, DC, USA, 1955. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Han, Y.; Zhang, S. Review on the beneficiation of Li, Be, Ta, Nb-bearing polymetallic pegmatite ores in China. Minerals 2023, 13, 865. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The lithium market, resources and processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives–A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Kozhukhova, N.; Kozhukhova, M.; Zhernovskaya, I.; Promakhov, V. The correlation of temperature-mineral phase transformation as a controlling factor of thermal and mechanical performance of fly ash-based alkali-activated binders. Materials 2020, 13, 5181. [Google Scholar] [CrossRef] [PubMed]
- Kasatkin, A.V.; Plášil, J.; Pekov, I.J.; Belakovskiy, D.I.; Nestola, F.; Čejka, J.; Vigasina, M.F.; Zorzi, F.; Thorne, B. Karpenkoite, Co3(V2O7)(OH)2·2H2O, a cobalt analogue of martyite from the Little Eva mine, Grand County, Utah, USA. J. Geosci. 2015, 60, 251–257. [Google Scholar] [CrossRef]
- Bailey, J.C. Formation of cryolite and other aluminofluorides: A petrologic review. Bull. Geol. Soc. Den. 1980, 29, 1–45. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Abirami, M.; Kim, J.; Go, W.; Hwang, S.M.; Kim, Y. Sodium-ion hybrid electrolyte battery for sustainable energy storage applications. J. Power Sources 2017, 341, 404–410. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid. State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukherjee, P.P. Electrolytes and interfaces driven thermal stability of sodium-ion batteries. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022; p. 501. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Ritchie, A.G. Recent developments and future prospects for lithium rechargeable batteries☆. J. Power Sources 2001, 96, 1–4. [Google Scholar] [CrossRef]
- Xie, K.; Wei, W.; Yu, H.; Deng, M.; Ke, S.; Zeng, X.; Li, Z.; Shen, C.; Wang, J.; Wei, B. Use of a novel layered titanoniobate as an anode material for long cycle life sodium ion batteries. RSC Adv. 2016, 6, 35746–35750. [Google Scholar] [CrossRef]
- Roscher, M.A.; Assfalg, J.; Bohlen, O.S. Detection of utilizable capacity deterioration in battery systems. IEEE Trans. Veh. Technol. 2011, 60, 98–103. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Fang, Y.; Zhou, M.; Liang, L.; Singh, S.; Zhao, H.; Schober, A.; Lei, Y. Extended π-Conjugated System for Fast-Charge and -Discharge Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, G.-L.; Che, H.; Wang, H.; Yang, K.; Yang, X.; Guo, F.; Ren, Y.; Chen, Z.; Amine, K.; et al. Probing thermal and chemical stability of NaxNi1/3Fe1/3Mn1/3O2 cathode material toward safe sodium-ion batteries. Chem. Mater. 2018, 30, 4909–4918. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Z.; Li, Q.; Wang, X.; Xu, X.; Meng, J.; Ren, W.; Zhang, X.; Huang, Y.; Mai, L. 3.0 V high energy density symmetric sodium-ion battery: Na4V2(PO4)3∥Na3V2(PO4)3. ACS Appl. Mater. Interfaces 2018, 10, 10022–10028. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338. [Google Scholar] [CrossRef]
- Quantifying Battery Raw Material Demand. Available online: https://www.woodmac.com/news/opinion/quantifying-battery-raw-material-demand/ (accessed on 3 April 2022).
- Ferraro, M.; Tumminia, G. Techno-economics analysis on sodium-ion batteries: Overview and prospective. In Emerging Battery Technologies to Boost the Clean Energy Transition: Cost, Sustainability, and Performance Analysis; Passerini, S., Barelli, L., Baumann, M., Peters, J., Weil, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 259–266. [Google Scholar] [CrossRef]
- Reid, M. Is the Electric Vehicle and Battery Supply Chain Charged for Success? Available online: https://www.woodmac.com/news/opinion/electric-vehicle-battery-supply-chain/ (accessed on 3 August 2023).
