Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Laser Structuring
2.3. Cell Assembly and Electrochemical Testing
2.4. Post-Mortem Analysis
3. Results and Discussion
3.1. Electrode Characterization
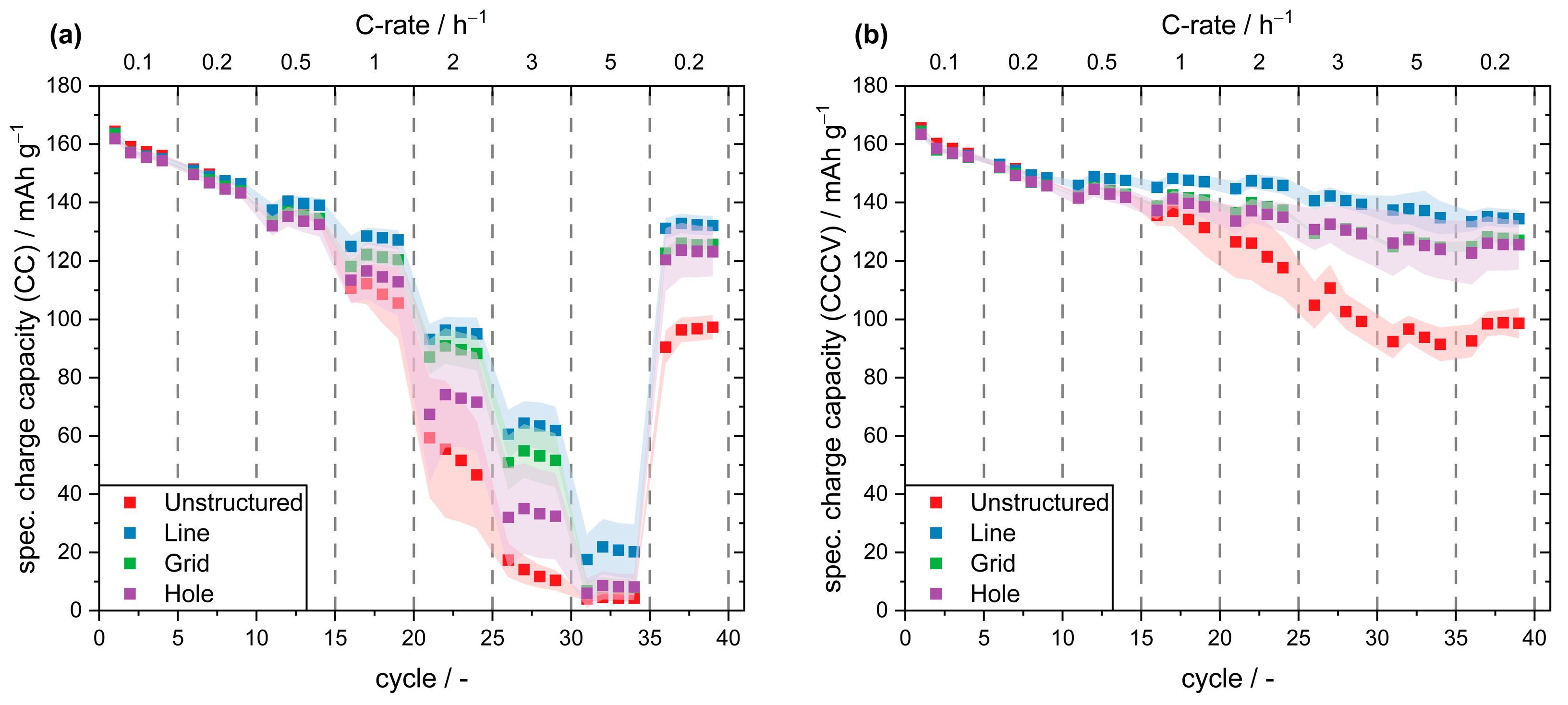
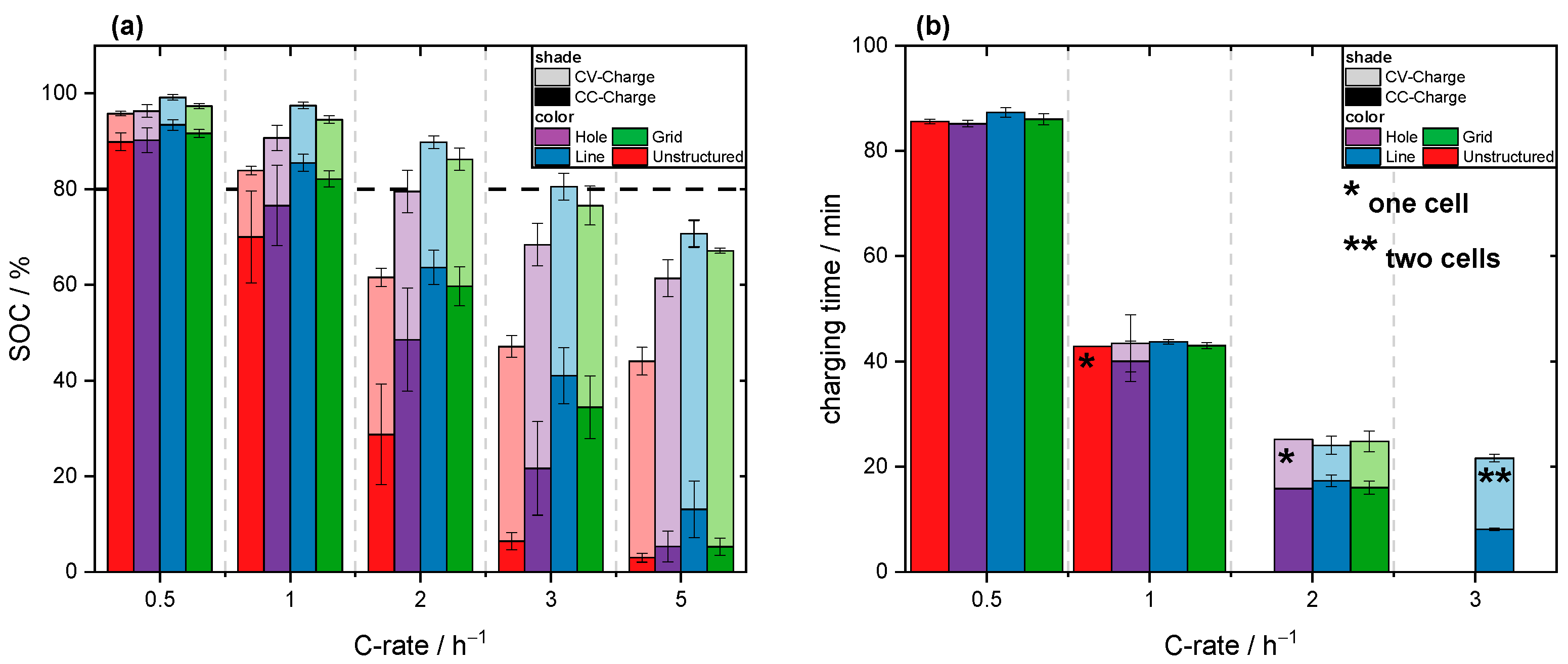
3.2. Fast-Charging Capability
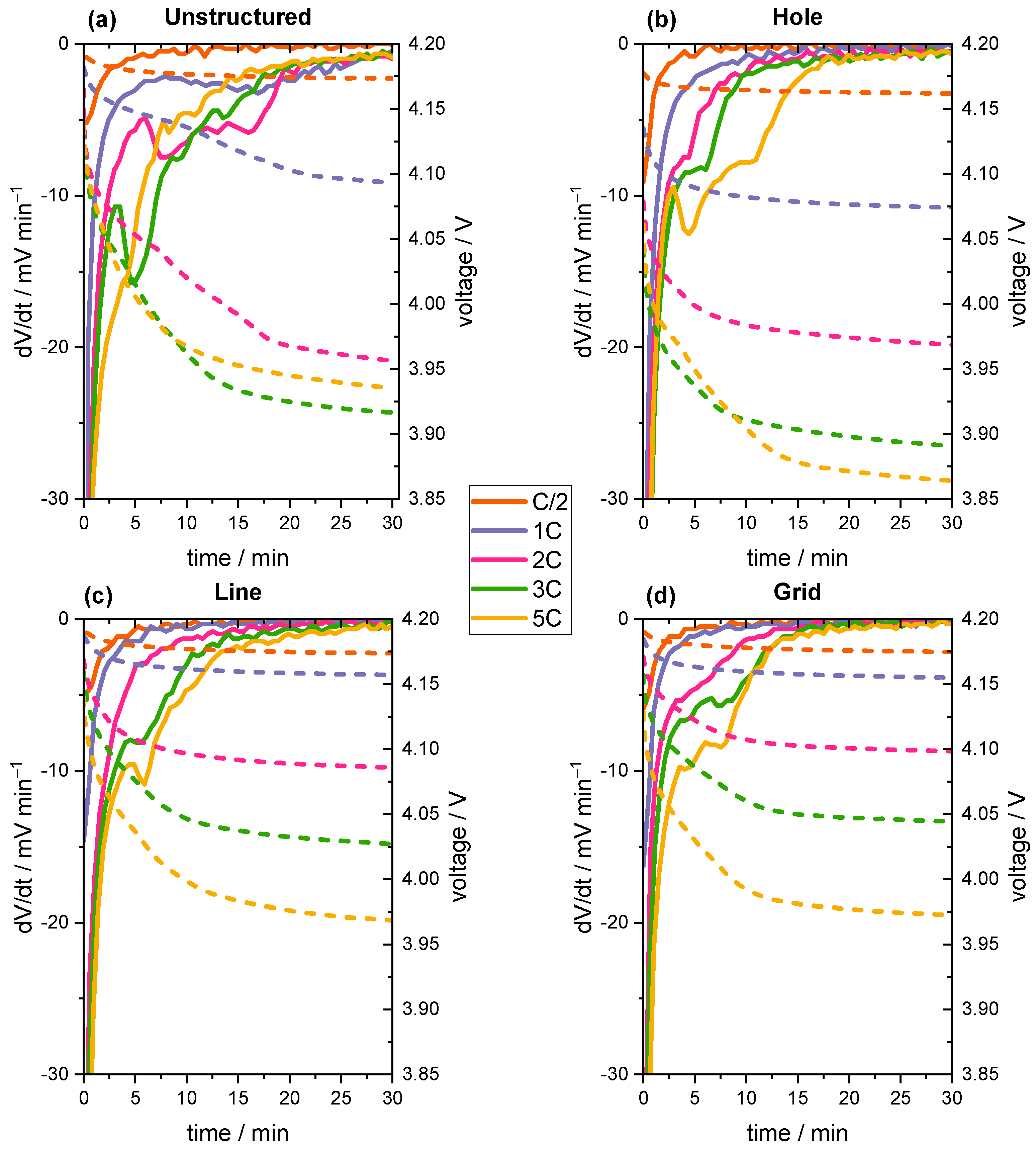
3.3. Voltage Relaxation Analyses
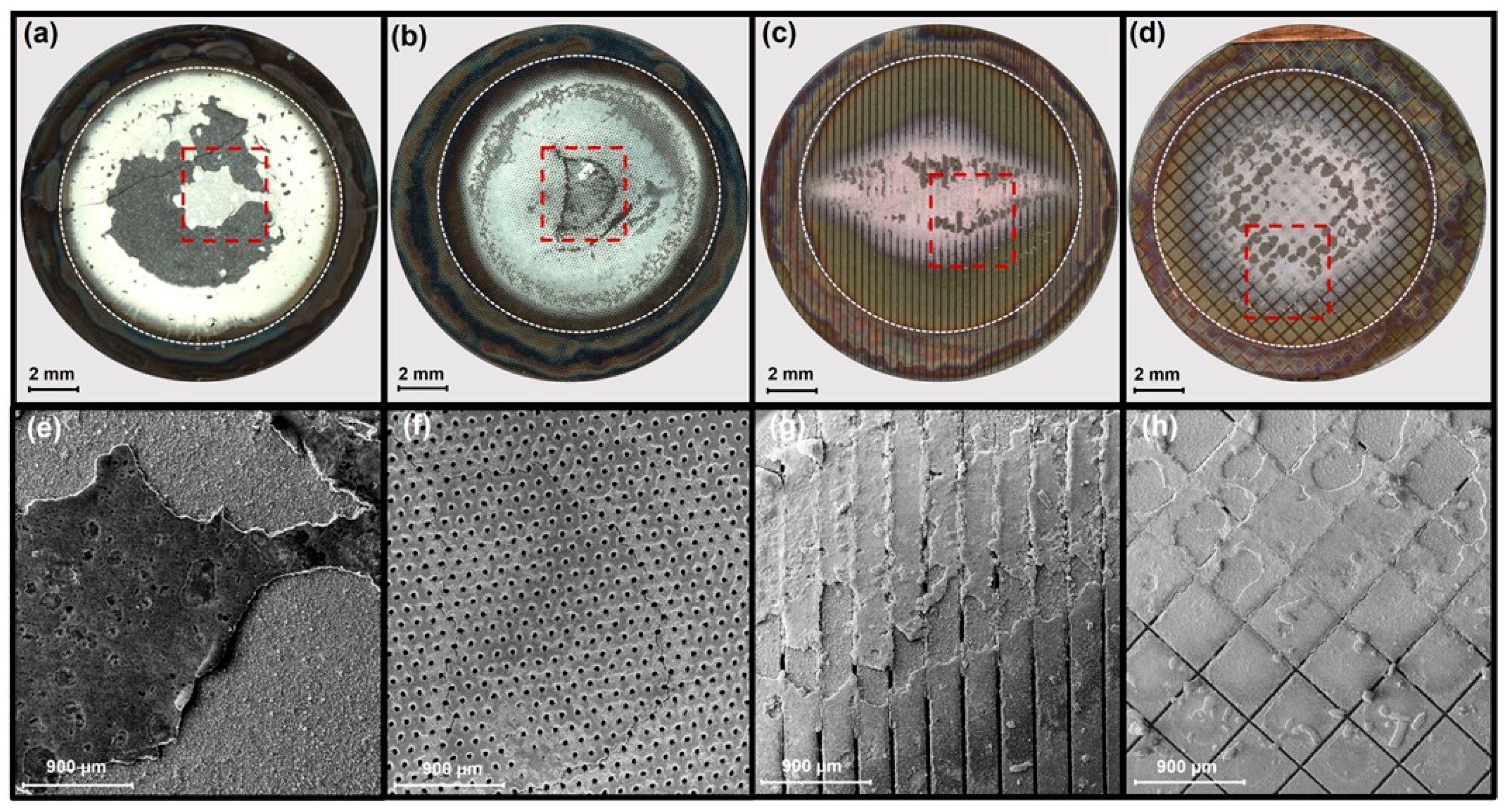
3.4. Post-Mortem Analyses
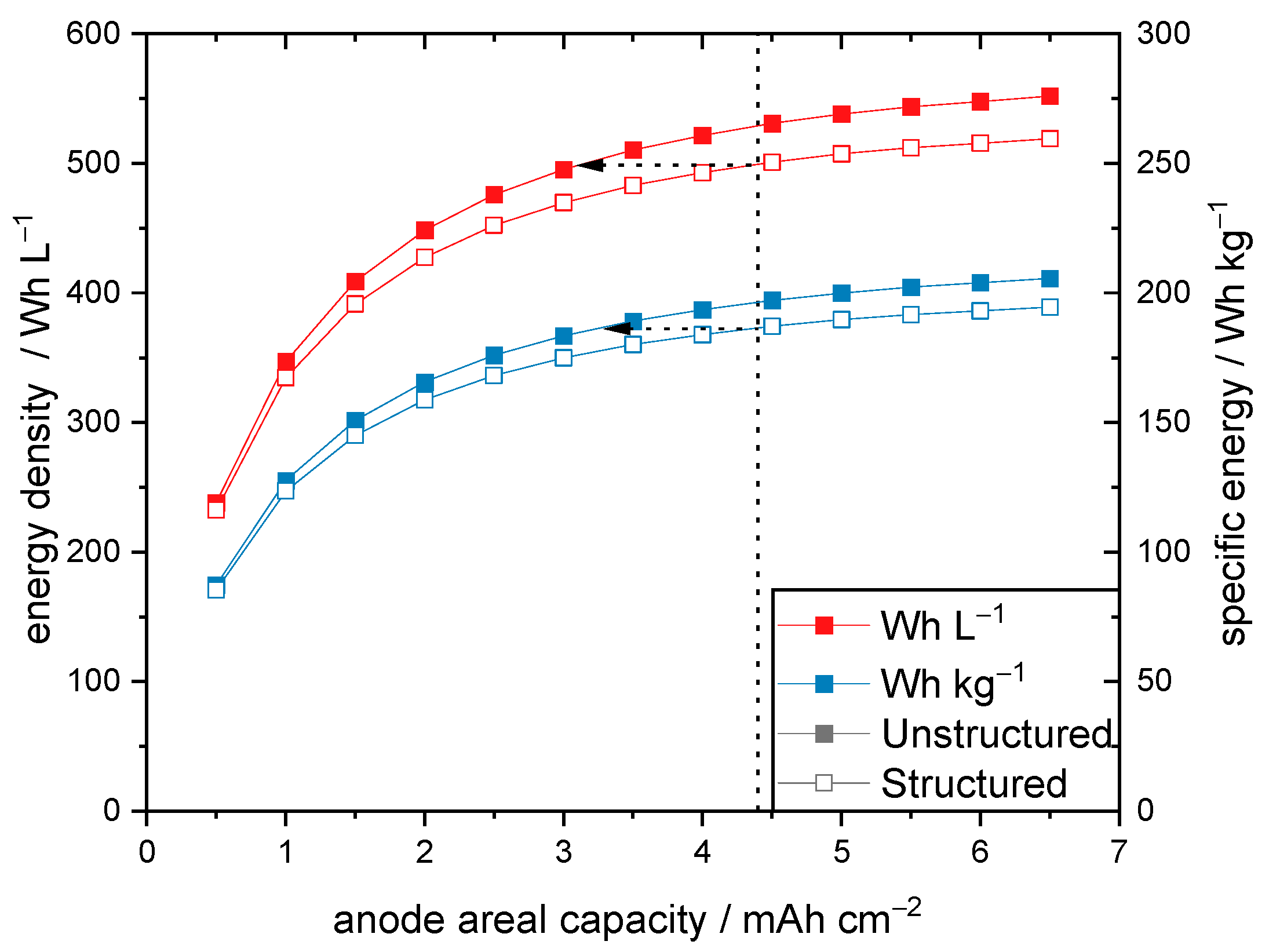
3.5. Extrapolation to Commercial Cell Formats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Link, S.; Neef, C.; Wicke, T. Trends in Automotive Battery Cell Design: A Statistical Analysis of Empirical Data. Batteries 2023, 9, 261. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2015, 163, A138–A149. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Michaelis, A. Diffusion-Limited C-Rate: A Fundamental Principle Quantifying the Intrinsic Limits of Li-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902523. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Dunlop, A.R.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Smith, K. Requirements for Enabling Extreme Fast Charging of High Energy Density Li-Ion Cells while Avoiding Lithium Plating. J. Electrochem. Soc. 2019, 166, A1412–A1424. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.-H.; Sanchez, A.J.; Kazyak, E.; Goel, V.; Gorlin, Y.; Christensen, J.; Thornton, K.; Dasgupta, N.P. Operando video microscopy of Li plating and re-intercalation on graphite anodes during fast charging. J. Mater. Chem. A 2021, 9, 23522–23536. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Konz, Z.M.; McShane, E.J.; McCloskey, B.D. Detecting the Onset of Lithium Plating and Monitoring Fast Charging Performance with Voltage Relaxation. ACS Energy Lett. 2020, 5, 1750–1757. [Google Scholar] [CrossRef]
- Chen, K.H.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C Fast Charging of Li-Ion Batteries with Graphite/Hard Carbon Hybrid Anodes. Adv. Energy Mater. 2020, 11, 2003336. [Google Scholar] [CrossRef]
- Gottschalk, L.; Strzelczyk, N.; Adam, A.; Kwade, A. Influence of different anode active materials and blends on the performance and fast-charging capability of lithium-ion battery cells. J. Energy Storage 2023, 68, 107706. [Google Scholar] [CrossRef]
- Strzelczyk, N.; Gottschalk, L.; Müller, J.; Kwade, A. The Influence of Calendering on the Fast Charging Performance and Lithium Plating of Hard Carbon Blend Anodes. Energy Technol. 2022, 11, 2200865. [Google Scholar] [CrossRef]
- Gottschalk, L.; Oertel, C.; Strzelczyk, N.; Müller, J.; Krüger, J.; Haselrieder, W.; Kwade, A. Improving the Performance of Lithium-Ion Batteries Using a Two-Layer, Hard Carbon-Containing Silicon Anode for Use in High-Energy Electrodes. Energy Technol. 2022, 11, 2200858. [Google Scholar] [CrossRef]
- Gottschalk, L.; Müller, J.; Schoo, A.; Baasch, E.; Kwade, A. Spherical Graphite Anodes: Influence of Particle Size Distribution and Multilayer Structuring in Lithium-Ion Battery Cells. Batteries 2024, 10, 40. [Google Scholar] [CrossRef]
