Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed Li(Ni0.6Mn0.2Co0.2)O2 Cathodes with High Mass Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Laser Structuring Process
2.3. Cell Assembly and Electrochemical Analyses
3. Results and Discussion
3.1. Electrodes and Laser Structuring Parameters
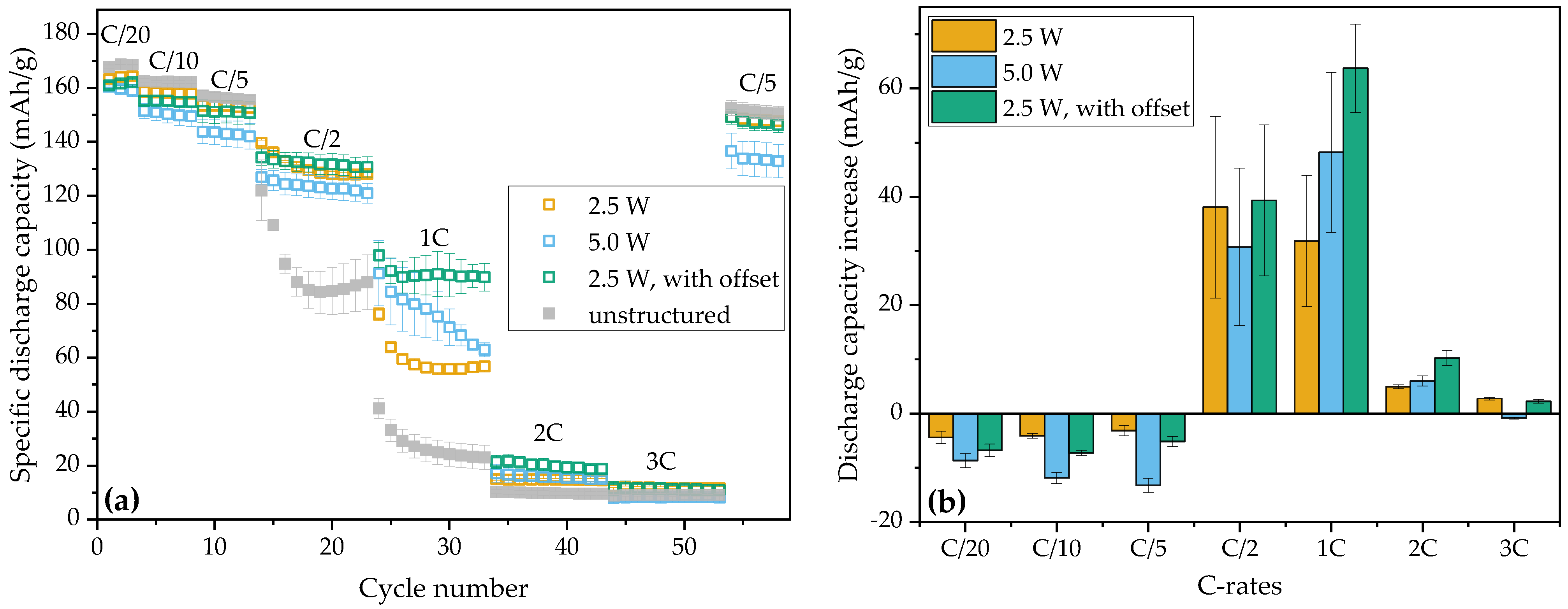
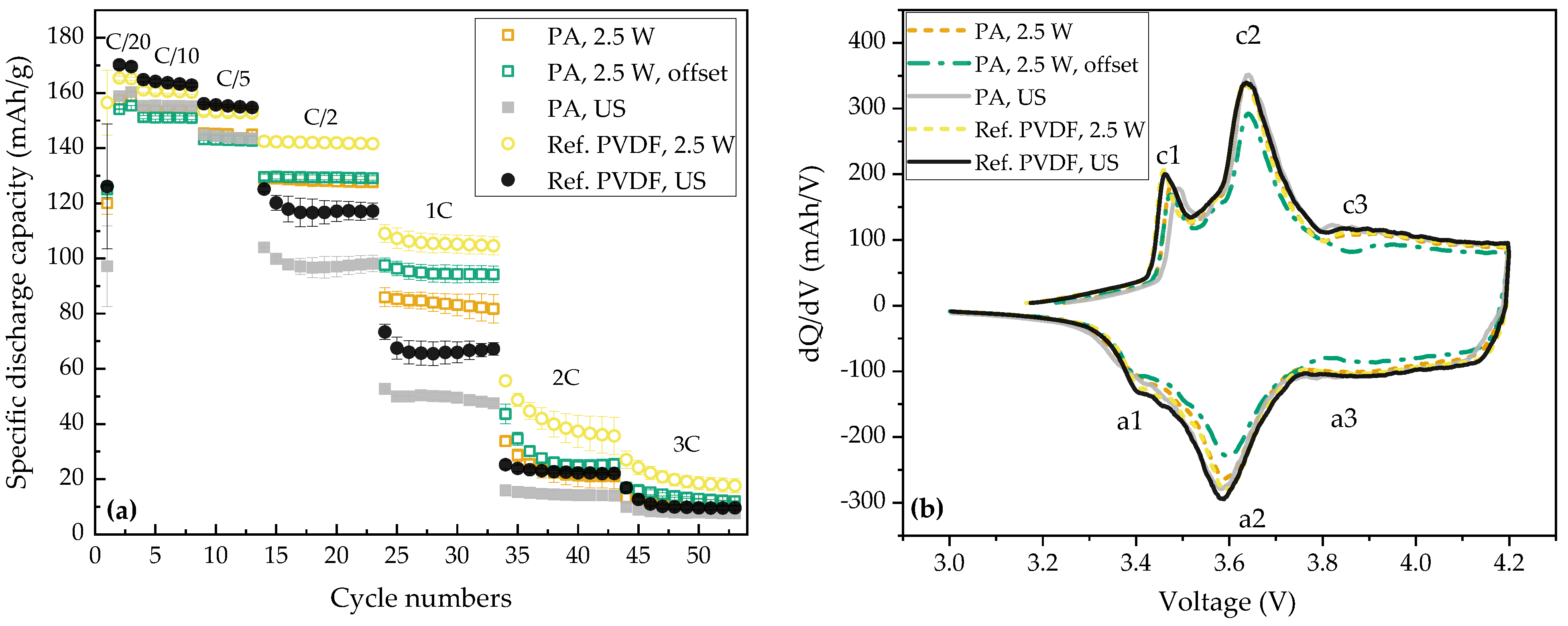
3.2. Electrochemical Analyses
3.2.1. Coin Cells (Half-Cells and Symmetric Cells)
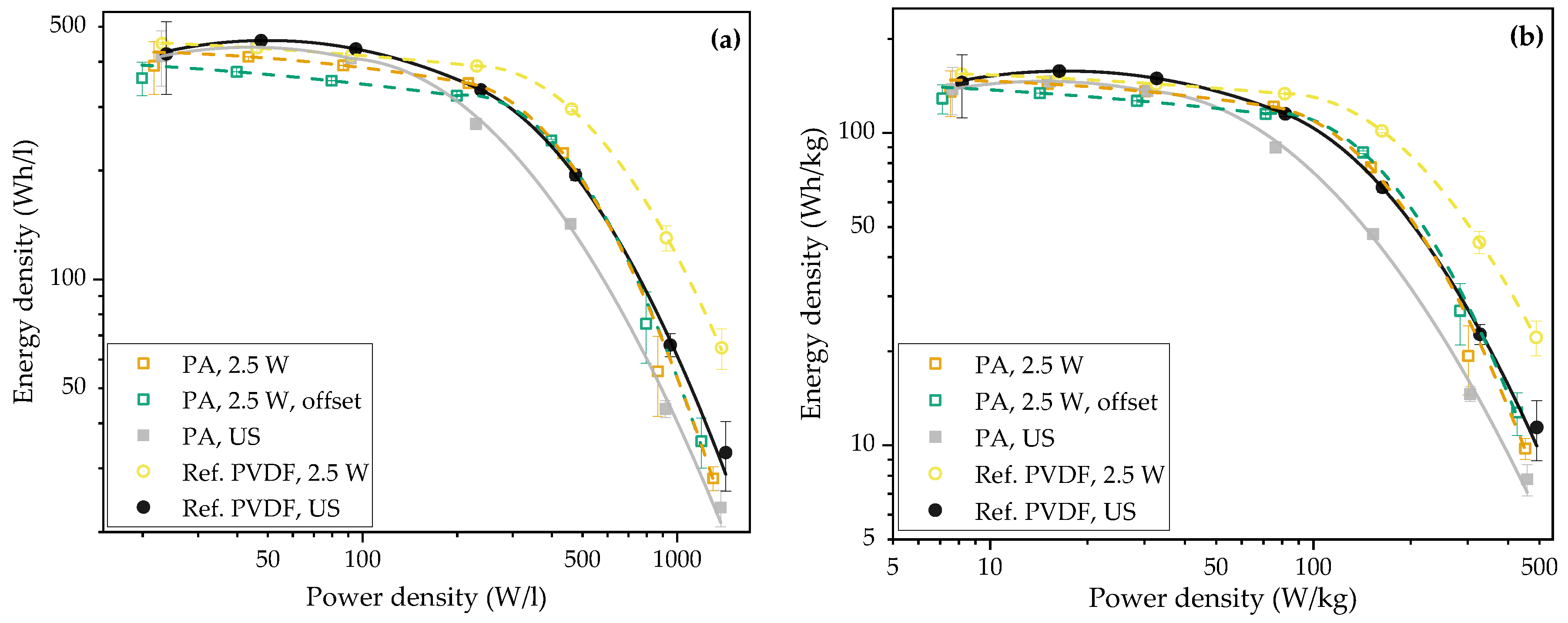
3.2.2. Pouch Cells (Full-Cells)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Calculation of Energy and Power Density of Pouch Cells
References
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Ismail, S.A.; Song, Y.; Irfan, R.M.; Yang, S.; Zhou, S.; Zhao, J.; Gao, L. A review of the energy storage aspects of chemical elements for lithium-ion based batteries. Energy Mater. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Andwari, A.M.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A review of Battery Electric Vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Zhou, W.; Cleaver, C.J.; Dunant, C.F.; Allwood, J.M.; Lin, J. Cost, range anxiety and future electricity supply: A review of how today’s technology trends may influence the future uptake of BEVs. Renew. Sustain. Energy Rev. 2023, 173, 113074. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Daniel, C.; Kalnaus, S.; Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Park, K.-Y.; Park, J.-W.; Seong, W.M.; Yoon, K.; Hwang, T.-H.; Ko, K.-H.; Han, J.-H.; Jaedong, Y.; Kang, K. Understanding capacity fading mechanism of thick electrodes for lithium-ion rechargeable batteries. J. Power Sources 2020, 468, 228369. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Susarla, N.; Ahmed, S.; Dees, D.W. Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 2018, 378, 660–670. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles–Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.; Daniel, C.; Wood, D., III. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Gilbert, J.A.; Phillips, P.J.; Klie, R.; Haasch, R.T.; Bareno, J.; Abraham, D.P. Chemical weathering of layered Ni-rich oxide electrode materials: Evidence for cation exchange. J. Electrochem. Soc. 2017, 164, A1489. [Google Scholar] [CrossRef]
