Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
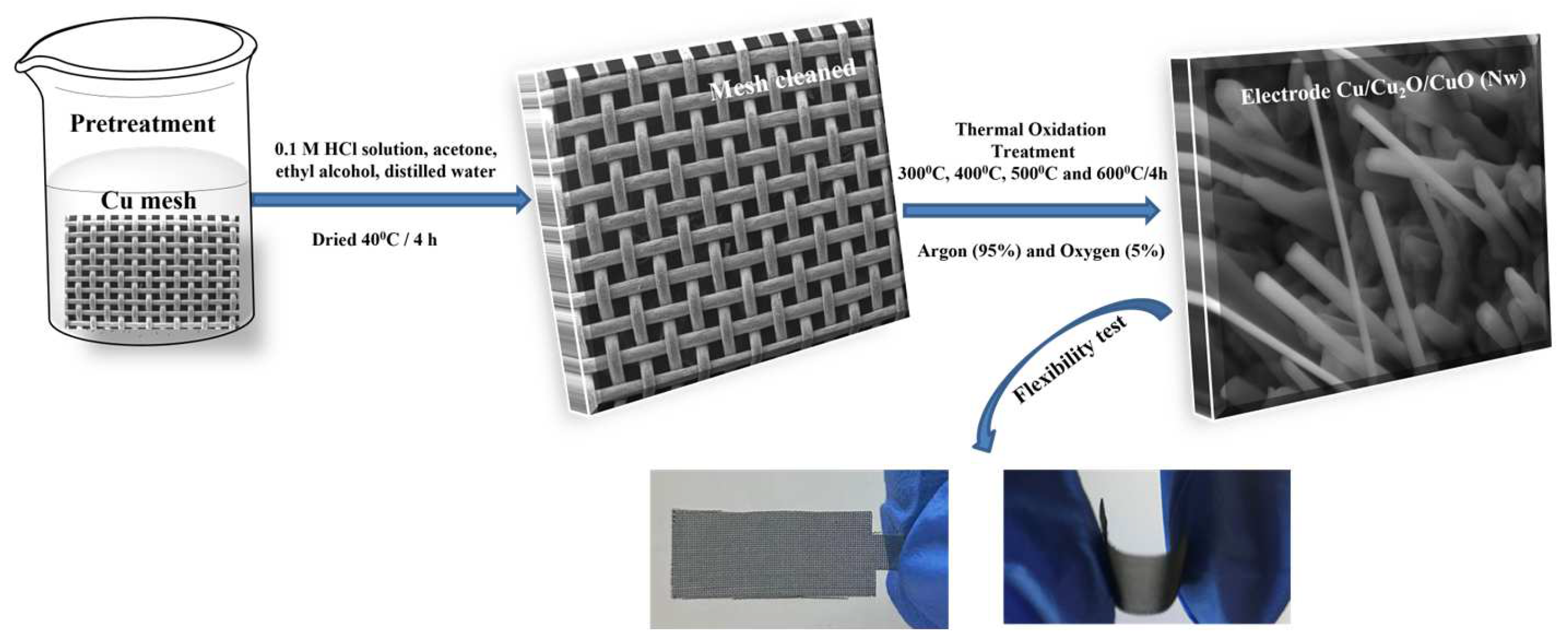
2.2. Synthesis Method of the Cu/Cu2O/CuO (Nw) Electrode
2.3. Cu/Cu2O/CuO (Nw) Electrodes Characterization
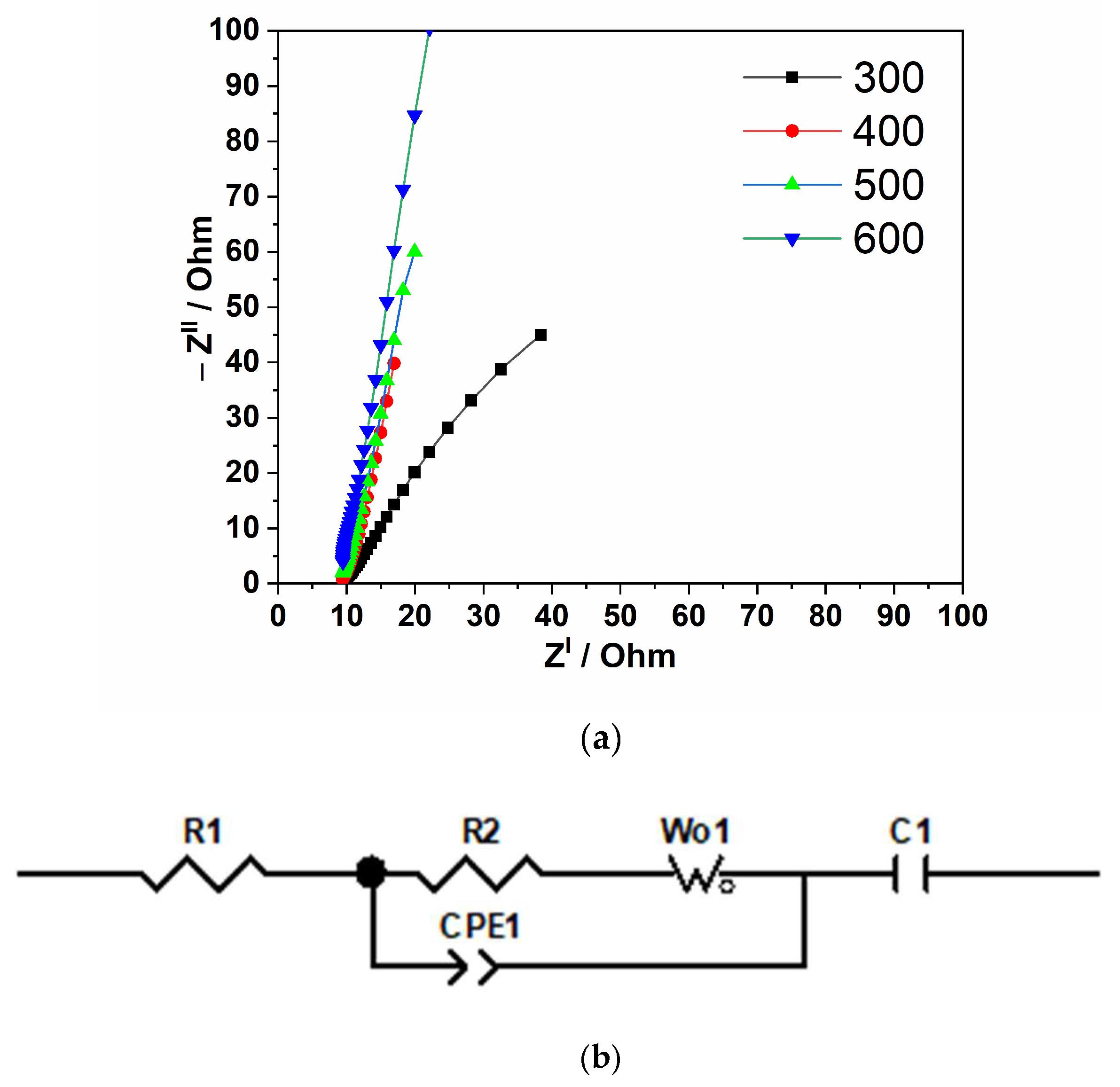
3. Results and Discussion
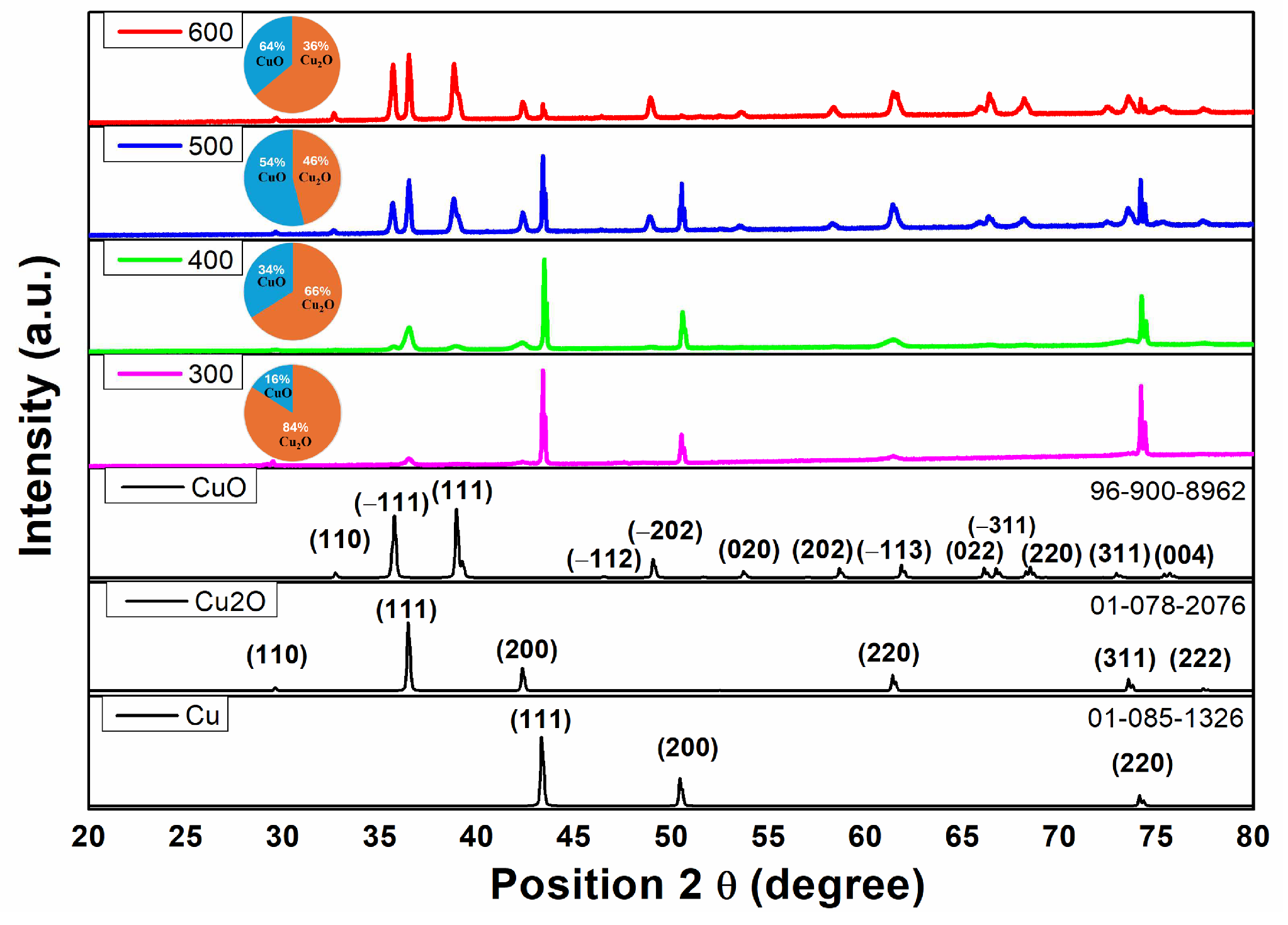
3.1. Structural and Morphological Investigations
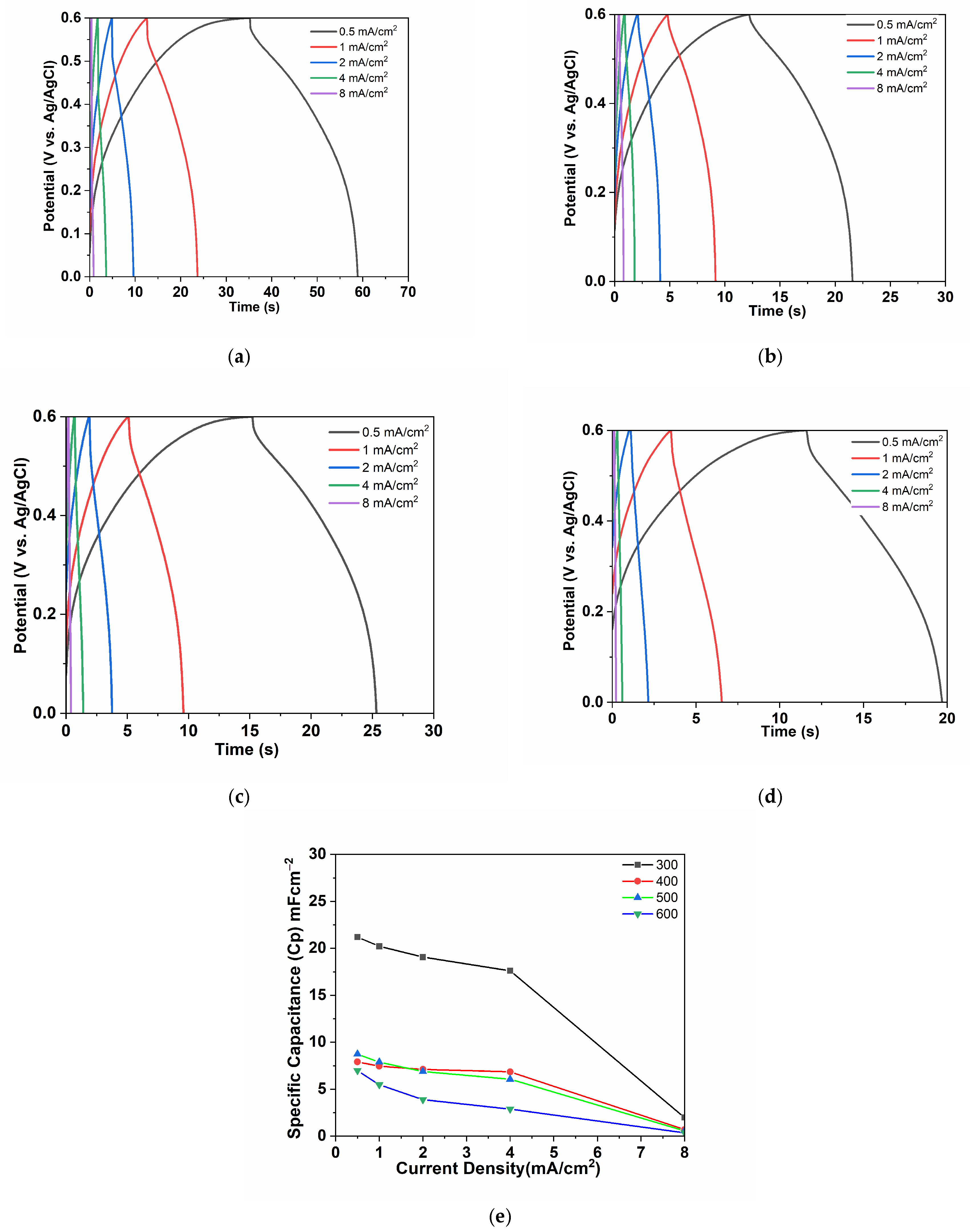
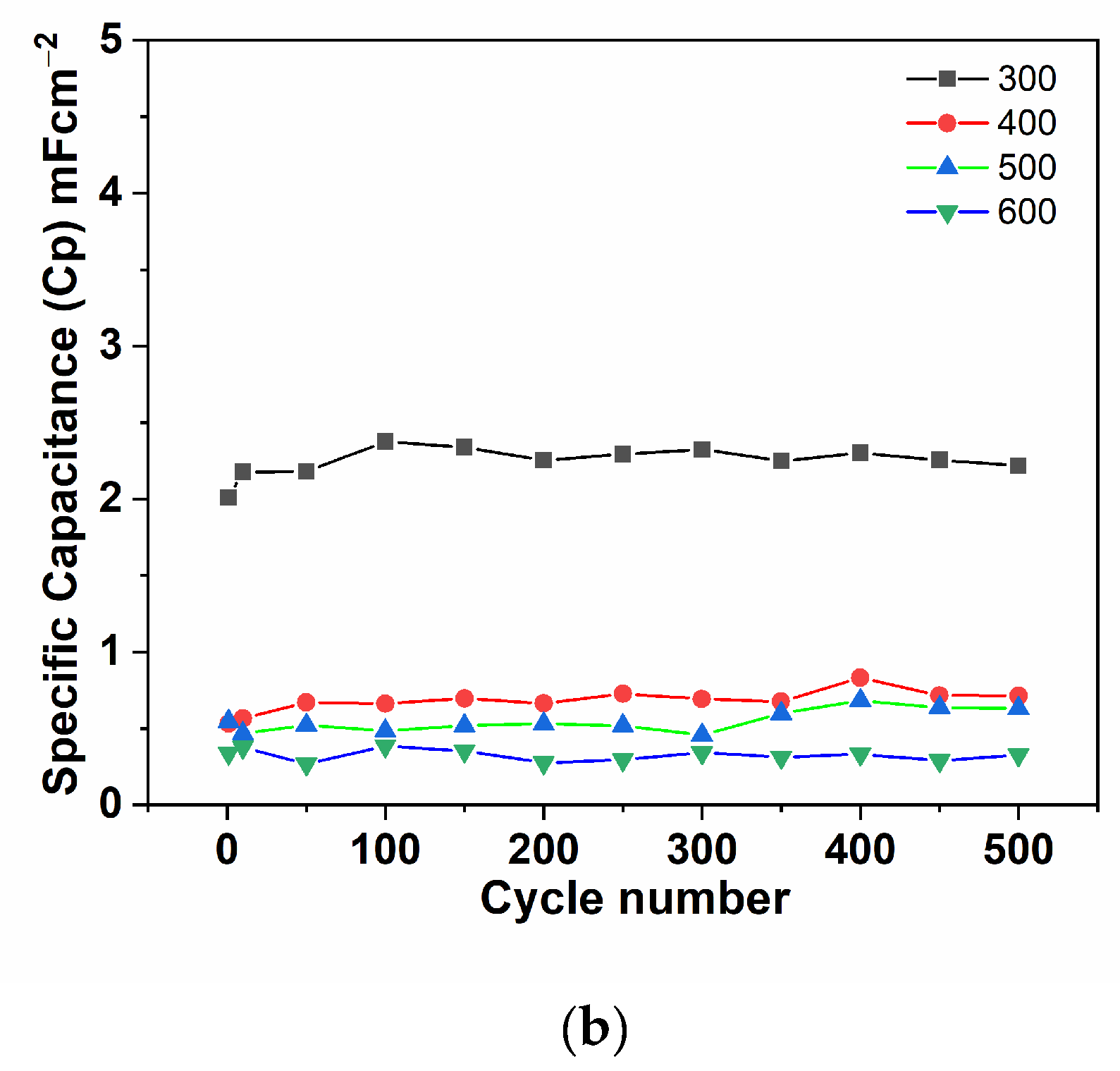
3.2. Electrochemical Performance of the Cu/Cu2O/CuO (Nw) Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Li, J.; Sun, H.; Guo, X.; Xu, J.; Zhang, H.; Zhang, X. In situ growth of Cu2O/CuO nanosheets on Cu coating carbon cloths as a binder-free electrode for asymmetric supercapacitors. Front. Chem. 2019, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Scherer, M.R.; Bower, C.; Andrew, P.; Ryhänen, T.; Steiner, U. A nanostructured electrochromic supercapacitor. Nano Lett. 2012, 12, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Kiruthika, S.; Kulkarni, G.U. Smart electrochromic supercapacitors made of metal mesh electrodes with polyaniline as charge storage indicator. Energy Technol. 2020, 8, 1901364. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ghodake, G.S.; Maile, N.C.; Kadam, A.A.; Sung Lee, D.; Fulari, V.J.; Shinde, S.K. Chemical synthesis of hierarchical NiCo2S4 nanosheets like nanostructure on flexible foil for a high performance supercapacitor. Sci. Rep. 2017, 7, 9764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Liu, Y.; Zhu, Y.; Zhuang, G.; Zheng, W.; Cai, Z.; Yang, P. Advanced binder-free electrodes based on CoMn2O4 @Co3O4 core/shell nanostructures for high-performance supercapacitors. RSC Adv. 2018, 8, 31594–31602. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wei, X.Q.; Deng, X.L.; Xu, X.J. Synthesis and property of spinel porous ZnMn2O4 microspheres. Appl. Surf. Sci. 2015, 356, 1127–1134. [Google Scholar] [CrossRef]
- Chiam, S.L.; Lim, H.N.; Hafiz, S.M.; Pandikumar, A.; Huang, N.M. Electrochemical performance of supercapacitor with stacked copper foils coated with graphene nanoplatelet. Sci. Rep. 2018, 8, 3093. [Google Scholar] [CrossRef] [PubMed]
