Enhanced Electrochemical Performance of Lithium Iron Phosphate Cathodes Using Plasma-Assisted Reduced Graphene Oxide Additives for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Plasma-Assisted LFP/Reduced GO Composite (LFP@rGO)
2.3. Morphology and Molecule Structure Analysis
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Fabrication of the LFP@rGO Composite
3.2. Electrochemical Analysis of LFP@rGO Cathodes
3.3. Practical LIB Application of the LFP@rGO//Graphite Full-Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Xie, C.; Chang, J.; Shang, J.; Wang, L.; Gao, Y.; Huang, Q.; Zheng, Z. Hybrid lithium-ion/metal electrodes enable long cycle stability and high energy density of flexible batteries. Adv. Funct. Mater. 2022, 32, 2203242. [Google Scholar] [CrossRef]
- Khan, F.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device configurations and future prospects of flexible/stretchable lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.S.; Hang, S.K.; Choi, S.; Ryu, J.H.; Song, H.K. The current move of lithium ion batteries towards the next phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Hu, Q.; Guo, S.; Yu, L.; Li, Q.; Liu, Q.; Hu, X. Safer lithium-ion batteries from the separator aspect: Development and future perspectives. Energy Environ. Mater. 2021, 4, 336–362. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Z. Effects of a separator on the electrochemical and thermal performances of lithium-ion batteries: A numerical study. Energy Fuels 2020, 34, 14915–14923. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Z.; Chen, W.; Sun, X.; Zhang, X.; Wang, K.; Ma, Y. Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives. Batteries 2023, 9, 74. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.; Kang, K. Redox-active organic compounds for future sustainable energy storage system. Adv. Energy Mater. 2020, 10, 2001445. [Google Scholar] [CrossRef]
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Kotal, M.; Jakhar, S.; Roy, S.; Sharma, H.K. Cathode materials for rechargeable lithium batteries: Recent progress and future prospects. J. Energy Storage 2022, 47, 103534. [Google Scholar] [CrossRef]
