Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Super P–Sulfur Cathode Fabrication
2.3. Solid State Electrolyte Synthesis
2.4. Ionic Liquid Preparation
2.5. Cathode Characterization
2.6. Battery Assembly
2.7. Electrochemical Testing
2.8. X-ray Photoelectron Spectroscopy
2.9. Ab Initio Molecular Dynamics Simulations
3. Results
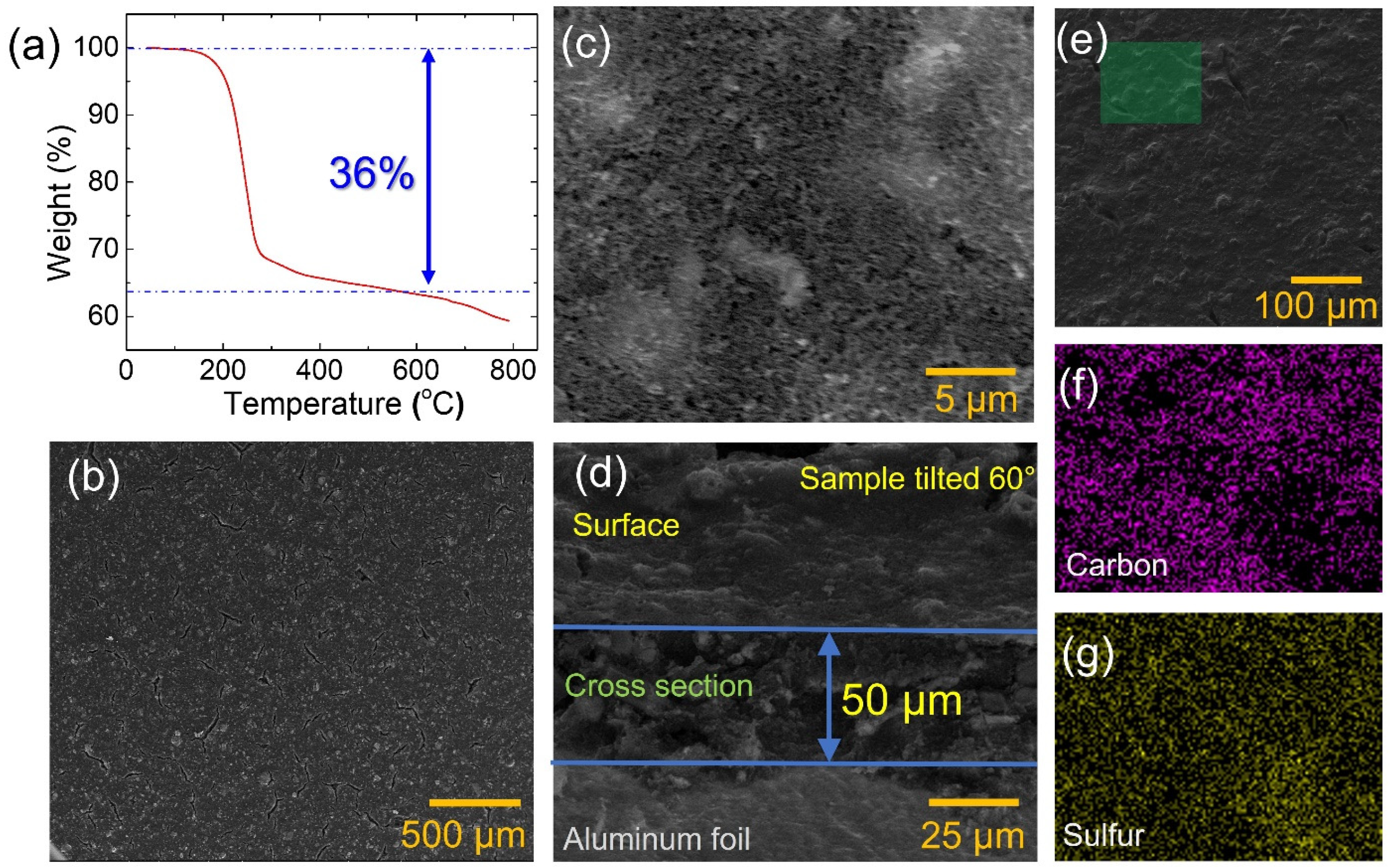
3.1. Cathode Characterization
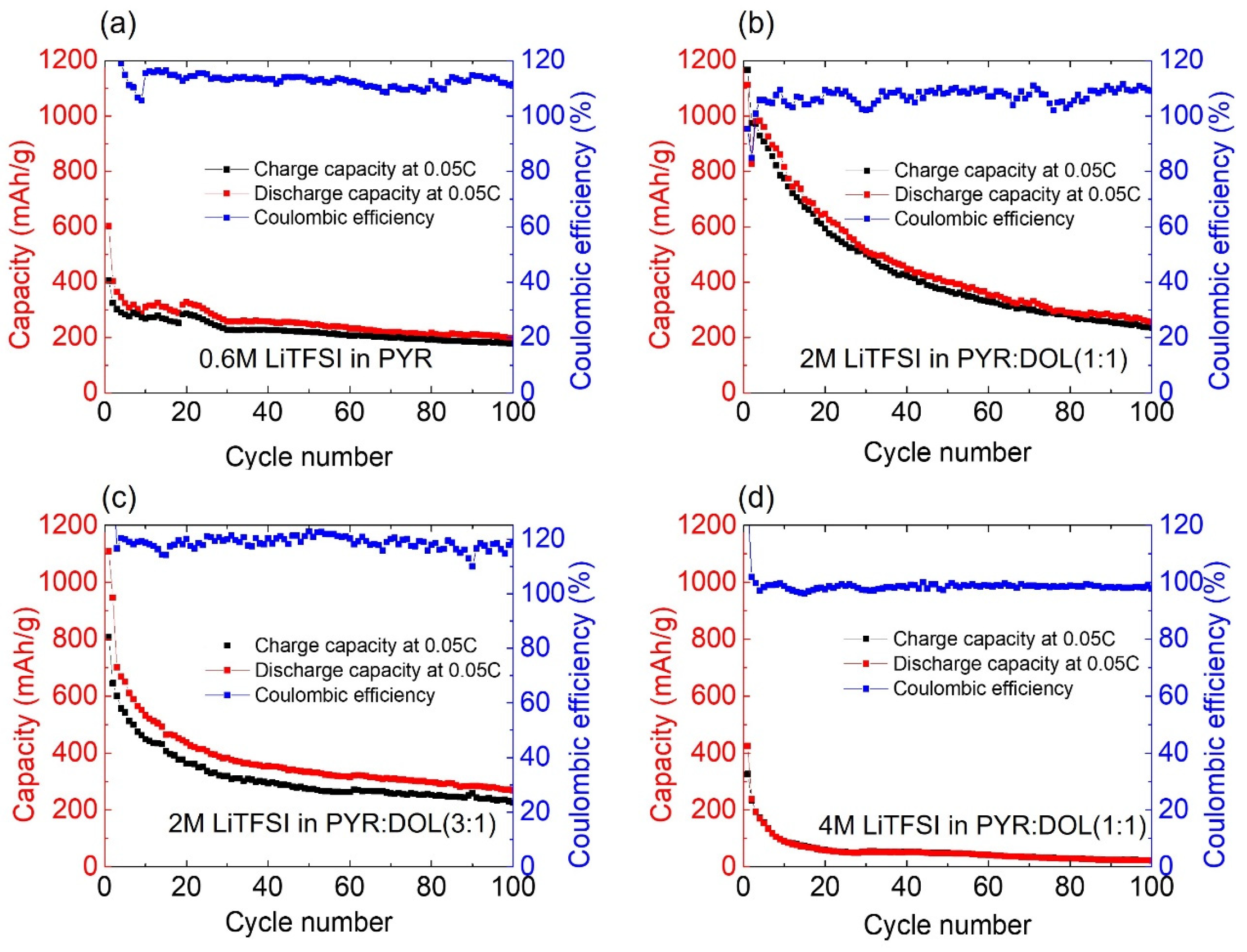
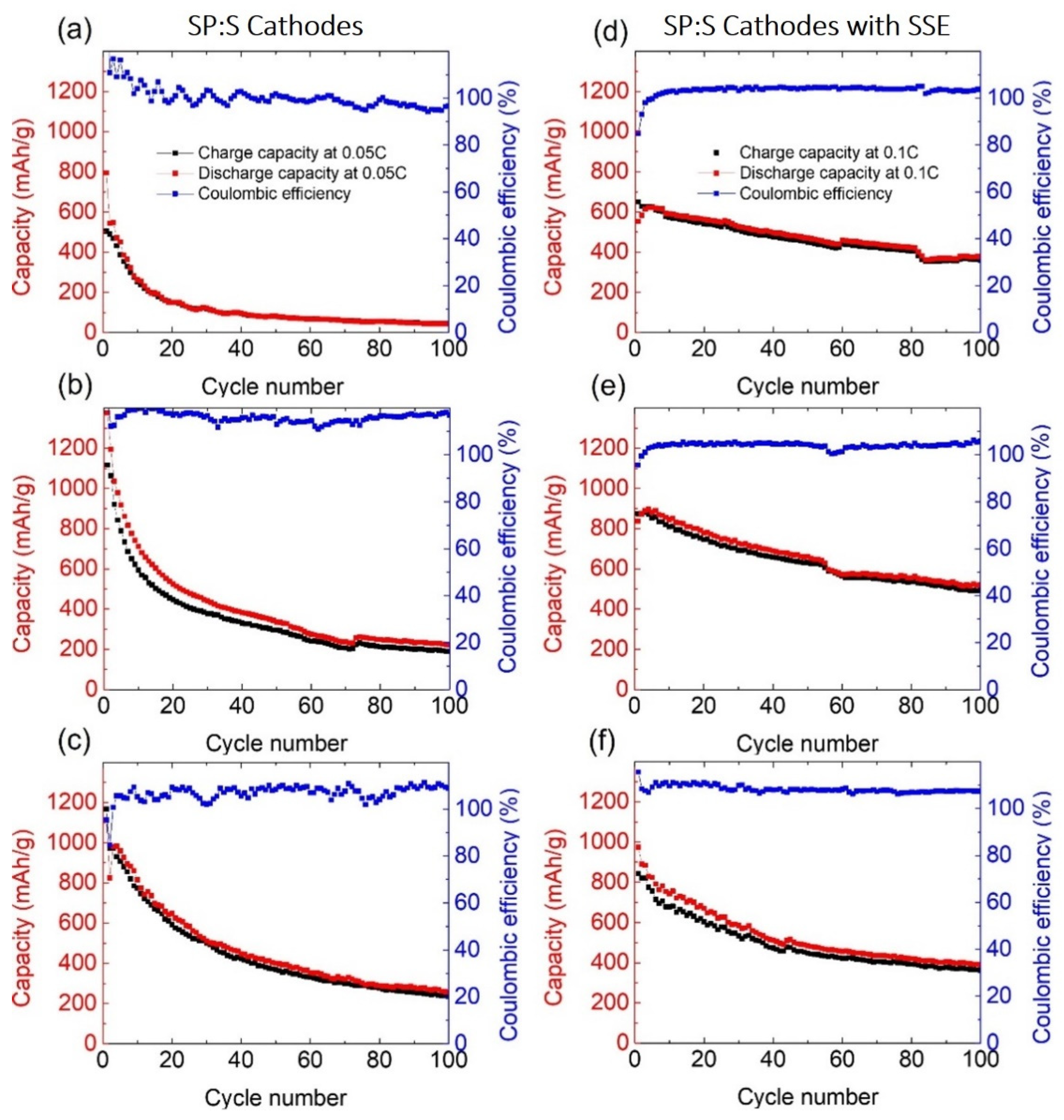
3.2. Electrochemical Testing
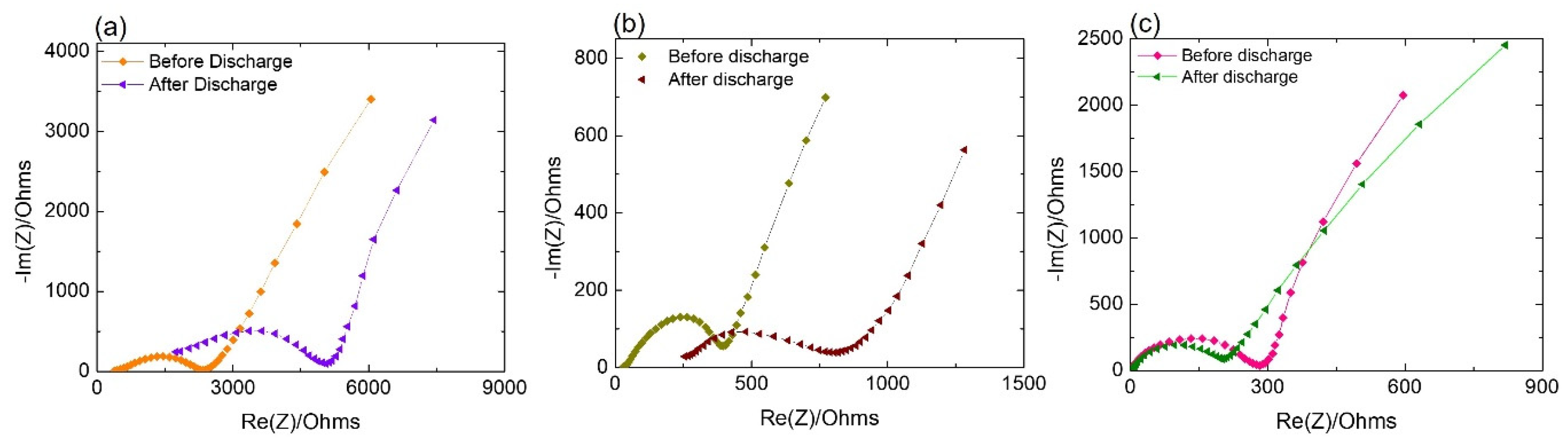
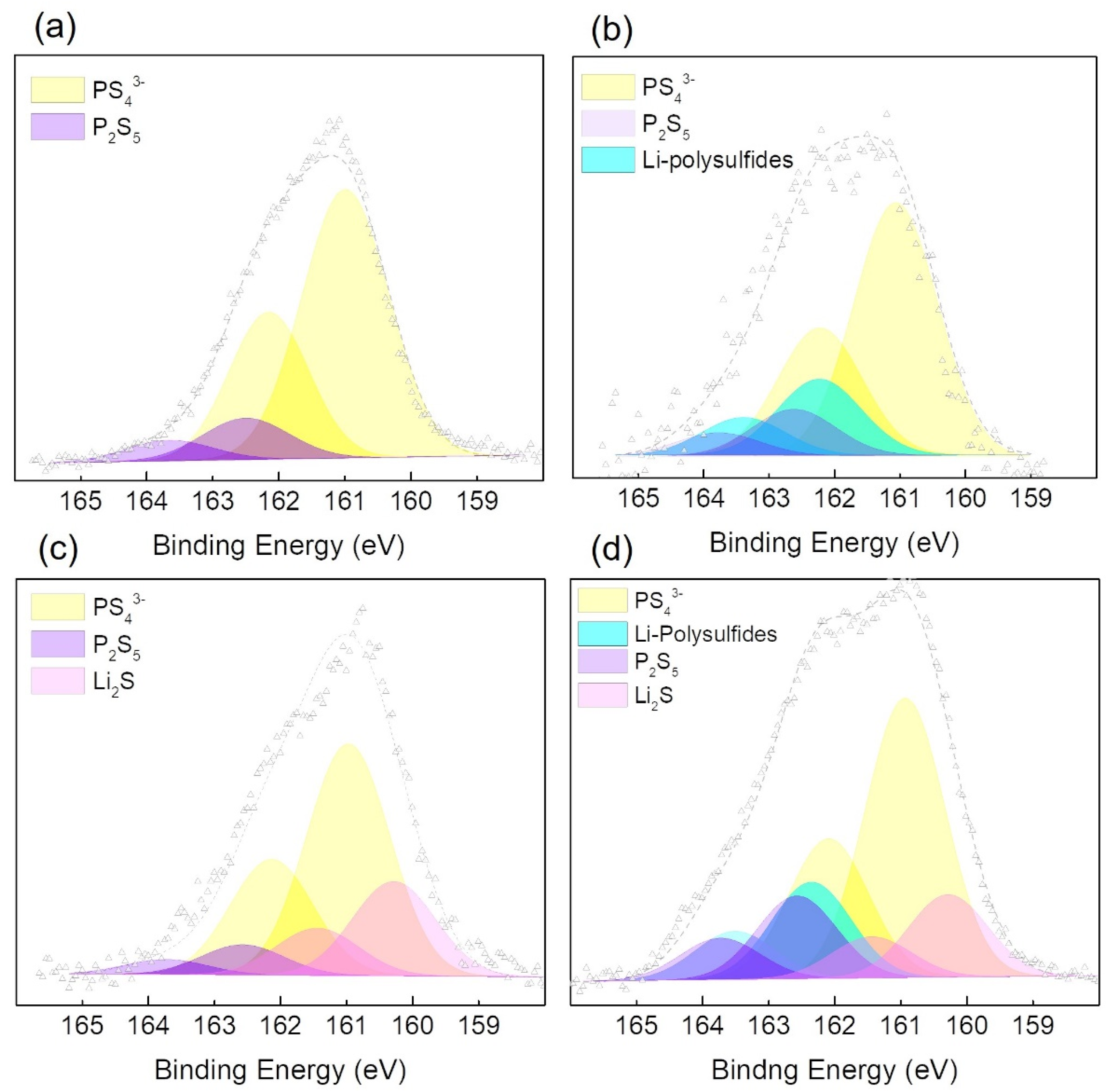
3.3. XPS Results
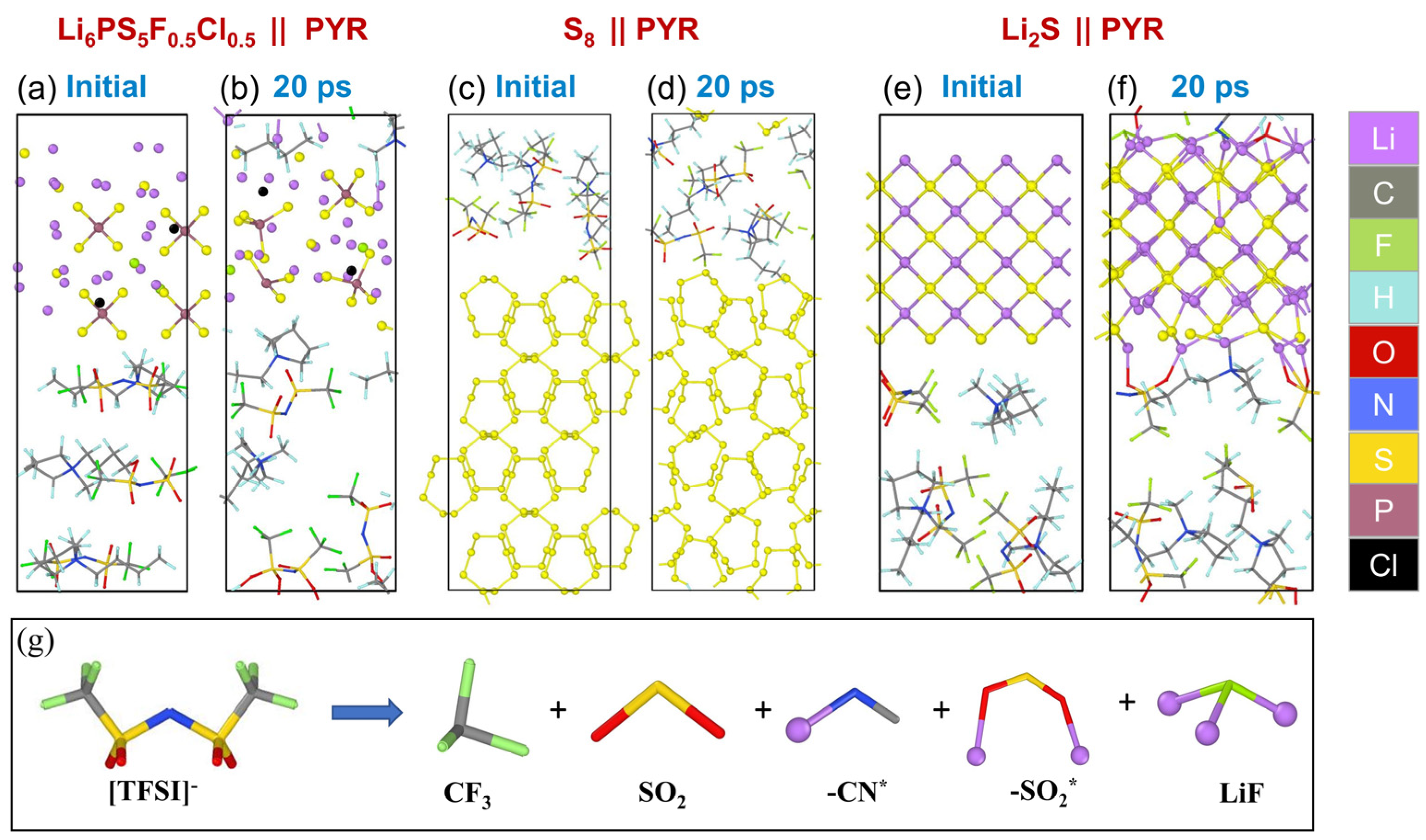
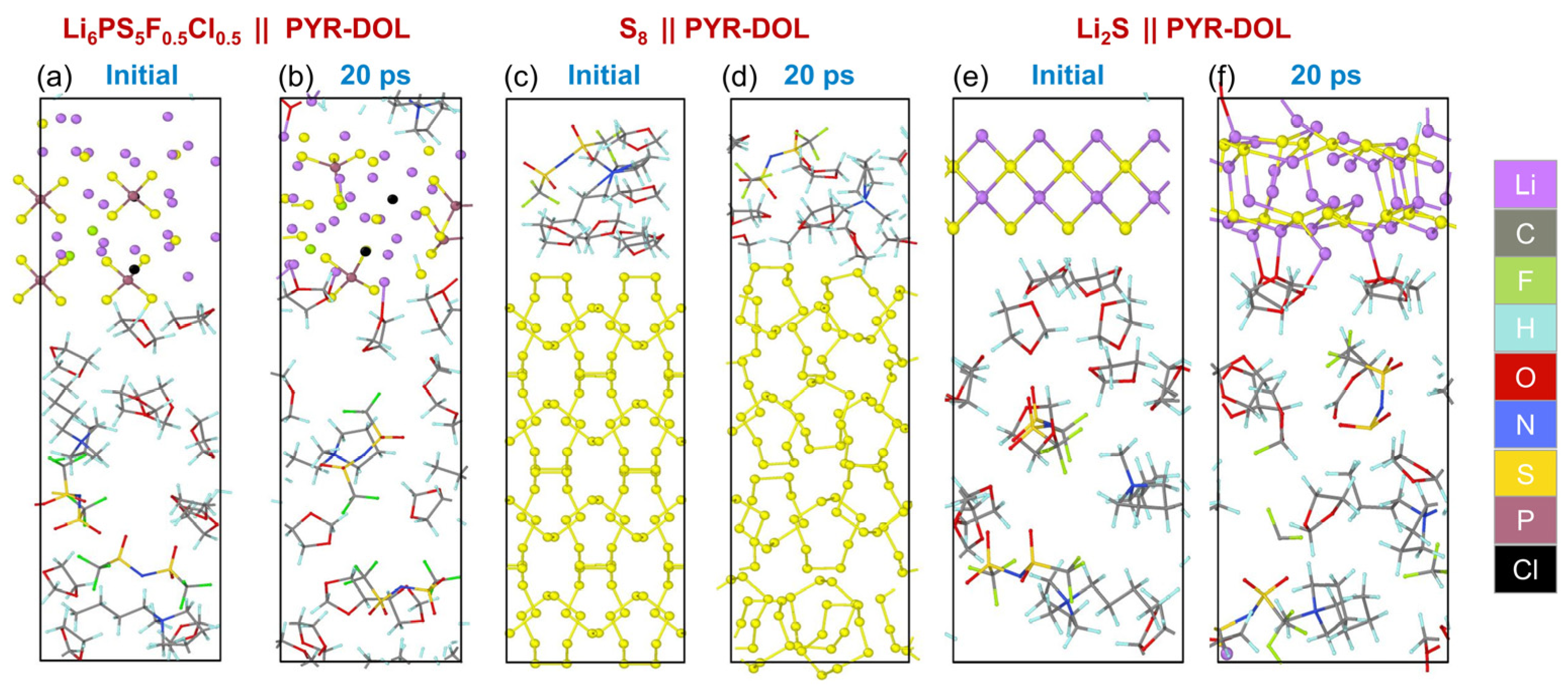
3.4. AIMD Simulation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, K.; Kalaiselvi, N. High sulfur loaded carbon aerogel cathode for lithium–sulfur batteries. RSC Adv. 2015, 5, 34008–34018. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-conductivity argyrodite Li6PS5Cl solid electrolytes prepared via optimized sintering processes for all-solid-state lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural design of lithium–sulfur batteries: From fundamental research to practical application. Electrochem. Energy Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Barchasz, C.; Molton, F.; Duboc, C.; Leprêtre, J.C.; Patoux, S.; Alloin, F. Lithium/sulfur cell discharge mechanism: An original approach for intermediate species identification. Anal. Chem. 2012, 84, 3973–3980. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969. [Google Scholar] [CrossRef]
- Ai, W.; Zhou, W.; Du, Z.; Chen, Y.; Sun, Z.; Wu, C.; Zou, C.; Li, C.; Huang, W.; Yu, T. Nitrogen and phosphorus co-doped hierarchically porous carbon as an efficient sulfur host for Li-S batteries. Energy Storage Mater. 2017, 6, 112–118. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Lee, J.H.; Koo, H.Y.; Shakoor, R.A.; Kahraman, R.; Jo, Y.N.; Park, M.-S.; Choi, J.W. Hierarchical porous carbon by ultrasonic spray pyrolysis yields stable cycling in lithium–sulfur battery. Nano Lett. 2014, 14, 4418–4425. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, Q.; Yang, X.; Liang, J.; Koo, A.; Li, W.; Liang, J.; Wang, J.; Li, R.; Holness, F.B.; et al. Toward a remarkable Li-S battery via 3D printing. Nano Energy 2019, 56, 595–603. [Google Scholar] [CrossRef]
- Bauer, I.; Thieme, S.; Brückner, J.; Althues, H.; Kaskel, S. Reduced polysulfide shuttle in lithium–sulfur batteries using Nafion-based separators. J. Power Sources 2014, 251, 417–422. [Google Scholar] [CrossRef]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Linford, R.G.; Hackwood, S. Physical techniques for the study of solid electrolytes. Chem. Rev. 1981, 81, 327–364. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef] [PubMed]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bi, Z.; Zhao, F.; Manthiram, A. Polysulfide-shuttle control in lithium-sulfur batteries with a chemically/electrochemically compatible NASICON-type solid electrolyte. Adv. Energy Mater. 2016, 6, 1601392. [Google Scholar] [CrossRef]
- Yu, X.; Bi, Z.; Zhao, F.; Manthiram, A. Hybrid lithium–sulfur batteries with a solid electrolyte membrane and lithium polysulfide catholyte. ACS Appl. Mater. Interfaces 2015, 7, 16625–16631. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.R.; Drossel, T.; Leichtweiss, T.; Weber, D.A.; Falk, M.; Schneider, M.; Reich, M.-L.; Sommer, H.; Adelhelm, P.; Janek, J. Dynamic formation of a solid-liquid electrolyte interphase and its consequences for hybrid-battery concepts. Nat. Chem. 2016, 8, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Luo, J.; Sun, Q.; Yang, X.; Li, R.; Sun, X. Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019, 21, 308–334. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-solid-state garnet type sulfurized polyacrylonitrile/lithium-metal battery enabled by an inorganic lithium conductive salt and a bilayer electrolyte architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards high-performance solid-state Li–S batteries: From fundamental understanding to engineering design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Cairns, E.J. N-Methyl-(n-butyl) pyrrolidinium bis (trifluoromethanesulfonyl) imide-LiTFSI–poly (ethylene glycol) dimethyl ether mixture as a Li/S cell electrolyte. J. Power Sources 2008, 177, 537–545. [Google Scholar] [CrossRef]
- Meisner, Q.J.; Rojas, T.; Glossmann, T.; Hintennach, A.; Liu, Q.; Cao, J.; Redfern, P.C.; Ngo, A.T.; Curtiss, L.A.; Zhang, Z. Impact of co-solvent and LiTFSI concentration on ionic liquid-based electrolytes for Li-S battery. J. Electrochem. Soc. 2020, 167, 070528. [Google Scholar] [CrossRef]
- Dharmasena, R.; Thapa, A.K.; Hona, R.K.; Jasinski, J.; Sunkara, M.K.; Sumanasekera, G.U. Mesoporous TiO2 coating on carbon–sulfur cathode for high capacity Li–sulfur battery. RSC Adv. 2018, 8, 11622–11632. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.S.; Fu, Y.; Manthiram, A. Self-weaving sulfur–carbon composite cathodes for high rate lithium–sulfur batteries. Phys. Chem. Chem. Phys. 2012, 14, 14495–14499. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Chung, S.H.; Manthiram, A.; Kalra, V. A free-standing carbon nanofiber interlayer for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S batteries using amine-functionalized carbon nanotubes in the cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Zhang, Y.; Wang, Y.; Mentbayeva, A.; Bakenov, Z. Synthesis of carbon coated Fe3O4 grown on graphene as effective sulfur-host materials for advanced lithium/sulfur battery. J. Power Sources 2019, 437, 226901. [Google Scholar] [CrossRef]