- Aouini, S.; Bardaoui, A.; Santos, D.M.F.; Chtourou, R. Hydrothermal synthesis of CuMn2O4 spinel-coated stainless steel mesh as a supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2022, 33, 12726–12733. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Lee, I.; Ahmed, K.; Zhong, J.; Favors, Z.; Francisco, Z.; Mihrimah, O.; Ozkan, C.S. Hydrous ruthenium oxide nanoparticles anchored to graphene and carbon nanotube hybrid foam for supercapacitors. Sci. Rep. 2014, 4, 4452. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Huang, R.; Xia, X.; Ye, H.; Jiao, X.; Wang, L.; Lei, W.; Hao, Q. Hierarchical structure electrodes of NiO ultrathin nanosheets anchored to NiCo2O4 on carbon cloth with excellent cycle stability for asymmetric supercapacitors. Chem. Eng. J. 2019, 355, 416–427. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, I.H.; Kim, S.I.; Jang, J.H. Nickel hydroxide supercapacitor with a theoretical capacitance and high rate capability based on hollow dendritic 3D-nickel current collectors. Chem. Asian J. 2017, 12, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Li, S.; Chen, P.; Dong, K.; Wu, Q.; Wu, T. Mesoporous Cubic Nanocages Assembled by Coupled Monolayers With 100% Theoretical Capacity and Robust Cycling. ACS Cent. Sci. 2024, 10, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, X.; Mu, H.; Tian, Q.; Li, Q.; Liu, Y.; Zhang, X.; Zhao, Z.; Su, X. Construction of hierarchical sea urchin-like manganese substituted nickel cobaltite@tricobalt tetraoxide core-shell microspheres on nickel foam as binder-free electrodes for high performance supercapacitors. J. Colloid Interface Sci. 2021, 596, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.; Zhang, L.L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; You, W.; Yu, J. Core-shell nitrogen-doped carbon hollow spheres/Co3O4 nanosheets as advanced electrode for high performance supercapacitor. Small 2018, 14, 1702407. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qu, G.; Chen, M.; Ma, W.; Li, W.; Tang, Y. Effective NaBH4- exfoliated ultrathin multilayer Co(OH)2 nanosheets arrays and sulfidation for energy storage. Nanotechnology 2018, 29, 295403. [Google Scholar] [CrossRef]
- Foo, C.Y.; Sumboja, A.; Tan, D.J.H.; Wang, J.; Lee, P.S. Flexible and highly scalable V2O5-rGO electrodes in an organic electrolyte for supercapacitor devices. Adv. Energy Mater. 2014, 4, 1400236. [Google Scholar] [CrossRef]
- Bu, I.Y.Y.; Huang, R. Fabrication of CuO-decorated reduced graphene oxide nanosheets for supercapacitor applications. Ceram. Int. 2017, 43, 45–50. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, Q.; Javed, M.S.; Liu, Q.; Xu, W.; Hu, C.; Wei, D. Ultra-fine CuO nanoparticles embedded in three-dimensional graphene network nanostructure for high-performance flexible supercapacitors. Electrochim. Acta 2017, 234, 63–70. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Jiang, D.; Jia, D.; Liu, J. Hierarchical CuO nanorod arrays in situ generated on three-dimensional copper foam via cyclic voltammetry oxidization for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 10474–10483. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, Z.; Chun, A.; Yoo, J.; Diao, G.; Kim, Y.S.; Piao, Y. Rose rock-shaped nano Cu2O anchored graphene for high-performance supercapacitors via solvothermal route. J. Power Sources 2016, 318, 66–75. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Liu, X.; Yuen, M.M.F.; Fu, X.Z.; Yang, Y.; Sun, R.; Wong, C.P. 3D porous Cu@Cu2O films supported Pd nanoparticles for glucose electrocatalytic oxidation. Electrochim. Acta 2017, 248, 299–306. [Google Scholar] [CrossRef]
- Vidyadharan, B.; Misnon, I.I.; Ismail, J.; Yusoff, M.M.; Jose, R. High performance asymmetric supercapacitors using electrospun copper oxide nanowires anode. J. Alloys Compd. 2015, 633, 22–30. [Google Scholar] [CrossRef]
- Bandas, C.; Nicolaescu, M.; Popescu, M.I.; Orha, C.; Căprărescu, S.; Lazau, C. One-step microwave-assisted hydrothermal preparation of Zn-ZnO(Nw)-rGO electrodes for supercapacitor applications. Materials 2023, 16, 4536. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, A.; Kaplan, K.; Bedir, M. Copper oxide coated stainless steel mesh for flexible electrodes. J. Phys. Chem. Solids 2021, 150, 109824. [Google Scholar] [CrossRef]
- Nicolaescu, M.; Vajda, M.; Lazau, C.; Orha, C.; Bandas, C.; Serban, V.A.; Codrean, C. Fabrication of flexible supercapacitor electrode materials by chemical oxidation of iron-based amorphous ribbons. Materials 2023, 16, 2820. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Shen, C.M.; Yang, H.T.; Li, H.L.; Gao, H.J. Controlled synthesis of highly ordered CuO nanowire arrays by template-based sol-gel route. Trans. Nonferrous Met. Soc. China 2007, 17, 783. [Google Scholar] [CrossRef]
- Chen, J.T.; Zhang, F.; Wang, J.; Zhang, G.A.; Miao, B.B.; Fan, X.Y.; Yan, D.; Yan, P.X.J. CuO nanowires synthesized by thermal oxidation route. J. Alloys Compd. 2008, 454, 268–273. [Google Scholar] [CrossRef]
- Yao, W.T.; Yu, S.H.; Zhou, Y.; Jiang, J.; Wu, Q.S.; Zhang, L.; Jiang, J. Formation of uniform CuO manorods by spontaneous aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles by a solid−liquid phase arc discharge process. J. Phys. Chem. B 2005, 109, 14011–14016. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Chen, C.N.; Hao, L.Y.; Hu, Y.; Chen, Z.Y. In-situ preparation of 1D CuO nanostructures using Cu2(OH)2CO3 nanoribbons as precursor for sacrifice-template via heat-treatment. J. Cryst. Growth 2004, 263, 473. [Google Scholar] [CrossRef]