- Kim, J.S.; Lim, S.; Ingole, R.S.; Munakata, H.; Kim, S.S.; Kanamura, K. Improving the high-rate performance of LCO cathode by metal oxide coating: Evaluation using single particle measurement. J. Electroanal. Chem. 2023, 933, 117190. [Google Scholar] [CrossRef]
- Cai, W.F.; Chen, K.C. Influence of the Calendar Aging on the Cycle Aging of LiNiMnCoO2 lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 120525. [Google Scholar] [CrossRef]
- Joos, J.; Buchele, A.; Schmidt, A.; Weber, A.; Ivers-Tiffée, E. Virtual Electrode Design for Lithium-Ion Battery Cathodes. Energy Technol. 2021, 9, 2000891. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Zhou, T.; Sun, J.; Li, J.; Wei, S.; Chen, J.; Dang, S.; Tang, N.; Zhu, Y.; Lian, Y.; Gao, J.; et al. Toxicity, Emissions and Structural Damage from Lithium-Ion Battery Thermal Runaway. Batteries 2023, 9, 308. [Google Scholar] [CrossRef]
- Yi, M.; Li, W.; Manthiram, A. Delineating the roles of Mn, Al, and Co by comparing three layered oxide cathodes with the same nickel content of 70% for lithium-ion batteries. Chem. Mater. 2022, 34, 629–642. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Ziebert, C. Thermal and mechanical safety assessment of type 21700 lithium-ion batteries with NMC, NCA and LFP cathodes–Investigation of cell abuse by means of accelerating rate calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Olivine positive electrodes for Li-ion batteries: Status and perspectives. Batteries 2018, 4, 39. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Feng, B.; Long, T.; Wang, K.; Yu, Q.; Wang, Z.; Ding, Y.L. Enabling fast charging and all-climate Mn-containing olivine cathode via constructing hierarchically porous bulk architecture. J. Power Sources 2024, 614, 234996. [Google Scholar] [CrossRef]
- Suttison, S.; Pengpat, K.; Intatha, U.; Fan, J.; Zhang, W.; Eitssayeam, S. Preparation of LFP-based cathode materials for lithium-ion battery applications. Mater. Today Proc. 2022, 65, 2347–2350. [Google Scholar] [CrossRef]
- Andrews, J.L.; Brady, M.J.; McClure, E.T.; Melot, B.C. Impact of structural deformations on the performance of Li-ion insertion hosts. Chem. Mater. 2022, 34, 4809–4820. [Google Scholar] [CrossRef]