- Park, J.h.; Park, J.k.; Lee, J.w. Stability of LiNi0.6Mn0.2Co0.2O2 as a Cathode Material for Lithium-Ion Batteries against Air and Moisture. Bull. Korean Chem. Soc. 2016, 37, 344–348. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Huang, J.; Fu, T.; Sun, G.; Lai, S.; Zhou, R.; Li, K.; Zhao, J. The high-temperature and high-humidity storage behaviors and electrochemical degradation mechanism of LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. J. Power Sources 2017, 363, 168–176. [Google Scholar] [CrossRef]
- Hofmann, M.; Kapuschinski, M.; Guntow, U.; Giffin, G.A. Implications of aqueous processing for high energy density cathode materials: Part I. Ni-rich layered oxides. J. Electrochem. Soc. 2020, 167, 140512. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer III, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L., III. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Li, S.; Church, B. Effect of aqueous-based cathode slurry pH and immersion time on corrosion of aluminum current collector in lithium-ion batteries. Mater. Corros. 2016, 67, 978–987. [Google Scholar] [CrossRef]
- Loeffler, N.; Kim, G.T.; Mueller, F.; Diemant, T.; Kim, J.K.; Behm, R.J.; Passerini, S. In situ coating of Li[Ni0.33Mn0.33Co0.33]O2 particles to enable aqueous electrode processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef]
- Bauer, W.; Çetinel, F.A.; Müller, M.; Kaufmann, U. Effects of pH control by acid addition at the aqueous processing of cathodes for lithium ion batteries. Electrochim. Acta 2019, 317, 112–119. [Google Scholar] [CrossRef]
- Heidbüchel, M.; Schultz, T.; Placke, T.; Winter, M.; Koch, N.; Schmuch, R.; Gomez-Martin, A. Enabling Aqueous Processing of Ni-Rich Layered Oxide Cathode Materials by Addition of Lithium Sulfate. ChemSusChem 2022, 16, e202202161. [Google Scholar] [CrossRef] [PubMed]
- Sahni, K.; Ashuri, M.; He, Q.; Sahore, R.; Bloom, I.D.; Liu, Y.; Kaduk, J.A.; Shaw, L.L. H3PO4 treatment to enhance the electrochemical properties of Li(Ni1/3Mn1/3Co1/3)O2 and Li(Ni0.5Mn0.3Co0.2)O2 cathodes. Electrochim. Acta 2019, 301, 8–22. [Google Scholar] [CrossRef]
