1. Introduction
As society increasingly relies on devices powered by electrical energy, batteries have become ubiquitous [
1]. Energy-storage systems (ESS) and batteries, in particular, play a vital role in mitigating the adverse effects of climate change resulting from the continued dependence on fossil fuels. Therefore, they constitute a critical component of the energy mix [
2]. Li-ion batteries (LiBs) have become the primary choice for applications ranging from portable electronics to stationary storage and electric vehicles (EVs) [
3]. As indicated in [
4], the total capacity of LiBs introduced to the global market is projected to surge from 242 GWh to 2731 GWh between 2020 and 2030. This represents an extraordinary, more than 11-fold increase in LiB capacity within the global markets over a mere decade.
The automotive industry is a leading segment that relies on LiBs, as evidenced by the significant growth in global EV sales from just around half a million in 2016 to a whopping 14 million in 2023 [
5]. This trend is expected to persist, with a projected surge to 66 million sales for passenger EVs by 2040, according to Bloomberg predictions [
6].
An important facet of electric transportation involves battery capacity degradation, which diminishes battery performance and consequently reduces EV mileage. EV batteries are typically designed for an acceptable mileage range and longevity. Manufacturers employ extensive research and development to mitigate the battery performance decrement rate. Nevertheless, battery degradation remains an inherent aspect of EV batteries’ durability and reliability, and consideration for eventual replacement as a part of sustainable EV ownership is unavoidable. EV batteries are typically replaced once they reach a certain threshold of an acceptable mileage range, corresponding to a specific battery State of Health (SOH) to circumvent conflicts with customers [
7]. This threshold is typically defined by car manufacturers when the battery capacity has decreased by 20–30% relative to its initial capacity [
8,
9].
Multiple factors profoundly influence the sustainability of EVs concerning battery end-of-life (EOL). Firstly, there must be a clear delineation of responsibility, as appropriately addressing EOL demonstrates the genuine environmental commitment of EV manufacturers. Secondly, recycling involves various steps such as battery collection and transportation and additional strategies to recover primary materials like lithium, cobalt, nickel, etc. The eventual necessity to replace degraded batteries in EVs could pose a significant bottleneck and barrier to further growth in the EV market [
10]. According to a forecasting model outlined in [
11], there will be an estimated stockpile of approximately 16.8 million EV batteries available for repurposing between 2011 and 2040. Hence, effective battery EOL management becomes necessary, and utilizing the batteries in second-life applications should become a viable solution to extend the battery’s useful life in a different application.
Etxandi-Santolaya, Maite et al. [
12] underscore the imperative of aligning the surge in EV sales with sustainable practices, particularly concerning the EOL determination of LiBs in their initial life cycle. Their work raises concerns about the potential underutilization of batteries, especially as EVs evolve toward higher capacities, which could impede overall sustainability. Previous research studies have delved into various aspects of SLBs to provide a comprehensive understanding from diverse perspectives, elucidating their technical functionalities, economic viability, and real-world implementation [
13]. Martinez-Laserna et al. [
14] critically evaluate the concept of SLBs, transitioning from hope to reality and discussing their economic, technical, and environmental aspects. The paper poses essential questions from an economic viability standpoint, such as profitability, market potential, and the selling price of SLBs. In a perspective review conducted by Illa Font, Carlos Henrique et al. [
15] on SLBs, critical questions regarding challenges are highlighted, including the disassembly process, SLB classification, determination of the EOL threshold, and trade-off considerations between extending the first life and maximizing the remaining useful life (RUL) for second-life applications. The paper underscores the importance of current business model maturity for SLBs without delving into the specific challenges hindering them.
Hossain, Eklas et al. [
16] underscore the critical importance and opportunities associated with extending battery life through a second-use phase. Their study delineates the standard production procedure for SLBs, explores their diverse applications, and underscores the significant environmental and economic impacts of their utilization by addressing barriers such as technological challenges, safety concerns, cost competitiveness, and the necessity for robust business strategies and policies. Xu, Jianing et al. [
17] proposed a fast identification approach for microhealth parameters characterizing negative electrode material and electrolyte performance in retired LiFePO4 batteries. Microhealth parameters encompass the performance of both active materials and electrolytes within the battery, and changes in these parameters serve as indicators of the battery’s internal health state. The study demonstrates improved efficiency in assessing battery health for second-use applications, enhancing the performance consistency of regrouped retired batteries. Hu, Xiaosong et al. [
18] provide a review on repurposing retired LiBs specifically for stationary energy storage applications. The review elucidates the economic and operational aspects of SLBs, covering battery aging, repurposing processes, optimal sizing, and energy-management strategies. It underscores the imperative for future advancements in sorting methodologies, accurate aging models, and the potential market for SLBs in renewable energy storage. The utilization of batteries in their initial application is subject to significant variability, influenced by factors such as user behavior, environmental conditions, driving modes, storage methods, and annual mileage. This variability results in a wide range of aging patterns and performance levels, often diverging significantly from the battery’s initial performance characteristics. Matching batteries with compatible performance characteristics is a significant challenge that limits the SLB market.
Despite extensive investigations into the potential of retired EV batteries, encompassing economic viability, parameter inconsistencies, safety considerations, policy implications, and more, the field still requires further exploration and comprehensive study to mitigate uncertainties surrounding battery reusability. Our review paper endeavors to confront the challenges associated with battery reuse from both technical and socioeconomic perspectives. It is imperative to address these challenges to comprehend feasibility, identify potential barriers, and minimize uncertainties. Moreover, we advance beyond previous research by providing a thorough analysis of detailed measurement characterizations of SLBs, aiming to offer insights that transcend superficial overviews of research findings.
This paper’s structure is as follows: Initially, it presents the primary motivation behind the study, along with a comprehensive overview of the key challenges associated with repurposing SLBs. Subsequently, it evaluates the two primary scenarios at the conclusion of the battery’s initial life: recycling or reuse. The subsequent sections of this paper delve deeply into each of these main challenges, with each section emphasizing its significance, role, and current state-of-the-art techniques aimed at enhancing the suitability of retired EV batteries for stationary second-life applications. The focal point of this paper lies in addressing the challenges related to battery screening, wherein various measurement methods are scrutinized and compared based on their applicability, complexity, accuracy, and ease of implementation. Finally, this paper concludes by assessing the various assessment methods employed in the battery-screening process.
2. Motivation and Overview
The exponential proliferation of batteries in the market underscores the urgent need for implementing effective and sustainable strategies to manage their EOL phase. This is essential to mitigate potential environmental risks and optimize resource utilization. As batteries reach their EOL in primary applications, the imperative of managing and utilizing them efficiently becomes increasingly paramount. This could entail developing recycling technologies or exploring alternative uses for repurposed batteries [
19,
20]. Consequently, the realm of retired EV batteries has experienced a significant surge in scientific, business, and policy interest over the past decade.
Figure 1 illustrates the notable uptick in the publication of papers during this period.
Considering the significance of this subject and its considerable potential in promoting circular economy principles, sustainable resource management, and economic opportunities, it is imperative to address the challenges and opportunities associated with SLBs. This paper delves into various scenarios concerning the management of EOL batteries and elucidates the potential obstacles hindering the implementation of effective SLB solutions. The overarching aim is to provide informed and actionable recommendations aimed at improving comprehension and clarity regarding the concept of SLBs, along with its associated challenges. These recommendations are intended to guide researchers, decision makers, industries, and policymakers toward fostering a sustainable and energy-efficient future.
Figure 2 provides an overview of the main challenges related to battery repurposing, categorized into six groups:
Screening challenges: Repurposing batteries for new applications demands meticulous attention to technical factors, notably performance degradation over time. This necessitates a rigorous screening process applied to cells and modules sourced from various packs.
Safety challenges: The reuse of batteries may entail safety risks such as thermal runaway, fire, and explosion. It is imperative to prioritize safety throughout the reuse process, meticulously evaluating all potential safety hazards for the second application.
Uncertainties from the first life: arises from the fact that batteries utilized in prior applications may have undergone varying usage patterns and environmental conditions, thus impacting their performance in a new application.
Battery management challenges: Originate from the battery-management system (BMS), which needs to be customized for the battery pack in its second life. The primary challenges faced by the BMS include battery diagnostics and prognostics concerning the battery’s state of charge (SOC), SOH, and balancing of cells that may not be uniformly aged.
Application challenges: the appropriateness of a previously used battery system for a specific application will be influenced by factors such as the compatibility of the battery with the new application and the anticipated cycle life of the battery.
Techno-economic challenges: Incorporates challenges pertaining to the economic assessment and feasibility analysis of battery reuse. Understanding the primary costs associated with battery dismantling, collection, transportation, storage, and maintenance is crucial, as is analyzing the diminished performance of batteries in comparison to new ones.
