Quantitative Analysis of Lithium-Ion Battery Eruption Behavior in Thermal Runaway
Abstract
1. Introduction
2. Materials and Methods
2.1. Battery Samples
2.2. Experimental Setup
2.3. Experimental Setup Calculation of Flame Area and Velocity
3. Results and Discussion
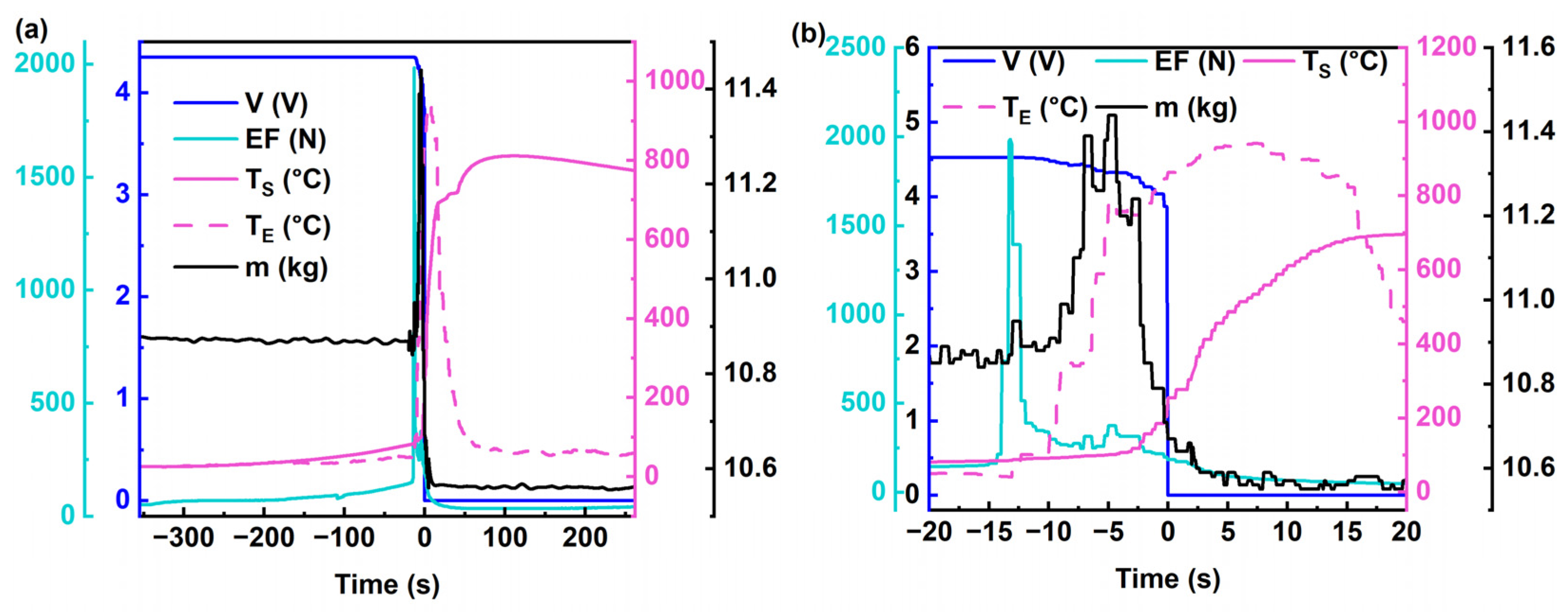
3.1. Temperature and Force Dynamics during TR
- At the −110 s mark: Fluctuations in expansion force are observed, decreasing from 92 N to 77 N;
- At the −15 s mark: Expansion force initiates a sharp ascent, reaching a peak value of 1980 N at −13.5 s, representing a 12-fold increase within a duration of 2.5 s. Figure A1 shows the rate of change in the expansion force;
- At the −13 s mark: Expansion force peaks and begins a rapid decline, registering at 1395 N, accompanied by a rapid rise in values and a slight increase in mass;
- At the −12 s mark: Voltage begins to decline, coinciding with the first appearance of sparks resembling those depicted in Figure 4a;
- At the −5 s mark: Mass (m) reaches its maximum value of 11.44 kg; within the −13 s to −5 s timeframe, exhibits the highest rate of temperature rise, reaching 93 °C/s;
- At the 0 s mark: Voltage reaches zero;
- At the 1.5 s mark: Intense eruption sparks are observed, as depicted in Figure 4a;
- At the 7 s mark: attains its maximum value of 941.2 °C, as depicted in Figure 3b;
- At the 11 s mark: Mass (m) reaches its minimum value of 10.55 kg;
- At the 100 s mark: reaches its maximum value of 810 °C;
- Subsequently, the temperatures , , and gradually decrease, marking the cessation of thermal runaway.