- Li, T.; Bo, H.; Cao, H.; Lai, Y.; Liu, Y.; Huang, Z. Effects of carbon hosts on electrochemical properties of lithium-sulfur batteries. Int. J. Electrochem. Sci. 2017, 12, 5731–5741. [Google Scholar] [CrossRef]
- Cho, I.; Choi, J.; Kim, K.; Ryou, M.H.; Lee, Y.M. A comparative investigation of carbon black (Super-P) and vapor-grown carbon fibers (VGCFs) as conductive additives for lithium-ion battery cathodes. RSC Adv. 2015, 5, 95073–95078. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, K.; Li, L.; Huang, H.; Liang, C.; Gan, Y.; He, X.; Zhang, W.; Zhang, J. High-Performance All-Solid-State Lithium–Sulfur Batteries Enabled by Slurry-Coated Li6PS5Cl/S/C Composite Electrodes. Front. Energy Res. 2021, 8, 606494. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Sun, C.; Jin, J.; Wang, Q.; Chen, X.; Zha, W.; Wen, Z. Sustained release-driven formation of ultrastable sei between Li6PS5Cl and lithium anode for sulfide-based solid-state batteries. Adv. Energy Mater. 2021, 11, 2002545. [Google Scholar] [CrossRef]
- Fan, X.; Ji, X.; Han, F.; Yue, J.; Chen, J.; Chen, L.; Deng, T.; Jiang, J.; Wang, C. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 2018, 4, eaau9245. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Arnold, W.; Shreyas, V.; Li, Y.; Koralalage, M.K.; Jasinski, J.B.; Thapa, A.; Sumanasekera, G.; Ngo, A.T.; Narayanan, B.; Wang, H. Synthesis of Fluorine-Doped Lithium Argyrodite Solid Electrolytes for Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 11483–11492. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A mathematical model for a lithium–sulfur cell. J. Electrochem. Soc. 2008, 155, A576. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; de Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, J.; Xie, W.; Chen, J.; Ke, X.; Ma, J.; Shi, Z. Effect of LiTFSI and LiFSI on cycling performance of lithium metal batteries using thermoplastic polyurethane/halloysite nanotubes solid electrolyte. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 359–372. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Camacho-Forero, L.E.; Schwarz, A.M.; Shutthanandan, V.; Thevuthasan, S.; Balbuena, P.B.; Mueller, K.T.; Murugesan, V. In situ chemical imaging of solid-electrolyte interphase layer evolution in Li–S batteries. Chem. Mater. 2017, 29, 4728–4737. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.-Z.; Li, B.; Yan, C.; Zhu, L.; Guan, C.; Cheng, X.-B.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. Towards stable lithium-sulfur batteries: Mechanistic insights into electrolyte decomposition on lithium metal anode. Energy Storage Mater. 