3. A Comparative Analysis to Deal with EOL Batteries: Reusing and Recycling Approaches
3.1. Reusing Scenario
Battery reuse involves employing batteries that have fulfilled their original primary application, such as those in EVs. Although they are no longer viable for their initial purpose, these batteries still retain a considerable portion of their capacity and functionality [
22]. Consequently, they can be repurposed for less-demanding secondary applications, prolonging their useful lifespan and mitigating the environmental consequences of battery disposal.
Before repackaging or repurposing a LiB into a second-life application, a comprehensive technical and economic evaluation must be performed to determine its eligibility and profitability for a possible second-life application. A battery performance assessment is the first technical step in the evaluation process, which helps to identify feasible strategies for repurposing, such as direct reuse or disassembling, by evaluating the SOH of the battery pack [
23]. Following a positive performance assessment, the LiBs used in EVs can be repurposed for other applications following their first life use. The capacity remaining in SLBs varies depending on several factors, including the battery chemistry, usage conditions, and aging pattern in the first application [
24].
SLBs have the potential to be employed in distributed energy-storage solutions for residential and commercial properties, allowing individuals, industries, and utilities to store solar- and wind-generated power [
25,
26,
27]. However, practical implementation faces challenges beyond technical considerations. The transition from the first life of the battery to the second-life application requires qualification and regulatory approval, which is currently lacking. This absence of a regulatory framework hinders the adoption of SLBs in such applications. Furthermore, SLBs find utility in electric vehicle charging stations, which greatly helps to reduce grid peak loads and provide cost-effective charging solutions for EV owners [
28,
29,
30]. However, it is important to note that the effectiveness of SLBs in maintaining high power densities over time may be affected by battery aging. Additionally, reusing batteries can create new revenue streams for businesses, as they can be sold or leased for various applications, such as stationary ESS [
31,
32,
33,
34,
35,
36], off-grid and remote applications [
37,
38], and uninterruptible power supply (UPS) as backup power [
39], depending on their remaining capacity and performance characteristics. This can be particularly attractive for energy-storage providers and industries requiring large-scale battery systems. While several potential applications exist for SLBs, it is essential to consider their compatibility with grid-scale energy-storage systems. Although SLBs have been proposed as a solution for storing excess renewable energy and supporting fast frequency regulation [
40,
41,
42], it is crucial to acknowledge the challenges they pose in meeting the particular requirements of the electrical grid. Balancing the electrical grid requires a high level of service guarantee and a low failure rate, which require a high level of risk-management analysis for SLBs due to their less-controlled performance characteristics, including their lifespan, power, and capacity. In addition, using SLBs for large-scale ESS requires aggregating a large number of batteries to meet the grid’s high energy and power requirements. However, the diverse characteristics of SLBs pose challenges in effectively balancing and managing such a diverse array of batteries.
Table 1 illustrates several large-scale ESS projects conducted in Europe utilizing SLBs, demonstrating the successful implementation of retired EV batteries. The concept of repurposing retired EV batteries for extended applications is a novel and innovative research area. As a result, the existing literature predominantly highlights successful implementations rather than unsuccessful trials. This scarcity is attributed to the nascent stage of SLB exploration, with many studies and projects still in the experimental or pilot phase. Researchers have primarily focused on showcasing the feasibility of reusing used batteries, pinpointing potential applications, and refining associated processes.
3.2. Recycling Scenario
The recycling processes for EOL LiBs involve a series of complex processes to recover materials while minimizing environmental impacts [
45]. These processes include collecting and sorting used batteries, discharge to ensure safety during handling, and subsequent dismantling to separate individual battery components [
46]. The recovered components, such as cathodes, anodes, and electrolytes, are subjected to pyrometallurgical [
47], hydrometallurgical [
48], etc., processes to extract valuable metals [
49]. Additionally, techniques to recover electrolytes and plastics contribute to a more comprehensive recycling approach [
50].
A systematic review conducted by Lai, Xin et al. [
51] comprehensively investigates strategies for the echelon utilization and material recycling of retired LiBs to turn waste into wealth. The paper provides a thorough analysis of the current status, recycling modes, industrial chains, policies, and technical challenges associated with echelon utilization and recycling. Resource recovery is a central aspect of battery recycling, offering significant benefits such as reduced reliance on primary raw material sources and conserving valuable metals like cobalt, nickel, and lithium. Moreover, effective recycling mitigates potential environmental hazards associated with improper battery disposal, thereby preventing soil and water contamination [
52]. Life Cycle Assessment (LCA) methodologies are typically employed to quantify the environmental impact of recycling processes and compare them with primary production methods [
53].
Various recycling technologies employ different approaches to recover materials, ranging from mechanical processes to high-temperature treatments and chemical extractions. Some techniques focus on specific components, like lithium recovery or Direct Cathode Recycling, while others aim for a more comprehensive recovery of multiple valuable metals. In the following, several commercially viable LiB recycling technologies are discussed. These methods continuously evolve as researchers and industry experts seek more efficient, sustainable, and economically feasible ways to recycle LiBs.
Mechanical Separationis the initial step in most recycling processes. It involves shredding the LiBs into smaller pieces to facilitate further processing. The shredded materials are then screened and sorted based on their physical properties. Different technologies, such as sieving, magnetic separation, and eddy current separation, are used to separate metals, plastics, etc. [
54].
Direct Cathode Recycling is a promising technology that seeks to recover cathode materials directly from retired LiBs. The process involves several methodologies, with the prevalent approach being cathode material leaching, wherein active materials like lithium cobalt oxide or lithium nickel cobalt manganese oxide are separated from conductive additives and binders [
55]. Once the active materials are isolated, they are purified to ensure high quality and suitability for reintegration into battery manufacturing. This method has the potential to significantly reduce the environmental impact of battery production and decrease the reliance on mining for new cathode materials [
56].
Pyrometallurgical Recycling relies on high-temperature processes to break down the battery components [
57]. The shredded materials are placed in a furnace and heated to high temperatures (typically above 1000 °C). During this process, the organic materials, such as plastics and electrolytes, burn off, leaving behind metal oxides and other compounds. The metal oxides are then further processed through techniques like smelting and refining to extract cobalt, nickel, and copper [
58].
Hydrometallurgical Recycling is an environmentally friendly alternative to pyrometallurgical methods [
59]. It involves using chemical processes to extract metals from the battery components. The shredded materials are immersed in a suitable solvent or acid, allowing the metals to dissolve and form soluble compounds. Solvents commonly include sulfuric acid, hydrochloric acid, and citric acid [
60]. Once the metals are in the solution, they can be separated and purified using various techniques such as precipitation, solvent extraction, and ion exchange [
61].
Despite the promising potential of recycling EOL LiBs, several challenges and limitations exist. Ensuring safety during battery handling and recycling processes remains a critical concern due to the presence of hazardous materials. The establishment of an efficient and widespread battery collection infrastructure poses logistical challenges. Moreover, some recycling processes may still incur high costs and energy consumption, hindering widespread implementation. Establishing large-scale recycling facilities can significantly reduce processing costs, making recycling a financially attractive option. Government incentives and regulations play a crucial role in encouraging the adoption of recycling practices and fostering a sustainable market for recycled materials.
Table 2 summarizes the advantages and disadvantages of each method.
3.3. Reuse vs. Recycling
Recycling has become vital to a greener and more sustainable world, reducing the burden of electronic waste and bringing opportunities to enhance resource conservation and reduce the carbon footprint of battery production. Nevertheless, recycling is not the only solution to increase the sustainability of LiBs. Reusing the batteries, especially those with the potential of usability in less demanding applications, is beneficial by increasing the longevity of the batteries.
The concept of SLBs focuses on delaying the recycling process by repurposing used batteries in alternative applications. Extending the battery lifetime maximizes the energy-storage potential and minimizes the overall environmental impact associated with their production, disposal, and recycling. A comprehensive analysis is required to cover a wide range of assessments, from the energy efficiency and greenhouse gas emissions analysis, a systematic approach that evaluates the environmental impacts like the LCA, economic viability, and cost-effectiveness of each scenario, to the market potential for SLBs to compare different scenarios for EOL batteries. Moreover, in the context of large-scale energy storage applications, various factors such as cost-effectiveness, performance, environmental impact, scalability, reliability, and safety should be considered to have a comprehensive comparison between different types of ESSs. For instance, recent advancements have been made in cost-effective iron-based redox flow batteries (RFBs) [
62]. Comparing SLBs with iron-based RFBs would provide valuable insights into their respective strengths and weaknesses, guiding decision-making processes in battery end-of-life management strategies. The choice between different technologies depends on specific project requirements, including cost constraints, performance criteria, and regulatory considerations.
Research in [
63] investigated the life cycle processes of NiMH batteries, showing that reusing or recycling can reduce environmental burdens compared to non-recycling scenarios. The findings indicate that reusing or recycling a waste NiMH battery rather than sending it to a landfill can substantially reduce its overall environmental impact. Notably, in the reuse and recycle scenario, approximately 83 kg of CO
2 emissions, 1.37 kg of resource depletion, 0.044 m
3 of landfill volume, and 1611 MJ of energy consumption can be conserved for each NiMH battery.
