The Next Frontier in Energy Storage: A Game-Changing Guide to Advances in Solid-State Battery Cathodes
Abstract
:1. Introduction
Challenges in Solid-State Battery Development
2. Traditional Cathode Materials
Importance of Solid Electrolytes in Solid-State Batteries
3. Emerging Cathode Materials
4. Structural Optimization of Cathode Materials
4.1. Nanostructuring
4.2. Surface Coatings
4.3. Composite Approaches
4.4. Addressing Issues Like Low Conductivity and Structural Instability
4.5. Role of Electrical Properties (Electronic and Ionic Conductivity)
4.6. Role of Thermal Characteristics (Heat Dissipation and Thermal Stability) in Safety
4.7. Characterization Techniques in the Development of Solid-State Cathodes
5. Integration of Advanced Cathode Materials into SSBs
5.1. Scalable Manufacturing Techniques
5.2. Electrode–Electrolyte Interfaces
5.3. Current Collectors
5.4. Practical Considerations for Implementing Advanced Cathode Materials
6. Comparative Evaluation of Cathode Materials
6.1. Key Performance Indicators
6.1.1. Voltage Stability
6.1.2. Cycle Life
7. Identification of Research Gaps and Challenges
7.1. Existing Research Gaps in SSB Cathodes
7.2. Challenges in the Development and Commercialization of SSB Cathodes
Metal–Chalcogen Batteries (MCBs)
8. Future Directions in SSB Cathode Development
9. Evolution of All-Solid-State Battery Technology: Key Milestones and Developments from 1990 to 2022
10. Conclusions
- Global Energy Shift and SSBs’ Role:
- The global transition from fossil fuels to cleaner energy alternatives has heightened the need for high-performance energy storage systems.
- SSBs emerge as a promising successor to conventional lithium-ion batteries, offering enhanced energy density, superior safety, and extended service life.
- Foundational and Novel Cathode Materials:
- Traditional cathode materials like LiCoO2, LiMn2O4, and LiFePO4 have been instrumental in advancing SSB technology.
- Novel cathode materials, including sulfides, oxides, and air-based cathodes, present unique benefits and challenges, particularly in their interaction with solid electrolytes.
- Strategies for Cathode Optimization:
- Key strategies for improving cathode performance include nanostructuring, surface modifications, and composite formulations.
- These approaches aim to address issues such as limited conductivity and structural vulnerabilities.
- Critical Factors for Cathode Performance:
- Electronic and ionic conductivity, along with thermal attributes like heat dissipation and robustness, are crucial for battery safety and efficiency.
- Manufacturing and Practical Application:
- Scalable fabrication methods are needed for the successful integration of advanced cathode materials into SSBs.
- Focus on electrode–electrolyte interfaces and current collectors is essential for practical deployment.
- Comprehensive Assessment of Cathode Materials:
- A holistic evaluation based on performance metrics such as energy density, voltage stability, and cycle longevity is vital for understanding potential and areas for refinement.
- Future Insights and Research Directions:
- Despite significant progress, there are persistent research gaps and challenges in the evolution of SSB cathodes.
- Future research is optimistic, with potential breakthroughs in cathode materials and methodologies.