- de Meatza, I.; Urdampilleta, I.; Boyano, I.; Castrillo, I.; Landa-Medrano, I.; Sananes-Israel, S.; Eguia-Barrio, A.; Palomares, V. From Lab to Manufacturing Line: Guidelines for the Development and Upscaling of Aqueous Processed NMC622 Electrodes. J. Electrochem. Soc. 2023, 170, 010527. [Google Scholar] [CrossRef]
- Tolchard, J.R.; Vullum, P.E.; Arstad, B.; Wagner, N.P. New insights into orthophosphoric acid assisted rapid aqueous processing of NMC622 cathodes. RSC Sustain. 2023, 1, 378–387. [Google Scholar] [CrossRef]
- Kukay, A.; Sahore, R.; Parejiya, A.; Hawley, W.B.; Li, J.; Wood, D.L., III. Aqueous Ni-rich-cathode dispersions processed with phosphoric acid for lithium-ion batteries with ultra-thick electrodes. J. Colloid Interface Sci. 2021, 581, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, L.; Fröhlich, K.; Eshraghi, N.; Cupid, D.; Winter, F.; Jahn, M. Aqueous Manufacturing of Defect-Free Thick Multi-Layer NMC811 Electrodes. Nanomaterials 2022, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Trouillet, V.; Heißler, S.; Pfleging, W. Laser structuring of high mass loaded and aqueous acid processed Li(Ni0.6Mn0.2Co0.2)O2 cathodes for lithium-ion batteries. J. Energy Storage 2023, 66, 107401. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid electrolyte wetting of lithium-ion batteries containing laser structured electrodes: In situ visualization by neutron radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778. [Google Scholar] [CrossRef]
- Berhe, M.G.; Oh, H.G.; Park, S.-K.; Mondal, M.; Lee, D. Effect of laser-induced groove morphology on the wettability and performance of Lithium-ion batteries. Mater. Des. 2023, 231, 112020. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.-J.; Jeong, S.; Kim, H.-J. Challenges, laser processing and electrochemical characteristics on application of ultra-thick electrode for high-energy lithium-ion battery. J. Power Sources 2021, 482, 228948. [Google Scholar] [CrossRef]
- Zhu, P.; Seifert, H.J.; Pfleging, W. The ultrafast laser ablation of Li(Ni0.6Mn0.2Co0.2)O2 electrodes with high mass loading. Appl. Sci. 2019, 9, 4067. [Google Scholar] [CrossRef]
- Dunlap, N.; Sulas-Kern, D.B.; Weddle, P.J.; Usseglio-Viretta, F.; Walker, P.; Todd, P.; Boone, D.; Colclasure, A.M.; Smith, K.; de Villers, B.J.T. Laser ablation of Li-ion electrodes for fast charging: Material properties, rate capability, Li plating, and wetting. J. Power Sources 2022, 537, 231464. [Google Scholar] [CrossRef]
