Characterization of Lithium-Ion Battery Fire Emissions—Part 2: Particle Size Distributions and Emission Factors
Abstract
1. Introduction
2. Materials and Methods
3. Results
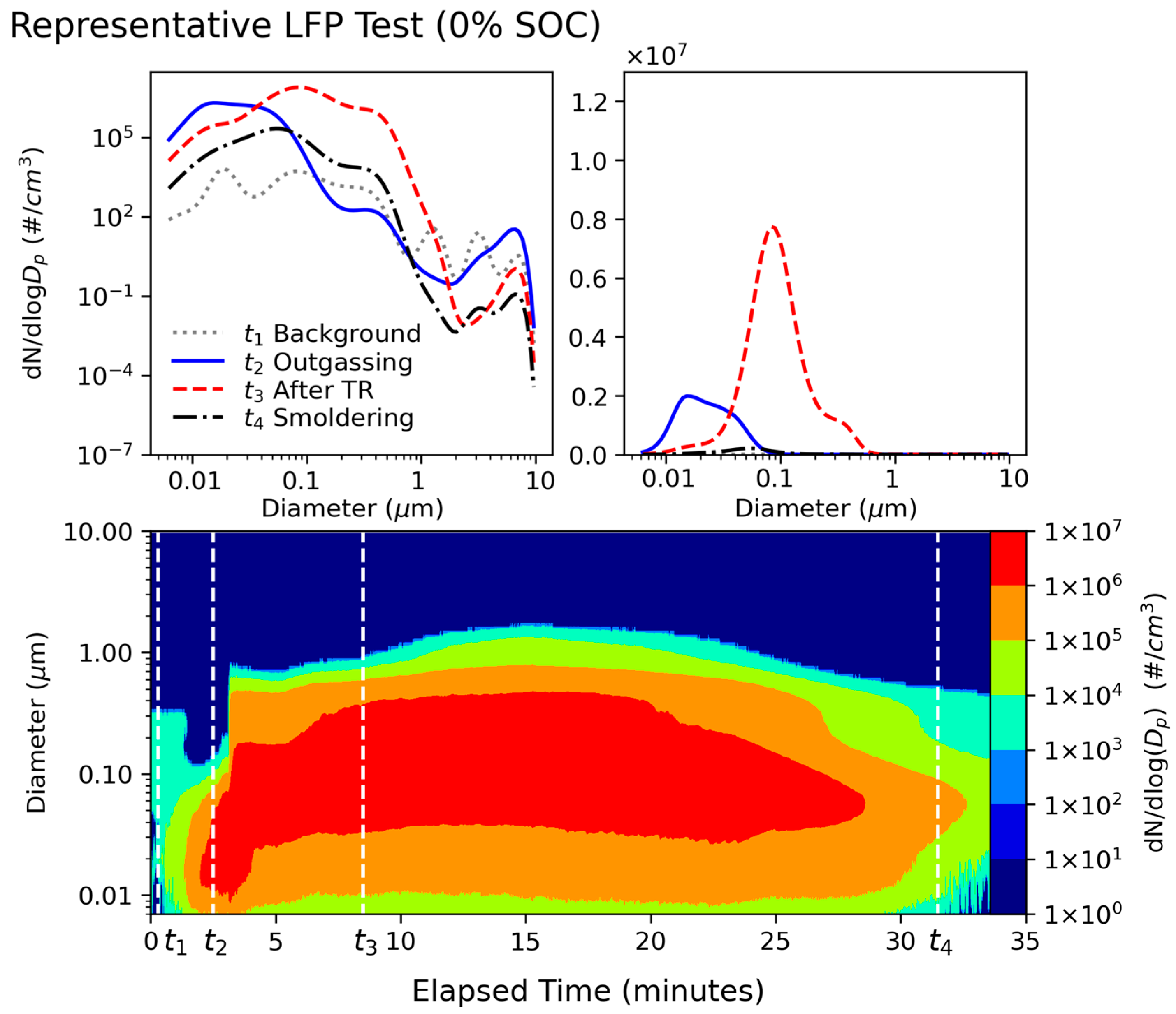
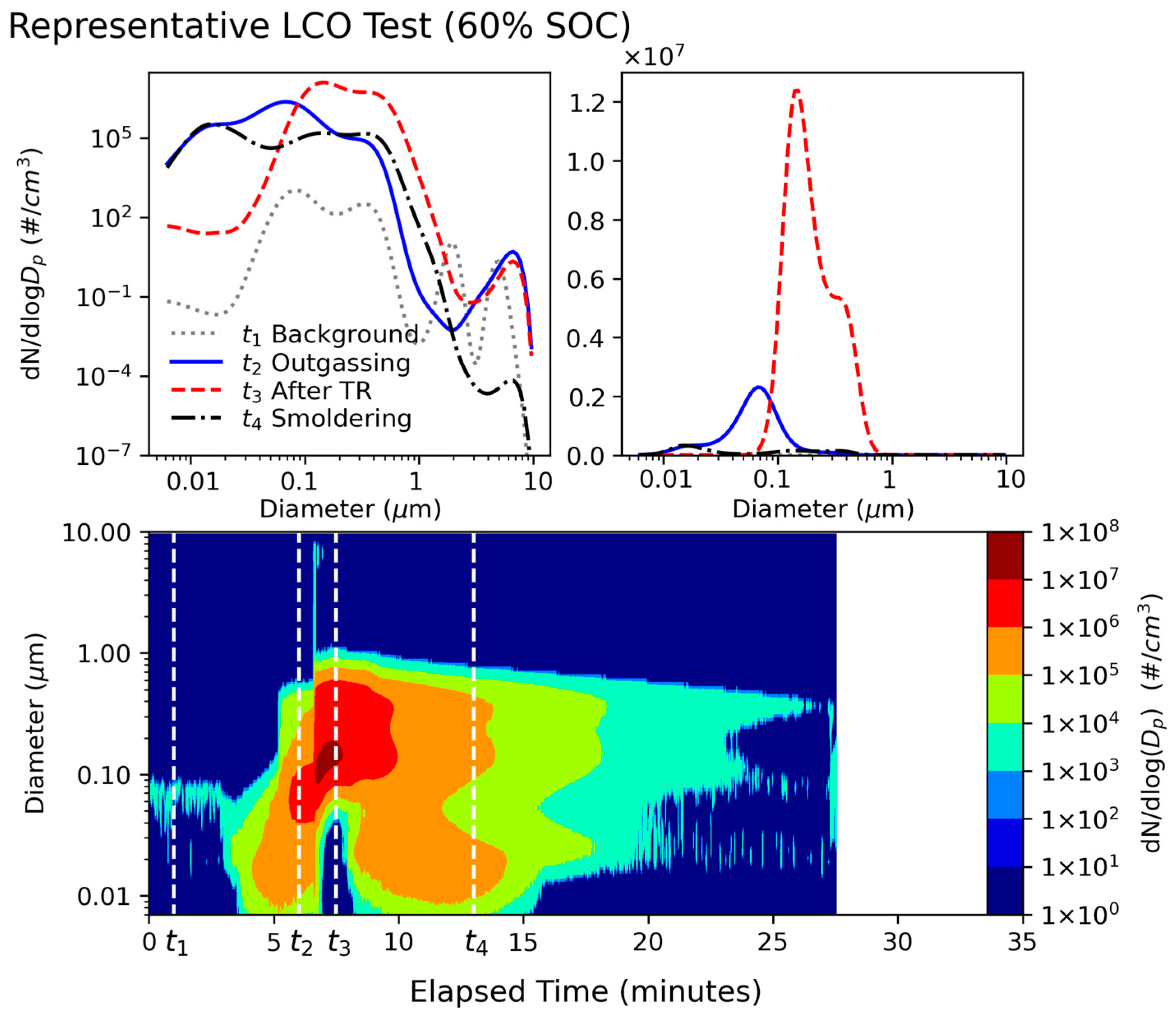
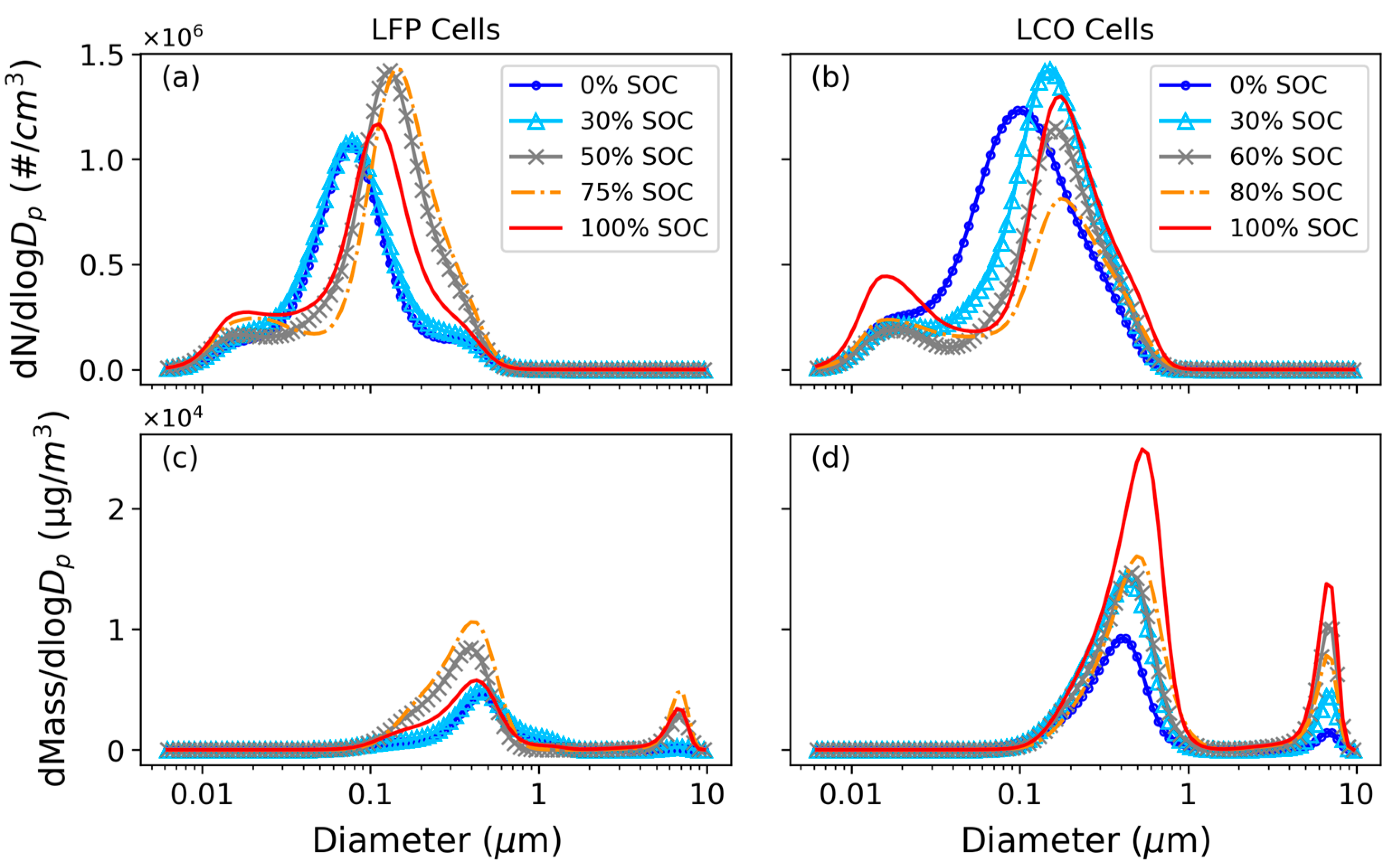
3.1. Particle Size Distributions
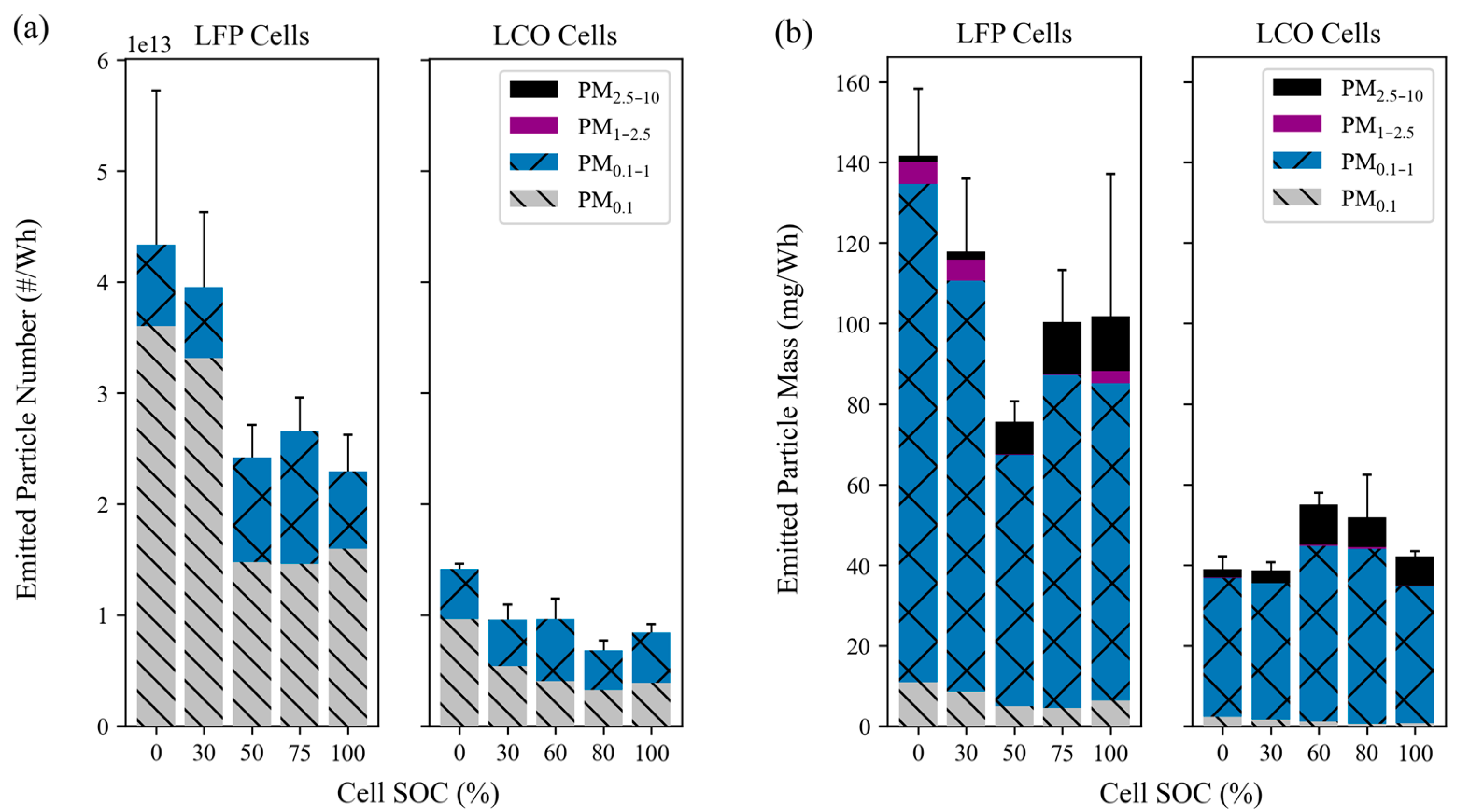
3.2. Emission Factors (EFs)
3.2.1. EFs for Particle Number and Mass
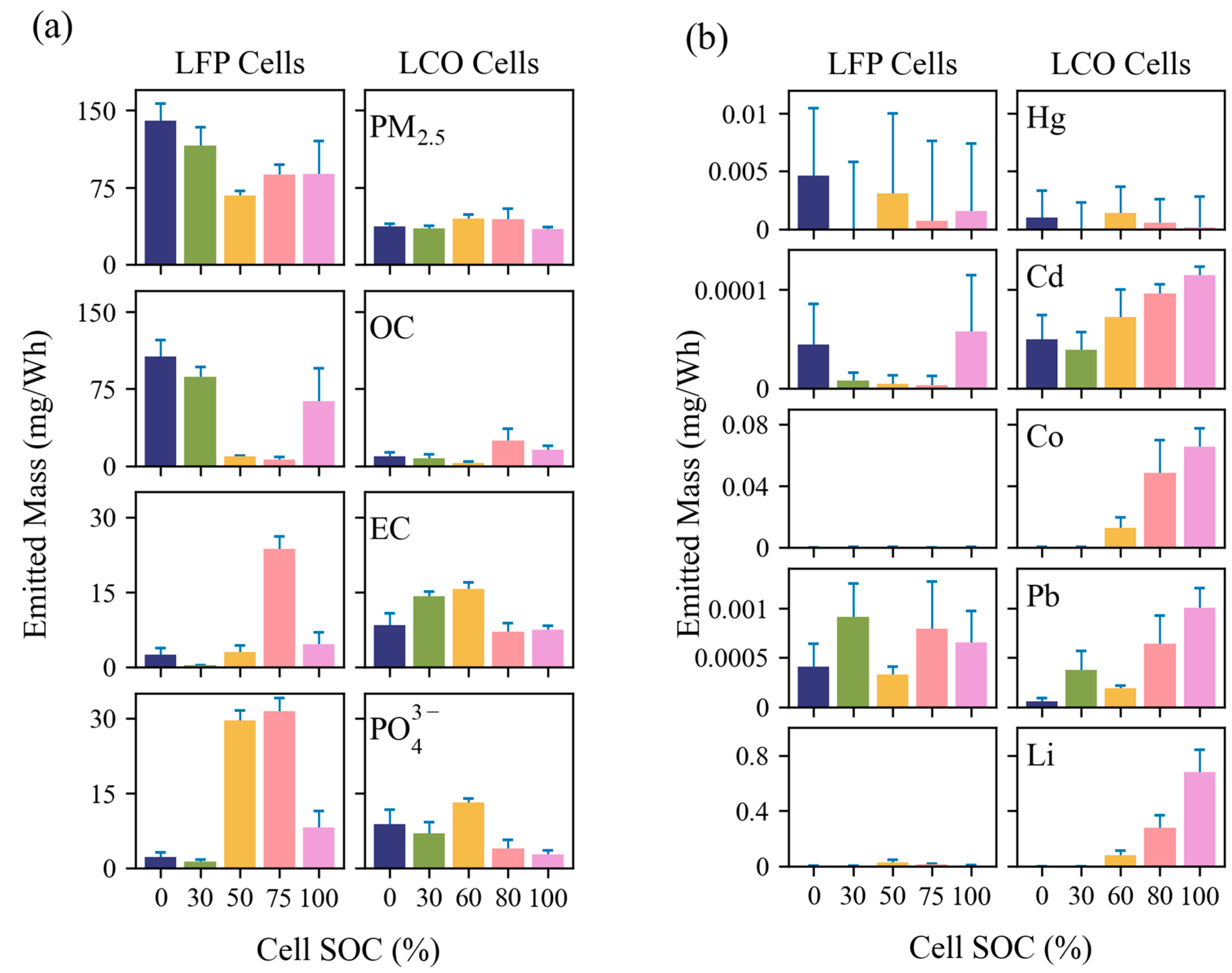
3.2.2. EFs for PM2.5, OC, EC, PO43−, and Toxic Metals
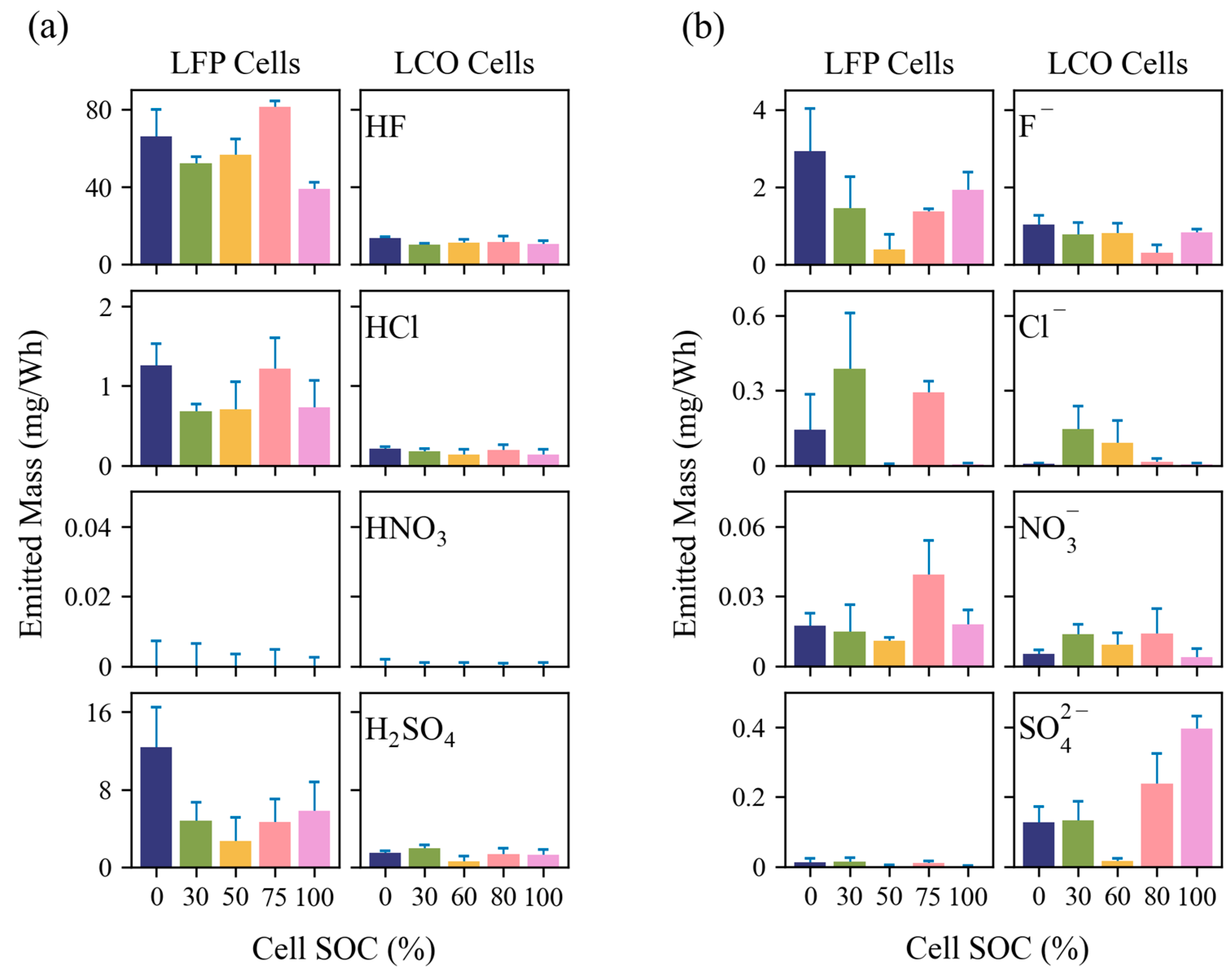
3.2.3. EFs for Anions and Acidic Gases