- Müller, D.; Fill, A.; Birke, K.P. Cycling of Double-Layered Graphite Anodes in Pouch-Cells. Batteries 2022, 8, 22. [Google Scholar] [CrossRef]
- Müller, D.; Landa-Medrano, I.; Eguia-Barrio, A.; Boyano, I.; Urdampilleta, I.; de Meatza, I.; Fill, A.; Birke, P. Electrochemical characterization of bi-layered graphite anodes combining high and low porosity in lithium-ion cells to improve cell performance. Electrochim. Acta 2021, 391, 138966. [Google Scholar] [CrossRef]
- Pfleging, W. Recent progress in laser texturing of battery materials: A review of tuning electrochemical performances, related material development, and prospects for large-scale manufacturing. Int. J. Extrem. Manuf. 2020, 3, 012002. [Google Scholar] [CrossRef]
- Keilhofer, J.; Schaffranka, L.W.F.; Wuttke, A.; Günter, F.J.; Hille, L.; Dorau, F.A.; Daub, R. Mechanical Structuring of Lithium-Ion Battery Electrodes Using an Embossing Roller. Energy Technol. 2023, 11, 2200869. [Google Scholar] [CrossRef]
- Delattre, B.; Amin, R.; Sander, J.; De Coninck, J.; Tomsia, A.P.; Chiang, Y.-M. Impact of Pore Tortuosity on Electrode Kinetics in Lithium Battery Electrodes: Study in Directionally Freeze-Cast LiNi0.8Co0.15Al0.05O2(NCA). J. Electrochem. Soc. 2018, 165, A388–A395. [Google Scholar] [CrossRef]
- Rist, U.; Falkowski, V.; Pfleging, W. Electrochemical Properties of Laser-Printed Multilayer Anodes for Lithium-Ion Batteries. Nanomaterials 2023, 13, 2411. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Hille, L.; Noecker, M.P.; Ko, B.; Kriegler, J.; Keilhofer, J.; Stock, S.; Zaeh, M.F. Integration of laser structuring into the electrode manufacturing process chain for lithium-ion batteries. J. Power Sources 2023, 556, 232478. [Google Scholar] [CrossRef]