- Cathode Innovation Makes Sodium-Ion Battery an Attractive Option for Electric Vehicles; Argonne National Laboratory, The US Department of Energy (DOE): Lemont, IL, USA, 2024.
- Jaffe, S. Vulnerable Links in the Lithium-Ion Battery Supply Chain. Joule 2017, 1, 225–228. [Google Scholar] [CrossRef]
- DOE. Sodium Batteries Technology Strategy Assessment; The US Department of Energy (DOE): Washington, DC, USA, 2023. [Google Scholar]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- König, A.; Nicoletti, L.; Schröder, D.; Wolff, S.; Waclaw, A.; Lienkamp, M. An Overview of Parameter and Cost for Battery Electric Vehicles. World Electr. Veh. J. 2021, 12, 21. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced organic electrode materials for rechargeable sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1601792. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef] [PubMed]
- United States Geological Survey. Mineral Commodity Summaries; United States Geological Survey: Reston, VA, USA, 2023. [Google Scholar]
- Ouerghi, D.; Li, Z. Chinese Lithium Prices Extend Uptrend amid Futures Strength; Other Markets in Stalemate. Available online: https://www.fastmarkets.com/insights/chinese-lithium-prices-extend-uptrend-amid-futures-strength-other-markets-in-stalemate/ (accessed on 10 May 2024).
- Zang, G.; Zhang, J.; Xu, S.; Xing, Y. Techno-economic analysis of cathode material production using flame-assisted spray pyrolysis. Energy 2021, 218, 119504. [Google Scholar] [CrossRef]
- Greenwood, M.; Wentker, M.; Leker, J. A bottom-up performance and cost assessment of lithium-ion battery pouch cells utilizing nickel-rich cathode active materials and silicon-graphite composite anodes. J. Power Sources Adv. 2021, 9, 100055. [Google Scholar] [CrossRef]
- Tapia-Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 roadmap for sodium-ion batteries. J. Phys. Energy 2021, 3, 031503. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, W.-H. Promising sustainable technology for energy storage devices: Natural protein-derived active materials. Electrochim. Acta 2023, 441, 141860. [Google Scholar] [CrossRef]
- Volta Foundation. Battery Report 2023; Volta Foundation: San Francisco, CA, USA, 2024. [Google Scholar]
- Wentker, M.; Greenwood, M.; Leker, J. A bottom-up approach to lithium-ion battery cost modeling with a focus on cathode active materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Srivastava, M.; M.R., A.K.; Zaghib, K. Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries 2024, 10, 268. [Google Scholar] [CrossRef]
- Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries . Batteries 2024, 10, 39. [Google Scholar] [CrossRef]
- Karabelli, D.; Singh, S.; Kiemel, S.; Koller, J.; Konarov, A.; Stubhan, F.; Miehe, R.; Weeber, M.; Bakenov, Z.; Birke, K.P. Sodium-based batteries: In search of the best compromise between sustainability and maximization of electric performance. Front. Energy Res. 2020, 8, 605129. [Google Scholar] [CrossRef]
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the safe shipping of lithium-ion and sodium-ion cells: A materials chemistry perspective. Energy Mater. Adv. 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Hara, R.; Kubota, K.; Paulsen, J.; Kumakura, S.; Komaba, S. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A 2014, 2, 16851–16855. [Google Scholar] [CrossRef]
- Chen, J.; Adit, G.; Li, L.; Zhang, Y.; Chua, D.H.C.; Lee, P.S. Optimization strategies toward functional sodium-ion batteries. Energy Environ. Mater. 2023, 6, e12633. [Google Scholar] [CrossRef]