- The advancement of SSB technology will play a pivotal role in achieving sustainable energy goals.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Feng, Q.; Lei, Y.; Tang, S.; Xu, L.; Xiong, Y.; Fang, G.; Wang, Y.; Yang, P.; Liu, J.; et al. Quasi-solid-state Zn-air batteries with an atomically dispersed cobalt electrocatalyst and organohydrogel electrolyte. Nat. Commun. 2022, 13, 3689. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, Y.; Ji, S.; Yuan, Z.; Liu, J.; Zhu, M. In Situ Construction a Stable Protective Layer in Polymer Electrolyte for Ultralong Lifespan Solid-State Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104277. [Google Scholar] [CrossRef]
- Zeng, D.-J.; Yao, J.; Zhang, L.; Xu, R.; Wang, S.; Yan, X.; Yu, C.; Wang, L. Promoting favorable interfacial properties in lithium-based batteries using chlorine-rich sulfide inorganic solid-state electrolytes. Nat. Commun. 2022, 13, 1909. [Google Scholar] [CrossRef]
- Su, Y.; Rong, X.; Gao, A.; Liu, Y.; Li, J.; Mao, M.; Qi, X.; Chai, G.; Zhang, Q.; Suo, L.; et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 2022, 13, 4181. [Google Scholar] [CrossRef]
- Minnmann, P.; Strauss, F.; Bielefeld, A.; Ruess, R.; Adelhelm, P.; Burkhardt, S.; Dreyer, S.L.; Trevisanello, E.; Ehrenberg, H.; Brezesinski, T.; et al. Designing Cathodes and Cathode Active Materials for Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2201425. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, F.; Shen, L.; Deng, S.; Wang, Z.; Li, M.; Yao, X. 20 μm-Thick Li6.4La3Zr1.4Ta0.6O12-Based Flexible Solid Electrolytes for All-Solid-State Lithium Batteries. Energy Mater. Adv. 2022, 2022, 9753506. [Google Scholar] [CrossRef]
- Albero Blanquer, L.; Marchini, F.; Seitz, J.R.; Daher, N.; Bétermier, F.; Huang, J.; Gervillié, C.; Tarascon, J. Optical sensors for operando stress monitoring in lithium-based batteries containing solid-state or liquid electrolytes. Nat. Commun. 2022, 13, 1153. [Google Scholar] [CrossRef]
- Castillo, J.; Qiao, L.; Santiago, A.; Judez, X.; Sáenz de Buruaga, A.; Jimenez, G.; Armand, M.; Zhang, H.; Li, C. Perspective of polymer-based solid-state Li-S batteries. Energy Mater. J. 2022, 2, 200003. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Meng, Y.; Jang, J. Scaling up high-energy-density sulfidic solid-state batteries: A lab-to-pilot perspective. Joule 2022, 6, 1755–1769. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Y.; Hao, F.; Kmiec, S.; Dong, H.; Xu, R.; Zhao, K.; Ai, Q.; Terlier, T.; Wang, L.; et al. electrochemically stable homogeneous glassy electrolyte formed at room temperature for all-solid-state sodium batteries. Nat. Commun. 2022, 13, 2854. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.-S.; Kim, S.; Yoon, K.; Han, S.; Lee, M.H.; Ko, Y.; Noh, J.; Kim, W.-Y.; Kang, K. Design of a lithiophilic and electron-blocking interlayer for dendrite-free lithium-metal solid-state batteries. Sci. Adv. 2022, 8, eabq0153. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lai, C.; Chen, K.; Wu, Q.; Gu, Y.; Wu, C.; Li, C. Dual fluorination of polymer electrolyte and conversion-type cathode for high-capacity all-solid-state lithium metal batteries. Nat. Commun. 2022, 13, 7914. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Teo, J.; Walther, F.; Ma, Y.; Zhang, R.; Mazilkin, A.; Tang, Y.; Goonetilleke, D.; Janek, J.; Bianchini, M.; et al. Advanced Nanoparticle Coatings for Stabilizing Layered Ni-Rich Oxide Cathodes in Solid-State Batteries. Adv. Funct. Mater. 2022, 32, 2111829. [Google Scholar] [CrossRef]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Cao, M.; Gu, Q.; Wang, H.; Yu, J.; Guo, Z.-H.; Zhou, X. Advanced inorganic/polymer hybrid electrolytes for all-solid-state lithium batteries. J. Adv. Ceram. 2022, 11, 835–861. [Google Scholar] [CrossRef]
