Comprehensive Review of Energy Storage Systems Characteristics and Models for Automotive Applications
Abstract
1. Introduction
2. Characteristics of Energy Storage Technologies for Automotive Systems
2.1. Batteries
2.2. Supercapacitors
2.3. Flywheels
2.4. Hydrogen Tank Storage
2.5. Compressed Air Energy Storage System
3. Power Electronic System Models for Energy Storage Systems
3.1. Batteries

3.2. Supercapacitors
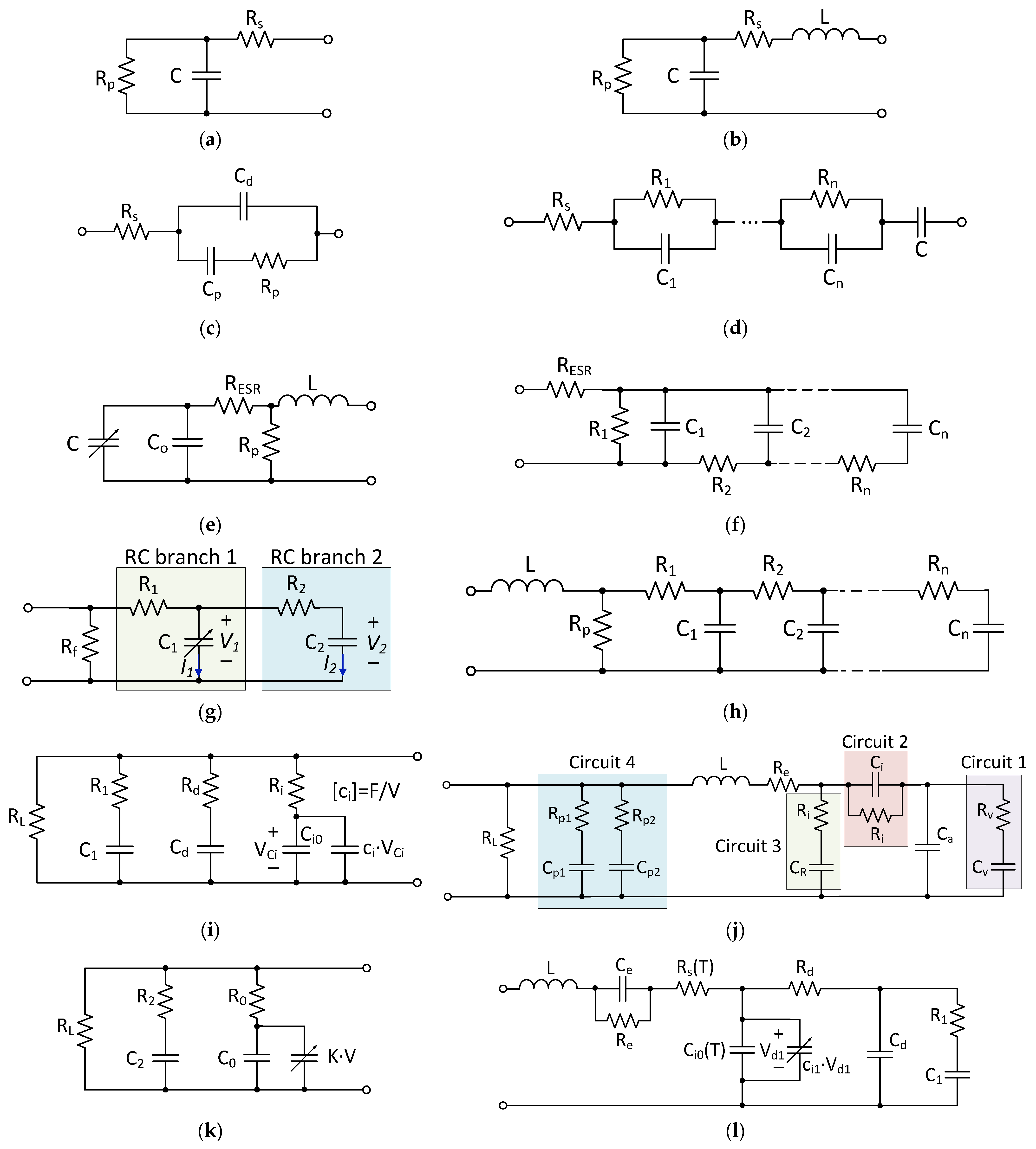
4. Hybrid Energy Storage Systems Applied to E-Mobility
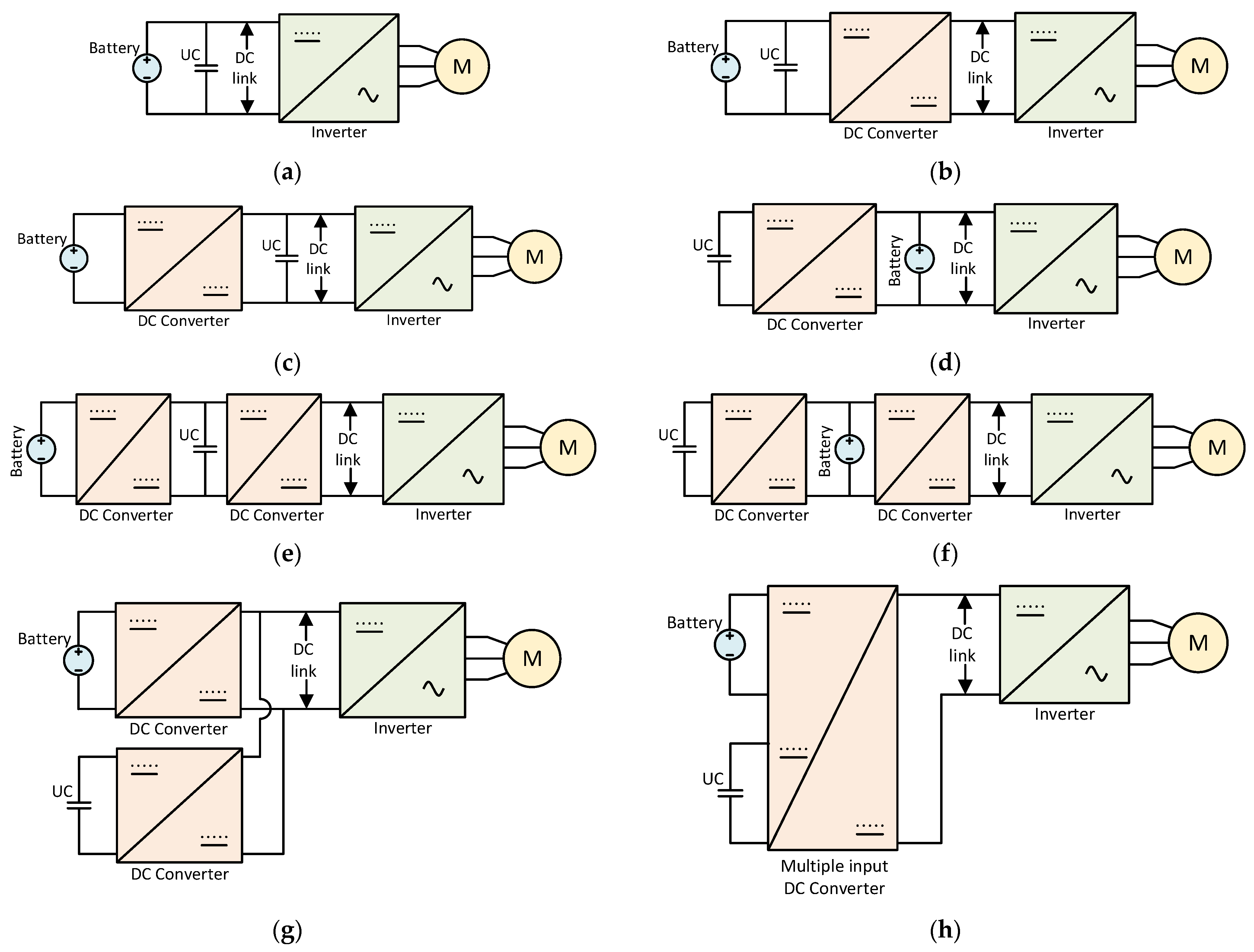
4.1. Battery and Ultracapacitor Hybrid Systems
4.2. Battery–Flywheel Hybrid System
5. Discussions
- ESS lifetime: there are currently limited data on the long-term performance of electric storage systems for e-mobility. This creates significant uncertainty from a social and economic point of view, making revenue forecasting difficult and leading to economic consequences. However, long ESS lifetimes is also an important feature to assist in the decrease in raw material extraction and ensure the possibility of reuse in second-life applications.