- Rkhis, M.; Laasri, S.; Touhtouh, S.; Hlil, E.K.; Hajjaji, A. Tailoring the electrochemical performance of olivine phosphate cathode materials for Li-Ion batteries by strain engineering: Computational experiments. ACS Appl. Energy Mater. 2023, 6, 7074–7082. [Google Scholar] [CrossRef]
- Chen, S.P.; Lv, D.; Chen, J.; Zhang, Y.H.; Shi, F.N. Review on defects and modification methods of LiFePO4 cathode material for lithium-ion batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Varzi, A.; Bresser, D.; von Zamory, J.; Müller, F.; Passerini, S. ZnFe2O4-C/LiFePO4-CNT: A novel high-power lithium-ion battery with excellent cycling performance. Adv. Energy Mater. 2014, 4, 1400054. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, T.R.; Yougbaré, S.; Lin, L.Y. Design of LiFePO4 and porous carbon composites with excellent High-Rate charging performance for Lithium-Ion secondary battery. J. Colloid Interface Sci. 2022, 607, 1457–1465. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Peng, J.M.; Chen, Z.Q.; Li, Y.; Hu, S.-J.; Pan, Q.-C.; Zheng, F.-H.; Wang, H.-Q.; Li, Q.-Y. Conducting network interface modulated rate performance in LiFePO4/C cathode materials. Rare Met. 2022, 41, 951–959. [Google Scholar] [CrossRef]
- Napolskiy, F.; Avdeev, M.; Yerdauletov, M.; Ivankov, O.; Bocharova, S.; Ryzhenkova, S.; Kaparova, B.; Mironovich, K.; Burlyaev, D.; Krivchenko, V. On the Use of Carbon Nanotubes in Prototyping the High Energy Density Li-ion Batteries. Energy Technol. 2020, 8, 2000146. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Liu, F.; Yang, J.; Feng, T. Variation of carbon coatings on the electrochemical performance of LiFePO4 cathodes for lithium ionic batteries. RSC Adv. 2017, 7, 44296–44302. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Cho, S.; Yoon, C.-M.; Lee, C.; Ryu, J.; Jang, J. Multidimensional polyaniline/reduced graphene oxide/silica nanocomposite for efficient supercapacitor electrodes. ChemNanoMat 2016, 2, 236–241. [Google Scholar] [CrossRef]