- Eckhardt, J.K.; Klar, P.; Janek, J.; Heiliger, C. Interplay of Dynamic Constriction and Interface Morphology between Reversible Metal Anode and Solid Electrolyte in Solid State Batteries. ACS Appl. Mater. Interfaces 2022, 14, 35545–35554. [Google Scholar] [CrossRef]
- Liang, J.; Maas, E.; Luo, J.; Li, X.; Chen, N.; Adair, K.; Li, W.; Li, J.Y.; Hu, Y.; Liu, J.; et al. A Series of Ternary Metal Chloride Superionic Conductors for High-Performance All-Solid-State Lithium Batteries. Adv. Energy Mater. 2022, 12, 2103921. [Google Scholar] [CrossRef]
- Fu, C.; Homann, G.; Grissa, R.; Rentsch, D.; Zhao, W.; Gouveia, T.; Falgayrat, A.; Lin, R.; Fantini, S.; Battaglia, C. A Polymerized-Ionic-Liquid-Based Polymer Electrolyte with High Oxidative Stability for 4 and 5 V Class Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2200412. [Google Scholar] [CrossRef]
- Payandeh, S.; Brezesinski, T.; Strauss, F.; Mazilkin, A.; Kondrakov, A. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Huang, Y. The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity. Interdiscip. Mater. 2022, 1, 323–329. [Google Scholar] [CrossRef]
- Wei, H.; Tian, Y.; An, Y.; Feng, J.; Xiong, S.; Qian, Y. Porous lithium cobalt oxide fabricated from metal–organic frameworks as a high-rate cathode for lithium-ion batteries. RSC Advances 2020, 10, 30000–30010. [Google Scholar] [CrossRef] [PubMed]
- Marincaş, A.-H.; Ilea, P. Enhancing Lithium Manganese Oxide Electrochemical Behavior by Doping and Surface Modifications. Coatings 2021, 11, 456. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Eilhardt, R.; Nappini, S.; Cococcioni, M.; Píš, I.; Dal Zilio, S.; Bondino, F.; Marzari, N.; Magnano, E.; Alff, L. Energy Level Alignment at the Cobalt Phosphate/Electrolyte Interface: Intrinsic Stability vs Interfacial Chemical Reactions in 5 V Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 57482–57493. [Google Scholar] [CrossRef]
- Lv, S.; Wang, X.; Lu, W.; Zhang, J.; Ni, H. The Influence of Temperature on the Capacity of Lithium-Ion Batteries with Different Anodes. Energies 2022, 15, 60. [Google Scholar] [CrossRef]
- Nguyen, Q.; Luu, V.T.; Nguyen, H.L.; Lee, Y.-W.; Cho, Y.; Kim, S.Y.; Jun, Y.-S.; Ahn, W. Li7La3Zr2O12 Garnet Solid Polymer Electrolyte for Highly Stable All-Solid-State Batteries. Front. Chem. 2021, 8, 619832. [Google Scholar] [CrossRef]
- Choi, H.; Lim, N.-g.; Lee, S.J.; Park, J. Feasibility Study for Sustainable Use of Lithium-Ion Batteries Considering Different Positive Electrode Active Materials under Various Driving Cycles by Using Cell to Electric Vehicle (EV) Simulation. Sustainability 2020, 12, 9764. [Google Scholar] [CrossRef]
- Bal, B.; Ozdogru, B.; Nguyen, D.T.; Li, Z.; Murugesan, V.; Çapraz, Ö.Ö. Probing the Formation of Cathode-Electrolyte Interphase on Lithium Iron Phosphate Cathodes via Operando Mechanical Measurements. ACS Appl. Mater. Interfaces 2023, 15, 42449–42459. [Google Scholar] [CrossRef]