Figure 3 shows various scenarios examined in this research and the corresponding outcomes regarding climate change impact, environmental burdens, and benefits.
A comprehensive investigation was conducted to assess the environmental impact of NMC SLBs compared to other battery types in [
64]. The study’s primary objective was to determine whether delaying the recycling of NMC batteries by giving them a second life is beneficial while considering the associated drawbacks of postponing the recycling. Within the study’s context, the NMC battery emerges as the more environmentally beneficial choice for most European regions than lithium iron phosphate (LFP) batteries. The study reveals that the choice of allocation and recycling input significantly affects the outcomes for NMC batteries. Under the assumption of 20% allocation to the second life and 10% recycling input, LFP batteries require less energy per equivalent full cycle (EFC) than second-life NMC batteries within a reasonable cycle life, suggesting that, based solely on energy demand, LFP outperforms second-life NMC batteries.
4. Screening Challenges
One of the main challenges in SLB implementation is ensuring battery cell consistency and stability in terms of capacity, voltage, and internal parameters [
65]. Furthermore, the history of the battery’s first life, including cyclic aging and the operating temperature, should ideally be considered to determine its suitability for SLB applications, but this information is usually unavailable, unfortunately. All these aspects make the screening process for second-life batteries complex and multi-disciplinary, requiring expertise in battery technology, electrical engineering, and data analysis.
There is variation in the internal parameters of cells in LiBs, even for fresh cells. Based on the outcome of the previous study and the measurements on 700 Li-Po cells [
66], it was observed that cells belonging to the same manufacturer and batch are not identical in terms of internal parameters, as was illustrated in
Figure 4. These inherent variations originate from the manufacturing process of the cells.
Table 3 shows a list of studies that investigated fresh cell-to-cell variations. Different aspects, like variations in the thickness of the electrodes, condensation of active materials, and different formation processes, can all cause these cell-to-cell variations.
The variation in cell properties within a battery pack will increase during battery aging. Temperature, different charges/discharge rates, time, etc., are the primary external stress factors that cause increased variations in cell properties [
72]. Since the temperature distribution is rarely uniform between the modules inside a battery pack and between the cells within a module, it can be considered the most influential factor in different battery aging, especially for their second life due to aging [
73,
74]. The battery degradation process is linked to irreversible chemical changes occurring within the battery over time. Each charge/discharge cycle of the battery changes a portion of the materials in different elements. The accumulation of this persistent degradation process constitutes the essence of the battery’s aging [
75].
Figure 5 outlines the primary aging mechanisms in the anode, cathode, and other inactive materials [
76].
Due to the complexity of degradation mechanisms based on chemistry (internal) and the influences of driving patterns (external), determining the battery SOH is challenging, especially in SLBs. Generally, the SOH can be estimated during the battery operation (in situ) or measured or estimated while the battery is not operating. The battery categorization for SLB applications means evaluating the battery’s performance in terms of its health condition. The battery’s SOH is usually defined as its capability to store energy compared to that of a fresh battery. It describes the battery’s current performance compared to its ideal situation as follows:
The SOH is an essential indicator for decision making in finding the proper SLB application. It is necessary to categorize the batteries correctly, which usually happens by selecting the battery cells with a similar SOH to form a module and subsequently combining modules with a similar SOH. There are different approaches to measuring the battery SOH directly or estimating it based on battery indices related to degradation. Since the number of batteries at their EOL is increasing rapidly from the EVs or HEVs, it is rational to use methods that can estimate the SOH expeditiously. Rapid methods include the assessment of battery parameters that indicate the health condition of the battery. An incremental capacity analysis (ICA), differential voltage analysis (DVA), differential thermal voltammetry (DTV), electrochemical impedance spectroscopy (EIS), etc., are some examples of such methods that are investigated in the remainder of this chapter.
4.1. Incremental Capacity Analysis
An ICA is an indirect battery SOH estimation method based on the capacity variation corresponding to the voltage change in the battery. It can be formulated based on the derivative of capacity concerning voltage:
The relation between the LiB’s voltage with respect to the capacity is almost like a flat curve, so a small error in the battery voltage measurement leads to a large estimation inaccuracy. By taking the derivative of the curve, the ICA method converts this flat curve into a curve with distinguishable peaks. Information related to the battery electrochemical degradation mechanism can be found in the resulting IC derivative curves. This means that different indicators that come from the IC curve, e.g., the information related to the peaks, such as the peak amplitude, location, and area under the peak, can be used to estimate the SOH of the battery. One of the major advantages of this technique is its capability to perform in situ measurements during the normal operation of the battery. The technique comes with considerable information about the internal phenomena of the battery. As an example,
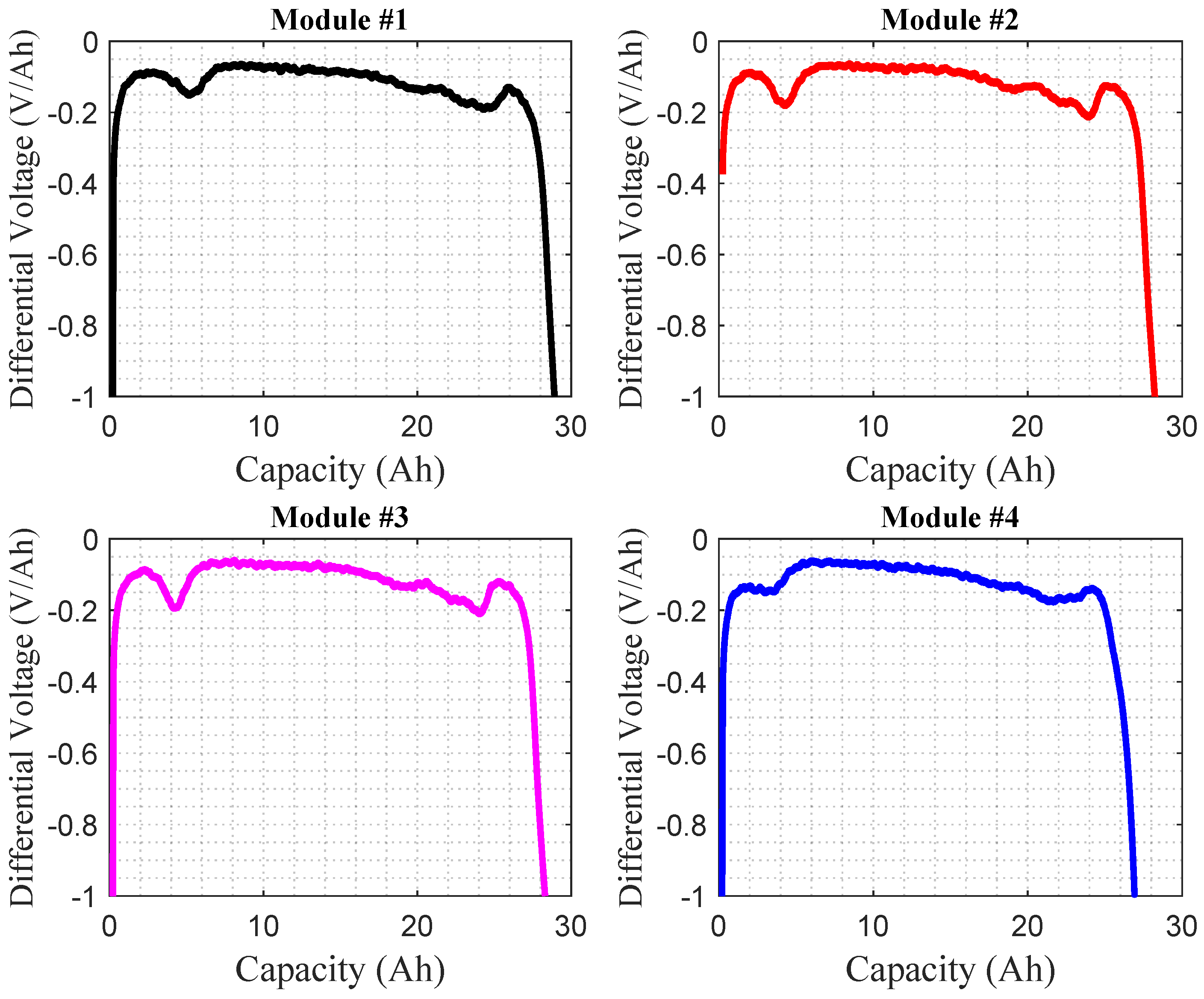
Figure 6 shows the IC curves of four modules from the same battery pack used for eight years in a hybrid electric vehicle (HEV). As expected from the capacity measurement of these four modules (module number 4 has the lowest capacity compared to other modules), module 4 has the lowest right-most peak. This means that this module experienced more loss of lithium inventory (LLI) than the other modules. The loss of active materials and the solid electrolyte interphase could be the potential causes of this peak. Ohmic resistance increases, usually happening when the battery ages and shifts the whole IC curve to the higher voltage [
77], and can be seen for modules 2 and 3 in this experiment.