- Meyer, A.; Zhu, P.; Smith, A.; Pfleging, W. Gaining a New Technological Readiness Level for Laser-Structured Electrodes in High-Capacity Lithium-Ion Pouch Cells. Batteries 2023, 9, 548. [Google Scholar] [CrossRef]
- Hille, L.; Kriegler, J.; Oehler, A.; Chaja, M.; Wagner, S.; Zaeh, M.F. Picosecond laser structuring of graphite anodes—Ablation characteristics and process scaling. J. Laser Appl. 2023, 35, 042054. [Google Scholar] [CrossRef]
- Kriegler, J.; Hille, L.; Stock, S.; Kraft, L.; Hagemeister, J.; Habedank, J.B.; Jossen, A.; Zaeh, M.F. Enhanced performance and lifetime of lithium-ion batteries by laser structuring of graphite anodes. Appl. Energy 2021, 303, 117693. [Google Scholar] [CrossRef]
- Chen, K.-H.; Namkoong, M.J.; Goel, V.; Yang, C.; Kazemiabnavi, S.; Mortuza, S.M.; Kazyak, E.; Mazumder, J.; Thornton, K.; Sakamoto, J.; et al. Efficient fast-charging of lithium-ion batteries enabled by laser-patterned three-dimensional graphite anode architectures. J. Power Sources 2020, 471, 228475. [Google Scholar] [CrossRef]
- Habedank, J.B.; Kriegler, J.; Zaeh, M.F. Enhanced Fast Charging and Reduced Lithium-Plating by Laser-Structured Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3940–A3949. [Google Scholar] [CrossRef]