- Safety issues: thermal runaway, stranded energy, toxic and flammable gas generation, and deep-seated fires are some examples of safety issues related to the use of ESS for e-mobility that still need to be addressed, even if important steps have been taken in recent decades. This issue is strongly related to the social acceptance of ESS technologies and environmental problems.
- Availability of materials: battery minerals are essential for ESS, particularly lithium, as well as for other applications related to the energy transition. Therefore, their demand will grow rapidly, and a responsible and sustainable development of these resources is a crucial necessity. The availability of materials has an impact on both economic and technological aspects. Huge efforts are being made by academics and industrials to increase the number of ESS solutions with different materials to those that are commonly used, but the socio-environmental impacts of lithium extraction, which is concentrated in a few locations worldwide, are still an unsolved problem [136].
- Recycling issues: even if different ESSs can be recycled, the processes are quite complex, with an important environmental impact, and, depending on the technology, the material recovery percentage is relatively low. Moreover, recycling costs have a huge impact from the economic sustainability perspective.
- E-waste: each ESS also includes several electronic components, which are mostly trashed after batteries’ first life. This issue must be addressed with a proper design, making the replacement and reuse of electronic devices, i.e., converters more feasible. This represents an important technological challenge.
- Reuse and second life: how to reuse batteries that were initially used in e-mobility in other applications, such as stationary storage systems, is often discussed. However, analyses of the state of health and price of second-life batteries are still under lacking, as well as discussions from the economic and technological perspectives. For instance, the European Union proposed the introduction of a patent stating the history of a battery to allow for the most suitable economic and environmental management.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Acronyms
| ESS | Energy Storage System |
| ICE | Internal Combustion Engine |
| EV | Electric Vehicle |
| SC | Supercapacitor |
| CAES | Compressed-Air Energy Storage |
| HESS | Hybrid Energy Storage System |
| RBS | Regenerative Braking System |
| BLDC | Brushless DC (motor) |
| ESD | Energy Storage Device |
| AZIB | Aqueous Zinc-Ion Battery |
| VLA | Vented Lead Acid |
| VRLA | Valve-Regulated Lead Acid |
| PV | Photovoltaic |
| NiCd | Nickel-Cadmium |
| NiMH | Nickel-Metal Hydride |
| NiFe | Nickel-Iron |
| NiZn | Nickel-Zinc |
| DoD | Depth of Discharge |
| EDLC | Electric Double-Layer Capacitor |
| UC | Ultracapacitors |
| PC | Pseudocapacitors |
| HSC | Hybrid Supercapacitors |
| FESS | Flywheel Energy Storage System |
| BEV | Battery Electric Vehicle |
| FC | Fuel Cell |
| PEMFC | Proton-Exchange Membrane Fuel Cells |
| SOFC | Solid-Oxide Fuel Cells |
| DMFC | Direct Methanol Fuel Cells |
| AFC | Alkaline Fuel Cells |
| MCFC | Molten Carbonate Fuel Cells |
| PAFC | Phosphoric Acid Fuel Cells |
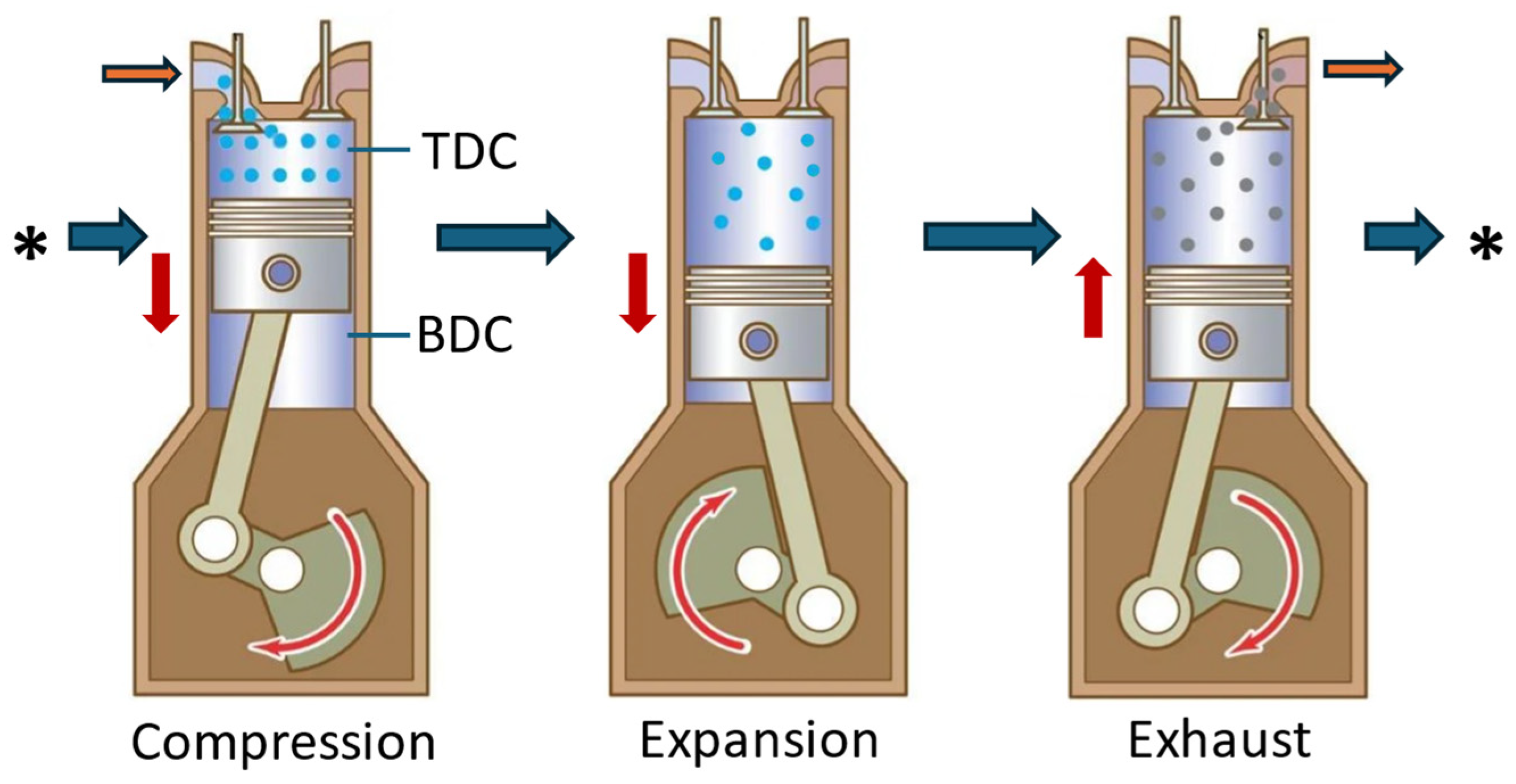
| TDC | Top Dead Centre |
| BDC | Bottom Dead Centre |
| MDI | Motor Development International |
| SoC | State of Charge |
| SoH | State of Health |
| DP | Dual-Polarization Model |
| PNGV | Partnership for a New Generation of Vehicle |
| VOC | Open-Circuit Voltage |
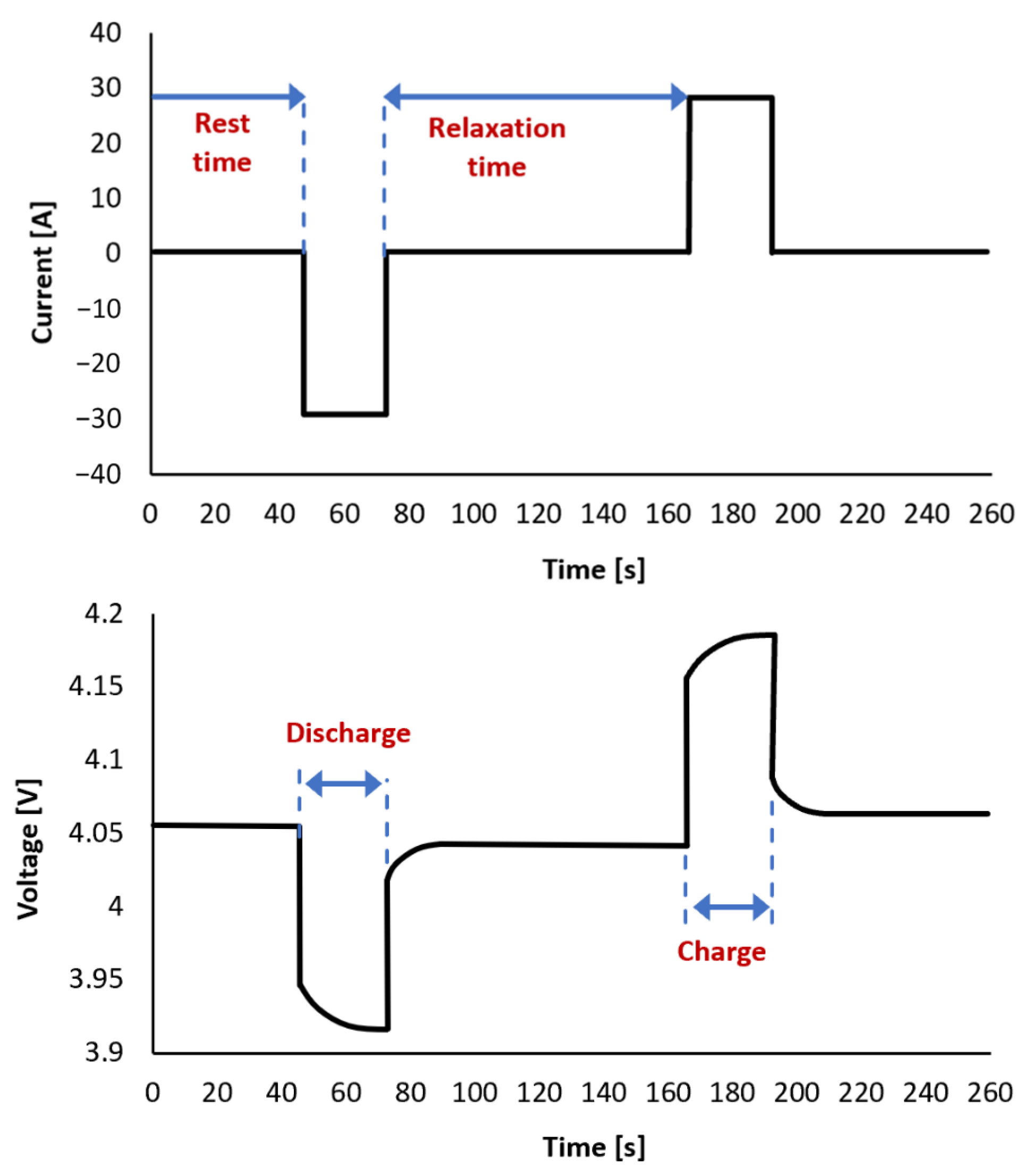
| HPPC | Hybrid Pulse Power Characterization |
| ANN | Artificial Neural Networks |
| ODE | Ordinary Differential Equations |