2017, 8, 194–201. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Liu, Q.; Cresce, A.; Schroeder, M.; Xu, K.; Mu, D.; Wu, B.; Shi, L.; Wu, F. Insight on lithium metal anode interphasial chemistry: Reduction mechanism of cyclic ether solvent and SEI film formation. Energy Storage Mater. 2019, 17, 366–373. [Google Scholar] [CrossRef]
- Merinov, B.V.; Zybin, S.V.; Naserifar, S.; Morozov, S.; Oppenheim, J.; Goddard, W.A., III; Lee, J.; Lee, J.H.; Han, H.E.; Choi, Y.C.; et al. Interface structure in Li-metal/[Pyr14][TFSI]-ionic liquid system from ab initio molecular dynamics simulations. J. Phys. Chem. Lett. 2019, 10, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Halacoglu, S.; Shreyas, V.; Arnold, W.; Guo, X.; Dou, Q.; Jasinski, J.B.; Narayanan, B.; Wang, H. Highly efficient interface stabilization for ambient-temperature quasi-solid-state sodium metal batteries. Chem. Eng. J. 2022, 434, 134679. [Google Scholar] [CrossRef]


| Ionic Liquid/Diluent | SSE/Cathode | Lattice Parameters (Å) | ||
|---|---|---|---|---|
| a | b | c | ||
| ([PYR14][TFSI]) | Li6PS5F0.5Cl0.5 | 10.186 | 10.186 | 28.636 |
| Li2S | 11.187 | 11.187 | 26.087 | |
| S8 | 10.559 | 12.945 | 38.170 | |
| DOL and DME (1:1) | Li6PS5F0.5Cl0.5 | 10.186 | 10.186 | 30.586 |