- Noh, J.; Jekal, S.; Yoon, C.-M. Polyaniline-Coated Mesoporous Carbon Nanosheets with Fast Capacitive Energy Storage in Symmetric Supercapacitors. Adv. Sci. 2023, 10, 2301923. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Li, H.; Xu, L.; Yan, C. Recent advances in graphene based materials as anode materials in sodium-ion batteries. J. Energy Chem. 2020, 42, 91–107. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Recent advancement made in the field of reduced graphene oxide-based nanocomposites used in the energy storage devices: A review. J. Energy Storage 2021, 33, 102032. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-inhibited 3D reduced graphene oxide for high performance supercapacitor electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef]
- Cui, C.; Qian, W.; Yu, Y.; Kong, C.; Yu, B.; Xiang, L.; Wei, F. Highly electroconductive mesoporous graphene nanofibers and their capacitance performance at 4 V. J. Am. Chem. Soc. 2014, 136, 2256–2259. [Google Scholar] [CrossRef]
- Lee, G.; Lee, C.; Yoon, C.-M.; Kim, M.; Jang, J. High-performance three-dimensional mesoporous graphene electrode for supercapacitors using lyophilization and plasma reduction. ACS Appl. Mater. Interfaces 2017, 9, 5222–5230. [Google Scholar] [CrossRef]
- Samal, S.; Blanco, I. An overview of thermal plasma arc systems for treatment of various wastes in recovery of metals. Materials 2022, 15, 683. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Nadolska, M.; Şahin, S.; Łapiński, M.; Prześniak-Welenc, M.; Sawczak, M.; Sadowskwi, W.; Żelechowska, K. Tailoring properties of reduced graphene oxide by oxygen plasma treatment. Appl. Surf. Sci. 2018, 440, 651–659. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, L.; Cong, H.; Zhang, X.; Lü, W.; Xue, C. Plasma Treatment for Nitrogen-Doped 3D Graphene Framework by a Conductive Matrix with Sulfur for High-Performance Li–S Batteries. Small 2019, 15, 1804347. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kim, C.-G.; Jekal, S.; Otgonbayar, Z.; Kim, J.; Ra, Y.-H.; Noh, J.; Oh, W.-C.; Yoon, C.-M. Synthesis of Plasma-Reduced Graphene Oxide/Lithium Titanate Oxide Composite and Its Application as Lithium-Ion Capacitor Anode Material. Batteries 2024, 10, 311. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Lee, K.; Jang, J. Enhanced Electrorheological Performance of Mixed Silica Nanomaterial Geometry. ACS Appl. Mater. Interface 2017, 9, 36358–36367. [Google Scholar] [CrossRef]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual external field-responsive polyaniline-coated magnetite/silica nanoparticles for smart fluid applications. Chem. Commun. 2017, 53, 6645–6648. [Google Scholar] [CrossRef]