Different chemistries of Li-ion batteries have different ICA characteristics, and changes in IC curves happen while the battery ages. Each peak corresponds to a different aging mechanism, e.g., loss of active material, loss of lithium inventory, and increase in battery impedance. Shifting the peak locations and changes in the magnitude of different peaks corresponding to the battery health condition makes the ICA method attractive in battery SOH measurement/estimation. The authors of [
78] suggested this method for accurate battery diagnostics and prognostics and noticed a strong correlation between the battery SOH and the peak’s shape, amplitude, and position. For example, a reduction in the amplitude of the first peak could indicate the loss of active material and increase the solid electrolyte interface, leading to the battery’s higher internal resistance. More information related to the degradation of the battery and its relation to battery IC curves can be found in [
79,
80].
In conclusion, as a non-destructive method, the ICA method is rich in information about the degradation processes inside the battery. This method’s main advantages are high accuracy, low computational burden, easiness of implementation, non-destructive nature, and applicability for different battery chemistries. On the other hand, this method is time-consuming and improper for second-life battery screening. Furthermore, the method’s applicability is mainly on the cell level, and there are still several uncertainties about pack-level SOH estimation. Finally, the accuracy of this method is very sensitive to the environmental operation of the battery.
4.2. Differential Voltage Analysis
The differential voltage analysis (DVA) methodology is similar to the ICA method. The unique aspect of this technique is that it assesses the battery features based on the voltage (V) response’s deviation concerning capacity (Q) as follows:
This method is usually used to analyze the battery degradation based on the main mechanisms, e.g., loss of active material, loss of lithium inventory, and loss of cathode materials.
Figure 7 shows the DV curves of the same modules used in the ICA analysis of
Figure 6. The curve reveals information related to battery aging and its SOH that can be interpreted from peak analyzing.
Like the IC curve, the location of the peak and magnitude can be used to indicate the health condition of the battery. ICA and DVA are both powerful tools for online battery SOH estimation. SOH estimations based on these methods are relatively simple since only voltage and capacity need to be measured and monitored. Even with its advantage of low complexity for SOH estimation, it is not considered for SLB screening due to the time-consuming nature of full charging and discharging.
4.3. Differential Thermal Voltammetry
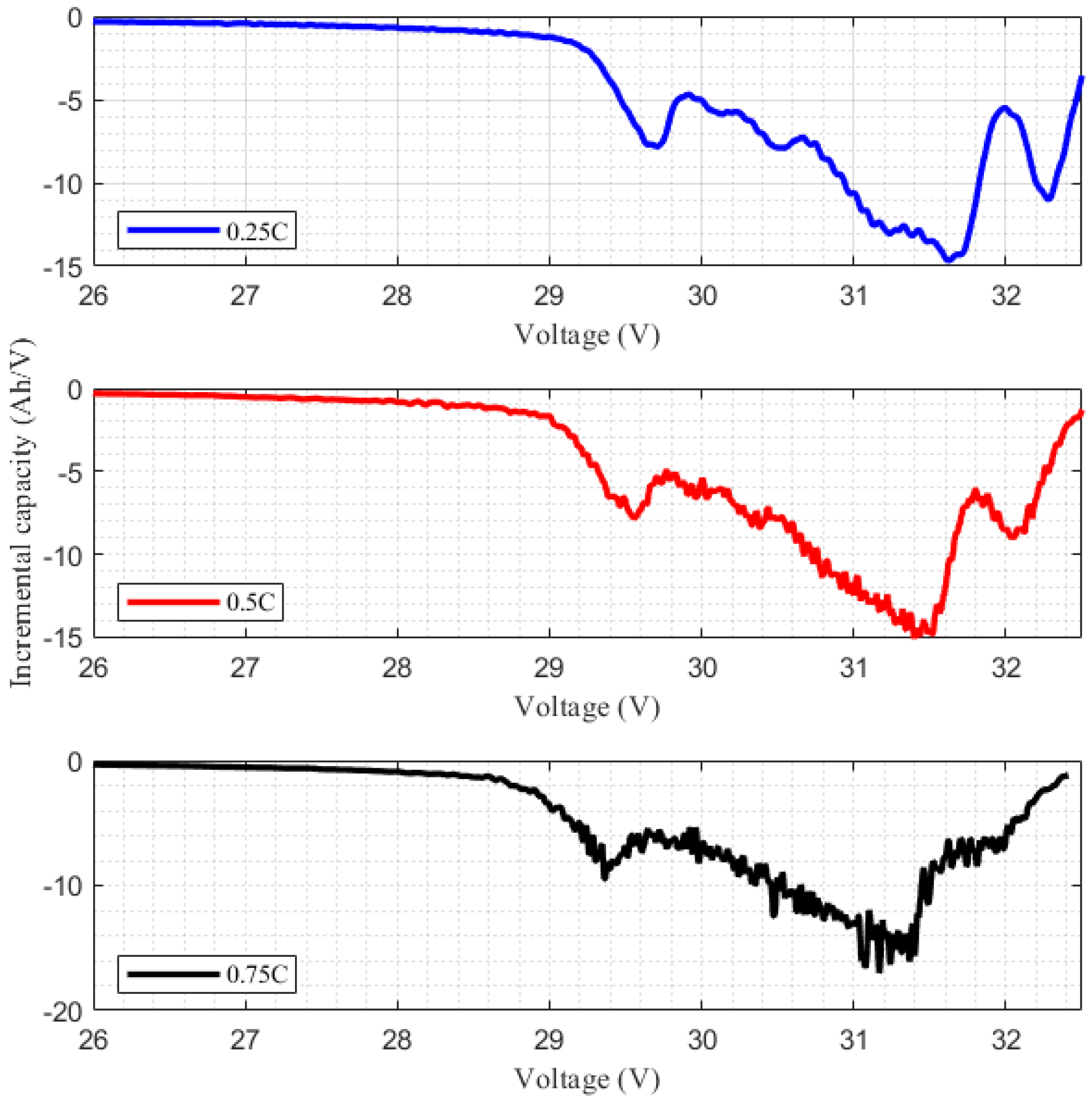
Our experience based on laboratory measurements has shown that improving the method’s accuracy and peak analysis in IC/DV curves requires measurements in low C
rate conditions, which takes several hours and is not feasible for battery repurposing.
Figure 8 illustrates the result of the battery capacity measurement at three different discharge current rates on the same lithium-ion manganese oxide (LMO) battery module as
Figure 6 and
Figure 7. Using a higher
Crate results in two significant changes in the IC curve. First, the increase in noise mandates the use of Gaussian filters, which leads to a loss of information, and more importantly, it becomes difficult to distinguish peaks. This was further observed in a subsequent measurement at high currents (voltages), where one peak became indistinguishable and therefore found missing.
Generally, loss of lithium inventory, loss of active materials, and the formation of solid electrolyte interphases are the main contributors to changes in the first right-most peak; as the battery ages, this peak fades significantly. The fading of the right-most peak is a proper indicator that shows the battery SOH [
81]. According to studies [
82,
83], each peak in the DVA curve can be linked to the electrochemistry inside the battery. The curve also shifts to a different voltage range during battery aging due to increases in the internal resistance.
Temperature is also an indicator that can be used to show the health condition of the battery. By increasing the C
rate during the battery charge/discharge, the temperature inside the battery and, consequently, on the surface, increases. The temperature profile is rich with information related to the battery’s entropic behavior. Like ICA and DVA methods, it is a relatively easy method and only requires surface temperature measurement and voltage monitoring [
84]. Differential thermal voltammetry can be formulated based on the temperature (T) derivative concerning voltage:
Differential thermal voltammetry (DTV) is an in situ method for battery SOH measurements with low complexity. The analysis is based on the peak parameters in the DT curve to quantify the battery degradation and estimate the SOH [
85]. DTV is a promising technique due to its simplicity, which does not require additional hardware within the battery pack or BMS. However, like most battery diagnostics techniques, the operation conditions affect the accuracy of the measurement and, consequently, the SOH estimation [
86].
To evaluate the applicability of this method, the authors monitored the temperature on four different modules from the same battery pack of an HEV. The experiment aimed to investigate the correlation and dependency of the DT curves on the module capacity. Each module contains eight cells, and a K-type thermocouple measured the surface temperature of each cell. During the temperature monitoring, it was noticed that the temperature of one cell inside a module is not only a function of the cell parameter itself but is also affected by the adjacent cells inside the module. The uncertainties related to the location of the cell within the module are always a big challenge for achieving good accuracy in the DTA method. It is also worth mentioning that the curves need data processing across all three differential methods to filter out high-frequency noise. During the filtering, some information will be lost, which again will affect the method’s reliability and accuracy.
Figure 9 shows the DT curves of eight cells inside a module concerning the voltage changes during the discharge at 1C.