- Gou, X.; Wang, G.; Yang, J.; Park, J.; Wexler, D. Chemical synthesis, characterisation and gas sensing performance of copper oxide nanoribbons. J. Mater. Chem. 2008, 18, 965–969. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Chen, G.; Zhang, H.; Chen, Y. Synthesis of Cu2O nano-whiskers by a novel wet-chemical route. Mater. Lett. 2008, 62, 886–888. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Y.; Li, M.; Li, L.; Dong, L. In situ synthesis and assembly of copper oxide nanocrystals on copper foil via a mild hydrothermal process. J. Mater. Chem. 2006, 16, 192–198. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Hahn, Y.B.; Kim, D.H.; Lee, K.S.; Jang, J.S.; Lee, J.S. Flower-shaped CuO nanostructures: Structural, photocatalytic and XANES studies. Catal. Commun. 2008, 10, 11–16. [Google Scholar] [CrossRef]
- Volanti, D.P.; Keyson, D.; Cavalcante, L.S.; Simões, A.Z.; Joya, M.R.; Longo, E.; Varela, J.A.; Pizani, P.S.; Souza, A.G. Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave. J. Alloys Compd. 2008, 459, 537–542. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Tao, Z.; Chen, J. CuO particles and plates: Synthesis and gas-sensor application. Mater. Res. Bull. 2008, 43, 2380–2385. [Google Scholar] [CrossRef]
- Chen, A.; Long, H.; Li, X.; Li, Y.; Yang, G.; Lu, P. Controlled growth and characteristics of single-phase Cu2O and CuO films by pulsed laser deposition. Vacuum 2009, 83, 927–930. [Google Scholar] [CrossRef]
- Jia, W.; Reitz, E.; Shimpi, P.; Rodriguez, E.G.; Gao, P.-X.; Lei, Y. Spherical CuO synthesized by a simple hydrothermal reaction: Concentration-dependent size and its electrocatalytic application. Mater. Res. Bull. 2009, 44, 1681–1686. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Fu, S.-Y.; Zhu, L.-P.; Li, Y.-Q.; Yang, G. Controlled synthesis and characterization of CuO nanostructures through a facile hydrothermal route in the presence of sodium citrate. Eur. J. Inorg. Chem. 2007, 14, 1966–1971. [Google Scholar] [CrossRef]
- Saadaldin, N.; Alsloum, M.N.; Hussain, N. Preparing of copper oxides thin films by chemical bath deposition (CBD) for using in environmental application. Energy Procedia 2015, 74, 1459–1465. [Google Scholar] [CrossRef][Green Version]