- Tran, M.X.; Smyrek, P.; Park, J.; Pfleging, W.; Lee, J.K. Ultrafast-Laser Micro-Structuring of LiNi0.8Mn0.1Co0.1O2 Cathode for High-Rate Capability of Three-Dimensional Li-ion Batteries. Nanomaterials 2022, 12, 3897. [Google Scholar] [CrossRef]
- Zheng, Y.; Seifert, H.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Dubey, R.; Zwahlen, M.D.; Shynkarenko, Y.; Yakunin, S.; Fuerst, A.; Kovalenko, M.V.; Kravchyk, K.V. Laser Patterning of High-Mass-Loading Graphite Anodes for High-Performance Li-Ion Batteries. Batter. Supercaps 2021, 4, 464–468. [Google Scholar] [CrossRef]
- Meyer, A.; Ball, F.; Pfleging, W. The Effect of Silicon Grade and Electrode Architecture on the Performance of Advanced Anodes for Next Generation Lithium-Ion Cells. Nanomaterials 2021, 11, 3448. [Google Scholar] [CrossRef]
- Tsuda, T.; Ando, N.; Matsubara, K.; Tanabe, T.; Itagaki, K.; Soma, N.; Nakamura, S.; Hayashi, N.; Gunji, T.; Ohsaka, T. Improvement of high-rate charging/discharging performance of a lithium ion battery composed of laminated LiFePO4 cathodes/graphite anodes having porous electrode structures fabricated with a pico-second pulsed laser. Electrochim. Acta 2018, 291, 267–277. [Google Scholar] [CrossRef]
- Habedank, J.B.; Kriegler, J.; Zaeh, M.F. Enhanced fast charging and reduced lithium-plating by laser-structured anodes for lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A3940. [Google Scholar] [CrossRef]
- Kriegler, J.; Hille, L.; Stock, S.; Kraft, L.; Hagemeister, J.; Habedank, J.B.; Jossen, A.; Zaeh, M.F. Enhanced performance and lifetime of lithium-ion batteries by laser structuring of graphite anodes. Appl. Energy 2021, 303, 117693. [Google Scholar] [CrossRef]
- Hille, L.; Toepper, H.-C.; Schriever, C.; Kriegler, J.; Keilhofer, J.; Noecker, M.P.; Zaeh, M.F. Influence of Laser Structuring and Calendering of Graphite Anodes on Electrode Properties and Cell Performance. J. Electrochem. Soc. 2022, 169, 060518. [Google Scholar] [CrossRef]
- Hille, L.; Kriegler, J.; Oehler, A.; Chaja, M.; Wagner, S.; Zaeh, M.F. Picosecond laser structuring of graphite anodes—Ablation characteristics and process scaling. J. Laser Appl. 2023, 35, 042054. [Google Scholar] [CrossRef]