| Li2S | 11.187 | 11.187 | 27.387 | |
| S8 | 10.559 | 12.945 | 33.800 | |
| ([PYR14][TFSI]) and DOL (1:1) | Li6PS5F0.5Cl0.5 | 10.186 | 10.186 | 36.986 |
| Li2S | 11.187 | 11.187 | 30.150 | |
| S8 | 10.559 | 12.945 | 39.500 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koralalage, M.K.; Shreyas, V.; Arnold, W.R.; Akter, S.; Thapa, A.; Narayanan, B.; Wang, H.; Sumanasekera, G.U.; Jasinski, J.B. Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study. Batteries 2024, 10, 155. https://doi.org/10.3390/batteries10050155
Koralalage MK, Shreyas V, Arnold WR, Akter S, Thapa A, Narayanan B, Wang H, Sumanasekera GU, Jasinski JB. Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study. Batteries. 2024; 10(5):155. https://doi.org/10.3390/batteries10050155
Chicago/Turabian StyleKoralalage, Milinda Kalutara, Varun Shreyas, William R. Arnold, Sharmin Akter, Arjun Thapa, Badri Narayanan, Hui Wang, Gamini U. Sumanasekera, and Jacek B. Jasinski. 2024. "Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study" Batteries 10, no. 5: 155. https://doi.org/10.3390/batteries10050155
APA StyleKoralalage, M. K., Shreyas, V., Arnold, W. R., Akter, S., Thapa, A., Narayanan, B., Wang, H., Sumanasekera, G. U., & Jasinski, J. B. (2024). Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study. Batteries, 10(5), 155. https://doi.org/10.3390/batteries10050155