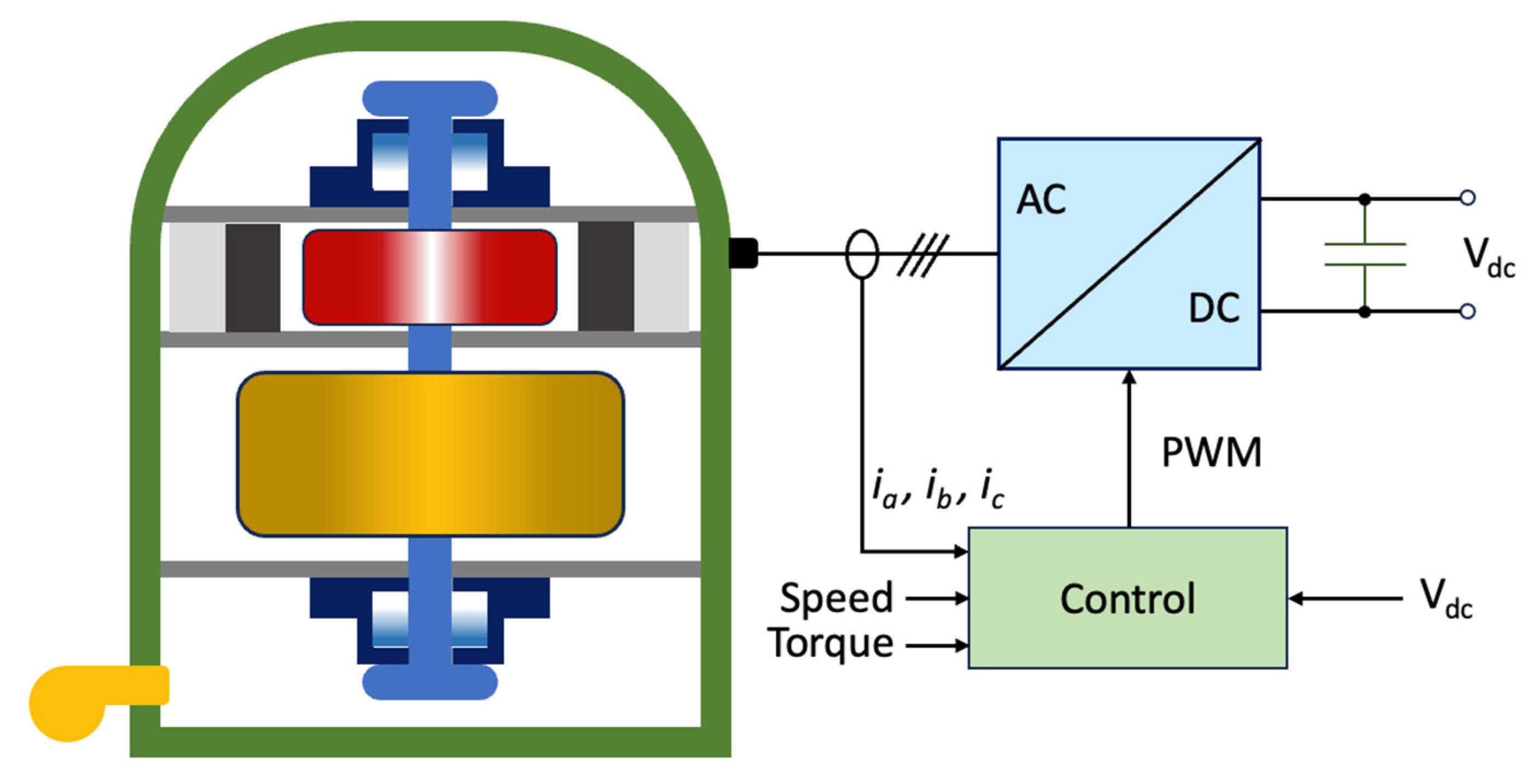
| FOC | Field-Oriented Control |
| DTC | Direct Torque Control |
| EMS | Energy Management System |
References
- Yu, X.; Jin, Y.; Liu, H.; Rai, A.; Kostin, M.; Chantzis, D.; Politis, D.J.; Wang, L. A Review of Renewable Energy and Storage Technologies for Automotive Applications. Int. J. Automot. Manuf. Mater. 2022, 1, 10. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, W.; Xu, S.; Spence, K. Review on Distributed Energy Storage Systems for Utility Applications. CPSS Trans. Power Electron. Appl. 2017, 2, 267–276. [Google Scholar] [CrossRef]
- Habib, A.; Hossain, S. Electric Vehicle Storage Energy System and Single Charge Balancing Circuit: Preview. Authorea 2023. [Google Scholar] [CrossRef]
- Odero, H.Z.; Wekesa, C.W.; Irungu, G.K. Comprehensive Review of Energy Storage Technologies: Types, Applications, Optimal Sizing and Siting in Power Systems. In Proceedings of the 2022 IEEE PES/IAS PowerAfrica, Kigali, Rwanda, 22–26 August 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–5. [Google Scholar]
- Kawakami, N.; Iijima, Y.; Li, H.; Ota, S. High Efficiency Power Converters for Battery Energy Storage Systems. In Proceedings of the 2014 International Power Electronics Conference (IPEC-Hiroshima 2014—ECCE ASIA), Hiroshima, Japan, 18–21 May 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 2095–2099. [Google Scholar]
- Ju, F.; Zhang, Q.; Deng, W.; Li, J. Review of Structures and Control of Battery-Supercapacitor Hybrid Energy Storage System for Electric Vehicles. In Proceedings of the 2014 IEEE International Conference on Automation Science and Engineering (CASE), New Taipei, Taiwan, 18–22 August 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 143–148. [Google Scholar]
- Zhang, L.; Xia, X.; Barzegar, F. Control of a Battery/Supercapacitor Hybrid Energy Storage System for Electric Vehicles. In Proceedings of the 2017 36th Chinese Control Conference (CCC), Dalian, China, 26–28 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 9560–9565. [Google Scholar]
- Bai, Y.; Li, J.; He, H.; Santos, R.C.D.; Yang, Q. Optimal Design of a Hybrid Energy Storage System in a Plug-In Hybrid Electric Vehicle for Battery Lifetime Improvement. IEEE Access 2020, 8, 142148–142158. [Google Scholar] [CrossRef]
- Lai, C.-M.; Cheng, Y.-H.; Hsieh, M.-H.; Lin, Y.-C. Development of a Bidirectional DC/DC Converter With Dual-Battery Energy Storage for Hybrid Electric Vehicle System. IEEE Trans. Veh. Technol. 2018, 67, 1036–1052. [Google Scholar] [CrossRef]
- Naseri, F.; Farjah, E.; Ghanbari, T. An Efficient Regenerative Braking System Based on Battery/Supercapacitor for Electric, Hybrid and Plug-In Hybrid Electric Vehicles with BLDC Motor. IEEE Trans. Veh. Technol. 2016, 65, 3724–3738. [Google Scholar] [CrossRef]
- Nguyen, N.-D.; Yoon, C.; Lee, Y. Il A Standalone Energy Management System of Battery/Supercapacitor Hybrid Energy Storage System for Electric Vehicles Using Model Predictive Control. IEEE Trans. Ind. Electron. 2023, 70, 5104–5114. [Google Scholar] [CrossRef]
- Riaz, A.; Sarker, M.R.; Saad, M.H.M.; Mohamed, R. Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations. Sensors 2021, 21, 5041. [Google Scholar] [CrossRef]
- Zhou, Z.; Benbouzid, M.; Frédéric Charpentier, J.; Scuiller, F.; Tang, T. A Review of Energy Storage Technologies for Marine Current Energy Systems. Renew. Sustain. Energy Rev. 2013, 18, 390–400. [Google Scholar] [CrossRef]
- Guo, R.; Shen, W. A Review of Equivalent Circuit Model Based Online State of Power Estimation for Lithium-Ion Batteries in Electric Vehicles. Vehicles 2021, 4, 1–29. [Google Scholar] [CrossRef]