- Chen, L.; Kishore, B.; Walker, M.; Dancer, C.E.; Kendrick, E. Undefined: Nanozeolite ZSM-5 electrolyte additive for long life sodium-ion batteries. Chem. Commun. 2020, 56, 11609–11612. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, S.Y.; Lee, T.K.; Park, M.W.; Lim, H.Y.; Sung, J.; Cho, J.; Kwak, S.K.; Hong, S.Y.; Choi, N.-S. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 2021, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Li, M.; Du, Z.; Khaleel, M.A.; Belharouak, I. Materials and engineering endeavors towards practical sodium-ion batteries. Energy Storage Mater. 2020, 25, 520–536. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2024; IEA: Paris, France, 2024. [Google Scholar]
- Ji, X.; Hou, H.; Zou, G. Sodium-Ion Batteries: Technologies and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Xu, S.; Yao, K.; Yang, D.; Chen, D.; Lin, C.; Liu, C.; Wu, H.; Zeng, J.; Liu, L.; Zheng, Y.; et al. Interfacial Engineering of Na3V2(PO4)2O2F Cathode for Low-Temperature (−40 °C) Sodium-Ion Batteries. ACS Appl. Mater. Interfaces. 2023, 15, 14329–14338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xu, S.; Yao, Y.; Chen, D.; Liu, L.; Xu, C.; Feng, Y.; Rui, X.; Yu, Y. Multi-component surface engineering of Na3V2(PO4)2O2F for low-temperature (−40 °C) sodium-ion batteries. Chem. Commun. 2022, 58, 10349–10352. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, K.; Lv, W.; Zhou, X.; Liang, Y.; Yang, F.; Chen, Z.; Zou, M.; Li, J.; Zhang, Y.; et al. Undefined: Materials insights into low-temperature performances of lithium-ion batteries. J. Power Sources 2015, 300, 29–40. [Google Scholar] [CrossRef]
- Mukai, K.; Inoue, T.; Kato, Y.; Shirai, S. Undefined: Superior low-temperature power and cycle performances of Na-ion battery over Li-ion battery. ACS Omega 2017, 2, 864–872. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Z.; Sun, Z.; Liu, P.; Jin, T.; Chen, X.; Li, H.; Lu, W.; Jiao, L.; Zhu, K.; et al. Inorganic electrolyte for low-temperature aqueous sodium ion batteries. Small 2022, 18, 2107662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, C.; Li, G. Fast-charging strategies for lithium-ion batteries: Advances and perspectives. ChemPlusChem 2022, 87, e202200155. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Xiao, J.; Ren, N.; Pan, B.; Chen, C.; Chen, C. Introducing a pseudocapacitive lithium storage mechanism into graphite by defect engineering for fast-charging lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 16279–16288. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeghan, S.M.N.; Lee, G. Boost charging lithium-ion battery using expanded graphite anode with enhanced performance. Mater. Lett. 2021, 299, 130077. [Google Scholar] [CrossRef]
- Li, M. Elevating the practical application of sodium-ion batteries through advanced characterization studies on cathodes. Energies 2023, 16, 8004. [Google Scholar] [CrossRef]
- He, M.; Mejdoubi, A.E.L.; Chartouni, D.; Morcrette, M.; Troendle, P.; Castiglioni, R. High power NVPF/HC-based sodium-ion batteries. J. Power Sources 2023, 588, 233741. [Google Scholar] [CrossRef]
- Rudola, A.; Sayers, R.; Wright, C.J.; Barker, J. Opportunities for moderate-range electric vehicles using sustainable sodium-ion batteries. Nat. Energy 2023, 8, 215–218. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus Prussian blue analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Okoshi, M.; Yamada, Y.; Yamada, A.; Nakai, H. Theoretical Analysis on De-Solvation of Lithium, Sodium, and Magnesium Cations to Organic Electrolyte Solvents. J. Electrochem. Soc. 2013, 160, A2160. [Google Scholar] [CrossRef]
- Komaba, S.; Takei, C.; Nakayama, T.; Ogata, A.; Yabuuchi, N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. Commun. 2010, 12, 355–358. [Google Scholar] [CrossRef]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680–3688. [Google Scholar] [CrossRef]
- Barker, J.; Heap, R. O3/p2 Mixed Phase Sodium-Containing Doped Layered Oxide Materials. U.S. Patent Application No. 17/045,660, 2021. Available online: https://patents.google.com/patent/US20210155501A1/en (accessed on 27 May 2021).