- Mallick, P.; Sahu, S. Structure, microstructure and optical absorption analysis of CuO nanoparticles synthesized by sol-gel route. Nanosci. Nanotechnol. 2012, 2, 71–74. [Google Scholar] [CrossRef]
- Li, C.; Yin, Y.; Hou, H.; Fan, N.; Yuan, F.; Shi, Y.; Meng, Q. Preparation and characterization of Cu(OH)2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid. State Commun. 2010, 150, 585–589. [Google Scholar] [CrossRef]
- Saravanan, V.; Shankar, P.; Mani, G.K.; Bosco, J.; Rayappan, B. Growth and characterization of spray pyrolysis deposited copper oxide thin films: Influence of substrate and annealing temperatures. J. Anal. Appl. Pyrol. 2015, 111, 272–277. [Google Scholar] [CrossRef]
- Wang, R.; Sui, Y.; Huang, S.; Pu, Y.; Cao, P. High-performance flexible all-solid-state asymmetric supercapacitors from nanostructured electrodes prepared by oxidation-assisted dealloying protocol. Chem. Eng. J. 2018, 331, 527–535. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaikh, A.; Dusane, R.O.; Parida, S. Copper oxide nanosheets and nanowires grown by one-step linear sweep voltammetry for supercapacitor application. J. Energy Storage 2020, 31, 101631. [Google Scholar] [CrossRef]
- Toboonsung, B.; Singjai, P. Formation of CuO nanorods and their bundles by an electrochemical dissolution and depo-sition process. J. Alloys Compd. 2011, 509, 4132–4137. [Google Scholar] [CrossRef]
- Wang, W.; Zhan, Y.; Wang, G. One-step, solid-state reaction to the synthesis of copper oxide nanorods in the presence of a suitable surfactant. Chem. Commun. 2001, 8, 727–728. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D. Thermal oxidation strategy towards porous metal oxide hollow architectures. Adv. Mater. 2008, 20, 2622–2627. [Google Scholar] [CrossRef]
- Singh, D.P.; Ali, N. Synthesis of TiO2 and CuO nanotubes and nanowires. Sci. Adv. Mater. 2010, 2, 295–335. [Google Scholar] [CrossRef]
- Xu, C.H.; Woo, C.H.; Shi, S.Q. Formation of CuO nanowires on Cu foil. Chem. Phys. Lett. 2004, 399, 62–66. [Google Scholar] [CrossRef]
- Liang, J.; Kishi, N.; Soga, T.; Jimbo, T. Cross-sectional characterization of cupric oxide nanowires grown by thermal oxidation of copper foils. App Surf. Sci. 2010, 257, 62–66. [Google Scholar] [CrossRef]
- Goncalves, A.M.B.; Campos, L.C.; Ferlauto, A.S.; Lacerda, R.G. On the growth and electrical characterization of CuO nanowires by thermal oxidation. J. Appl. Phys. 2009, 106, 034303. [Google Scholar] [CrossRef]