- Corti, F.; Gulino, M.-S.; Laschi, M.; Lozito, G.M.; Pugi, L.; Reatti, A.; Vangi, D. Time-Domain Circuit Modelling for Hybrid Supercapacitors. Energies 2021, 14, 6837. [Google Scholar] [CrossRef]
- Hu, S.; Liang, Z.; He, X. Ultracapacitor-Battery Hybrid Energy Storage System Based on the Asymmetric Bidirectional Z-Source Topology for EV. IEEE Trans. Power Electron. 2016, 31, 7489–7498. [Google Scholar] [CrossRef]
- Li, Z.; Khajepour, A.; Song, J. A Comprehensive Review of the Key Technologies for Pure Electric Vehicles. Energy 2019, 182, 824–839. [Google Scholar] [CrossRef]
- Yang, C.; Tong, Y.; Yang, Z.; Xiao, H.; Qi, H.; Chen, F. High-Performance Aqueous Zinc-Ion Battery Based on Laser-Induced Graphene. Nanomanuf. Metrol. 2023, 6, 16. [Google Scholar] [CrossRef]
- Grignon, E.; Battaglia, A.M.; Schon, T.B.; Seferos, D.S. Aqueous Zinc Batteries: Design Principles toward Organic Cathodes for Grid Applications. iScience 2022, 25, 104204. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Wang, G.; Wang, D.; Wang, M.; Gao, X.; Bai, Z.; Wang, N.; Yang, J.; Xing, Z.; Dou, S. Recent Progress on Zn Anodes for Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300606. [Google Scholar] [CrossRef]
- Oliveira Farias, H.E.; Neves Canha, L. Battery Energy Storage Systems (BESS) Overview of Key Market Technologies. In Proceedings of the 2018 IEEE PES Transmission & Distribution Conference and Exhibition—Latin America (T&D-LA), Lima, Peru, 18–21 September 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Ogunniyi, E.O.; Pienaar, H. Overview of Battery Energy Storage System Advancement for Renewable (Photovoltaic) Energy Applications. In Proceedings of the 2017 International Conference on the Domestic Use of Energy (DUE), Cape Town, South Africa, 4–5 April 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 233–239. [Google Scholar]
- EUROBAT Lithium-Based. Available online: https://www.eurobat.org/about-secondary-batteries/battery-technologies/lithium-based/ (accessed on 23 September 2023).
- BU-203; Nickel-Based Batteries. Battery University: Richmond, BC, Canada, 2021.
- Revankar, S.T. Chemical Energy Storage. In Storage and Hybridization of Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–227. [Google Scholar]
- Garimella, N.; Nair, N.-K.C. Assessment of Battery Energy Storage Systems for Small-Scale Renewable Energy Integration. In Proceedings of the TENCON 2009—2009 IEEE Region 10 Conference, Singapore, 23–26 January 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1–6. [Google Scholar]
- Kurzweil, P.; Garche, J. Overview of Batteries for Future Automobiles. In Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–96. [Google Scholar]
- Abdin, Z.; Khalilpour, K.R. Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–131. [Google Scholar]
- Hussain, F.; Rahman, M.Z.; Sivasengaran, A.N.; Hasanuzzaman, M. Energy Storage Technologies. In Energy for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–165. [Google Scholar]
- The Electropaedia Battery Knowledge Base Battery and Energy Technologies. Available online: https://www.mpoweruk.com/nickel_iron.htm (accessed on 25 December 2023).
- Thomas, B. Reddy Chapter 31: NICKEL-ZINC BATTERIES. Available online: https://www.globalspec.com/reference/67819/203279/chapter-31-nickel-zinc-batteries (accessed on 25 December 2023).
- Sundén, B. Battery Technologies. In Hydrogen, Batteries and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–79. [Google Scholar]
- Qiao, H.; Wei, Q. Functional Nanofibers in Lithium-Ion Batteries. In Functional Nanofibers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 197–208. [Google Scholar]
- Clean Energy Lithium-Ion Battery. Available online: https://www.cei.washington.edu/research/energy-storage/lithium-ion-battery/ (accessed on 25 December 2023).