- Stenina, K.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Rastabi, S.R.; Mamoory, R.S.; Biomquist, N.; Phadatare, M.; Olin, H. Synthesis of a NiMoO4/3D-rGO Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance. Batteries 2020, 6, 5. [Google Scholar] [CrossRef]
- Zou, G.; Chen, K.; Luo, X.; Fu, Q.; Wu, B. Crystal structure, morphology, and electrical properties of aluminum-doped LFP materials. Batteries 2024, 30, 2549–2563. [Google Scholar] [CrossRef]
- Stenina, I.; Safikanov, D.; Minakova, P.; Novikova, S.; Kulova, T.; Yaroslavtsev, A. Composite Cathodes Based on Lithium-Iron Phosphate and N-Doped Carbon Materials. Batteries 2022, 8, 256. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Wu, W.; Zeng, W.; Dai, H.; Du, Y.; Liu, Z.; Li, L.; Ji, H.; Zhu, Y. LiFePO4/reduced graphene oxide hybrid cathode for lithium ion battery with outstanding rate performance. J. Mater. Chem. A 2024, 2, 7812–7818. [Google Scholar] [CrossRef]
- Longoni, G.; Panda, J.K.; Gagliani, L.; Brescia, R.; Manna, L.; Bonaccorso, F.; Pellegrini, V. In situ LiFePO4 nano-particles grown on few-layer graphene flakes as high-power cathode nanohybrids for lithium-ion batteries. Nano Energy 2018, 51, 656–667. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.; Lee, K.; Yu, H.; Lee, J.W.; Yoon, C.-M.; Kim, S.G.; Kim, S.K. Fluorine plasma treatment on carbon-based perovskite solar cells for rapid moisture protection layer formation and performance enhancement. Chem. Commun. 2020, 56, 535–538. [Google Scholar] [CrossRef]
- Wunderlich, P.; Küpper, J.; Simon, U. Optimizing Discharge Capacity of Graphite Nanosheet Electrodes for Lithium–Oxygen Batteries. Batteries 2020, 6, 36. [Google Scholar] [CrossRef]
- Zhou, S.; Du, J.; Xiong, X.; Liu, L.; Wang, J.; Fu, L.; Ye, J.; Chen, Y.; Wu, Y. Direct recovery of scrapped LiFePO4 by a green and low-cost electrochemical re-lithiation method. Green Chem. 2022, 24, 6278–6286. [Google Scholar] [CrossRef]