- 1.
- Internal Chemical Reactions
- 2.
- Heat Release
- 3.
- Material Expansion
- 4.
- Structural Damage
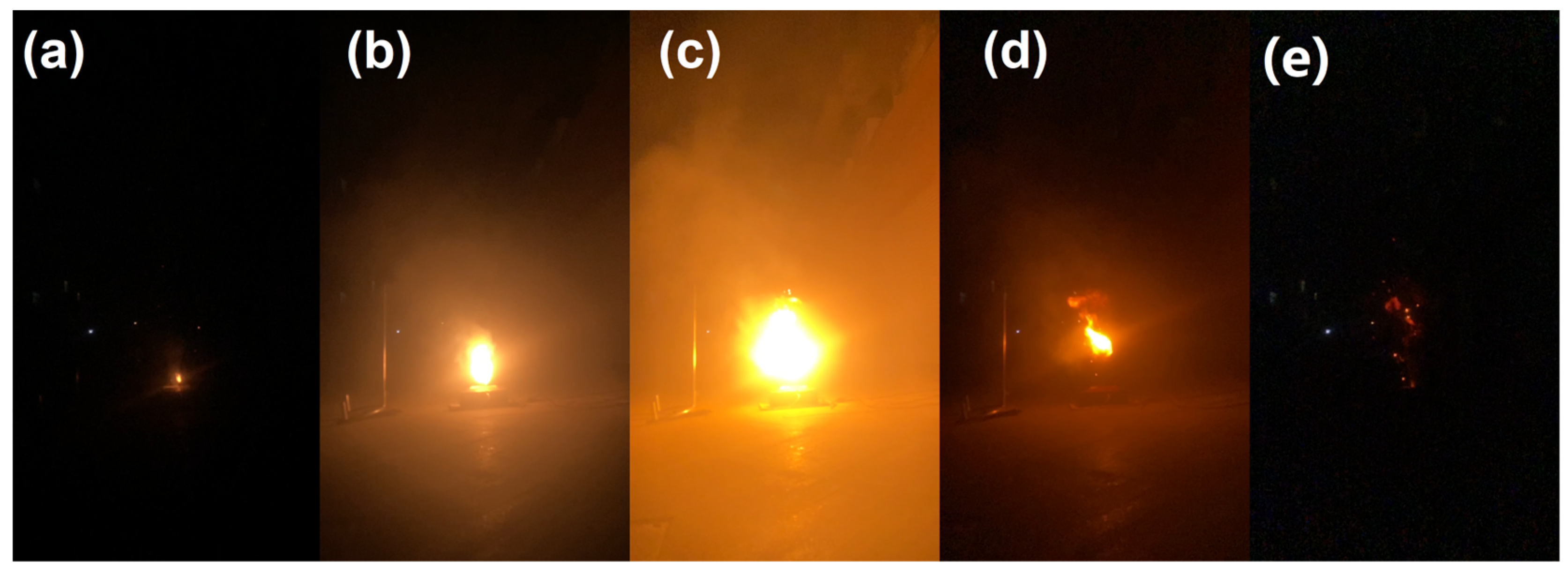
3.2. Characteristics of Eruption Flames

4. Conclusions
- From the onset of heating by the heating plate to −20 s, the rate of change in the expansion force remained below 0.3 N/s. At −110 s, a sudden decrease in the expansion force was observed, with a rate of change falling below −70 N/s. The maximum rate of change in the expansion force reached approximately 6100 N/s.
- The point at which the expansion force reached its maximum value and sharply declined, corresponding to the occurrence of the first jetting of sparks (as depicted in behavior shown in Figure 4a), was at the time point −(11.5 ± 1.5) s, concurrent with a 0.01 V decrease in voltage.
- The most intense jetting of sparks occurred at the time point of 1.4 ± 0.3 s, followed by the time period of 0.02 to 0.05 s, where the explosion flame reached its maximum radius, with an average rate of change exceeding 17 m/s. The distance of the explosion center from the safety valve height varied randomly in each explosion, yet it typically fell within a range of 0.1 to 0.5 m.