- Dunlap, N.; Sulas-Kern, D.B.; Weddle, P.J.; Usseglio-Viretta, F.; Walker, P.; Todd, P.; Boone, D.; Colclasure, A.M.; Smith, K.; Tremolet de Villers, B.J.; et al. Laser ablation for structuring Li-ion electrodes for fast charging and its impact on material properties, rate capability, Li plating, and wetting. J. Power Sources 2022, 537, 231464. [Google Scholar] [CrossRef]
- Dubey, R.; Zwahlen, M.D.; Shynkarenko, Y.; Yakunin, S.; Fuerst, A.; Kovalenko, M.V.; Kravchyk, K.V. Laser Patterning of High-Mass-Loading Graphite Anodes for High-Performance Li-Ion Batteries. Batter. Supercaps 2020, 4, 464–468. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.-J.; Jeong, S.; Kim, H.-J. Challenges, laser processing and electrochemical characteristics on application of ultra-thick electrode for high-energy lithium-ion battery. J. Power Sources 2021, 482, 228948. [Google Scholar] [CrossRef]
- Schweighofer, L.; Eschelmuller, B.; Frohlich, K.; Pfleging, W.; Pichler, F. Modelling and Optimisation of Laser-Structured Battery Electrodes. Nanomaterials 2022, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Seifert, H.J.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Meyer, A.; Ball, F.; Pfleging, W. The Effect of Silicon Grade and Electrode Architecture on the Performance of Advanced Anodes for Next Generation Lithium-Ion Cells. Nanomaterials 2021, 11, 3448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Ebert, B.; Smyrek, P.; Pfleging, W. The Impact of Structural Pattern Types on the Electrochemical Performance of Ultra-Thick NMC 622 Electrodes for Lithium-Ion Batteries. Batteries 2024, 10, 58. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technology 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Meyer, A.; Sterzl, Y.; Pfleging, W. High repetition ultrafast laser ablation of graphite and silicon/graphite composite electrodes for lithium-ion batteries. J. Laser Appl. 2023, 35, 042036. [Google Scholar] [CrossRef]
- Kouli, M.; Kandula, M.W.; Dilger, K.; Klotzbach, U.; Kling, R.; Watanabe, A. Laser-material-interactions between ultrashort pulse lasers and electrodes for lithium-ion batteries during micro-structuring the electrode surface. In Proceedings of the Laser-Based Micro- and Nanoprocessing XV, San Francisco, CA, USA, 27 October 2021; SPIE: Bellingham, WA, USA, 2021. [Google Scholar]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373–A1387. [Google Scholar] [CrossRef]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance Spectroscopy Characterization of Porous Electrodes under Different Electrode Thickness Using a Symmetric Cell for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. Npj Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Lasia, A. Modeling of Impedance of Porous Electrodes. In Modeling and Numerical Simulations I; Springer: New York, NY, USA, 2009; pp. 67–137. [Google Scholar]
- Schneider, L.; Klemens, J.; Herbst, E.C.; Müller, M.; Scharfer, P.; Schabel, W.; Bauer, W.; Ehrenberg, H. Transport Properties in Electrodes for Lithium-Ion Batteries: Comparison of Compact versus Porous NCM Particles. J. Electrochem. Soc. 2022, 169, 100553. [Google Scholar] [CrossRef]