- Nkhaili, L.; Narjis, A.; Agdad, A.; Tchenka, A.; El Kissani, A.; Outzourhit, A.; Oueriagli, A. A simple method to control the growth of copper oxide nanowires for solar cells and catalytic applications. Adv. Cond. Matter Phys. 2020, 2020, 5470817. [Google Scholar] [CrossRef]
- Castrejón-Sánchez, V.-H.; Solís, A.C.; López, R.; Encarnación-Gomez, C.; Morales, F.M.; Vargas, O.S.; Mastache-Mastache, J.E.; Sánchez, G.V. Thermal oxidation of copper over a broad temperature range: Towards the formation of cupric oxide (CuO). Mater. Res. Express 2019, 6, 075909. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the ‘Debye-Scherrer Equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Kosicek, M.; Zavasnik, J.; Baranov, O.; Batic, S.B.; Cvelbar, U. Understanding the Growth of Copper Oxide Nanowires and Layers by Thermal Oxidation over a Broad Temperature Range at Atmospheric Pressure. Cryst. Growth Des. 2022, 22, 6656–6666. [Google Scholar] [CrossRef]
- Lamberti, A.; Fontana, M.; Bianco, S.; Tresso, E. Flexible solid-state CuxO-based pseudo-supercapacitor by thermal oxidation of copper foils. Int. J. Hydrogen Energy 2016, 41, 11700–11708. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers. Batteries 2022, 9, 19. [Google Scholar] [CrossRef]
- Schoetz, T.; Gordon, L.W.; Ivanov, S.; Bund, A.; Mandler, D.; Messinger, R.J. Disentangling faradaic, pseudocapacitive, and capacitive charge storage: A tutorial for the characterization of batteries, supercapacitors, and hybrid systems. Electrochim. Acta 2022, 412, 140072. [Google Scholar] [CrossRef]
- Orgen, S.B.; Balela, M.D.L. Effect of Reaction Time on the Morphology of CuO Nanostructured Electrode for Pseudocapacitor Application. J. Phys. Conf. Ser. 2021, 1974, 012006. [Google Scholar] [CrossRef]
- Sun, B.; Yao, M.; Chen, Y.; Tang, X.; Hu, W.; Pillai, S.C. Facile fabrication of flower-like γ-Fe2O3 @PPy from iron rust for high-performing asymmetric supercapacitors. J. Alloy Compound. 2022, 922, 166055. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.; Sun, X.; Zhang, J.; Hou, L.; Li, L.; Yang, S.; Yuan, C. One-Dimensional Nanostructured Pseudocapacitive Materials: Design, Synthesis and Applications in Supercapacitors. Batter. Supercaps 2019, 2, 820–841. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Chodankar, N.R.; Cho, J.Y.; Gomez-Romero, P.; Park, C.; Lokhande, C.D. Low-cost flexible supercapacitors with high-energy density based on nanostructured MnO2 and Fe2O3 thin films directly fabricated onto stainless steel. Sci. Rep. 2015, 5, 12454. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-Based Quantum Dots for Supercapacitors: Recent Advances and Future Challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, S.G.; Shaikh, A.V.; Shinde, U.P.; Hiremath, P.; Naik, N. Copper oxide-based high-performance symmetric flexible supercapacitor: Potentiodynamic deposition. J. Mater. Sci. Mater. Electron. 2023, 34, 1361. [Google Scholar] [CrossRef]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Gomez-Romero, P.; Kim, S.; Fulari, V.J. Influence of Mn incorporation on the supercapacitive properties of hybrid CuO/Cu(OH)2 electrodes. RSC Adv. 2015, 5, 30478–30484. [Google Scholar] [CrossRef]
- Dey, R.S.; Hjuler, H.A.; Chi, Q. Approaching the theoretical capacitance of graphene through copper foam integrated three-dimensional graphene networks. J. Mater. Chem. A 2015, 3, 6324–6329. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Jiang, L.; Li, X.; Cao, J.; Wang, Q.; Wang, A.; Li, X.; Qu, L.; Lu, Y. High–performance 3D CuO/Cu flowers supercapacitor electrodes by femtosecond laser enhanced electrochemical anodization. Electrochim. Acta 2019, 293, 273–282. [Google Scholar] [CrossRef]
- He, D.; Wang, G.; Liu, G.; Suo, H.; Zhao, C. Construction of Leaf-Like CuO-Cu2O Nanocomposite on Copper Foam for High-Performance Supercapacitors. Dalton Trans. 2017, 46, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lau, K.S.; Chia, C.H.; Umar, A.A.; Mohamed, M.A.; Buyong, M.R.; Hamzah, A.A. Fabrication and Characterization of Supercapacitor Electrode with Amorphous CuO/Crystalline Cu2O/Porous Cu Composite Based on Copper Foil Substrate. 2023; book chapter. [Google Scholar] [CrossRef]
- Sepahvand, S.; Ghasemi, S.; Sanaee, Z. Electric Field Enhanced Synthesis of Copper Hydroxide Nanostructures for Supercapacitor Application. NANO Brief Rep. Rev. 2017, 12, 1750010. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, X.; Song, M.; Wu, Z.; Li, Z.; Wang, X.; Zhu, M.; Liu, D.; Zhao, J. Facile fabrication of Cu2O/Cu columnar array electrode through dealloying and in situ oxidation for supercapacitor applications. J. Solid State Electrochem. 2020, 24, 1313–1324. [Google Scholar] [CrossRef]