- Andreev, M.K. An Overview of Supercapacitors as New Power Sources in Hybrid Energy Storage Systems for Electric Vehicles. In Proceedings of the 2020 XI National Conference with International Participation (ELECTRONICA), Sofia, Bulgaria, 23–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Castro-Gutiérrez, J.; Celzard, A.; Fierro, V. Energy Storage in Supercapacitors: Focus on Tannin-Derived Carbon Electrodes. Front. Mater. 2020, 7, 217. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, Y.; Ma, Y. The Overview of Energy Storage Technology. In Proceedings of the 2015 IEEE International Conference on Mechatronics and Automation (ICMA), Beijing, China, 2–5 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 43–48. [Google Scholar]
- Mahatkar, T.K.; Bachawad, M.R. An Overview of Energy Storage Devices for Distribution Network. In Proceedings of the 2017 International Conference on Computation of Power, Energy Information and Commuincation (ICCPEIC), Melmaruvathur, India, 22–23 March 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 536–541. [Google Scholar]
- Luo, X.; Barreras, J.V.; Chambon, C.L.; Wu, B.; Batzelis, E. Hybridizing Lead–Acid Batteries with Supercapacitors: A Methodology. Energies 2021, 14, 507. [Google Scholar] [CrossRef]
- Timothy, A.; Natalie, R.S.; Zhiwei, M. Thermal, Mechanical, and Hybrid Chemical Energy Storage Systems; Brun, K., Allison, T., Dennis, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128198926. [Google Scholar]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Arabkoohsar, A. Classification of Energy Storage Systems. In Mechanical Energy Storage Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–12. [Google Scholar]
- Gupta, N.; Kaur, N.; Jain, S.K.; Singh Joshal, K. Smart Grid Power System. In Advances in Smart Grid Power System; Elsevier: Amsterdam, The Netherlands, 2021; pp. 47–71. [Google Scholar]
- Gao, D.W. Basic Concepts and Control Architecture of Microgrids. In Energy Storage for Sustainable Microgrid; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–34. [Google Scholar]
- Dongxu, H.; Xingjian, D.; Wen, L.; Yangli, Z.; Xuehui, Z.; Haisheng, C.; Zhilai, Z. A Review of Flywheel Energy Storage Rotor Materials and Structures. J. Energy Storage 2023, 74, 109076. [Google Scholar] [CrossRef]
- Berrada, A.; Loudiyi, K. Energy Storage. In Gravity Energy Storage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–23. [Google Scholar]
- Cheng, Q.; Zhang, R.; Shi, Z.; Lin, J. Review of Common Hydrogen Storage Tanks and Current Manufacturing Methods for Aluminium Alloy Tank Liners. Int. J. Lightweight Mater. Manuf. 2024, 7, 269–284. [Google Scholar] [CrossRef]
- Gómez, J.A.; Santos, D.M.F. The Status of On-Board Hydrogen Storage in Fuel Cell Electric Vehicles. Designs 2023, 7, 97. [Google Scholar] [CrossRef]
- Shin, H.K.; Ha, S.K. A Review on the Cost Analysis of Hydrogen Gas Storage Tanks for Fuel Cell Vehicles. Energies 2023, 16, 5233. [Google Scholar] [CrossRef]
- Bethoux, O. Hydrogen Fuel Cell Road Vehicles: State of the Art and Perspectives. Energies 2020, 13, 5843. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel Cell Application in the Automotive Industry and Future Perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Marvania, D.; Subudhi, S. A Comprehensive Review on Compressed Air Powered Engine. Renew. Sustain. Energy Rev. 2017, 70, 1119–1130. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, Y.; Roskilly, A.P.; Yu, X. A Review of Compressed Air Energy Systems in Vehicle Transport. Energy Strategy Rev. 2021, 33, 100583. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhang, H.; Yang, F.; Liang, J.; Yang, H.; Niu, K.; Liu, Z.; Wang, Y.; Wu, Y. Experimental Investigation of the Output Performance of Compressed-Air-Powered Vehicles with a Pneumatic Motor. Sustainability 2022, 14, 15377. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, H.; Yin, Z.; Liu, Y.; Zhang, X.; Xu, Y.; Chen, H. Advancements in Compressed Air Engine Technology and Power System Integration: A Comprehensive Review. Energy Rev. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Wasbari, F.; Bakar, R.A.; Gan, L.M.; Tahir, M.M.; Yusof, A.A. A Review of Compressed-Air Hybrid Technology in Vehicle System. Renew. Sustain. Energy Rev. 2017, 67, 935–953. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of Energy Storage Systems for Electric Vehicle Applications: Issues and Challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Mahanta, C. An Overview of Battery Based Electric Vehicle Technologies With Emphasis on Energy Sources, Their Configuration Topologies and Management Strategies. IEEE Trans. Intell. Transp. Syst. 2024, 25, 1087–1111. [Google Scholar] [CrossRef]
- Beardsall, J.C.; Gould, C.A.; Al-Tai, M. Energy Storage Systems: A Review of the Technology and Its Application in Power Systems. In Proceedings of the 2015 50th International Universities Power Engineering Conference (UPEC), Stoke on Trent, UK, 1–4 September 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Abbas, Q.; Mirzaeian, M.; Hunt, M.R.C.; Hall, P.; Raza, R. Current State and Future Prospects for Electrochemical Energy Storage and Conversion Systems. Energies 2020, 13, 5847. [Google Scholar] [CrossRef]
- Hylla, P.; Trawiński, T.; Polnik, B.; Burlikowski, W.; Prostański, D. Overview of Hybrid Energy Storage Systems Combined with RES in Poland. Energies 2023, 16, 5792. [Google Scholar] [CrossRef]
- Şahin, M.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Ramadan, M. Critical Review of Flywheel Energy Storage System. Energies 2021, 14, 2159. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, W.; Kang, J.; Yuan, T. The Necessity and Feasibility of Hydrogen Storage for Large-Scale, Long-Term Energy Storage in the New Power System in China. Energies 2023, 16, 4837. [Google Scholar] [CrossRef]
- Rząsa, M.; Łukasiewicz, E.; Wójtowicz, D. Test of a New Low-Speed Compressed Air Engine for Energy Recovery. Energies 2021, 14, 1179. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Z.; Jin, T.; Liu, L. Recent Advance of Hybrid Energy Storage Systems for Electrified Vehicles. In Proceedings of the 2018 14th IEEE/ASME International Conference on Mechatronic and Embedded Systems and Applications (MESA), Oulu, Finland, 2–4 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–2. [Google Scholar]
- Roiu, D.; Primon, A.; Rossella, M.; Ornato, M. 12V Battery Modeling: Model Development, Simulation and Validation. In Proceedings of the 2017 International Conference of Electrical and Electronic Technologies for Automotive, Turin, Italy, 15–16 June 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–5. [Google Scholar]
- Arianto, S.; Yunaningsih, R.Y.; Astuti, E.T.; Hafiz, S. Development of Single Cell Lithium Ion Battery Model Using Scilab/Xcos. In Proceedings of the International Symposium on Frontier of Applied Physics (ISFAP) 2015, Bandung, Indonesia, 5–7 October 2015. [Google Scholar]
- Hussein, A.A. An Overview and Practical Considerations of Common Lithium-Ion Battery Cell Models. In Proceedings of the 2021 4th International Symposium on Advanced Electrical and Communication Technologies (ISAECT), Alkhobar, Saudi Arabia, 6–8 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–5. [Google Scholar]
- Einhorn, M.; Conte, V.F.; Kral, C.; Fleig, J.; Permann, R. Parameterization of an Electrical Battery Model for Dynamic System Simulation in Electric Vehicles. In Proceedings of the 2010 IEEE Vehicle Power and Propulsion Conference, Lille, France, 1–3 September 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–7. [Google Scholar]
- Saidani, F.; Hutter, F.X.; Scurtu, R.-G.; Braunwarth, W.; Burghartz, J.N. Lithium-Ion Battery Models: A Comparative Study and a Model-Based Powerline Communication. Adv. Radio Sci. 2017, 15, 83–91. [Google Scholar] [CrossRef]
- Kai, S.; Qifang, S. Overview of the Types of Battery Models. In Proceedings of the 30th Chinese Control Conference, Yantai, China, 22–24 July 2011; pp. 3644–3648. [Google Scholar]
- Kim, Y.-H.; Ha, H. -D. Design of Interface Circuits with Electrical Battery Models. IEEE Trans. Ind. Electron. 1997, 44, 81–86. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Lee, J.; Cho, B.H. State-of-Charge and Capacity Estimation of Lithium-Ion Battery Using a New Open-Circuit Voltage versus State-of-Charge. J. Power Sources 2008, 185, 1367–1373. [Google Scholar] [CrossRef]
- Tjandra, R.; Thanagasundram, S.; Tseng, K.J.; Jossen, A. Improved Lithium-Ion Battery Model with Hysteresis Effect. In Proceedings of the 2014 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 15–18 June 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–8. [Google Scholar]