3.2.4. Relationship between Emission Factors and Combustion Temperatures
3.3. Cell Mass Losses
4. Discussion and Conclusions
- (1)
- LIB fires emit high concentrations of fine and ultrafine particles. The particle number distributions showed a dominant mode centered around 100 nm, with additional modes centered around 20 nm and 300 nm. The particle mass distributions showed that the venting-only LFP tests in this study with 0% and 30% SOCs had one mode centered around 460 nm, while all other tests had bimodal distributions, with one fine particle mode centered around 400 nm and a coarse particle mode centered around 8 µm. PM0.1 and PM0.1–1 together accounted for >99.9% of PM10 numbers, while PM0.1–1 dominated the PM10 mass at 77–89%. Super-micron particles have non-negligible mass contributions, with PM1–2.5 and PM2.5–10 accounting for up to 4% and 18% of PM10 mass, respectively. PM1–2.5 was emitted during cell venting, after TR and when flaming did not occur, while PM2.5–10 was emitted just before and during TR onset for all tests.
- (2)
- Venting-only combustion (LFP tests at 0% SOC, 30% SOC, and some at 100% SOC) showed smooth evolution of PSDs, while the onset of TR caused a discontinuity in particle size distribution by increasing both mode diameter and concentration and releasing coarse mode particles (1–10 µm). Particles from LCO tests in this study (GNMD 90–130 nm; GMMD 387–673 nm) were generally larger than those from LFP tests (GNMD 74–114 nm; GMMD 415–568 nm). The GMMD increased with SOC.
- (3)
- LFP tests had higher EFs for particle number and mass than LCO tests. The EFs for ultrafine particles (PM0.1) ranged from 1.5 × 1013 to 3.6 × 1013 particles/Wh for LFP and 3.2 × 1012 to 9.6 × 1012 particles/Wh for LCO tests. LFP tests had PM2.5 EFs between 67 and 140 mg/Wh with more variability at different SOCs, whereas LCO tests had lower and more consistent PM2.5 mass EFs between 35 and 45 mg/Wh. Even though PM2.5 only accounted for 0.25–1.5% of the total cell mass and a small fraction of total PM mass emitted from LIB fires, it accounts for the most particle numbers that can be inhaled by humans and presents great health risks.
- (4)
- LIB fires emit acidic gases, such as HF, HCl, and H2SO4. Gaseous HF ranged from 39 to 81 mg/Wh for LFP tests and 10 to 14 mg/Wh for LCO tests. These toxic and corrosive gases may represent great hazards to people and properties.