In conclusion, DTV is a valuable tool for assessing the internal condition of the battery at the cell level with information for battery SOH monitoring. It is a minimally destructive method that only requires straightforward temperature and voltage measurement, which makes it suitable for in situ measurement. Since the temperature is highly correlated with the battery’s internal resistance (figure of merit of this technique), it can be a very reliable technique for the battery SOH at the cell level in controlled operation conditions. On the other hand, this method strongly depends on accurate measurements, generally leading to limited accuracy. Also, the applicability of the method at the module and pack level is very dependent on the thermal management system (TMS) of the battery pack; as was mentioned above, the location of the cell inside the module and the location of the module inside the battery pack seriously affects the reliability of this method [
87].
4.4. Electrochemical Impedance Spectroscopy
A powerful technique in battery diagnostics and prognostics is electrochemical impedance spectroscopy (EIS). This method has been getting more attention recently due to its non-destructive nature and the rich amount of information that can be derived from it on key parameters that are linked to the battery’s degradation and health condition [
88]. There are several ways to make a graphical demonstration of the measured impedance over the frequency range used, but the most favorable way is the Nyquist plot. To interpret the EIS results from its Nyquist plot to meaningful information related to the battery degradation and also use it for battery SOC, SOH, and RUL estimation, it is essential to model the battery with parameters that can explain the electrochemical nature of the battery, as illustrated in
Figure 10. This equivalent circuit model (ECM) is one of the most commonly used techniques for interpreting the EIS result. In this approach, the impedance from wide-range frequency spectra is represented by electrical elements that model the physics and chemistry of the battery. The circuit model will be obtained by finding a good circuit topology that can be an effective ECM with all important elements, e.g., series resistance in combination with RC networks.
The ECM model can be used to link each electrical element with the three common degradation modes of the battery, each reflected in a response in a different frequency range [
75,
78]. The high-frequency region (kHz range) is usually modeled with series resistance and an inductor. The degradation mechanism affecting this region is the loss of lithium inventory, causing electrolyte decomposition. In the mid-frequency region, commonly modeled by RC networks, the degradation mechanisms that will lead to a change in the ECM are loss of active materials in the anode and cathode and the growth of a solid electrolyte interface (SEI). Finally, the low-frequency range shows the diffusion process, which is represented in the ECM model with a Warburg or a constant phase element (CPE). The diffusion process is related to the battery degradation by loss of active material in the cathode that will lead to a change in the electrode’s formation [
89].
Degradation processes in LiBs have distinct effects on each of the three regions in the EIS spectrum, which thus can be used to understand the degradation mechanisms. The links between EIS spectra and degradation are well established, with certain degradation processes leading to specific changes in the EIS spectrum. There is a strong correlation between the ECM parameters obtained from EIS spectra with the battery SOH [
90]. The authors of [
89] conducted a comprehensive review on the link between the degradation process and ECM elements to analyze the strengths and limitations of EIS as a diagnostics tool for battery health monitoring. An acceptable correlation index between the battery ohmic and charge transfer resistance in the ECM with SOH was achieved in [
88].
To illustrate the power of the EIS tool, we show the results of cycling aging for an NMC cell under a specific charge and discharge pattern. During the cycling, an EIS measurement is performed to analyze the degradation behavior regarding an increasing cycle index. The results given in
Figure 11 show a clearly visible shift in the EIS spectra while the battery ages, with the mid-frequency region as the main link between the changing battery SOH and the changes in the EIS spectra.
Most of the research based on impedance-based models and EIS has been performed at cell level to find the effect of different aging patterns and temperatures on the battery model. EIS at cell level has been investigated in [
91,
92], and it shows a well-distinguishable SOH related to the second-life battery resistance parameters. This finding is crucial, especially for SLBs, due to their rapid growth and the fact that dismantling the battery to cell level is very time-consuming, so it is almost impossible to access each individual cell inside a battery pack. Furthermore, the fast measurement, which is one of the advantages of the EIS method, may be compromised if it is not applicable for module and pack levels on SLBs. The number of studies focusing on EIS measurements at the battery module level is very limited [
93,
94].
In general, the EIS method is getting increased attention as a non-destructive and fast method with high accuracy, especially for SLBs that do not require on-site measurement [
91]. Still, time is an important factor in decreasing the labor cost of evaluating the battery for a possible new life in a second application. Accuracy, time efficiency, and the large amount of information related to the battery’s internal degradation are the main advantages of the EIS method. On the other hand, this method requires specialized equipment, making the measurement technique complex and expensive.
Table 4 presents an extensive comparison between the investigated methods in this study.
5. Safety Challenges
Safety remains a paramount concern in the realm of LiBs, given their status as a relatively young technology. Due to their high energy density, LiBs have the potential to release a significant amount of energy in a short span, posing substantial safety risks. Despite notable advancements in safety-oriented battery design in recent years, incidents related to battery hazards continue to occur with some frequency.
Based on data from the National Fire Protection Association (NFPA) and the U.S. Department of Transportation, “there is approximately one vehicle fire per 30 million kilometers for petrol cars”. In comparison with Tesla, this number is one Tesla fire per 330 million kilometers, based on data from 2012 until 2020 [
95]. These data show that EVs are significantly safer than other types of vehicles. However, there is always a potential fire risk with LiBs due to their high energy density. Based on reports from the NFPA journal, the failure rate of Li-ion batteries is between 1 in 10 million and 40 million, considering both the higher and lower end of the spectrum, respectively [
96]. Recent battery fire incidents in EVs have been reviewed in [
97] to investigate varied and robust designs considering system safety features and architectures.
Safety is one of the main reasons that EV car manufacturers define an SOH close to 80% as the threshold for battery replacement. A safe charge/discharge operation window exists for Li-ion batteries to achieve optimal performance and life from the battery. This window must be stricter for the battery’s second life as the battery-safe operation window depends on non-linear battery aging and related detrimental effects [
98].
The operation window of the battery is usually defined by factors such as temperature, current, and voltage/SOC. These parameters have a recommended range for most batteries that can vary depending on the battery’s chemistry and intended application. Operating the battery outside its recommended range can reduce performance, lead to a short lifespan, or pose a safety risk. It is important to note that the safe operation window for batteries can be affected by other factors as well, such as the operating environment, the battery’s design, its age, and its usage history. It is always best to consult the manufacturer’s specifications for the specific battery to determine its safe operating conditions [
99].
Identifying the degradation mechanism in Li-ion batteries is complicated as the electrochemical phenomenon in action depends on several parameters. This field is still nascent and requires further experimental and simulation studies [
100]. The main improvements are expected to occur at the cell or BMS level. Furthermore, the battery aging mechanism is not linear, and this non-linearity is more critical in the battery’s second life [
101,
102]. Further, in [
103], the influence of battery cyclic aging on thermal runaways has been investigated at low-pressure conditions. The results showed that with increasing cycling, the thermal runaway surface decreases, leading to conditions that accelerate thermal runaway.
Table 5 presents some relatively recent incidents involving Li-ion batteries.
Generally, the BMS within the battery pack has the prominent role of guaranteeing the optimal and safe operation of the pack [
104]. The role of the BMS in SLBs is more critical due to increased uncertainty and reduced reliability in these batteries. The BMS represents the intelligence of a battery pack, and it is involved in all battery safety control systems, charge and discharge, thermal management, cell balancing, and battery state estimation [
105]. In SLBs, accurate SOH estimation and RUL prediction are more difficult due to the lack of information about aging in their first use. Furthermore, SOH estimations are typically defined for fresh batteries based on their first-life data. Any optimistic or pessimistic estimate in battery states by the BMS in a second-life application can strongly decrease the battery lifetime, or even, in the worst case, can cause a fire/explosion.
Table 6 shows the reusing challenges of Li-ion batteries with respect to potential hazards. Implementing appropriate safety measures to mitigate potential risks is crucial in avoiding these hazards. Robust standards, as discussed in the techno-economic section, beyond their significant impact on market growth, can play a crucial role in the safe operation condition of SLBs. It is also worth mentioning that investing in user education and training is equally important beyond all technical aspects of the safe usage of SLBs due to all the above-mentioned risks and hazards.
Table 5.
Some of the most well-known fire incidents involving Li-ion batteries illustrate the potential hazards associated with Li-ion batteries and the importance of proper safety system design.
Table 5.