- De Lauri, V.; Krumbein, L.; Hein, S.; Prifling, B.; Schmidt, V.; Danner, T.; Latz, A. Beneficial Effects of Three-Dimensional Structured Electrodes for the Fast Charging of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 13847–13859. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid electrolyte wetting of lithium-ion batteries containing laser structured electrodes: In situ visualization by neutron radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778. [Google Scholar] [CrossRef]
- Sterzl, Y.; Pfleging, W.; Kling, R.; Pfleging, W.; Sugioka, K. An electrode design study: Laser structuring of anodes for fast-charging of batteries. In Proceedings of the Laser-Based Micro- and Nanoprocessing XVIII, San Francisco, CA, USA, 27 January–1 February 2024; SPIE: Bellingham, WA, USA, 2024. [Google Scholar]
- Von Lüders, C.; Zinth, V.; Erhard, S.V.; Osswald, P.J.; Hofmann, M.; Gilles, R.; Jossen, A. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction. J. Power Sources 2017, 342, 17–23. [Google Scholar] [CrossRef]
- Günter, F.J.; Wassiliadis, N. State of the Art of Lithium-Ion Pouch Cells in Automotive Applications: Cell Teardown and Characterization. J. Electrochem. Soc. 2022, 169, 030515. [Google Scholar] [CrossRef]



| Material | Anode Mass Fraction/wt.% | Cathode Mass Fraction/wt.% |
|---|---|---|
| graphite | 93 | - |
| CB | 1.4 | 3 |
| CMC | 1.87 | - |
| SBR | 3.73 | - |
| NMC622 | - | 92 |
| PVDF | - | 3 |
| conductive graphite | - | 2 |
| solid content/wt.% | 51.2 | 66.7 |
| Charging CC | 0.1C | 0.2C | 0.5C | 1C | 2C | 3C | 5C |
| Cut-off CV | 0.05C | 0.1C | 0.1C | 0.1C | 0.1C | 0.1C | 0.1C |