- (5)
- Emissions are highly dependent on cell type, SOC, and combustion temperatures. The emitted PM2.5 mass can depend on cell SOC by a factor of two, while the emitted OC, EC, and PO43− can differ by a factor of 10 or more. PO43− increased with combustion temperature, particularly when it reached >500 °C. Toxic metal emissions increased with SOC, but only for LCO tests, with no trend for LFP tests. Acidic gas emissions depended primarily on cell type, indicating that cell design is crucial to lowering emissions of HF and other corrosive compounds. The emission dependence on LIB cell properties should be considered when evaluating the overall hazard that each LIB pack presents. For example, while LFP cells are known to be more thermally stable, results from this study show that they may release more HF and can generate higher particulate concentrations than LCO cells.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ∆t | sampling duration |
| C | concentration |
| Cd | cadmium |
| Cl− | chloride |
| Co | cobalt |
| Dp | particle diameter |
| E | battery cell energy capacity |
| EC | elemental carbon |
| EEPS | engine exhaust particle sizer |
| EF | emission factor |
| ELPI | electrical low-pressure impactor |
| F− | fluoride |
| GMMD | geometric mass mean diameter: mean diameter of a particle mass distribution in logarithmic scale |
| GNMD | geometric number mean diameter: mean diameter of a particle number distribution in logarithmic scale |
| GNSD | geometric number standard deviation: standard deviation of a particle number distribution in logarithmic scale |
| HCl | hydrochloric acid |
| Hg | mercury |
| HF | hydrofluoric acid |
| HNO3 | nitric acid |
| H2SO4 | sulfuric acid |
| IC | ion chromatography |
| ICP-MS | inductively coupled plasma mass spectrometry |
| KOH | potassium hydroxide |
| LCO | lithium cobalt oxide |
| Li | lithium |
| Li+ | lithium ion |
| LIB | lithium-ion battery |
| LFP | lithium iron phosphate |
| NMC | nickel manganese cobalt |
| NO3− | nitrate |
| OC | organic carbon |
| P | phosphorus |
| Pb | lead |
| PM | particulate matter |
| PMx | particles with aerodynamic diameters ≤x µm |
| PO43− | phosphate |
| PSD | particle size distribution |
| Q | flow rate |
| SMPS | scanning mobility particle sizer |
| SO42− | sulfate |
| SOC | state of charge |
| TR | thermal runaway |
| Wh | watt hours |
| XRF | X-ray fluorescence |
References
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of gas emissions from lithium-ion battery thermal runaway failure—Considering toxic and flammable compounds. J. Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Li, W.; Xue, Y.; Feng, X.; Rao, S.; Zhang, T.; Gao, Z.; Guo, Y.; Zhou, H.; Zhao, H.; Song, Z.; et al. Characteristics of particle emissions from lithium-ion batteries during thermal runaway: A review. J. Energy Storage 2024, 78, 109980. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Chen, X.; Pang, K.; Deng, B.; Han, Z.; Shao, J.; Qian, K.; Chen, D. Thermal runaway and soot production of lithium-ion batteries: Implications for safety and environmental concerns. Appl. Therm. Eng. 2024, 248, 123193. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Chen, D. Soot formation and its hazards in battery thermal runaway. J. Aerosol Sci. 2024, 181, 106420. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries. eTransportation 2019, 2, 100031. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, W.; Li, C.; Ouyang, M. Particles released by abused prismatic Ni-rich automotive lithium-ion batteries. WSEAS Trans. Syst. Control 2020, 15, 30–38. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Jin, C.; Xu, C.; Zhao, Y.; Li, Y.; Zhong, C.; Feng, X. Detailed characterization of particle emissions due to thermal failure of batteries with different cathodes. J. Hazard. Mater. 2023, 458, 131646. [Google Scholar] [CrossRef]
- Wang, G.; Kong, D.; Ping, P.; Wen, J.; He, X.; Zhao, H.; He, X.; Peng, R.; Zhang, Y.; Dai, X. Revealing particle venting of lithium-ion batteries during thermal runaway: A multi-scale model toward multiphase process. eTransportation 2023, 16, 100237. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, D.; Maleki, A.; Kohzadi, S.; Liu, Y.; Chen, Y.; Liu, R.; Gao, G.; Zhi, J. Characterization of Thermal-Runaway Particles from Lithium Nickel Manganese Cobalt Oxide Batteries and Their Biotoxicity Analysis. ACS Appl. Energy Mater. 2021, 4, 10713–10720. [Google Scholar] [CrossRef]
- Held, M.; Tuchschmid, M.; Zennegg, M.; Figi, R.; Schreiner, C.; Mellert, L.D.; Welte, U.; Kompatscher, M.; Hermann, M.; Nachef, L. Thermal runaway and fire of electric vehicle lithium-ion battery and contamination of infrastructure facility. Renew. Sustain. Energy Rev. 2022, 165, 112474. [Google Scholar] [CrossRef]
- Bordes, A.; Papin, A.; Marlair, G.; Claude, T.; El-Masri, A.; Durussel, T.; Bertrand, J.-P.; Truchot, B.; Lecocq, A. Assessment of Run-Off Waters Resulting from Lithium-Ion Battery Fire-Fighting Operations. Batteries 2024, 10, 118. [Google Scholar] [CrossRef]
- ICRP. Human Respiratory Tract Model for Radiological Protection. ICRP Publication 66. Ann. ICRP 24 (1-3). 1994. Available online: https://www.icrp.org/publication.asp?id=icrp%20publication%2066 (accessed on 2 July 2024).