- Kim, H. Sodium-ion battery: Can it compete with Li-ion? ACS Mater. Au 2023, 3, 571–575. [Google Scholar] [CrossRef]
- Yan, G.; Mariyappan, S.; Rousse, G.; Jacquet, Q.; Deschamps, M.; David, R.; Mirvaux, B.; Freeland, J.W.; Tarascon, J.M. Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO4)2F3 material. Nat. Commun. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.F.; Baumann, M.; Binder, J.R.; Weil, M. On the environmental competitiveness of sodium-ion batteries under a full life cycle perspective-a cell-chemistry specific modelling approach. Sustain. Energy Fuels 2021, 5, 6414–6429. [Google Scholar] [CrossRef]
- Wickerts, S.; Arvidsson, R.; Nordelöf, A.; Svanström, M.; Johansson, P. Prospective life cycle assessment of sodium-ion batteries made from abundant elements. J. Ind. Ecol. 2024, 28, 116–129. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Mela, G.; Temporelli, A.; Brivio, E.; Girardi, P. Sodium-Ion Batteries with Ti1 Al1 TiC1.85 MXene as Negative Electrode: Life Cycle Assessment and Life Critical Resource Use Analysis. Sustainability 2022, 14, 5976. [Google Scholar] [CrossRef]
- Serrano-Luján, L.; Víctor-Román, S.; Toledo, C.; Sanahuja-Parejo, O.; Mansour, A.E.; Abad, J.; Amassian, A.; Benito, A.M.; Maser, W.K.; Urbina, A. Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl. Sci. 2019, 1, 179. [Google Scholar] [CrossRef]
- Rey, I.; Iturrondobeitia, M.; Akizu-Gardoki, O.; Minguez, R.; Lizundia, E. Environmental impact assessment of Na3V2(PO4)3 cathode production for sodium-ion batteries. Adv. Energy Sustain. Res. 2022, 3, 2200049. [Google Scholar] [CrossRef]
- Lai, X.; Chen, J.; Chen, Q.; Han, X.; Lu, L.; Dai, H.; Zheng, Y. Comprehensive assessment of carbon emissions and environmental impacts of sodium-ion batteries and lithium-ion batteries at the manufacturing stage. J. Clean. Prod. 2023, 423, 138674. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.S.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Zuo, W.; Zhou, S.; Zeng, G.; Zhang, B.; Zhang, H.; Huang, Z.; Zheng, L.; Xu, J.; et al. Sustainable layered cathode with suppressed phase transition for long-life sodium-ion batteries. Nat. Sustain. 2024, 7, 348–359. [Google Scholar] [CrossRef]
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Boorboor Ajdari, F.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Gomes Chagas, L.; Kuenzel, M.; Gonzalo, E.; Rojo, T.; Passerini, S.; Muñoz-Márquez, M.Á. Toward stable electrode/electrolyte interface of P2-layered oxide for rechargeable Na-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 28885–28893. [Google Scholar] [CrossRef]
- Lin, Z.; Xia, Q.; Wang, W.; Li, W.; Chou, S. Recent research progresses in ether- and ester-based electrolytes for sodium-ion batteries. InfoMat 2019, 1, 376–389. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-X.; Zhang, X.-L.; Zhao, X.-X.; Zhao, Y.-Y.; Aravindan, V.; Liu, Y.-H.; Geng, H.; Wu, X.-L. Electrode/electrolyte additives for practical sodium-ion batteries: A mini review. Inorg. Chem. Front. 2023, 10, 37–48. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Yang, C.; Xin, S.; Mai, L.; You, Y. Materials design for high-safety sodium-ion battery. Adv. Energy Mater. 2021, 11, 2000974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).