- Zhu, P.; Ebert, B.; Smyrek, P.; Pfleging, W. The Impact of Structural Pattern Types on the Electrochemical Performance of Ultra-Thick NMC 622 Electrodes for Lithium-Ion Batteries. Batteries 2024, 10, 58. [Google Scholar] [CrossRef]
- Sterzl, Y.; Pfleging, W. Optimizing Structural Patterns for 3D Electrodes in Lithium-Ion Batteries for Enhanced Fast-Charging Capability and Reduced Lithium Plating. Batteries 2024, 10, 160. [Google Scholar] [CrossRef]
- Park, D.; Lee, D. Effect of fluence and multi-pass on groove morphology and process efficiency of laser structuring for 3D electrodes of lithium-ion batteries. Materials 2021, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Habedank, J.B.; Schwab, D.; Kiesbauer, B.; Zaeh, M.F. Paving the way for industrial ultrafast laser structuring of lithium-ion battery electrodes by increasing the scanning accuracy. J. Laser Appl. 2020, 32, 022053. [Google Scholar] [CrossRef]
- Yamada, M.; Soma, N.; Tsuta, M.; Nakamura, S.; Ando, N.; Matsumoto, F. Development of a roll-to-roll high-speed laser micro processing machine for preparing through-holed anodes and cathodes of lithium-ion batteries. Int. J. Extrem. Manuf. 2023, 5, 035004. [Google Scholar] [CrossRef]
- Zhu, P.; Han, J.; Pfleging, W. Characterization and laser structuring of aqueous processed Li(Ni0.6Mn0.2Co0.2)O2 thick-film cathodes for lithium-ion batteries. Nanomaterials 2021, 11, 1840. [Google Scholar] [CrossRef]
- Stock, S.; Hagemeister, J.; Grabmann, S.; Kriegler, J.; Keilhofer, J.; Ank, M.; Dickmanns, J.L.; Schreiber, M.; Konwitschny, F.; Wassiliadis, N. Cell teardown and characterization of an automotive prismatic LFP battery. Electrochim. Acta 2023, 471, 143341. [Google Scholar] [CrossRef]
- Ank, M.; Sommer, A.; Gamra, K.A.; Schöberl, J.; Leeb, M.; Schachtl, J.; Streidel, N.; Stock, S.; Schreiber, M.; Bilfinger, P. Lithium-ion cells in automotive applications: Tesla 4680 cylindrical cell teardown and characterization. J. Electrochem. Soc. 2023, 170, 120536. [Google Scholar] [CrossRef]
- Sterzl, Y.; Pfleging, W. Extending the 3D-battery concept: Large areal ultrashort pulsed laser structuring of multilayered electrode coatings. In Proceedings of the Laser-based Micro-and Nanoprocessing XVII, San Francisco, CA, USA, 31 January–2 February 2023; pp. 114–122. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; He, M.; Jiang, Y.; Zhang, B.; Hang, W.; Huang, B. Laser-induced plasma temperature. Spectrochim. Acta Part B At. Spectrosc. 2014, 97, 13–33. [Google Scholar] [CrossRef]