- Song, Y.-W.; Kang, S.-W.; Heo, K.; Lee, J.; Kim, M.; Hwang, D.; Kim, S.-J.; Kim, J.; Lim, J. Effect of Nanoparticles in LiFePO4 Cathode Material Using Organic/Inorganic Composite Solid Electrolyte for All-Solid-State Batteries. Langmuir 2023, 39, 45–52. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Gao, Y.; Zhao, Y.; Kong, X.; Rong, Q.; Xiong, J.; Yu, D.; Ding, S. Multiple Dynamic Bonds-Driven Integrated Cathode/Polymer Electrolyte for Stable All-Solid-State Lithium Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202307255. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Zhao, S.; Zhao, F.; Xiong, S.; Brett, D.J.L.; He, G.; Parkin, I.P. An Anti-Aging Polymer Electrolyte for Flexible Rechargeable Zinc-Ion Batteries. J. Mater. Chem. A 2020, 8, 22637–22644. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Wei, H.-X.; Tang, L.-B.; Huang, Y.-D.; Wang, Z.-Y.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.; Zheng, J.-C. Nickel-rich and cobalt-free layered oxide cathode materials for lithium-ion batteries. Energy Storage Mater. 2022, 50, 274–307. [Google Scholar] [CrossRef]
- Ran, L.; Li, M.; Cooper, E.; Luo, B.; Gentle, I.; Wang, L.; Knibbe, R. Enhanced Safety and Performance of High-Voltage Solid-State Sodium Battery through Trilayer, Multifunctional Electrolyte Design. Energy Storage Mater. 2021, 41, 8–13. [Google Scholar] [CrossRef]
- Dong, S.; Sheng, L.; Wang, L.; Liang, J.; Zhang, H.; Chen, Z.; Xu, H.; He, X. Challenges and Prospects of All-Solid-State Electrodes for Solid-State Lithium Batteries. Adv. Funct. Mater. 2023, 33, 2304371. [Google Scholar] [CrossRef]
- Yang, X.; Adair, K.R.; Gao, X.; Sun, X. Recent advances and perspectives on thin electrolytes for high-energy-density solid-state lithium batteries. Energy Environ. Sci. 2021, 14, 643–671. [Google Scholar] [CrossRef]
- Lee, T.; Char, K.; Kim, K.J.; Choi, J.W. Issues and Advances in Scaling up Sulfide-Based All-Solid-State Batteries. Acc. Chem. Res. 2021, 54, 3390–3402. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Xie, Z.; Qu, W.; Freschi, D.J.; Liu, J. Progress and Perspectives of Lithium Aluminum Germanium Phosphate-Based Solid Electrolytes for Lithium Batteries. Adv. Funct. Mater. 2023, 33, 2300973. [Google Scholar] [CrossRef]
- Sun, Z.; Lai, Y.; Lv, N.; Hu, Y.; Li, B.; Jing, S.; Jiang, L.; Jia, M.; Li, J.; Chen, S.; et al. Boosting the Electrochemical Performance of All-Solid-State Batteries with Sulfide Li6PS5Cl Solid Electrolyte Using Li2WO4-Coated LiCoO2 Cathode. Adv. Mater. Interfaces 2021, 8, 2100624. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Tang, L.; Han, F.; Zhang, Y.; Xia, Y.; Wang, L.; Lu, S. Review of the electrochemical performance and interfacial issues of high-nickel layered cathodes in inorganic all-solid-state batteries. Int. J. Miner. Metall. Mater. 2022, 29, 1003–1018. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Feng, W.; Kang, F. Interface Improvement of Li6.4La3Zr1.6Ta0.6O12@La2Sn2O7 and Cathode Transfer Printing Technology with Splendid Electrochemical Performance for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39414–39423. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Zhao, S.; Song, J.; Liao, Y.; Tang, H. Sulfur/carbon cathode composite with LiI additives for enhanced electrochemical performance in all-solid-state lithium-sulfur batteries. Adv Compos Hybrid Mater 2023, 6, 162. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Hemmelmann, H.; Walther, F.; Bianchini, M.; Kondrakov, A.; Janek, J.; Brezesinski, T. Atomic Layer Deposition Derived Zirconia Coatings on Ni-Rich Cathodes in Solid-State Batteries: Correlation Between Surface Constitution and Cycling Performance. Small Sci. 2023, 3, 2200073. [Google Scholar] [CrossRef]