Some of the most well-known fire incidents involving Li-ion batteries illustrate the potential hazards associated with Li-ion batteries and the importance of proper safety system design.
| Country | Description | Year | Ref. |
|---|
| United States | Boeing 787 Dreamliner was grounded due to issues with the lithium-ion batteries in the auxiliary power unit. Smoke and flames originated from the batteries, causing safety concerns. The grounding lasted for several months as the batteries were prone to overheating.
| 2013 | [106] |
| Global Recall | Samsung recalled millions of Galaxy Note 7 smartphones to prevent further safety hazards. The problem related to the battery, specifically the insulation material inside, and a design flaw. These issues caused the phone to overheat and, in some cases, catch fire.
| 2016 | [107] |
| Belgium | | 2017 | [108] |
| South Korea | Fire at a cement plant in Jecheon, North Chungcheong Province (South Korea). USD 3.63 million in damage. LG Chem and LG CNS structure the ESS.
| 2018 | [109] |
| United States | Explosion in a 2.16 MWh Li-ion ESS in Surprise, Arizona, USA. The predominant factor was the malfunction of a single cell within the battery. Caused a series of thermal runaways that affected the whole system.
| 2019 | [110] |
| China | Incident for a Tesla Model S in a parking garage. Based on the results of a subsequent investigation, failure in a module positioned at the front of the car caused a severe fire.
| 2019 | [111] |
| Netherlands | Fire in a dealership showroom in Tilburg, North Brabant province. The cause of the fire for the BMW i8 has not been identified thus far. The firefighters dropped the car in a massive water container to extinguish the fire.
| 2019 | [112] |
Degradation processes within the LiBs are characterized by various chemical, electrochemical, and physical phenomena throughout the battery’s lifespan [
113]. As LiBs experience cycling and aging, various degradation mechanisms gradually compromise their performance and reliability. During the early stages of battery life, degradation progresses relatively slowly and predictably. Electrode material degradation, electrolyte decomposition, and the formation of the solid–electrolyte interphase layer lead to capacity loss and internal resistance increase during this stage [
114]. These mechanisms operate relatively linearly during this period of battery operation [
115]. However, as the battery cycles and ages, secondary degradation mechanisms become more prominent, especially phenomena such as lithium plating as an unwanted side reaction [
116]. In this stage, which is called the aging knee, there is a notable acceleration in degradation rates, leading to accelerated capacity fade and internal resistance rise [
115,
117]. Operating conditions such as high/low temperatures, overvoltage/charge, deep discharge/undervoltage, and a high C
rate play a significant role in influencing the aging knee [
117].
Operating batteries beyond the aging knee poses increased safety risks, and identifying the aging knee is crucial to determining the EOL of LiBs, particularly in second-life applications repurposed from their first EV cycling/calendar aging. Understanding the factors influencing the aging knee and its implications is essential to mitigate the risk of SLB implementation and safe operation in various applications. Fan, Wenjun et al. [
118] present a novel knee-point prediction method for predicting non-linear degradation in LiBs during long-term usage. Martinez-Laserna, Egoitz et al. [
119] conducted a comprehensive investigation into the non-linear performance and degradation behavior of SLBs. The study emphasized the necessity of considering the first-life aging performance of batteries when evaluating their suitability for second-life applications. The authors noted that upon reaching the knee point in the first life, there was no deceleration in the aging trend of the retired batteries when used in second-life applications. An interesting finding of the study was related to the conventional criterion of 70%-80% remaining capacity as a standard automotive battery retirement threshold. It was demonstrated that relying solely on the remaining capacity as a benchmark for EOL might not effectively evaluate the applicability of the battery for its second life.
Identifying the retirement point for SLBs is crucial for the effective use of these batteries while ensuring safety and sustainability [
120]. Capacity degradation is always an important endpoint criterion [
121]. Unlike first-life batteries, which often come with manufacturer-specified guidelines, SLBs undergo diverse usage histories with different degrees of degradation before entering their second life, which makes it difficult to define a common SOH as an EOL threshold. Defining specific safe endpoints can be complex and highly dependent on various factors, including battery chemistry, usage conditions, and intended second-life application.
6. Uncertainties from the First Life
Battery degradation occurs in different proportions, subject to every operating condition to which the cells within modules/packs are exposed. Operating temperatures (high/low), overvoltage/charge, deep discharge/undervoltage, and a high C-rate are the main factors that accelerate the aging of batteries. Battery degradation is a complicated subject due to the electrochemical reactions occurring within the anode, cathode, and electrolyte, each impacting the performance and longevity of the battery [
122,
123]. Even a simple battery operation (charge/discharge) impacts the battery’s lifetime and the internal degradation processes [
124]. The cycle life is a vital durability indicator, representing the battery’s number of full charges and discharges under a specific operating condition. This number corresponds to the battery performance while still in its acceptable range of capacity fade [
125,
126]. Since battery health, lifetime, and efficiency are decremental over the lifetime, a deep knowledge of the aging process of different battery chemistries paves the way for more extended longevity and safer batteries.
Batteries in EVs face a wide range of operating conditions; generally, batteries in EVs experience harsh operation conditions. Different battery utilization patterns in different EVs mean each battery has its unique degradation mechanism. With many batteries reaching their EOL in EVs, thousands of spent packs and modules become available that have experienced different aging processes. This makes it exceedingly difficult to perform reliable SLB diagnostics and prognostics.
The lack of data from a battery’s first life or the unavailability of accurate information about its history poses significant challenges to its utilization for second-life applications. The diverse driving habits, charge and discharge patterns, ambient temperature, and cell chemistry uncertainties all contribute to battery degradation and alter its internal parameters. These variations in degradation patterns result in different lifetimes for batteries, and the absence of historical data makes it arduous to predict the battery pack’s lifetime. Moreover, measuring the battery’s SOH is a challenge, as even cells with the same capacity can have different internal parameters. These complexities underscore the difficulty in repurposing batteries for second-life applications.
The unavailability of data from the first application will bring uncertainties in battery model parameters and measurements from the battery offline health estimation. Consequently, this will also impact the real-time battery state estimation. These uncertainties also make it difficult to predict the rate of aging and degradation, leading to unreliable performance for second-life applications.
Without proper information, it is also challenging to determine the compatibility of the SLBs with the new applications and systems, leading to potential performance issues. These uncertainties surrounding the battery’s first life can lead to a trade-off between the price and reliable performance, which is also a techno-economic barrier to SLB utilization.
In recent years, there have been some reports of the use of intelligent battery systems to continuously measure the critical parameters of the battery and process these over the cloud [
127,
128]. This intelligent battery passport program allows EV manufacturers to continuously monitor battery aging patterns for improved battery reliability. Having access to such data is crucial for performing both diagnostics and prognostics of EV batteries, and at the same time, accessing BMS data poses significant challenges. Manufacturers often secure and encrypt BMS data, making it difficult to obtain without agreements with battery or car manufacturers. It is still unclear when and whether these data will be available for the companies working on SLBs. Data from the first life of the battery that provides the battery SOH without further measurements will decrease the prospective measurement uncertainties and make the utilization of the SLBs more affordable.
Table 7 provides a concise overview of the main challenges regarding the uncertainties from the lack of information from the first life.
7. Battery-Management System Challenges
The battery-management system (BMS), often likened to the brain within the battery pack, is a sophisticated electronic component with pivotal responsibilities for the monitoring and management of the cells and modules that comprise the battery pack. It plays a prominent role in optimizing battery performance, regulating charge/discharge processes, ensuring safety, and extending battery longevity. Given the high energy density of LiBs, customizing a BMS for different applications is imperative to ensure safety, protect the battery against abnormal situations, and minimize the likelihood of potential hazards [
129].
There is an evident necessity for enhancement across all aforementioned functions of BMS, particularly in light of the increasing utilization of new battery types boasting higher energy densities, necessitating elevated safety measures [
130]. During the battery’s initial life cycle, several challenges remain unconsidered. These encompass issues in battery diagnostics and prognostics pertaining to SOC, SOH, and RUL prediction, as well as challenges associated with balancing the battery during the charge and discharge processes. Furthermore, challenges persist in safeguarding the battery during rapid charge/discharge conditions, managing thermal dynamics, and mitigating issues arising from non-uniform temperature distributions within battery modules/packs.
Azizighalehsari et al. [
124] present a state-of-the-art review focusing on the primary challenges encountered in battery diagnostics and prognostics concerning battery state estimation. Numerous unresolved issues persist in battery state estimation due to the intricate electrochemical behavior of batteries. Various techniques for battery state estimation have been explored, and a comparative analysis of these methods has been conducted based on their individual strengths and weaknesses. The comparison encompasses considerations such as computational burden, implementation complexity, accuracy, and suitability for onboard applications.