- Yao, P.; Li, C.; Yu, J.; Zhang, S.; Zhang, M.; Liu, H.; Ji, M.; Cong, G.; Zhang, T.; Zhu, C. High performance flexible energy storage device based on copper foam supported NiMoO4 nanosheets-CNTs-CuO nanowires composites with core–shell holey nanostructure. J. Mater. Sci. Technol. 2021, 85, 87–94. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, M.D.; Lohar, G.M.; Jadhav, S.T.; Fulari, V.J. Supercapacitive properties of CuO thin films using modified SILAR method. Ionics 2016, 23, 1259–1266. [Google Scholar] [CrossRef]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Crystallite Size/nm | |||
|---|---|---|---|---|
| 300 °C | 400 °C | 500 °C | 600 °C | |
| Cu2O | 23.4 | 26.4 | 73.4 | 110.2 |
| CuO | 13.8 | 14.2 | 41.7 | 62.4 |
| Nanowire Average Width/nm | ||||
| CuO | 29.13 | 56.85 | 270.55 | 437.68 |
| Materials | Structure | Specific Capacitance | Scan Rate | Specific Capacitance | Current Density | References |
|---|---|---|---|---|---|---|
| 3D CuO/Cu flowers | Electrode | - | - | 3348.57 mF cm−2 | 1 mA cm−2 | [69] |
| CuO-Cu2O | Nanocomposite Electrode | 1954 mF cm−2 | 2 mV s−1 | - | - | [70] |
| Cu2O/CuO@ Cu-CCs | Electrode | - | - | 1710 mF cm−2 | 10 mA cm−2 | [1] |
| Cu2O/CuO | Composite Electrode | - | - | 1280 mF cm−2 | 40 mA cm−2 | [71] |
| CuO | Nanostructured Electrode | 231 mF cm−2 | 2 mV s−1 | - | - | [61] |
| Copper hydroxide nanostructures | Electrode | 42 mF cm−2 | 20 mV s−1 | - | - | [72] |
| Cu2O/Cu columnar array | Electrode | 128.6 mF cm−2 | 5 mV s−1 | - | - | [73] |
| NiMoO4 NSs-CNTs-CuO NWAs/Cu foam | Electrode | - | - | 23.40 F cm−2 | 2 mA cm−2 | [74] |
| Cu/Cu2O/CuO (Nw) | Electrode | 26.158 mF cm−2 | 5 mV s−1 | 21.198 mF cm−2 | 0.5 mA cm−2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morariu, M.-I.; Nicolaescu, M.; Hulka, I.; Duţeanu, N.; Orha, C.; Lăzău, C.; Bandas, C. Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications. Batteries 2024, 10, 246. https://doi.org/10.3390/batteries10070246
Morariu M-I, Nicolaescu M, Hulka I, Duţeanu N, Orha C, Lăzău C, Bandas C. Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications. Batteries. 2024; 10(7):246. https://doi.org/10.3390/batteries10070246
Chicago/Turabian StyleMorariu (Popescu), Mina-Ionela, Mircea Nicolaescu, Iosif Hulka, Narcis Duţeanu, Corina Orha, Carmen Lăzău, and Cornelia Bandas. 2024. "Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications" Batteries 10, no. 7: 246. https://doi.org/10.3390/batteries10070246
APA StyleMorariu, M.-I., Nicolaescu, M., Hulka, I., Duţeanu, N., Orha, C., Lăzău, C., & Bandas, C. (2024). Fabrication of Cu2O/CuO Nanowires by One-Step Thermal Oxidation of Flexible Copper Mesh for Supercapacitor Applications. Batteries, 10(7), 246. https://doi.org/10.3390/batteries10070246