- Zhang, W.; Zhan, Y.; Gao, X.; Li, R.; Zhu, W.; Xu, H.; Liu, B.; Fang, X.; Xu, Y.; Ding, T. Effect of oxygen functionalities of graphene oxide on polymerization and thermal properties of reactive benzoxazine nanocomposites. Macromol. Res. 2017, 26, 77–84. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.-G. RGO–TiO2–ZnO composites: Synthesis, characterization, and application to photocatalysis. Appl. Catal. A-Gen. 2015, 491, 52–57. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Latha, D.; Karthikeyan, V.; Narayanan, V. Enhanced photocatalytic performance of ZnSnO3/rGO nanocomposite. Chem. Phys. Lett. 2020, 739, 137050. [Google Scholar] [CrossRef]
- Zhou, D.; Qiu, X.; Liang, F.; Cao, S.; Yao, Y.; Huang, X.; Ma, W.; Yang, B.; Dai, Y. Comparison of the effects of FePO4 and FePO4·2H2O as precursors on the electrochemical performances of LiFePO4/C. Ceram. Int. 2017, 43, 13254–13263. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, R.-G.; Liao, X.; Ma, Z.-M.; Wang, S.-D.; Xu, D. LiFePO4/C cathode material prepared with sphere mesoporous-FePO4 as precursors for lithium-ion batteries. J. Electroanal. Chem. 2018, 820, 18–23. [Google Scholar] [CrossRef]
- Apachitei, G.; Hidalgo, M.; Dogaru, D.; Lain, M.; Heymer, R.; Marco, J.; Copley, M. Optimisation of Industrially Relevant Electrode Formulations for LFP Cathodes in Lithium Ion Cells. Batteries 2023, 9, 192. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jekal, S.; Kim, D.-H.; Noh, J.; Kim, J.; Kim, H.-Y.; Kim, C.-G.; Chu, Y.-R.; Oh, W.-C. 3D Hierarchically Structured Tin Oxide and Iron Oxide-Embedded Carbon Nanofiber with Outermost Polypyrrole Layer for High-Performance Asymmetric Supercapacitor. Nanomaterials 2023, 13, 1614. [Google Scholar] [CrossRef]
- Seidl, C.; Thieme, S.; Frey, M.; Nikolowski, K.; Michaelis, A. Comparison of Electronic Resistance Measurement Methods and Influencing Parameters for LMFP and High-Nickel NCM Cathodes. Batteries 2024, 10, 105. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jekal, S.; Kim, C.-G.; Chu, Y.-R.; Noh, J.; Kim, M.S.; Lee, N.; Song, W.-J.; Yoon, C.-M. Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte. Gels 2023, 9, 351. [Google Scholar] [CrossRef]