- Tian, L.W.; Kim, J.W.; Hong, S.B.; Ryu, H.H.; Kim, U.H.; Sun, Y.K.; Kim, D.W. All-solid-state lithium batteries featuring hybrid electrolytes based on Li+ ion-conductive Li7La3Zr2O12 framework and full-concentration gradient Ni-rich NCM cathode. J. Chem. Eng. 2022, 450, 138043. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Li, X.; Luo, J.; Deng, S.; Zhao, Y.; Sun, Y.; Wu, D.; Hu, Y.; Li, W.; et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.X.; Wang, J.J. Solid-state batteries: From fundamental interface characterization to realize sustainable promise. Rare Met. 2020, 39, 743–744. [Google Scholar] [CrossRef]
- Morchhale, A.; Tang, Z.; Yu, C.; Farahati, R.; Kim, J.-H. Coating Materials and Processes for Cathodes in Sulfide-Based All Solid-State Batteries. Curr. Opin. Electrochem. 2023, 39, 101251. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, B.; Han, F. Interfacial Challenges in All-Solid-State Lithium Batteries. Curr. Opin. Electrochem. 2022, 33, 100933. [Google Scholar] [CrossRef]
- Gannett, C.N.; Peterson, B.M.; Melecio-Zambrano, L.; Trainor, C.Q.; Fors, B.P.; Abruña, H.D. Performance optimization and fast rate capabilities of novel polymer cathode materials through balanced electronic and ionic transport. J. Mater. Chem. A 2021, 9, 5657–5663. [Google Scholar] [CrossRef]
- Lou, S.; Liu, Q.; Zhang, F. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 2020, 11, 5700. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; He, T.; Yan, X.; Yu, J.; Bi, J.; Ye, D.; Yao, W.; Tang, Y.; Zhao, H.; et al. Chevrel Phase Mo6S8 Nanosheets Featuring Reversible Electrochemical Li-Ion Intercalation as Effective Dynamic-Phase Promoter for Advanced Lithium-Sulfur Batteries. Small 2023, 19, 2300042. [Google Scholar] [CrossRef]
- Bashirpour-Bonab, H. Thermal behavior of lithium batteries used in electric vehicles using phase change materials. Int. J. Energy Res. 2020, 44, 12583–12591. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yu, T.-Y.; Yeh, S.-C.; Wu, N.-L.; Jeng, R.-J. Spiro-Twisted Benzoxazine Derivatives Bearing Nitrile Group for All-Solid-State Polymer Electrolytes in Lithium Batteries. Polymers 2022, 14, 2869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, H.; Dang, Y.; Zhuang, Q.; Arandiyan, H.; Wang, Y.; Cheng, N.; Sun, H.; Pérez Garza, H.H.; Zheng, R.; et al. Recent advances in in situ and operando characterization techniques for Li7La3Zr2O12-based solid-state lithium batteries. Mater. Horiz. 2023, 10, 1479–1538. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.; Fitch, B.; Xia, J. Industrial Perspectives on Innovation: Sustainability, Safety, Scalability, and Advanced Performance. Meet. Abstr. 2022, MA2022-02, 218. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, M.; Rao, A.; Lin, X.; Doyle-Davis, K.; Liang, J.; Yu, C.; Li, R.; Zhao, S.; Zhang, L.; et al. Fast-Charging Halide-Based All-Solid-State Batteries by Manipulation of Current Collector Interface. Adv. Funct. Mater. 2022, 32, 2200767. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, C.; Yuan, H.; Lu, Y.; Hu, J.; Huang, J.; Zhang, Q. Multiscale Understanding of High-Energy Cathodes in Solid-State Batteries: From Atomic Scale to Macroscopic Scale. Materials Futures 2022, 1, 012101. [Google Scholar] [CrossRef]