- Tran, M.-K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative Study of Equivalent Circuit Models Performance in Four Common Lithium-Ion Batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51. [Google Scholar] [CrossRef]
- Johnson, V.H. Battery Performance Models in ADVISOR. J. Power Sources 2002, 110, 321–329. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Feng, J. Research on Equivalent Circuit Model of Lithium-Ion Battery for Electric Vehicles. In Proceedings of the 2020 3rd World Conference on Mechanical Engineering and Intelligent Manufacturing (WCMEIM), Shanghai, China, 4–6 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 492–496. [Google Scholar]
- Zhou, W.; Zheng, Y.; Pan, Z.; Lu, Q. Review on the Battery Model and SOC Estimation Method. Processes 2021, 9, 1685. [Google Scholar] [CrossRef]
- Saldaña, G.; San Martín, J.I.; Zamora, I.; Asensio, F.J.; Oñederra, O. Analysis of the Current Electric Battery Models for Electric Vehicle Simulation. Energies 2019, 12, 2750. [Google Scholar] [CrossRef]
- Yanga, X.; Wanga, S.; Xua, W.; Fernandezb, C.; Yua, C.; Fana, Y.; Caoa, W. A Novel Improved PNGV Model Parameter Identification of Lithium Battery Based on Double Exponential Fitting. Open Access J. Biosens. Renew. Sources 2020, 1, 43–47. [Google Scholar]
- Hinz, H. Comparison of Lithium-Ion Battery Models for Simulating Storage Systems in Distributed Power Generation. Inventions 2019, 4, 41. [Google Scholar] [CrossRef]
- Chen, M.; Rincon-Mora, G.A. Accurate Electrical Battery Model Capable of Predicting Runtime and I-V Performance. IEEE Trans. Energy Convers. 2006, 21, 504–511. [Google Scholar] [CrossRef]
- Kharisma, M.D.; Ridwan, M.; Ilmiawan, A.F.; Ario Nurman, F.; Rizal, S. Modeling and Simulation of Lithium-Ion Battery Pack Using Modified Battery Cell Model. In Proceedings of the 2019 6th International Conference on Electric Vehicular Technology (ICEVT), Bali, Indonesia, 18–21 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 25–30. [Google Scholar]
- Yao, L.W.; Aziz, J.A.; Kong, P.Y.; Idris, N.R.N. Modeling of Lithium-Ion Battery Using MATLAB/Simulink. In Proceedings of the IECON 2013—39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1729–1734. [Google Scholar]
- Knauff, M.; McLaughlin, J.; Dafis, C.; Niebur, D.; Singh, P.; Kwatny, H.; Nwankpa, C. Simulink Model of a Lithium-Ion Battery for the Hybrid Power System Testbed. In Proceedings of the ASNE Intelligent Ships Symposium, Arlington, VA, USA, 21–23 May 2007; p. 8. [Google Scholar]
- Zhang, X.; Zhang, W.; Lei, G. A Review of Li-Ion Battery Equivalent Circuit Models. Trans. Electr. Electron. Mater. 2016, 17, 311–316. [Google Scholar] [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of Lithium-Ion Battery Equivalent Circuit Models for State of Charge Estimation by an Experimental Approach. Energies 2011, 4, 582–598. [Google Scholar] [CrossRef]
- Thanagasundram, S.; Arunachala, R.; Makinejad, K.; Teutsch, T.; Jossen, A. A Cell Level Model for Battery Simulation. In Proceedings of the EEVC European Electric Vehicle Congress, Brussels, Belgium, 19–22 November 2012; pp. 1–13. [Google Scholar]
- Niu, R.; Yang, H. Modeling and Identification of Electric Double-Layer Supercapacitors. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; IEEE: Piscataway, NJ, USA, May 2011; pp. 1–4. [Google Scholar]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A Review of Supercapacitor Modeling, Estimation, and Applications: A Control/Management Perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Naseri, F.; Karimi, S.; Farjah, E.; Schaltz, E. Supercapacitor Management System: A Comprehensive Review of Modeling, Estimation, Balancing, and Protection Techniques. Renew. Sustain. Energy Rev. 2022, 155, 111913. [Google Scholar] [CrossRef]
- Jiya, I.; Gurusinghe, N.; Gouws, R. Electrical Circuit Modelling of Double Layer Capacitors for Power Electronics and Energy Storage Applications: A Review. Electronics 2018, 7, 268. [Google Scholar] [CrossRef]
- Cahela, D.R.; Tatarchuk, B.J. Overview of Electrochemical Double Layer Capacitors. In Proceedings of the IECON’97 23rd International Conference on Industrial Electronics, Control, and Instrumentation (Cat. No.97CH36066), New Orleans, LA, USA, 14 November 1997; IEEE: Piscataway, NJ, USA, 1997; pp. 1068–1073. [Google Scholar]
- Helseth, L.E. Modelling Supercapacitors Using a Dynamic Equivalent Circuit with a Distribution of Relaxation Times. J. Energy Storage 2019, 25, 100912. [Google Scholar] [CrossRef]
- Cultura, A.B.; Salameh, Z.M. Modeling, Evaluation and Simulation of a Supercapacitor Module for Energy Storage Application. In Proceedings of the International Conference on Computer Information Systems and Industrial Applications, Bangkok, Thailand, 28–29 June 2015. [Google Scholar]
- Nelms, R.M.; Cahela, D.R.; Tatarchuk, B.J. Using a Debye Polarization Cell to Predict Double-Layer Capacitor Performance. IEEE Trans. Ind. Appl. 2001, 37, 4–9. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Hu, X.; Sun, F.; Dorrell, D.G. A Comparative Study of Equivalent Circuit Models of Ultracapacitors for Electric Vehicles. J. Power Sources 2015, 274, 899–906. [Google Scholar] [CrossRef]
- Nelms, R.M.; Cahela, D.R.; Tatarchuk, B.J. Modeling Double-Layer Capacitor Behavior Using Ladder Circuits. IEEE Trans. Aerosp. Electron. Syst. 2003, 39, 430–438. [Google Scholar] [CrossRef]
- Cabrane, Z.; Ouassaid, M.; Maaroufi, M. Analysis and Evaluation of Battery-Supercapacitor Hybrid Energy Storage System for Photovoltaic Installation. Int. J. Hydrogen Energy 2016, 41, 20897–20907. [Google Scholar] [CrossRef]
- Yang, H. Evaluation of Cell Balancing Circuits for Supercapacitor-Based Energy Storage Systems. In Proceedings of the 2019 IEEE Transportation Electrification Conference and Expo (ITEC), Detroit, MI, USA, 19–21 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Zubieta, L.; Bonert, R. Characterization of Double-Layer Capacitors for Power Electronics Applications. IEEE Trans. Ind. Appl. 2000, 36, 199–205. [Google Scholar] [CrossRef]
- Rafik, F.; Gualous, H.; Gallay, R.; Crausaz, A.; Berthon, A. Frequency, Thermal and Voltage Supercapacitor Characterization and Modeling. J. Power Sources 2007, 165, 928–934. [Google Scholar] [CrossRef]
- Faranda, R.; Gallina, M.; Son, D.T. A New Simplified Model of Double-Layer Capacitors. In Proceedings of the 2007 International Conference on Clean Electrical Power, Capri, Italy, 21–23 May 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 706–710. [Google Scholar]
- Kai, W.; Baosen, R.; Liwei, L.; Yuhao, L.; Hongwei, Z.; Zongqiang, S. A Review of Modeling Research on Supercapacitor. In Proceedings of the 2017 Chinese Automation Congress (CAC), Jinan, China, 20–22 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 5998–6001. [Google Scholar]
- Devillers, N.; Jemei, S.; Péra, M.-C.; Bienaimé, D.; Gustin, F. Review of Characterization Methods for Supercapacitor Modelling. J. Power Sources 2014, 246, 596–608. [Google Scholar] [CrossRef]
- Argyrou, M.C.; Christodoulides, P.; Marouchos, C.C.; Kalogirou, S.A. Hybrid Battery-Supercapacitor Mathematical Modeling for PV Application Using Matlab/Simulink. In Proceedings of the 2018 53rd International Universities Power Engineering Conference (UPEC), Glasgow, UK, 4–7 September 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar]
- Vural, B.; Uzunoglu, M.; Erdinc, O.; Onar, O.C. A Dynamic Ultra-Capacitor Model for Vehicular Applications. In Proceedings of the 2009 International Conference on Clean Electrical Power, Capri, Italy, 9–11 June 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 595–598. [Google Scholar]
- Du, L. Study on Supercapacitor Equivalent Circuit Model for Power Electronics Applications. In Proceedings of the 2009 2nd International Conference on Power Electronics and Intelligent Transportation System (PEITS), Shenzhen, China, 19–20 December 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 51–54. [Google Scholar]
- Şahin, M.; Blaabjerg, F.; Sangwongwanİch, A. Modelling of Supercapacitors Based on Simplified Equivalent Circuit. CPSS Trans. Power Electron. Appl. 2021, 6, 31–39. [Google Scholar] [CrossRef]
- Seim, L.H. Modeling, Control and Experimental Testing of a Supercapacitor/Battery Hybrid System: Passive and Semi-Active Topologies; Norwegian University of Life Sciences: Ås, Norway, 2012. [Google Scholar]
- Navarro, G.; Nájera, J.; Torres, J.; Blanco, M.; Santos, M.; Lafoz, M. Development and Experimental Validation of a Supercapacitor Frequency Domain Model for Industrial Energy Applications Considering Dynamic Behaviour at High Frequencies. Energies 2020, 13, 1156. [Google Scholar] [CrossRef]
- Supercapacitor Electrical Equivalent Model. Available online: https://www.garmanage.com/atelier/index.cgi?path=public/Energy_storage/Supercapacitors/Model (accessed on 25 December 2023).