- Bolsinger, M.; Weller, M.; Ruck, S.; Kaya, P.; Riegel, H.; Knoblauch, V. Selective surface treatment by means of IR-laser–A new approach to enhance the rate capability of cathodes for Li-ion batteries. Electrochim. Acta 2020, 330, 135163. [Google Scholar] [CrossRef]
- Ku, H.H. Notes on the use of propagation of error formulas. J. Res. Natl. Bur. Stand. 1966, 70, 263–273. [Google Scholar] [CrossRef]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance spectroscopy characterization of porous electrodes under different electrode thickness using a symmetric cell for high-performance lithium-ion batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. npj Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Neidhart, L.; Fröhlich, K.; Boz, B.; Winter, F.; Jahn, M. Layer by Layer: Improved Tortuosity in High Loading Aqueous NMC811 Electrodes. J. Electrochem. Soc. 2024, 171, 050532. [Google Scholar] [CrossRef]
- Landesfeind, J.; Gasteiger, H.A. Temperature and concentration dependence of the ionic transport properties of lithium-ion battery electrolytes. J. Electrochem. Soc. 2019, 166, A3079–A3097. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, P.; Pfleging, W.; Sun, J. Electrochemical performance of thick-film Li(Ni0.6Mn0.2Co0.2)O2 cathode with hierarchic structures and laser ablation. Nanomaterials 2021, 11, 2962. [Google Scholar] [CrossRef]
- Hawley, W.B.; Parejiya, A.; Bai, Y.; Meyer, H.M., III; Wood, D.L., III; Li, J. Lithium and transition metal dissolution due to aqueous processing in lithium-ion battery cathode active materials. J. Power Sources 2020, 466, 228315. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 2018, 11, 527–543. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; Eguia-Barrio, A.; Sananes-Israel, S.; Lijó-Pando, S.; Boyano, I.; Alcaide, F.; Urdampilleta, I.; de Meatza, I. In situ analysis of NMC∣graphite Li-ion batteries by means of complementary electrochemical methods. J. Electrochem. Soc. 2020, 167, 090528. [Google Scholar] [CrossRef]