- From the onset of the first occurrence of spark jetting to the cessation of the jetting behavior, the phenomena depicted in Figure 4a,b manifested in multiple alternating sequences. The ejected sparks, which represent the combustion of solid particulates, persisted throughout the entire ejection process. Hence, when calculating the flammability limits of battery ejecta, the presence of solid particulates cannot be disregarded. Observations from video footage indicate that explosive phenomena are initiated by the ignition of the ejected sparks, which ignite the combustible gases expelled during the jetting process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| m | Mass, kg |
| Cell side surface center temperature, °C | |
| Cell jet zone temperatures near the cell safety valve, °C | |
| Heating plate temperature, °C | |
| DMC | Dimethyl carbonate, C3H6O3 |
| EMC | Methyl ethyl carbonate, C4H8O3 |
| EV | Electric vehicle |
| SOC | State of charge |
| LIB | Lithium-ion battery |
| BMS | Battery management system |
| BTMS | Battery thermal management system |
| EF | Expansion force, N |
| CC–CV | Constant current–constant voltage |
| SEI | Solid electrolyte interphase |
Appendix A


References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Xu, C.; Jin, C.; Li, K.; Du, Z.; Wang, Q.; Ouyang, M.; Feng, X. Dynamic Thermophysical Modeling of Thermal Runaway Propagation and Parametric Sensitivity Analysis for Large Format Lithium-Ion Battery Modules. J. Power Sources 2022, 520, 230724. [Google Scholar] [CrossRef]
- Wei, N.; Li, M. Experimental Study of Thermal Runaway Process of 50 Ah Prismatic Nickel-Rich Battery. Energies 2023, 16, 5967. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Q.; Wang, G.; Lu, P.; Zhao, M.; Bao, W. A Review on Research Status and Key Technologies of Battery Thermal Management and Its Enhanced Safety. Int. J. Energy Res. 2018, 42, 4008–4033. [Google Scholar] [CrossRef]
- Ding, S.; Dong, C.; Zhao, T.; Koh, L.; Bai, X.; Luo, J. A Meta-Learning Based Multimodal Neural Network for Multistep Ahead Battery Thermal Runaway Forecasting. IEEE Trans. Ind. Inform. 2021, 17, 4503–4511. [Google Scholar] [CrossRef]
- Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. A 3D Thermal Runaway Propagation Model for a Large Format Lithium Ion Battery Module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Li, H.; Gao, Q.; Wang, Y. Experimental Investigation of the Thermal Runaway Propagation Characteristics and Thermal Failure Prediction Parameters of Six-Cell Lithium-Ion Battery Modules. Energies 2023, 16, 5172. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, W.; Gao, Z.; Zhang, B.; Ouyang, M. Experimental Study on the Cell-Jet Temperatures of Abused Prismatic Ni-Rich Automotive Batteries under Medium and High States of Charge. Appl. Therm. Eng. 2022, 202, 117859. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Modeling Li-Ion Battery Temperature and Expansion Force during the Early Stages of Thermal Runaway Triggered by Internal Shorts. J. Electrochem. Soc. 2019, 166, A2431–A2443. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, Y.; Cheng, L.; Wu, Y.; Feng, X.; Lu, L.; Ouyang, M. Thermal Oxidation Characteristics for Smoke Particles from an Abused Prismatic Li(Ni0.6Co0.2Mn0.2)O2 Battery. J. Energy Storage 2021, 39, 102639. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Lei, N.; Zhou, F.; Sun, C. An Experimental Study on the Mechanical Characteristics of Li-ion Battery during Overcharge-induced Thermal Runaway. Int. J. Energy Res. 2021, 45, 19985–20000. [Google Scholar] [CrossRef]
- Self, J.; Aiken, C.P.; Petibon, R.; Dahn, J.R. Survey of Gas Expansion in Li-Ion NMC Pouch Cells. J. Electrochem. Soc. 2015, 162, A796–A802. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal Runaway of Commercial 18650 Li-Ion Batteries with LFP and NCA Cathodes—Impact of State of Charge and Overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Ostanek, J.K.; Li, W.; Mukherjee, P.P.; Crompton, K.R.; Hacker, C. Simulating Onset and Evolution of Thermal Runaway in Li-Ion Cells Using a Coupled Thermal and Venting Model. Appl. Energy 2020, 268, 114972. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Wang, L.; Lu, L.; Ren, D.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748–A3765. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Shi, C.; Wang, Y.; Li, Y.; Ouyang, M. A Fitting Method to Characterize the Gaseous Venting Behavior of Lithium–Ion Batteries in a Sealed Chamber during Thermal Runaway. Energies 2023, 16, 7874. [Google Scholar] [CrossRef]
- Coman, P.T.; Mátéfi-Tempfli, S.; Veje, C.T.; White, R.E. Modeling Vaporization, Gas Generation and Venting in Li-Ion Battery Cells with a Dimethyl Carbonate Electrolyte. J. Electrochem. Soc. 2017, 164, A1858–A1865. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, B.; Zhao, Q.; Wang, J.; An, W.; Zhai, H. External Heating-Induced Thermal Runaway and Gas Venting Characteristics of Polymer Lithium-Ion Cells with LiNixCoyMnzO2 Cathode. Process Saf. Environ. Prot. 2023, 174, 745–755. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, L.; Woolley, R.; Zhang, Y. Investigation of Ignition Process from Visible to Infrared by a High Speed Colour Camera. Fuel 2016, 185, 500–507. [Google Scholar] [CrossRef]
- Huang, H.W.; Zhang, Y. Digital Colour Image Processing Based Measurement of Premixed CH4+air and C2H4+air Flame Chemiluminescence. Fuel 2011, 90, 48–53. [Google Scholar] [CrossRef]