At the BMS level, the precision of the battery state estimation method, such as the SOC and SOH, inherently depends on the application’s sensitivity [
131]. An accurate SOC estimation is crucial for effectively utilizing SLBs in various applications. Traditional SOC estimation methods, such as voltage-based methods [
132], suffer from inaccuracies caused by non-linear voltage responses and capacity degradation over time. Similarly, coulomb counting methods [
133] rely on integrating charge and discharge currents, but they may encounter challenges in accurately tracking capacity changes and accounting for aging effects. Model-based approaches [
134], including equivalent circuit models and electrochemical models, offer higher accuracy but require detailed knowledge of battery characteristics and may be computationally intensive. Additionally, data-driven methods [
135], such as machine learning algorithms, have gained attention for their ability to learn complex battery behaviors from data, but they may require extensive training datasets and computation burden [
134]. Hybrid approaches [
136,
137], combining multiple methods, aim to leverage the strengths of different approaches while mitigating their limitations. However, integrating these methods for SOC estimation in SLBs remains a significant challenge, requiring careful consideration of battery aging effects, heterogeneity, and environmental conditions. Recently, there has been interest in employing approaches involving non-destructive methods, such as ultrasonic wave-based techniques [
138], which have shown potential for detecting SOC and temperature simultaneously, providing valuable insights into the internal dynamics of batteries without causing damage. Ultrasonic wave-based methods, as demonstrated by Zhang et al. [
138], utilize piezoelectric transducers to emit and receive ultrasonic signals, which propagate through the battery and reflect back to the transducer. Analyzing the characteristics of these reflected waves, including their amplitude, frequency, and phase, makes it possible to infer both the SOC and temperature levels within the battery. In general, there is a perpetual trade-off between the complexity of the method and its accuracy [
139]. Still, there is a tendency for more precise battery state estimation as this helps prolong the battery lifespan by maintaining it within its optimal operating range.
In
Section 4, we extensively explored various measurement techniques and indirect methods for assessing battery SOH based on battery indices associated with degradation. These methods offer insights into the health condition of batteries by analyzing key parameters or indicators that are indicative of degradation processes occurring within the battery [
140]. Similar to SOC estimation, numerous methods have been proposed for estimating the SOH of batteries. Model-based methods [
141] and data-driven methods [
142,
143,
144] have gained attention for SLBs as a promising approach compared to traditional capacity-based SOH estimation methods, which often require extensive testing times [
145]. Model-based methods (physics-based models/empirical models) utilize mathematical models that describe the physical and chemical processes occurring inside the battery, providing insights into the underlying mechanisms of degradation. These methods often require an understanding of battery physics and chemistry, and with that understanding, they are capable of achieving high accuracy in SOH estimation. In contrast, data-driven methods do not rely on detailed knowledge of battery physics but instead utilize large datasets of aging data. The effectiveness of data-driven methods heavily relies on the quantity and quality of the available data and the extent to which they cover all relevant operating conditions.
Transitioning batteries into a second life poses unique challenges in battery management, requiring sophisticated strategies to address the complex interplay between the battery’s past usage, current condition, and intended application. One key challenge involves developing effective techniques to accurately assess retired batteries’ health and remaining capacity. End-of-first-life battery performance can vary significantly based on usage patterns, operating conditions, and degradation profiles from their primary application. As a result, BMSs designed for first-life applications may lack the precision needed to optimize performance in a second-life scenario.
In SLBs, BMS designs must prioritize reduced complexity to meet application requirements and lower pack costs. However, achieving an acceptable accuracy range for battery state estimation poses challenges due to accelerated aging or degradation compared to the first life. Increased aging also raises the risk of battery failure, and overly optimistic or pessimistic estimations may lead to complete battery failure, exacerbating safety concerns, or unnecessarily reducing the remaining useful second life. Unfortunately, the majority of battery state estimation research focuses on batteries in their first life, with models typically based on cycling data until 70–80% SOH. Limited historical battery information and limited insight into the impact of aging conditions further complicate RUL prediction for SLBs. Consequently, diagnosing and prognosticating battery health in their second life proves more challenging than for fresh batteries.
SLBs, after reaching the EOL in the first application, always suffer from imbalanced impedance values between the cells and between modules due to different aging patterns that originate from non-uniform temperature distribution [
68,
146]. The impedance mismatch in turn causes heterogeneous temperature distributions that will lead to different aging patterns (also depending on the cell’s position inside the pack), making it more difficult for the thermal management system (TMS) functionality of the BMS in the second application to assure the reliable operation of the SLB [
147]. As an example,
Figure 12 shows the temperature discrepancy on the surface of the cells inside an aged module from an HEV during a discharge process.
The work in [
148] comprehensively reviewed challenges in LiB thermal management systems. The review analyzed thermal management technologies separately and described the advantages and disadvantages of each method and the need for more investigation and improvement in the efficiency of the systems for both cooling and heating of the battery.
Table 8 shows the analyzed methods and their pros and cons. Battery performance in terms of energy efficiency, lifetime, and safety is a function of the operating temperature. The temperature significantly affects battery degradation, leading to capacity fading of the battery. Any non-uniform temperature distribution between the cells within a module will increase the failure probability of the battery pack [
149]. A proper TMS is needed to guarantee an acceptable safety level for LiB operation. With an increasing battery charge/discharge rate, the battery’s temperature increases as well, and the failure risk will be higher. It is also essential for the battery’s second life to have the battery pack temperature as uniform as possible. If the pack is not tailored with an efficient TMS, the aging pattern of the cells and modules within the pack will be different, creating many challenges for the BMS in the second application.
The temperature changes in the cells during the discharge process at the same
are more significant than those during the charging process. Charging happens for LiBs in constant current–constant voltage mode (CC-CV), which means that when the voltage reaches the cut-off limit, the constant current in the first charging part is decreased and charging is continued in a CV mode. Based on our initial work [
87] and the infrared image of
Figure 12, the location to monitor the surface temperature was chosen in the middle of the cell. There is an almost 2 °C temperature discrepancy between cell number 2 and cell number 6 in this aged module that underlines the necessity for a proper TMS in an SLB. It is noted that degraded cells can affect their adjacent cells and increase the working temperature (and thus aging) of these neighboring cells.
8. Application Challenges
SLBs, with their potential to delay the recycling process and reduce the need for manufacturing new batteries, play a significant role in mitigating climate change and are thus an intriguing component of the energy transition. In addition to considering the advantages and challenges presented at various levels of the battery structure, from cell to module and pack, it is crucial to carefully assess the applications for which these batteries will be utilized.
The eligibility of the retired battery for a particular second-life application and the longevity of the battery for a new application are two important characteristics that need special attention in SLB analysis. In [
14], a technical assessment of the performance of an SLB was performed based on the aging history of the battery in its first life. The two applications considered in this study are the residential demand response and power smoothing integration. The result indicates that the aging pattern of the battery in the first life is a missing link between the first and second life and significantly affects its performance and technical viability.
Cycle life is a notable indicator to assess the durability of the SLB; it means it is necessary to know how many full cycles the battery can be used or deliver power and energy before reaching its failure threshold. It is always crucial for the customer of the SLB to know the remaining useful life and health condition of the battery.
RUL methods aim to predict the future state of the battery to prevent any failure within the battery that comes from battery degradation [
150]. An accurate estimation of the replacement time of the battery for the safe operation of the battery will decrease the failure risk. Naturally, accurate battery aging data are key to the RUL prediction. These data can be gathered from the current operation of the battery, which is compared with historical data gathered on the same battery, or it can be performed based on the data that have been collected from the different aging patterns and operational conditions in the laboratory.
Based on the type of data and the comparison methodology, the RUL prediction approaches can be categorized into four groups, i.e., physics model-based, statistical model-based, artificial intelligence, and hybrid approaches [
151]. The work in [
152] comprehensively compared these methods for RUL predictions in first-life battery applications. The accuracy of different approaches is related to the method’s complexity. Finding a trade-off between the accuracy and complexity of the method is always challenging. To achieve maximum accuracy, hybrid approaches with accurate models and high-quality data are to be used [
87].
The impact of the application in RUL prediction of the battery comes from the operating condition of the SLB, depth of discharge (DOD), temperature, C
rate rate, and calendar aging. Less DOD will lead to a higher lifetime: the study of [
153] showed a strong potential to increase the battery lifetime just by avoiding a high SOC and not keeping the battery charged while storing it to prevent short calendar aging periods. The impact of different stress factors on the battery lifetime was investigated in [
154]. The results show that temperature has the highest effect on the battery lifetime and then
and DOD, consequently, on prismatic Li-ion cells.
The capacity data for a lithium manganese oxide/graphite battery, obtained from thirty-two used modules and ten new modules from [
19], is illustrated in
Figure 13. In this study, the total difference between the modules from the average capacity for second-life modules is 11.3%, whereas this parameter for fresh modules is 3%. This figure calculates the coefficient variation by dividing the standard deviation by the average capacity. According to their capacity measurement, the range is the capacity difference between the best and worst modules. The result shows the importance of the first-life history for the modules and proper characterization at the beginning of their second life.
The cycling aging of six second-life cells from a Nissan LEAF was performed in [
115]. The results show that these cells can be used at least for 2033 equivalent complete cycles before their EOL, which is equal to at least five years of usage in a normal photovoltaic system with daily charge and discharge usage. In this study, EOL was defined as the time that the cells reach their aging knee. This study’s experimental results show that the cells’ aging is not linear in their second life. The DC internal resistance (DCIR) and capacity fading of the cells as degradation indicators demonstrate a linear behavior in the first life. Then, the DCIR will go to the non-linear stage; consequently, the capacity fading will increase and go to the non-linear stage.