- Yoon, J.H.; Cho, W.-J.; Kang, T.H.; Lee, M.; Yi, G.-R. Nanostructured Polymer Electrolytes for Lithium-Ion Batteries. Macromol. Res. 2021, 29, 509–518. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Lu, Y.C.; Hou, Y.; Li, Q. Electrophoretic lithium iron phosphate/reduced graphene oxide composite for lithium ion battery cathode application. J. Power Sources 2015, 284, 236–244. [Google Scholar] [CrossRef]
- Ha, S.H.; Lee, Y.J. Core-Shell LiFePO4/carbon-coated reduced graphene oxide hybrids for high-power lithium-ion battery cathodes. Chem.–A Eur. J. 2015, 21, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Mwizerwa, J.P.; Liu, C.; Xu, K.; Zhao, N.; Li, Y.; Ndagijimana, P.; Chen, Z.; Shen, J. Activated carbon/reduced graphene oxide wrapped LiFePO4 cathode for Li-ion batteries with ultrahigh capacities and high specific energy density. FlatChem 2022, 34, 100393. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Ryu, K.-S. Study of the lithium diffusion properties and high rate performance of TiNb6O17 as an anode in lithium secondary battery. Sci. Rep. 2017, 7, 16617. [Google Scholar] [CrossRef]
- Shaji, N.; Jiang, F.; Sung, J.Y.; Nanthagopal, M.; Kim, T.; Jeong, B.J.; Jung, S.P.; Lee, C.W. Heteroatoms-doped carbon effect on LiFePO4 cathode for Li-ion batteries. J. Energy Storage 2023, 72, 108710. [Google Scholar] [CrossRef]
- Dong, G.H.; Mao, Y.Q.; Li, Y.Q.; Huang, P.; Fu, S.Y. MXene-carbon nanotubes-Cellulose-LiFePO4 based self-supporting cathode with ultrahigh-area-capacity for lithium-ion batteries. Electrochim. Acta 2022, 420, 140464. [Google Scholar] [CrossRef]
- Xia, Z.; Li, Z.; Xu, J.; Sasidharan, S.; Sanchez, J.S.; Palermo, V.; Asp, L.E. Green synthesis of positive electrodes for high performance structural batteries-A study on graphene additives. Compos. Sci. Technol. 2024, 251, 110568. [Google Scholar] [CrossRef]
- Guo, F.; Kong, Z.; Wang, T.; Liu, X.; Xu, Z.; Fu, A.; Li, Y.; Guo, P.; Guo, Y.G.; Li, H. Porous microspheres consisting of carbon-modified LiFePO4 grains prepared by a spray-drying assisted approach using cellulose as carbon source. Ionics 2020, 26, 2737–2746. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, R.; Xia, Y.; Li, Q. Improved electrochemical performance of LiFePO4/carbon cathode for lithium-ion batteries. Ionics 2022, 28, 4579–4585. [Google Scholar] [CrossRef]