- Berrueta, A.; San Martín, I.; Hernández, A.; Ursúa, A.; Sanchis, P. Electro-Thermal Modelling of a Supercapacitor and Experimental Validation. J. Power Sources 2014, 259, 154–165. [Google Scholar] [CrossRef]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Nielson, G.; Emadi, A. Hybrid Energy Storage Systems for High-Performance Hybrid Electric Vehicles. In Proceedings of the 2011 IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 6–9 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–6. [Google Scholar]
- Behjati, H.; Davoudi, A. Comparative Reliability Study of Hybrid Energy Storage Systems in Hybrid Electric Vehicles. In Proceedings of the 2012 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 18–20 June 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–6. [Google Scholar]
- Dhand, A.; Pullen, K. Review of Battery Electric Vehicle Propulsion Systems Incorporating Flywheel Energy Storage. Int. J. Automot. Technol. 2015, 16, 487–500. [Google Scholar] [CrossRef]
- Emadi, A. Advanced Electric Drive Vehicles, 1st ed.; Emadi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781315215570. [Google Scholar]
- Sankarkumar, R.S.; Natarajan, R. Energy Management Techniques and Topologies Suitable for Hybrid Energy Storage System Powered Electric Vehicles: An Overview. Int. Trans. Electr. Energy Syst. 2021, 31, e12819. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid Battery/Supercapacitor Energy Storage System for the Electric Vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Zimmermann, T.; Keil, P.; Hofmann, M.; Horsche, M.F.; Pichlmaier, S.; Jossen, A. Review of System Topologies for Hybrid Electrical Energy Storage Systems. J Energy Storage 2016, 8, 78–90. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Y.; Hu, S.; Wang, W. A New Topology and Control Strategy for a Hybrid Battery-Ultracapacitor Energy Storage System. Energies 2014, 7, 2874–2896. [Google Scholar] [CrossRef]
- Wang, E.; Yang, F.; Ouyang, M. A Hybrid Energy Storage System for a Coaxial Power-Split Hybrid Powertrain. In Hybrid Electric Vehicles; InTech: London, UK, 2017. [Google Scholar]
- Lencwe, M.J.; Chowdhury, S.P.D.; Olwal, T.O. Hybrid Energy Storage System Topology Approaches for Use in Transport Vehicles: A Review. Energy Sci. Eng. 2022, 10, 1449–1477. [Google Scholar] [CrossRef]
- Song, Z.; Hofmann, H.; Li, J.; Han, X.; Zhang, X.; Ouyang, M. A Comparison Study of Different Semi-Active Hybrid Energy Storage System Topologies for Electric Vehicles. J. Power Sources 2015, 274, 400–411. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, W.; Wei, S.; Lin, H.; Jia, Z. An Improved Energy Management Strategy for Hybrid Energy Storage System in Light Rail Vehicles. Energies 2018, 11, 423. [Google Scholar] [CrossRef]
- Ostadi, A.; Kazerani, M.; Chen, S.-K. Hybrid Energy Storage System (HESS) in Vehicular Applications: A Review on Interfacing Battery and Ultra-Capacitor Units. In Proceedings of the 2013 IEEE Transportation Electrification Conference and Expo (ITEC), Detroit, MI, USA, 16–19 June 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1–7. [Google Scholar]
- Choi, M.-E.; Kim, S.-W.; Seo, S.-W. Energy Management Optimization in a Battery/Supercapacitor Hybrid Energy Storage System. IEEE Trans. Smart Grid 2011, 3, 463–472. [Google Scholar] [CrossRef]