| Discharge CC | 0.1C | 0.2C | 0.2C | 0.2C | 0.2C | 0.2C | 0.2C |
| Repetitions | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Unstructured | Hole | Line | Grid | |
|---|---|---|---|---|
| Areal capacity anode/mAh cm−2 | 4.33 ± 0.01 | 4.52 ± 0.04 | 4.38 ± 0.01 | 4.45 ± 0.01 |
| Cell capacity/mAh | 4.13 ± 0.04 | 4.18 ± 0.05 | 4.07 ± 0.03 | 4.09 ± 0.01 |
| N/P-ratio | 1.14 ± 0.00 | 1.17 ± 0.00 | 1.16 ± 0.00 | 1.17 ± 0.00 |
| ICE/% | 85.75 ± 1.75 | 83.48 ± 4.56 | 86.39 ± 0.23 | 86.37 ± 0.19 |
| Mass loss (anode)/% | - | 9.7 ± 0.6 | 12.4 ± 0.1 | 11.0 ± 0.2 |
| Unstructured | Hole | Line | Grid | New Ref. | |
|---|---|---|---|---|---|
| dV/dt onset of lithium plating | 1C | 2C | 3C | 2C | 2C |
| Charging time to SOC 80%/min | 85.57 (C/2) | 43.43 (1C) | 24.07 (2C) | 42.99 (1C) | 43.5 (1C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterzl, Y.; Pfleging, W. Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating. Batteries 2024, 10, 160. https://doi.org/10.3390/batteries10050160
Sterzl Y, Pfleging W. Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating. Batteries. 2024; 10(5):160. https://doi.org/10.3390/batteries10050160
Chicago/Turabian StyleSterzl, Yannic, and Wilhelm Pfleging. 2024. "Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating" Batteries 10, no. 5: 160. https://doi.org/10.3390/batteries10050160
APA StyleSterzl, Y., & Pfleging, W. (2024). Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating. Batteries, 10(5), 160. https://doi.org/10.3390/batteries10050160