- Qian, F.; Wang, H.; Li, M.; Li, C.; Shen, H.; Wang, J.; Li, Y.; Ouyang, M. Thermal Runaway Vent Gases from High-Capacity Energy Storage LiFePO4 Lithium Iron. Energies 2023, 16, 3485. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability Characteristics of the Battery Vent Gas: A Case of NCA and LFP Lithium-Ion Batteries during External Heating Abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. Thermal Runaway of Large Automotive Li-Ion Batteries. RSC Adv. 2018, 8, 40172–40186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lv, Y.; Zhou, Z.; Cao, F. A Novel Deep Learning Algorithm for Incomplete Face Recognition: Low-Rank-Recovery Network. Neural Netw. 2017, 94, 115–124. [Google Scholar] [CrossRef]
- Wei, N.; Li, M. Experimental Study of Thermal Runaway Process of 256 Ah Prismatic Nickel-Rich Battery. Front. Energy Res. 2023, 11, 1230429. [Google Scholar] [CrossRef]
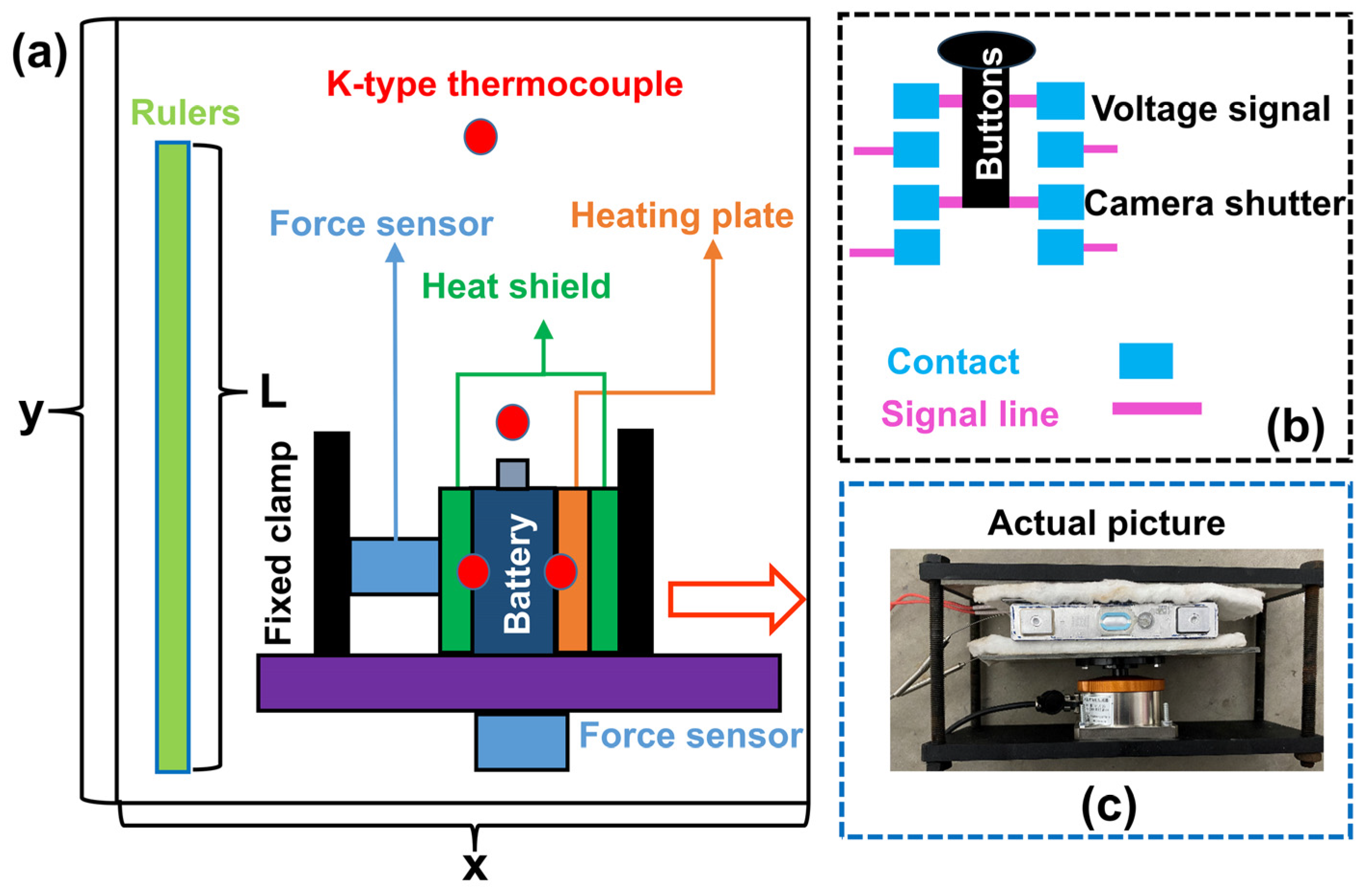



| Items | Parameter | Remarks |
|---|---|---|
| Cell mass (g) | 926 ± 15 | / |
| Width (mm) × Thickness (mm) × Height (mm) | 148.24 × 26.66 × 102.8 | / |
| Rated Capacity (Ah) | 58 | Standard Discharge * |
| 58 | 1/3C Discharge | |
| Rated Voltage (V) | 3.67 | Standard Discharge |
| 3.73 | 1/3C Discharge | |
| Rated Energy (Wh) | 212.86 | Standard Discharge |
| 216.34 | 1/3C Discharge | |
| Mass Energy Density (Wh/kg) | 233 | 1/3C Discharge |
| Voltage Range (V) | 2.75~4.35 | 0 °C ≤ T ≤ 55 °C |
| 2.20~4.35 | −30 °C ≤ T < 0 °C | |
| Main components of electrolyte | DMC, EMC | / |
| Cathode active material | Li(Ni0.8Co0.1Mn0.1)O2 | / |
| Anode active material | Graphite | / |
| Cathode current collector | Aluminum foil | / |
| Anode current collector | Copper foil | / |
| Shell material | Aluminum alloy | / |
| Parameters | Value |
|---|---|
| (N) | 1900~2000 |
| Temporal range of explosion (s) | 0.054~0.179 |
| Maximum ejection velocity (m/s) | ≥90 |
| The mass loss rate (%) | 32.4 ± 2 |
| The maximum flame propagation velocity (m/s) | >17 |
| The maximum flame area (m2) | 0.79 |
| The distance from the explosion center to the safety valve (m) | 0.1~0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Wei, N.; Li, M. Quantitative Analysis of Lithium-Ion Battery Eruption Behavior in Thermal Runaway. Batteries 2024, 10, 182. https://doi.org/10.3390/batteries10060182
Xing Y, Wei N, Li M. Quantitative Analysis of Lithium-Ion Battery Eruption Behavior in Thermal Runaway. Batteries. 2024; 10(6):182. https://doi.org/10.3390/batteries10060182
Chicago/Turabian StyleXing, Yu, Ningning Wei, and Minghai Li. 2024. "Quantitative Analysis of Lithium-Ion Battery Eruption Behavior in Thermal Runaway" Batteries 10, no. 6: 182. https://doi.org/10.3390/batteries10060182
APA StyleXing, Y., Wei, N., & Li, M. (2024). Quantitative Analysis of Lithium-Ion Battery Eruption Behavior in Thermal Runaway. Batteries, 10(6), 182. https://doi.org/10.3390/batteries10060182