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM(2.5))-Mediated Toxicity in Humans: An Overview. Int. J. Env. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Premnath, V.; Wang, Y.; Wright, N.; Khalek, I.; Uribe, S. Detailed characterization of particle emissions from battery fires. Aerosol Sci. Technol. 2022, 56, 337–354. [Google Scholar] [CrossRef]
- Wang, X.L.; Grose, M.A.; Avenido, A.; Stolzenburg, M.R.; Caldow, R.; Osmondson, B.L.; Chow, J.C.; Watson, J.G. Improvement of Engine Exhaust Particle Sizer (EEPS) size distribution measurement—I. Algorithm and applications to compact-shape particles. J. Aerosol Sci. 2016, 92, 95–108. [Google Scholar] [CrossRef]
- Wang, X.L.; Grose, M.A.; Caldow, R.; Osmondson, B.L.; Swanson, J.J.; Chow, J.C.; Watson, J.G.; Kittelson, D.B.; Li, Y.; Xue, J.; et al. Improvement of Engine Exhaust Particle Sizer (EEPS) size distribution measurement—II. Engine exhaust particles. J. Aerosol Sci. 2016, 92, 83–94. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology, Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; Wiley: Los Angeles, CA, USA, 1999. [Google Scholar]
- Goupil, V.; Gaya, C.; Boisard, A.; Robert, E. Effect of the heating rate on the degassing and combustion of cylindrical Li-Ion cells. Fire Saf. J. 2022, 133, 103648. [Google Scholar] [CrossRef]
- Wang, X.L.; Chancellor, G.; Evenstad, J.; Farnsworth, J.E.; Hase, A.; Olson, G.M.; Sreenath, A.; Agarwal, J.K. A Novel Optical Instrument for Estimating Size Segregated Aerosol Mass Concentration in Real Time. Aerosol Sci. Technol. 2009, 43, 939–950. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, H.; Arnott, W.P.; Meyer, M.E.; Taylor, S.; Firouzkouhi, H.; Moosmüller, H.; Chow, J.C.; Watson, J.G. Evaluation of gas and particle sensors for detecting spacecraft-relevant fire emissions. Fire Saf. J. 2020, 113, 102977. [Google Scholar] [CrossRef]
- Lee, J.; He, S.; Song, G.; Hogan, C.J. Size distribution monitoring for chemical mechanical polishing slurries: An intercomparison of electron microscopy, dynamic light scattering, and differential mobility analysis. Powder Technol. 2022, 396, 395–405. [Google Scholar] [CrossRef]
- Wang, X.L.; Firouzkouhi, H.; Chow, J.C.; Watson, J.G.; Carter, W.; De Vos, A.S. Characterization of gas and particle emissions from open burning of household solid waste from South Africa. Atmos. Chem. Phys. 2023, 23, 8921–8937. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.-E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [PubMed]
- Claassen, M.; Bingham, B.; Chow, J.C.; Watson, J.G.; Wang, Y.; Wang, X. Characterization of Lithium-Ion Battery Fire Emissions—Part 1: Chemical Composition of Fine Particles (PM2. 5). Batteries 2024, 10, 301. [Google Scholar] [CrossRef]
- Lithiumwerks. APR18650M1B Nanophosphate® Technology Data Sheet. Austin, Texas. 2023. Available online: https://lithiumwerks.com/products/lithium-ion-18650-cells/ (accessed on 2 July 2024).
- AA Portable Power Corp. Model 544792 Polymer Lithium-Ion Battery Data Sheet. Available online: https://www.batteryspace.com/prod-specs/3175.pdf (accessed on 2 July 2024).
- Tian, J.; Chow, J.C.; Cao, J.; Han, Y.; Ni, H.; Chen, L.-W.A.; Wang, X.; Huang, R.; Moosmu, H.; Watson, J.G. A biomass combustion chamber: Design, evaluation, and a case study of wheat straw combustion emission tests. Aerosol Air Qual. Res. 2015, 15, 2104–2114. [Google Scholar] [CrossRef]
- U.S. EPA. Method 1—Sample and Velocity Traverses for Stationary Sources. Code of Federal Regulations, Title 40, Part 60, Appendix A-1. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-C/part-60/appendix-Appendix%20A-1%20to%20Part%2060 (accessed on 6 August 2024).