- Plattard, T.; Barnel, N.; Assaud, L.; Franger, S.; Duffault, J.-M. Combining a fatigue model and an incremental capacity analysis on a commercial NMC/graphite cell under constant current cycling with and without calendar aging. Batteries 2019, 5, 36. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361. [Google Scholar] [CrossRef]
- Weng, C.; Cui, Y.; Sun, J.; Peng, H. On-board state of health monitoring of lithium-ion batteries using incremental capacity analysis with support vector regression. J. Power Sources 2013, 235, 36–44. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhao, J.; Ma, Z.; Tan, Y.; Xue, J.; Xu, H.; Nan, J. Aqueous Lithium Carboxymethyl Cellulose and Polyacrylic Acid/Acrylate Copolymer Composite Binder for the LiNi0.5Mn0.3Co0.2O2 Cathode of Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 010513. [Google Scholar] [CrossRef]
- Liang, L.; Hu, G.; Jiang, F.; Cao, Y. Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J. Alloys Compd. 2016, 657, 570–581. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Du, Z.; Daniel, C.; Wood, D.L., III. Fast formation cycling for lithium ion batteries. J. Power Sources 2017, 342, 846–852. [Google Scholar] [CrossRef]
- Heinrich, M.; Wolff, N.; Harting, N.; Laue, V.; Röder, F.; Seitz, S.; Krewer, U. Physico-chemical Modeling of a lithium-ion battery: An ageing study with electrochemical impedance spectroscopy. Batter. Supercaps 2019, 2, 530–540. [Google Scholar] [CrossRef]
- Westerhoff, U.; Kurbach, K.; Lienesch, F.; Kurrat, M. Analysis of lithium-ion battery models based on electrochemical impedance spectroscopy. Energy Technol. 2016, 4, 1620–1630. [Google Scholar] [CrossRef]
- Middlemiss, L.A.; Rennie, A.J.; Sayers, R.; West, A.R. Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep. 2020, 6, 232–241. [Google Scholar] [CrossRef]

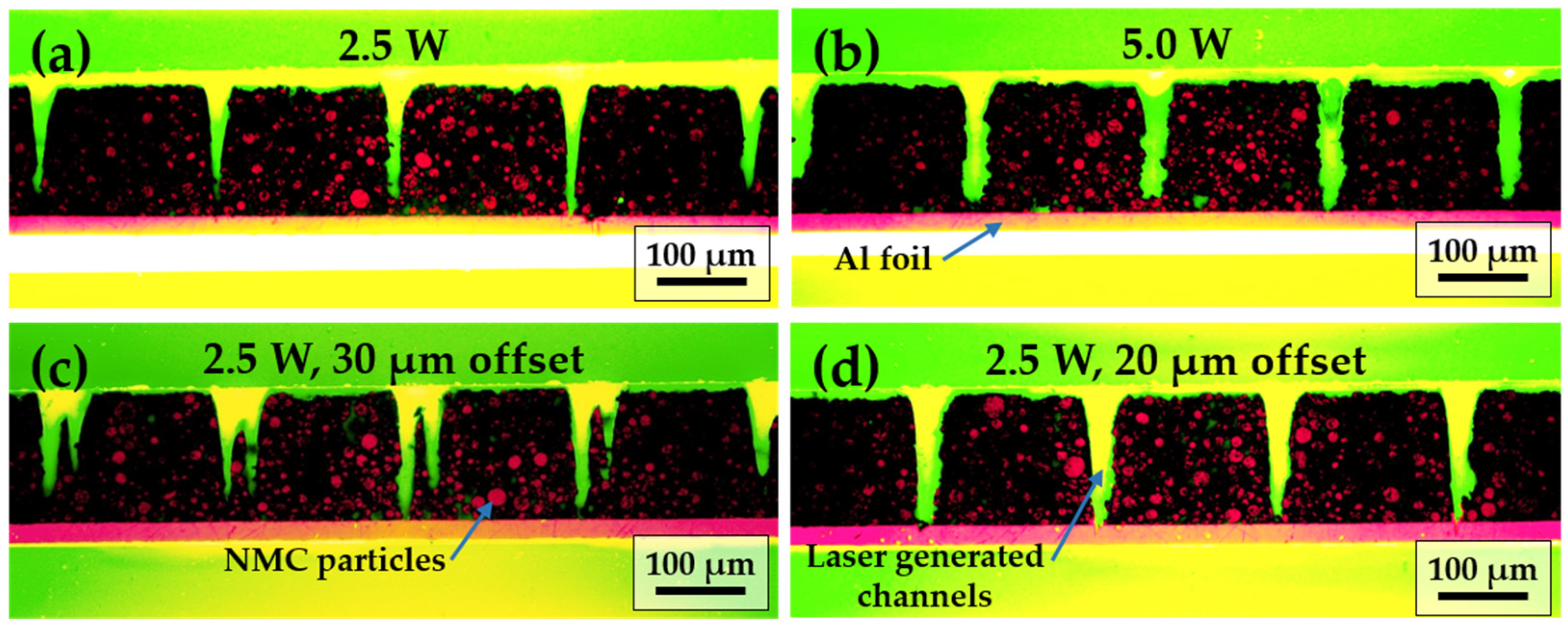


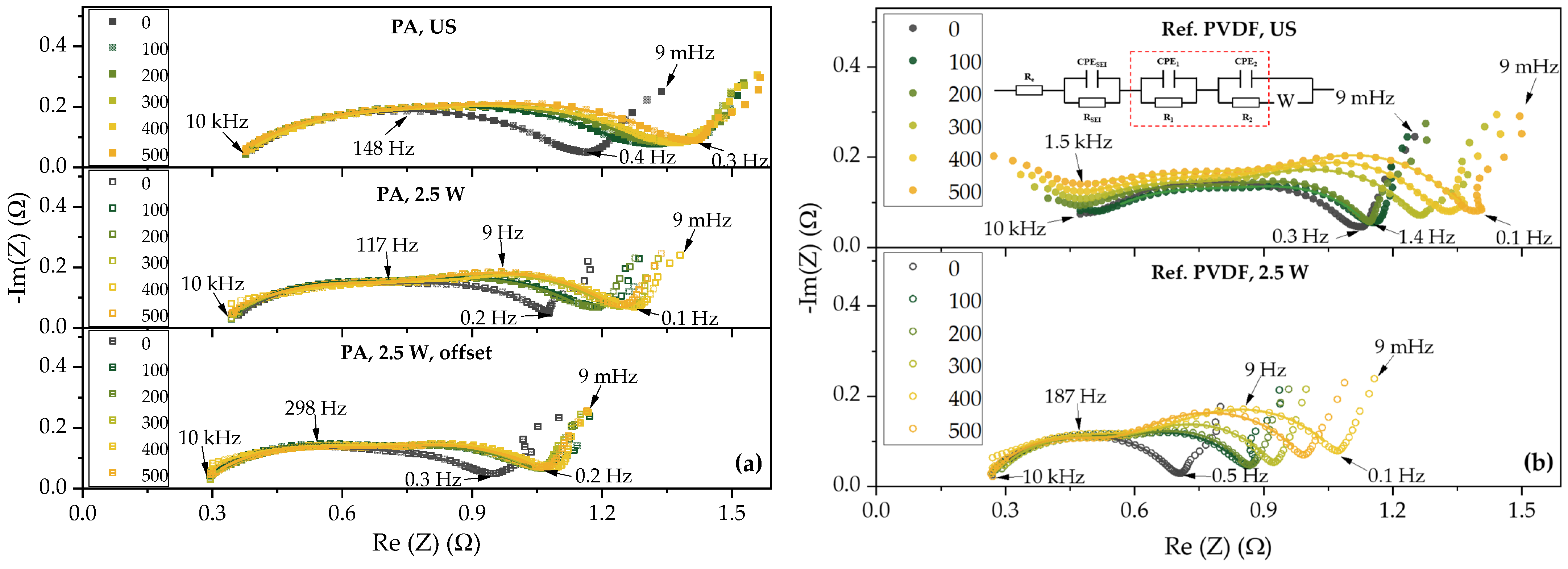
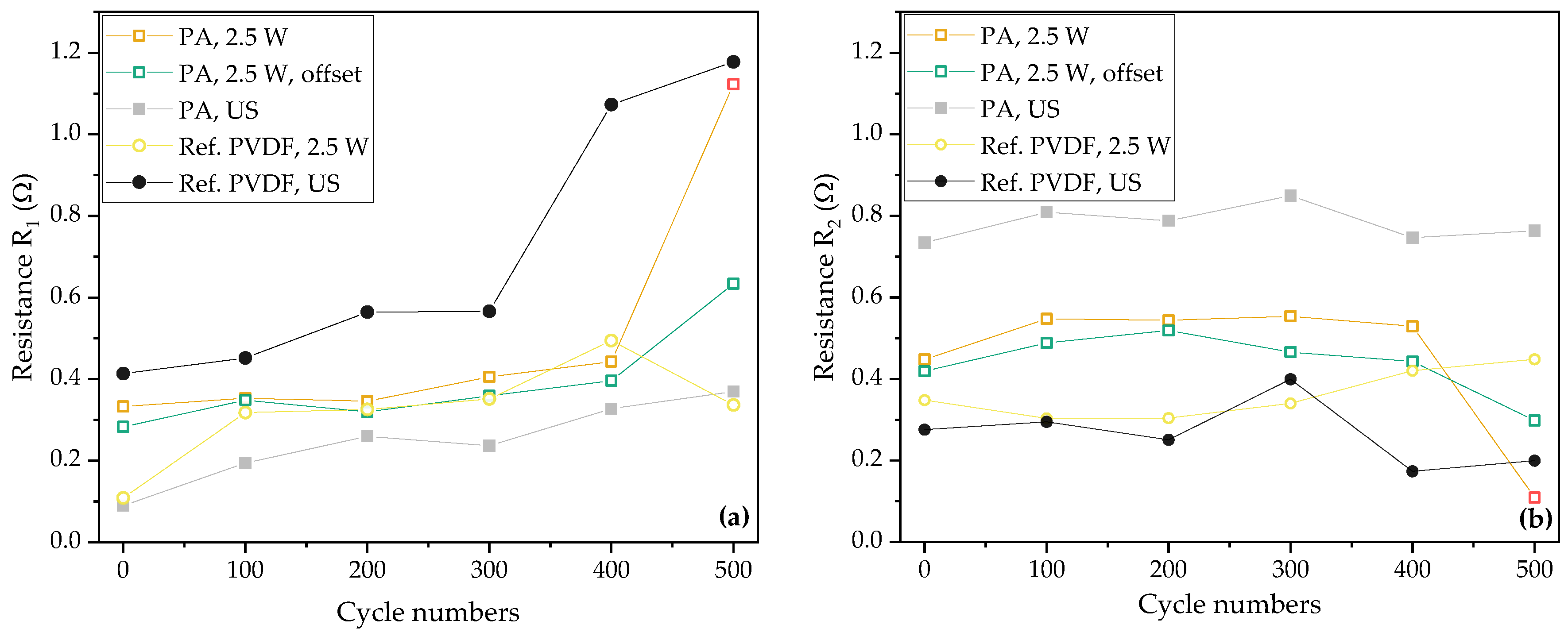
| Electrodes | Average Laser Power [W] | Laser Scan Speed [mm/s] | Laser Repetition Rate [kHz] | Line Energy [J/m] | Laser Scans [-] | Pitch [µm] |
|---|---|---|---|---|---|---|
| NMC 622 cathodes with PVDF as binder | 2.5 | 500 | 500 | 5 | 22 | 200 |
| Aqueous processed NMC 622 cathodes | 2.5 | 500 | 500 | 5 | 13 | 200 |
| Aqueous processed NMC 622 cathodes | 5.0 | 500 | 500 | 10 | 7 | 200 |