- Jayasawal, K.; Karna, A.K.; Thapa, K.B. Topologies for Interfacing Supercapacitor and Battery in Hybrid Electric Vehicle Applications: An Overview. In Proceedings of the 2021 International Conference on Sustainable Energy and Future Electric Transportation (SEFET), Hyderabad, India, 21–23 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Abuaish, A. Assessment of Battery Capacity Fading in Partially-Decoupled Battery-Supercapacitor Hybrid Energy Storage System Topologies for Electric Vehicles; University of Waterloo: Waterloo, ON, Canada, 2016. [Google Scholar]
- Chirkin, V.G.; Lezhnev, L.Y.; Petrichenko, D.A.; Papkin, I.A. A Battery-Supercapacitor Hybrid Energy Storage System Design and Power Management. Int. J. Pure Appl. Math. 2018, 119, 2621–2625. [Google Scholar]
- Jaafar, A.; Akli, C.R.; Sareni, B.; Roboam, X.; Jeunesse, A. Sizing and Energy Management of a Hybrid Locomotive Based on Flywheel and Accumulators. IEEE Trans. Veh. Technol. 2009, 58, 3947–3958. [Google Scholar] [CrossRef]
- Wang, J.; Su, J.; Lai, J.; Zhang, J.; Wang, S. Research on Control Method for Flywheel Battery Energy Storage System. In Proceedings of the 2016 IEEE 8th International Power Electronics and Motion Control Conference (IPEMC-ECCE Asia), Hefei, China, 22–26 May 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1006–1010. [Google Scholar]
- Zhang, J.W.; Wang, Y.H.; Liu, G.C.; Tian, G.Z. A Review of Control Strategies for Flywheel Energy Storage System and a Case Study with Matrix Converter. Energy Rep. 2022, 8, 3948–3963. [Google Scholar] [CrossRef]
- Agusdinata, D.B.; Liu, W.; Eakin, H.; Romero, H. Socio-Environmental Impacts of Lithium Mineral Extraction: Towards a Research Agenda. Environ. Res. Lett. 2018, 13, 123001. [Google Scholar] [CrossRef]
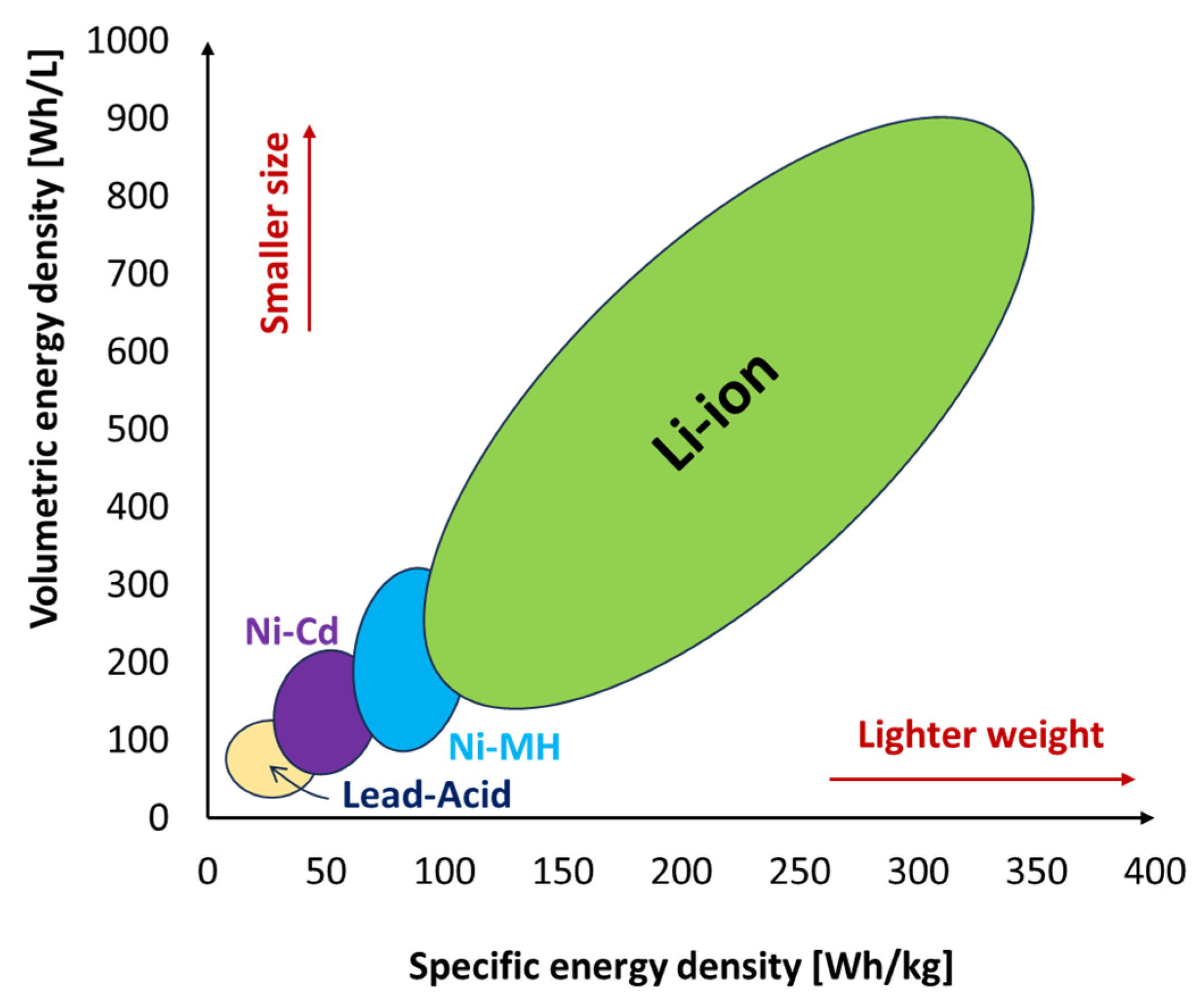

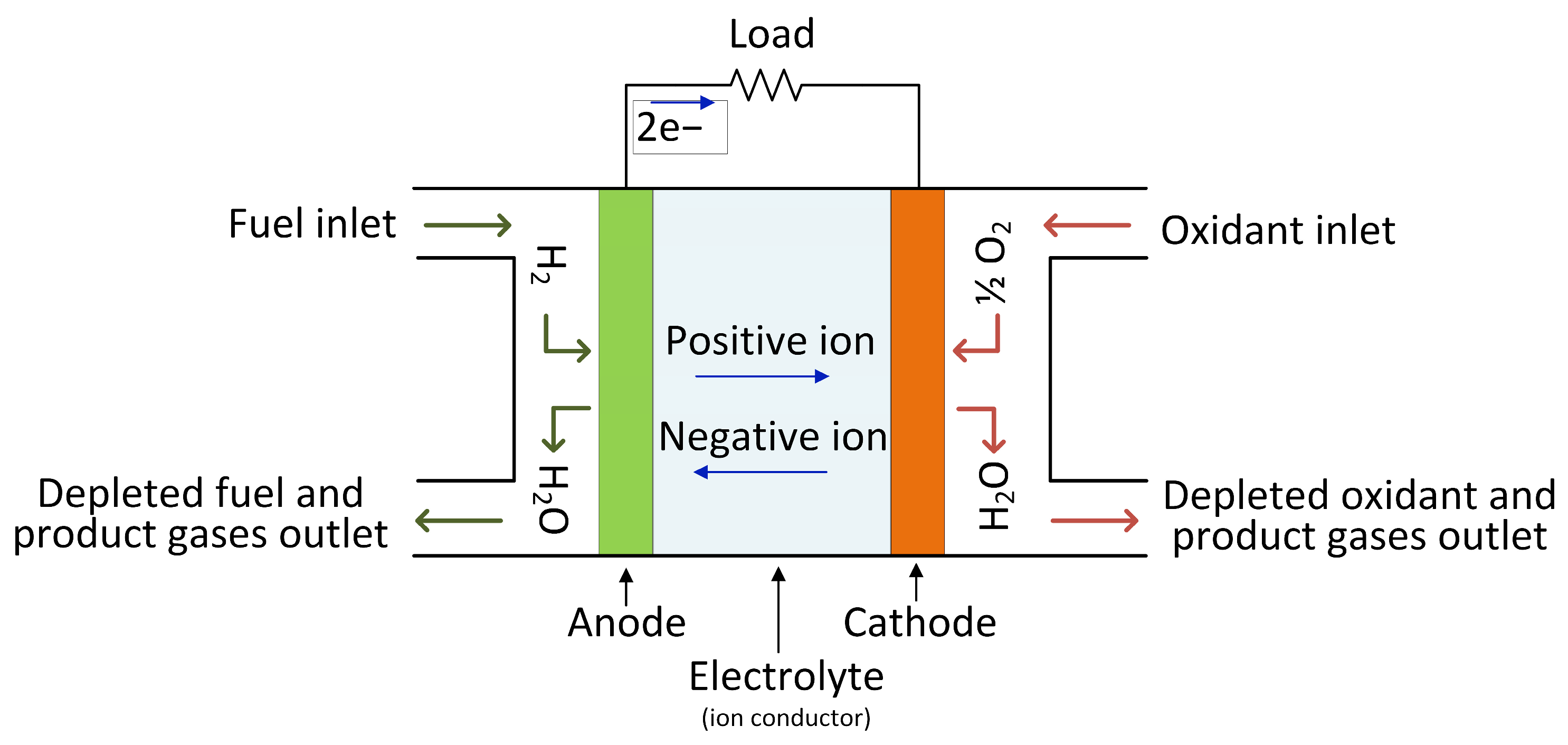
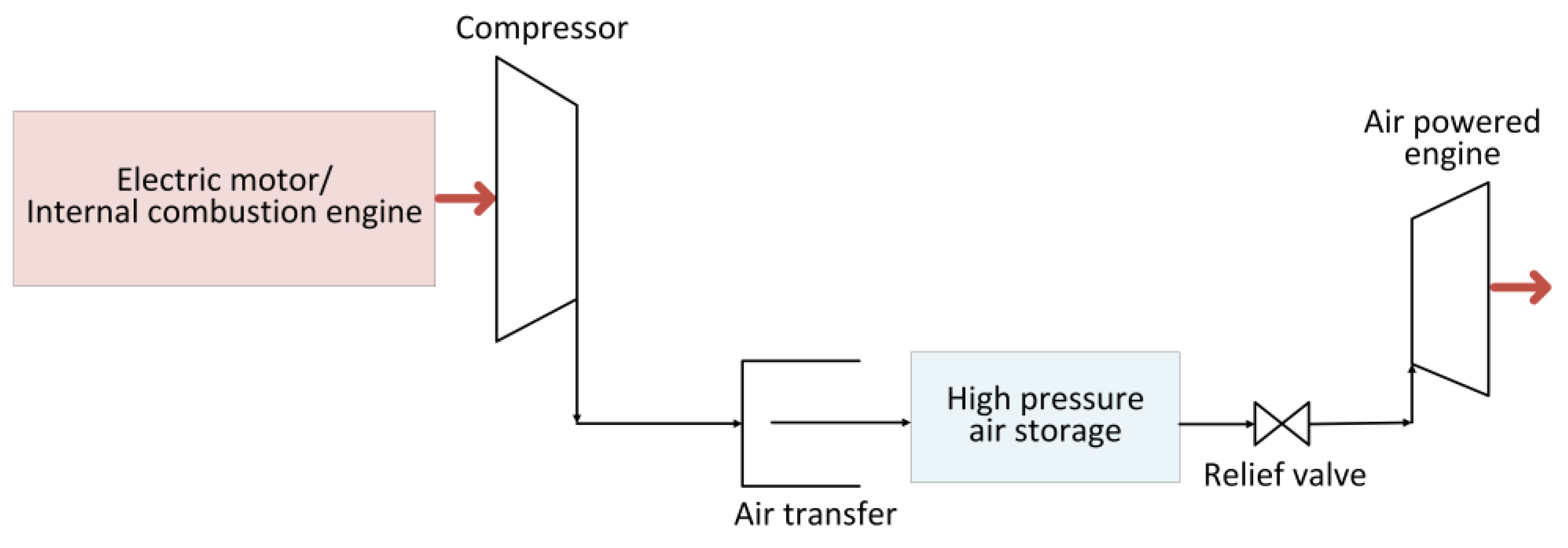
| Energy Storage Type | Energy Density (Wh/kg) | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Battery | Lead acid | 25–50 |
|
| [57,58,59,60] |
| NiCd | 40–75 |
|
| [57,58,60] | |
| NiMH | 70–100 |
|
| [57,58,59,60] | |
| NiFe | <50 |
|
| [57,59] | |
| NiZn | 50–60 |
|
| [57,58,59] | |
| Li-ion | 150–350 |
|
| [57,58,59,60] | |
| Supercapacitor | 2–5 |
|
| [61,62] | |
| Flywheel | Low speed | <5 |
|
| [61,63] |
| High Speed | >100 | ||||
| Hydrogen storage | 33.3 × 103 |
|
| [61,64] | |
| Compressed air storage | 20–85 |
|
| [65] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkembi, A.A.; Simonazzi, M.; Santoro, D.; Cova, P.; Delmonte, N. Comprehensive Review of Energy Storage Systems Characteristics and Models for Automotive Applications. Batteries 2024, 10, 88. https://doi.org/10.3390/batteries10030088
Nkembi AA, Simonazzi M, Santoro D, Cova P, Delmonte N. Comprehensive Review of Energy Storage Systems Characteristics and Models for Automotive Applications. Batteries. 2024; 10(3):88. https://doi.org/10.3390/batteries10030088
Chicago/Turabian StyleNkembi, Armel Asongu, Marco Simonazzi, Danilo Santoro, Paolo Cova, and Nicola Delmonte. 2024. "Comprehensive Review of Energy Storage Systems Characteristics and Models for Automotive Applications" Batteries 10, no. 3: 88. https://doi.org/10.3390/batteries10030088
APA StyleNkembi, A. A., Simonazzi, M., Santoro, D., Cova, P., & Delmonte, N. (2024). Comprehensive Review of Energy Storage Systems Characteristics and Models for Automotive Applications. Batteries, 10(3), 88. https://doi.org/10.3390/batteries10030088