- Järvinen, A.; Aitomaa, M.; Rostedt, A.; Keskinen, J.; Yli-Ojanperä, J. Calibration of the new electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 69, 150–159. [Google Scholar] [CrossRef]
- Saari, S.; Arffman, A.; Harra, J.; Rönkkö, T.; Keskinen, J. Performance evaluation of the HR-ELPI+ inversion. Aerosol Sci. Technol. 2018, 52, 1037–1047. [Google Scholar] [CrossRef]
- Marjamäki, M.; Keskinen, J.; Chen, D.-R.; Pui, D.Y.H. Performance Evaluation of the Electrical Low-Pressure Impactor (ELPI). J. Aerosol Sci. 2000, 31, 249–261. [Google Scholar] [CrossRef]
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360. [Google Scholar] [CrossRef]
- Maricq, M.M.; Podsiadlik, D.H.; Chase, R.E. Size Distributions of Motor Vehicle Exhaust PM: A Comparison Between ELPI and SMPS Measurements. Aerosol Sci. Technol. 2000, 33, 239–260. [Google Scholar] [CrossRef]
- Wang, X.; Firouzkouhi, H.; Chow, J.C.; Watson, J.G.; Ho, S.S.H.; Carter, W.; De Vos, A.S. Chemically speciated air pollutant emissions from open burning of household solid waste from South Africa. Atmos. Chem. Phys. 2023, 23, 15375–15393. [Google Scholar] [CrossRef]
- Eldering, A.; Solomon, P.A.; Salmon, L.G.; Fall, T.; Cass, G.R. Hydrochloric acid: A regional perspective on concentrations and formation in the atmosphere of Southern California. Atmos. Environ. Part A Gen. Top. 1991, 25, 2091–2102. [Google Scholar] [CrossRef]
- Sturges, W.T.; Harrison, R.M. The use of nylon filters to collect HCl: Efficiencies, interferences and ambient concentrations. Atmos. Environ. (1967) 1989, 23, 1987–1996. [Google Scholar] [CrossRef]
- U.S. EPA. Initial List of Hazardous Air Pollutants with Modifications; Air Toxics Assessment Group, U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2020. Available online: https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications (accessed on 6 August 2024).
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Andersson, P.; Blomqvist, P.; Lorén, A.; Larsson, F. Investigation of Fire Emissions from Li-Ion Batteries; SP Technical Research Institute of Sweden: Borås, Sweden, 2013. [Google Scholar]
- Larsson, F. Lithium-Ion Battery Safety-Assessment by Abuse Testing, Fluoride Gas Emissions and Fire Propagation; Chalmers Tekniska Hogskola: Göteborg, Sweden, 2017. [Google Scholar]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Sturk, D.; Rosell, L.; Blomqvist, P.; Ahlberg Tidblad, A. Analysis of li-ion battery gases vented in an inert atmosphere thermal test chamber. Batteries 2019, 5, 61. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Herreyre, S.; Papin, A.; Desprez, P.; Lecocq, A. New insight on the risk profile pertaining to lithium-ion batteries under thermal runaway as affected by system modularity and subsequent oxidation regime. J. Energy Storage 2022, 52, 104790. [Google Scholar] [CrossRef]
- Willstrand, O.; Pushp, M.; Andersson, P.; Brandell, D. Impact of different Li-ion cell test conditions on thermal runaway characteristics and gas release measurements. J. Energy Storage 2023, 68, 107785. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]



| Cell Type | SOC (%) | GNMD (nm) | GMMD (nm) |
|---|---|---|---|
| LFP | 0 | 74 (72–77) | 415 (409–424) |
| 30 | 74 (72–77) | 426 (403–453) | |
| 50 | 110 (109–111) | 425 (415–435) | |
| 75 | 114 (106–129) | 479 (434–530) | |
| 100 | 84 (71–93) | 568 (536–611) | |
| LCO | 0 | 90 (78–102) | 387 (371–405) |
| 30 | 120 (111–137) | 474 (470–477) | |
| 60 | 130 (128–132) | 663 (553–751) | |
| 80 | 118 (109–128) | 673 (651–704) | |
| 100 | 116 (96–129) | 668 (602–778) |
| Source | Cell Type | HF (mg/Wh) | HCl (mg/Wh) |
|---|---|---|---|
| This study | LFP | 39–81 | 0.7–1.3 |
| [40] | LFP | 350 | 125 |
| [25] | LFP | 12–24 | |
| [41] | LFP | 40–125 | |
| This study | LCO | 10–14 | 0.14–0.21 |
| [40] | LCO | 30 | 8 |
| [25] | LCO | 30–40 | |
| [42] | Unknown | 20–200 | |
| [43] | LMO | 40–70 | |
| [44] | NMC/LFP | 23–36 | |
| [45] | NMC | 1–10 | |
| [46] | NMC | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claassen, M.; Bingham, B.; Chow, J.C.; Watson, J.G.; Chu, P.; Wang, Y.; Wang, X. Characterization of Lithium-Ion Battery Fire Emissions—Part 2: Particle Size Distributions and Emission Factors. Batteries 2024, 10, 366. https://doi.org/10.3390/batteries10100366
Claassen M, Bingham B, Chow JC, Watson JG, Chu P, Wang Y, Wang X. Characterization of Lithium-Ion Battery Fire Emissions—Part 2: Particle Size Distributions and Emission Factors. Batteries. 2024; 10(10):366. https://doi.org/10.3390/batteries10100366
Chicago/Turabian StyleClaassen, Matthew, Bjoern Bingham, Judith C. Chow, John G. Watson, Pengbo Chu, Yan Wang, and Xiaoliang Wang. 2024. "Characterization of Lithium-Ion Battery Fire Emissions—Part 2: Particle Size Distributions and Emission Factors" Batteries 10, no. 10: 366. https://doi.org/10.3390/batteries10100366
APA StyleClaassen, M., Bingham, B., Chow, J. C., Watson, J. G., Chu, P., Wang, Y., & Wang, X. (2024). Characterization of Lithium-Ion Battery Fire Emissions—Part 2: Particle Size Distributions and Emission Factors. Batteries, 10(10), 366. https://doi.org/10.3390/batteries10100366