9. Techno-Economic Challenges
The techno-economic analysis holds significant importance for several reasons, primarily to assess the feasibility of commercially exploiting SLBs. Understanding the technical and financial benefits of battery repurposing is crucial to determine its viability on an industrial scale and ensure widespread acceptance.
Analyzing the market potential, price, and environmental benefits gives insight into the techno-economics of SLBs. SLBs offer a cost advantage compared to new batteries since they are supposed to be significantly cheaper, as recycling and repurposing costs are expected to be lower than producing new batteries from raw materials [
155]. From the ecological perspective, SLBs, by decreasing the demand to mine raw materials and supporting renewable energy resources, can help reduce the environmental impact, including greenhouse gas emissions. There is a vast market potential for SLBs, which can significantly promote sustainability and reduce the energy sector’s environmental impact.
A study released by the Sandia National Laboratory highlights the analysis of SLBs and their associated costs [
156]. This study identifies critical contributors to SLB expenses, including funds for production, labor costs, administrative overhead, and packaging materials. As illustrated in
Figure 14, the retired battery procurement is the most significant cost, encompassing a substantial 56% of the total SLB expenses. Labor cost also has a big share in this cost breakdown, which mainly comes from the technical assessment of the batteries at their EOL. The modular design of the battery within its first life can extend the economic viability of the batteries by facilitating the complex technical process from the repackaging, screening, and cell/module replacement, which can significantly reduce the labor cost.
A comprehensive feasibility analysis on SLBs regarding their lifetime, economic impact, and environmental effect was performed in [
158] and proved that SLBs would provide a considerable profit besides their significant influence on mitigating environmental issues. A performance evaluation and economic assessment on SLBs for peak shaving as an ESS were executed in [
159] to make the impact of reusing batteries on global warming more visible. The work concludes that using SLBs for power peak shaving is technically and economically feasible, and the batteries have enough capacity and lifetime for the application.
A performance evaluation on six different batteries from different EVs to be used for frequency regulation purposes was conducted in [
40] to weigh different SLBs based on their performance metrics for different specific frequency regulation cases. The results show that repurposed batteries have a sufficient capacity and reliability for grid frequency regulation and can provide this service for several years. Therefore, this application shows that using SLBs is a promising solution to reduce costs and support a more sustainable energy system.
The finding of the research on reusing batteries and its impact on the circular economy shows that it can lead to significant environmental benefits, including reducing CO
2 emissions and decreasing the demand for raw materials [
160]. The result of evaluating the environmental impact of reusing LFP batteries in different scenarios, including frequency regulation, energy storage, and power peak shaving, shows the positive impact of reusing LFP batteries in different scenarios in SLB applications [
161]. Additionally, the study finds that various SLB applications have varying levels of environmental impact, with frequency regulation having the lowest and energy storage having the highest environmental impact.
A recent experimental cycle aging appraisal on six Li-ion modules from different Nissan LEAF cars was performed in [
19]. The results show that they can be used for at least five years on a PV-based storage application with daily cycling, and in some cases, the SLBs can even be employed for up to 11 years. The implementation of second-life batteries in microgrids by proposing four configurations was investigated in [
162]. This work proves the cost-effectiveness and feasibility of SLBs compared to the use of new Li-ion batteries. The results show that a grid-connected system could reduce energy consumption by more than 70% compared to the off-grid models. The most economically viable model consisted of PV modules, floating photovoltaic modules, wind turbines, and a biogas generator connected to the grid using fresh Li-ion batteries. Further analysis of the same model with second-life Li-ion batteries showed that using 0.8 kWh SLBs was their study’s most economically viable solution. The McKinsey Center for Future Mobility predicts that the extent of SLBs in use in stationary applications could go beyond 200 GWh/year by 2030 and believes that SLBs have the potential to be considered the latest value pool in energy storage [
163]. The results of the EU Joint Research Center technical report on the sustainability assessment of SLBs [
164] also shows that it is technically viable and environmentally beneficial to use EV batteries in second applications. In addition, the battery lifespan would increase by 35% by repackaging batteries in residential buildings [
165]. A further study proves that the battery’s second life can accelerate the electrification process by reducing the costs of EVs upfront [
166]. A recent paper published by Nature [
167] revealed that integrating SLBs significantly reduced the cost of electricity by 5.6% to 35.3% in most cases compared to using new batteries. Using SLBs also speeds up the time it takes for the system to pay for itself, making it financially beneficial. The quickest payback period was about 2.9 years for a setup with 5 kW solar panels and 5 kWh storage. The study demonstrates that SLBs can be an economical and effective option, providing a similar service to new batteries mainly in regions with limited access to energy while also helping to reduce waste.
Different studies demonstrate a significant pricing range for SLBs depending on factors such as the particular market conditions and application [
168,
169]. According to a study conducted for Global Battery Alliance, used batteries are traded at varying prices, typically between USD 60 and USD 300 per kWh, depending on the market and how they have been used. This study predicts that by 2030, these prices could drop to around 43 USD/kWh. This drop aligns with the overall trend of lowering battery costs, like the prices of new batteries [
170]. Technological advancements have led to a considerable reduction in battery costs, making new, latest-generation batteries more cost-effective than ever before. Pricing remains a critical concern within the SLBs market, particularly given the notable decrease in cost per kilowatt-hour observed over the past decade for latest-generation batteries.
To highlight specific industrial implementations, RWE, a German energy company, is working on a project that uses EOL batteries from Audi e-Tron cars for energy storage applications since they believe that next to its ecological advantages, it is cheaper than new batteries [
171]. In addition, Audi also employs SLBs in its high-power charging hub in Nuremberg [
30]. The number of projects using EV batteries for stationary applications is rapidly growing, as was discussed in
Table 1 in
Section 3. However, this market has uncertainties that necessitate techno-economical investigations to find a strong case for the specific development in question.
Figure 15 shows the potential impact of a lack of standardization and certification as an important techno-economic challenge for the market growth of SLBs. As illustrated, a lack of standardization and accreditation can create various challenges and risks (safety concerns, compatibility issues, regularity, legal challenges, etc.), leading to limited market growth. Global standardization can increase the compatibility between different manufacturers’ SLBs with an acceptable range of safety and reliability, leading to increased market trust.
In conclusion, while second-life batteries can offer cost and environmental benefits, there are several technical, economic, market, and regulatory challenges and uncertainties to reusing batteries for a second application that need to be overcome for the technology to reach its full potential [
14].
10. Conclusions
Addressing the urgent need for sustainable solutions to mitigate the environmental consequences of batteries highlights the critical importance of developing a mechanism to manage the vast quantity of batteries reaching the end of their first application-related lifespan.
While SLBs present clear advantages, reusing EV batteries for second-life applications poses inherent complexities. Various challenges demand our focus and creative thinking, yet overcoming these hurdles often paves the way for innovation and progress within the field. This study extensively explores the complexities associated with these challenges to grasp feasibility and potential barriers. It aims to enhance the utilization of these batteries in SLB applications, offering a cutting-edge perspective on the significance of diverse techniques to enhance SLB viability and overcome bottlenecks.
A significant portion of this review is dedicated to the challenges associated with screening SLBs. Various measurements and analysis techniques can be applied to a single battery module, each differing in applicability, complexity, time consumption, and accuracy. The methods under scrutiny include battery capacity measurement, incremental capacity analysis, differential voltage analysis, differential thermal voltammetry, and electrochemical impedance spectroscopy. The accuracy of different techniques for estimating SOH and their complexity varies depending on the second-life application and its sensitivity to precise measurement. Often, a trade-off between different performance indicators is crucial to ensure sufficient applicability. Individual methods or hybrid approaches combining multiple techniques can be employed to optimize the efficiency of the evaluation process.
Research on SLBs is still in its infancy, with anticipation for further exploration in the years ahead to address existing gaps. A key aspect in bridging these gaps lies in gaining a deeper understanding of battery degradation characterization across different chemistries, which is crucial for mitigating challenges impacting the long-term performance, reliability, and stability of SLBs. Since battery degradation is significantly influenced by its prior usage history, accessing data from its initial life cycle is pivotal, acting as a catalyst for the growth of the SLB market. The future of SLBs undeniably hinges on technological advancements and collaborative efforts among stakeholders to establish transparent protocols for collecting, analyzing, and sharing data among researchers, industry players, and policymakers.
In essence, reusing EV batteries has become a pressing concern, elevating the importance of addressing associated challenges within the EV industry. Additionally, upcoming trends such as solid-state batteries (SSBs), silicon-based anodes, and Co-free/low-Co cathodes paired with Li-metal anodes, extending beyond conventional Li-ion chemistry (Na-ion, etc.) hold promise for cost reduction, enhanced safety, performance, and increased energy density. These advancements will undoubtedly impact performance, environmental aspects, and the potential gains in second-life applications, which need further studies, including impact assessments and life-cycle evaluations.