| Graphite anodes | 37 | 20,000 | 1000 | 1.85 | 92 | 350 |
| Electrodes Type | Film Thickness * [µm] | Active Mass Loading [mg/cm2] | Areal Capacity [mAh/cm2] |
|---|---|---|---|
| Ref. cathodes with PVDF | 150 ± 5 | 35.4 ± 0.6 | 6.1 ± 0.1 |
| Cathodes with PA addition | 149 ± 4 | 35.5 ± 0.3 | 5.9 ± 0.1 |
| Graphite anodes (thin) | 171 ± 3 | 19.5 ± 0.3 | 6.4 ± 0.1 |
| Graphite anodes (thick) | 195 ± 4 | 22.0 ± 0.4 | 7.3 ± 0.2 |
| Electrode Type | Max. Width [µm] | FWHM [µm] | Active Mass Loading [mg/cm2] | Areal Capacity [mAh/cm2] | Mass Loss [%] |
|---|---|---|---|---|---|
| Cathodes with PA addition, 2.5 W | 30 ± 3 | 13 ± 1 | 34.0 ± 0.1 | 5.6 ± 0.1 | 4.2 ± 1.3 |
| Cathodes with PA addition, 5 W | 43 ± 4 | 26 ± 2 | 32.3 ± 0.3 | 5.3 ± 0.1 | 9.0 ± 1.5 |
| Cathodes with PA addition, 2.5 W, with offset | 50 ± 4 | 25 ± 3 | 31.9 ± 0.2 | 5.3 ± 0.1 | 10.2 ± 1.9 |
| Ref. cathodes with PVDF, 2.5 W | 33 ± 2 | 10 ± 2 | 33.6 ± 0.2 | 5.8 ± 0.2 | 3.4 ± 2.2 |
| Anode (thick) | 46 ± 5 | 28 ± 2 | 19.4 ± 0.4 | 6.4 ± 0.1 | 12.0 ± 3.3 |
| Cathode Type | Initial CE [%] | Initial Discharge Capacity [mAh/g] |
|---|---|---|
| PA, 2.5 W | 81.7 ± 0.7 | 120 ± 4 |
| PA, 2.5 W, offset | 84.3 ± 3.3 | 125 ± 3 |
| PA, US | 83.8 ± 1.6 | 97 ± 15 |
| Ref. PVDF, 2.5 W | 86.0 ± 3.2 | 157 ± 12 |
| Ref. PVDF, US | 83.3 ± 3.1 | 126 ± 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Sterzl, Y.; Pfleging, W. Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed Li(Ni0.6Mn0.2Co0.2)O2 Cathodes with High Mass Loading. Batteries 2024, 10, 354. https://doi.org/10.3390/batteries10100354
Zhu P, Sterzl Y, Pfleging W. Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed Li(Ni0.6Mn0.2Co0.2)O2 Cathodes with High Mass Loading. Batteries. 2024; 10(10):354. https://doi.org/10.3390/batteries10100354
Chicago/Turabian StyleZhu, Penghui, Yannic Sterzl, and Wilhelm Pfleging. 2024. "Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed Li(Ni0.6Mn0.2Co0.2)O2 Cathodes with High Mass Loading" Batteries 10, no. 10: 354. https://doi.org/10.3390/batteries10100354
APA StyleZhu, P., Sterzl, Y., & Pfleging, W. (2024). Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed Li(Ni0.6Mn0.2Co0.2)O2 Cathodes with High Mass Loading. Batteries, 10(10), 354. https://doi.org/10.3390/batteries10100354