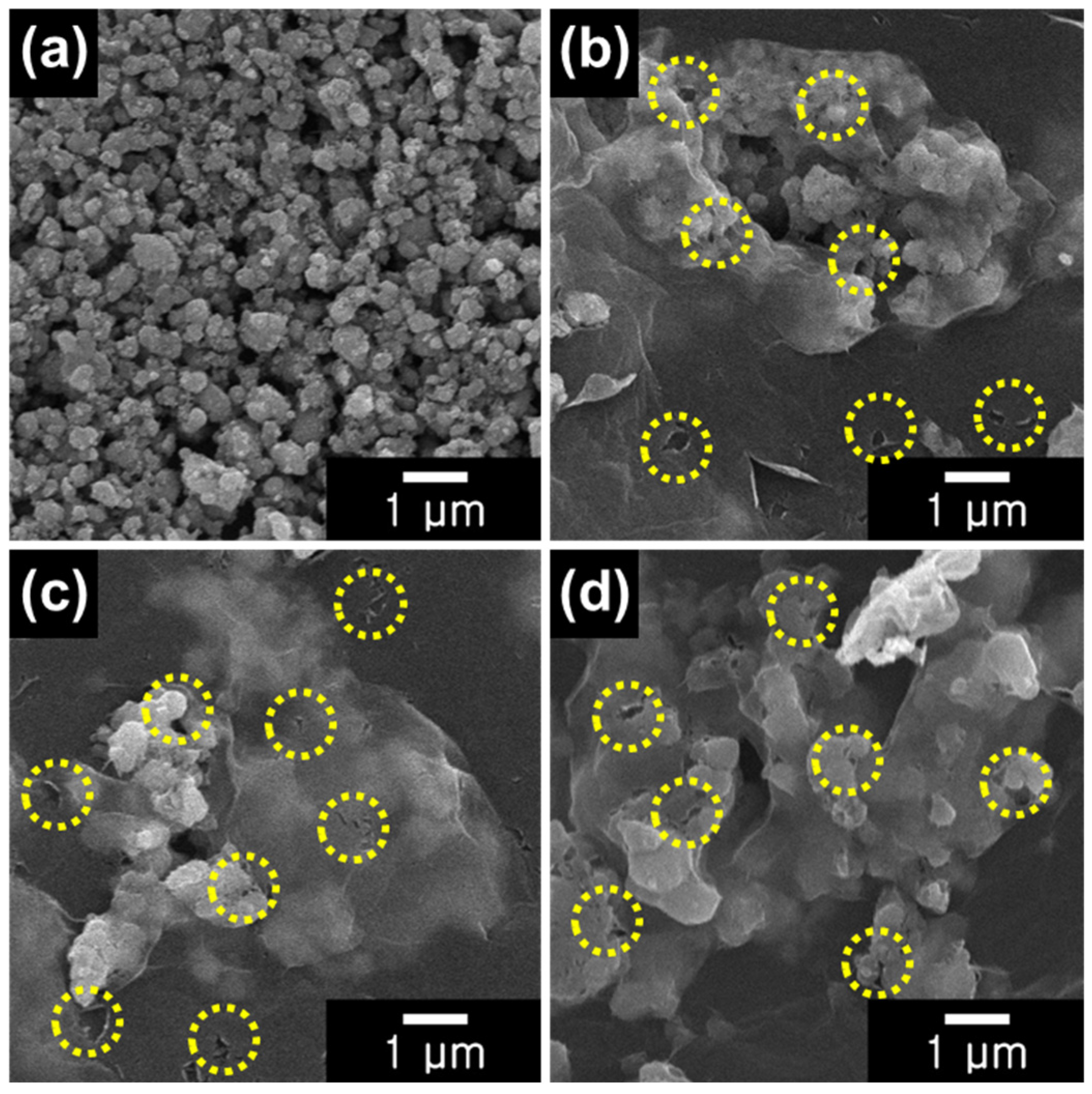

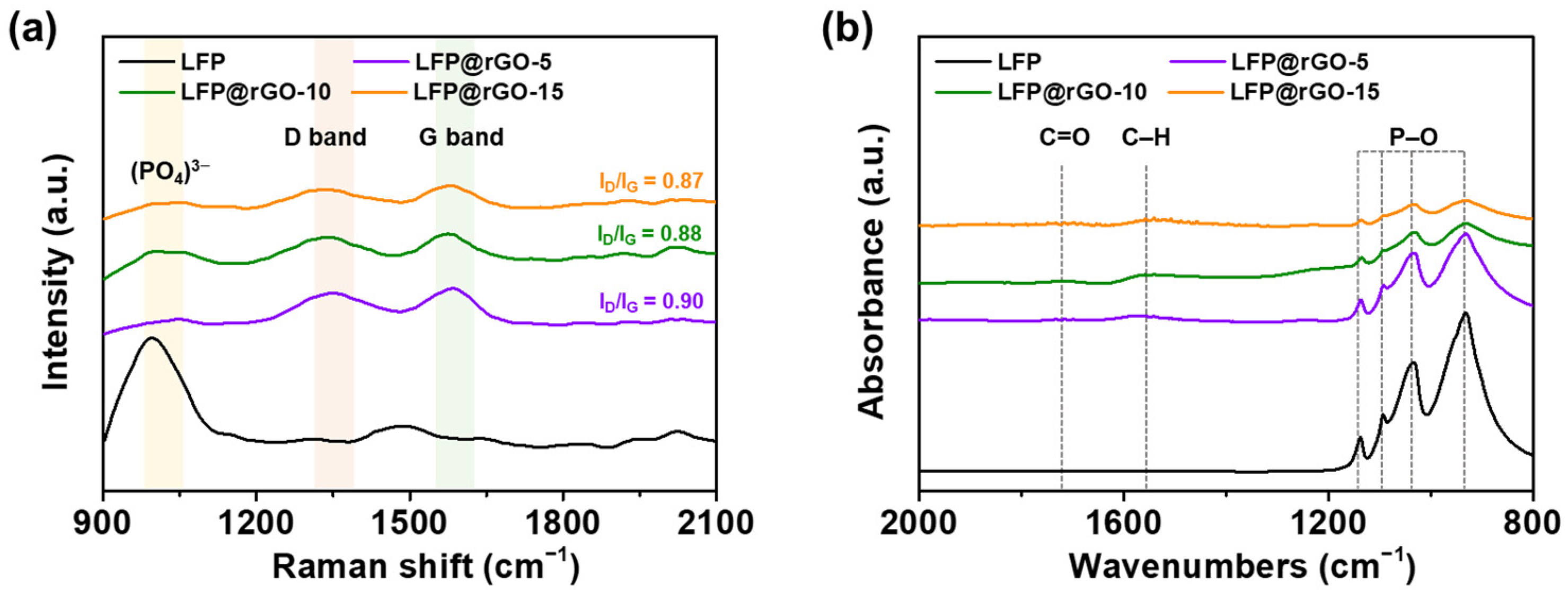
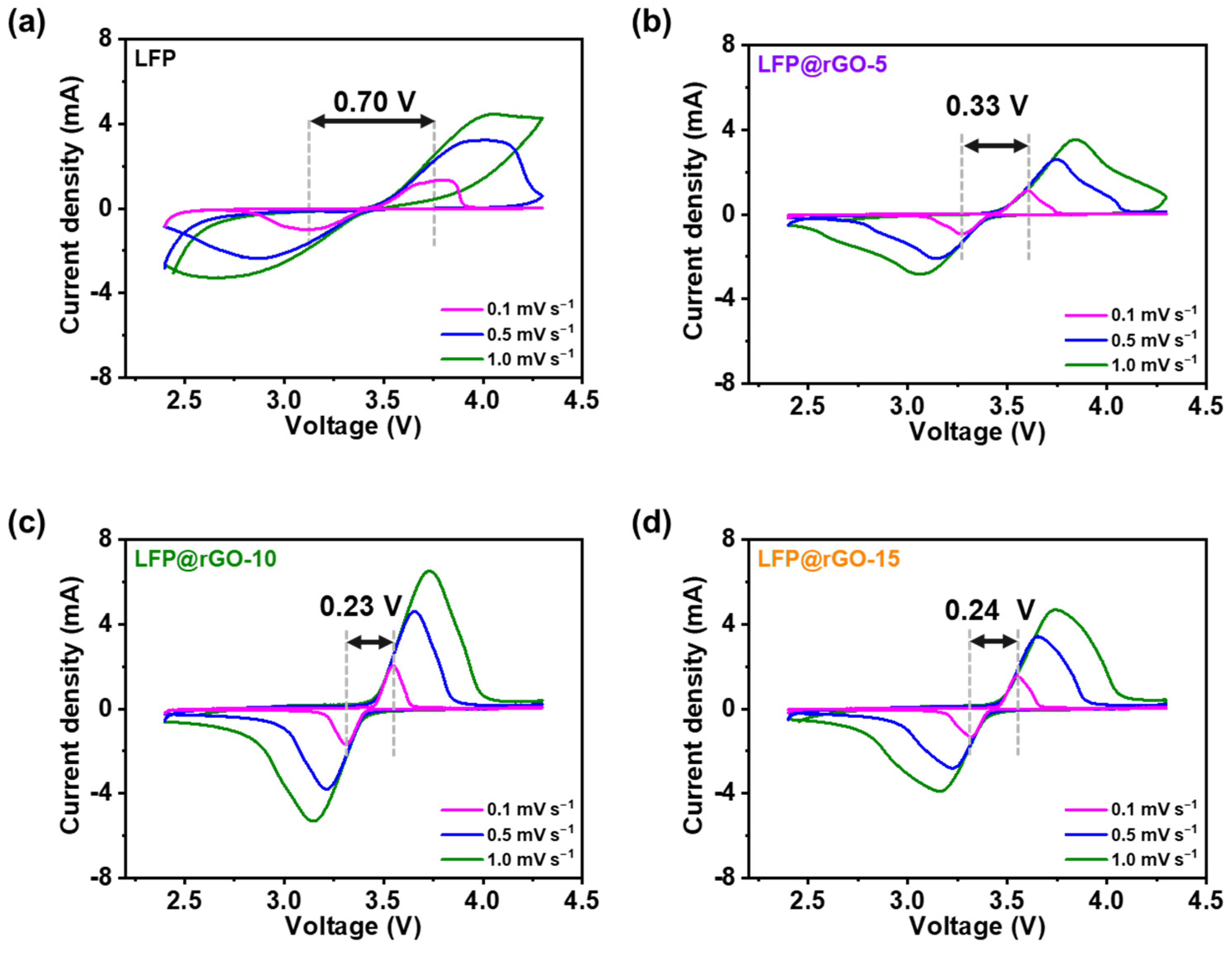
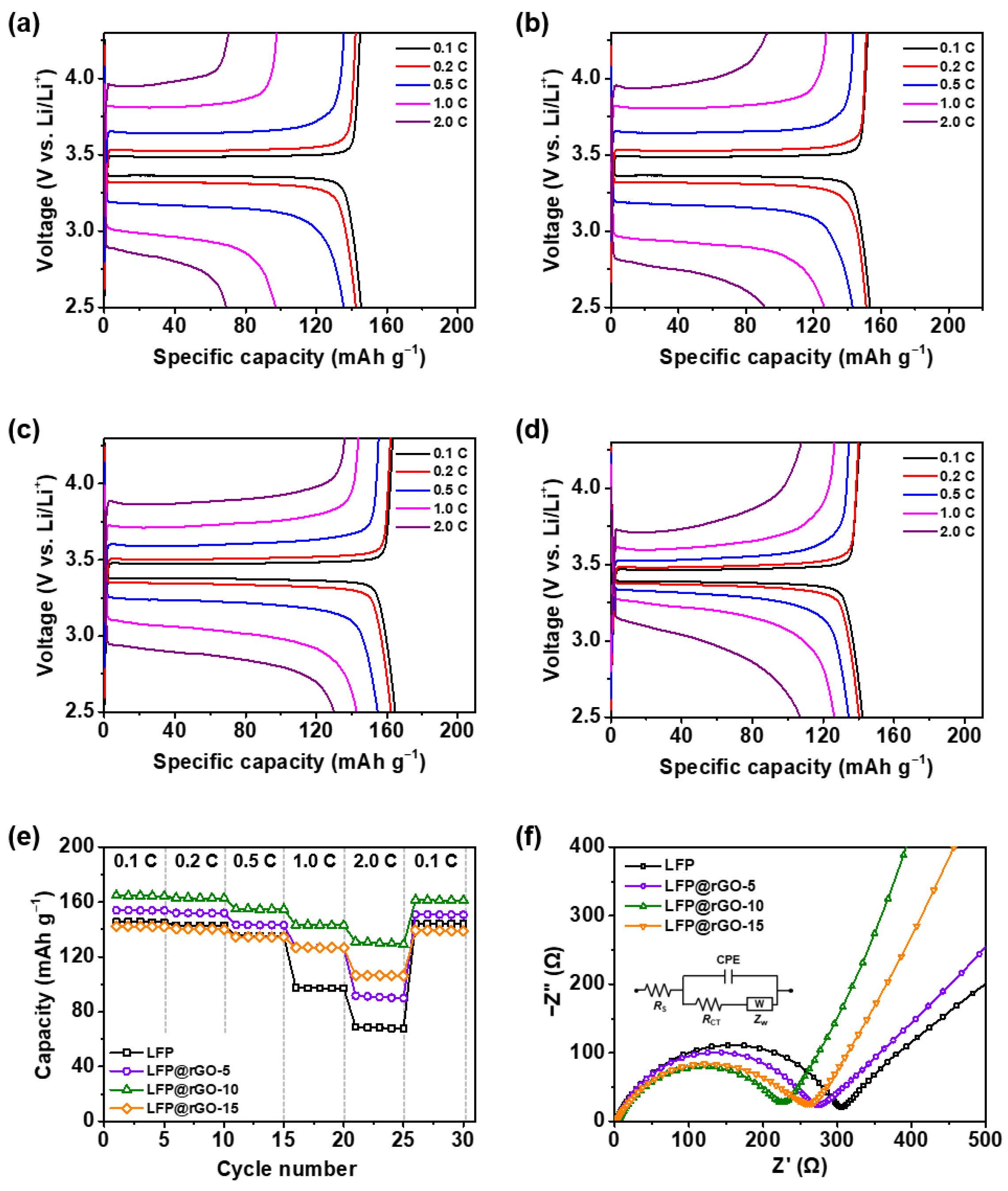


| Sample | Element (Atomic %) | ||||
|---|---|---|---|---|---|
| Li | Fe | P | O | C | |
| LFP | - | 34.0 | 17.0 | 49.0 | - |
| LFP@rGO-5 | - | 32.5 | 16.1 | 46.7 | 4.7 |
| LFP@rGO-10 | - | 30.7 | 14.9 | 44.5 | 9.9 |
| LFP@rGO-15 | - | 28.9 | 13.1 | 43.4 | 14.6 |
| Electrode Material | Precursor | Capacity (mAh g−1) | Ref. |
|---|---|---|---|
| NSC@LFP | Methionine | 103.0 at 2.0 C | [76] |
| 3D-MCC-LFP10 | MXene and CNTs | 85.9 at 5.0 C | [77] |
| LFP/EGO | Exfoliated graphene oxide | 61.7 at 2.0 C | [78] |
| C/LCP-2 | Cellulose | 90 at 2.0 C | [79] |
| LFP/C-15 | Glucose | 124.9 at 2.0 C | [80] |
| LFP@rGO-10 | rGO | 94.3 at 2.0 C | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jekal, S.; Kim, C.-G.; Kim, J.; Kim, H.-Y.; Chu, Y.-R.; Ra, Y.-H.; Otgonbayar, Z.; Yoon, C.-M. Enhanced Electrochemical Performance of Lithium Iron Phosphate Cathodes Using Plasma-Assisted Reduced Graphene Oxide Additives for Lithium-Ion Batteries. Batteries 2024, 10, 345. https://doi.org/10.3390/batteries10100345
Jekal S, Kim C-G, Kim J, Kim H-Y, Chu Y-R, Ra Y-H, Otgonbayar Z, Yoon C-M. Enhanced Electrochemical Performance of Lithium Iron Phosphate Cathodes Using Plasma-Assisted Reduced Graphene Oxide Additives for Lithium-Ion Batteries. Batteries. 2024; 10(10):345. https://doi.org/10.3390/batteries10100345
Chicago/Turabian StyleJekal, Suk, Chan-Gyo Kim, Jiwon Kim, Ha-Yeong Kim, Yeon-Ryong Chu, Yoon-Ho Ra, Zambaga Otgonbayar, and Chang-Min Yoon. 2024. "Enhanced Electrochemical Performance of Lithium Iron Phosphate Cathodes Using Plasma-Assisted Reduced Graphene Oxide Additives for Lithium-Ion Batteries" Batteries 10, no. 10: 345. https://doi.org/10.3390/batteries10100345
APA StyleJekal, S., Kim, C.-G., Kim, J., Kim, H.-Y., Chu, Y.-R., Ra, Y.-H., Otgonbayar, Z., & Yoon, C.-M. (2024). Enhanced Electrochemical Performance of Lithium Iron Phosphate Cathodes Using Plasma-Assisted Reduced Graphene Oxide Additives for Lithium-Ion Batteries. Batteries, 10(10), 345. https://doi.org/10.3390/batteries10100345