- Culver, S.P.; Koerver, R.; Zeier, W.G.; Janek, J. On the Functionality of Coatings for Cathode Active Materials in Thiophosphate-Based All-Solid-State Batteries. Adv. Energy Mater. 2019, 9, 1900626. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Tan, C.; Fan, X.; Pan, Q.; Chu, Y.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Stabilizing Reaction Interface in Ni-rich Layered Oxides Cathode for High-Performance Lithium-Ion Batteries at a High Cutoff Voltage. Chem. Eng. J. 2022, 430, 132985. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, T.T.; Kwok, C.Y.; Kim, S.Y.; Assoud, A.; Zhang, Q.; Janek, J.; Nazar, L.F. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 2022, 7, 83–93. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Meng, Y.; Plamthottam, R.; Tjiu, W.W.; Zhang, C.; Liu, T. Ultrathin Polypyrrole Layers Boosting MoO3 as Both Cathode and Anode Materials for a 2.0 V High-Voltage Aqueous Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, Y.; Chu, C.; Chang, L.; Wang, Z.; Zhang, D.; Liu, W.; Zhuang, Z.; Wang, L. Stabilizing effects of atomic Ti doping on high-voltage high-nickel layered oxide cathode for lithium-ion rechargeable batteries. Nano Res. 2022, 15, 4091–4099. [Google Scholar] [CrossRef]
- Haruna, A.B.; Mwonga, P.; Barrett, D.; Rodella, C.B.; Forbes, R.P.; Venter, A.; Sentsho, Z.; Fletcher, P.J.; Marken, F.; Ozoemena, K.I. Defect-Engineered β-MnO2−δ Precursors Control the Structure–Property Relationships in High-Voltage Spinel LiMn1.5Ni0.5 O4−δ. ACS Omega 2021, 6, 25562–25573. [Google Scholar] [CrossRef] [PubMed]
- Trzciński, K.; Zarach, Z.; Szkoda, M. Controlling Crystallites Orientation and Facet Exposure for Enhanced Electrochemical Properties of Polycrystalline MoO3 Films. Sci. Rep. 2023, 13, 16668. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, X.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Cheng, Y. Gospel for Improving the Lithium Storage Performance of High-Voltage High-Nickel Low-Cobalt Layered Oxide Cathode Materials. ACS Appl. Mater. Interfaces 2021, 13, 58871–58884. [Google Scholar] [CrossRef]
- Wu, E.A.; Banerjee, S.; Tang, H.; Richardson, P.M.; Doux, J.M.; Qi, J.; Zhu, Z. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries. Nat. Commun. 2021, 12, 1256. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, F.; Wang, S.; Liang, J.; Wang, J.; Wang, C.; Zhang, H.; Adair, K.; Li, W.; Li, M.; et al. Advanced High-Voltage All-Solid-State Li-Ion Batteries Enabled by a Dual-Halogen Solid Electrolyte. Adv. Energy Mater. 2021, 11, 2100836. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Song, H.; Richardson, P.M.; Doux, J.M.; Qi, J.; Zhu, Z. Insights Into the Interfacial Degradation of High-Voltage All-Solid-State Lithium Batteries. Nano-Micro Lett. 2022, 14, 191. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Liu, X.; Zhao, M. Novel Conductive Metal–Organic Framework for a High-Performance Lithium–Sulfur Battery Host: 2D Cu-Benzenehexathial (BHT). ACS Appl. Mater. Interfaces 2018, 10, 15012–15020. [Google Scholar] [CrossRef]
- Song, C.-L.; Li, Z.; Ma, L.-Y.; Li, M.-Z.; Huang, S.-L.; Hong, X.-J.; Cai, Y.; Lan, Y. Single-Atom Zinc and Anionic Framework as Janus Separator Coatings for Efficient Inhibition of Lithium Dendrites and Shuttle Effect. ACS Nano 2021, 15, 13436–13443. [Google Scholar] [CrossRef]
- Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. A Seamless Metal-Organic Framework Interphase with Boosted Zn2+ Flux and Deposition Kinetics for Long-Living Rechargeable Zn Batteries. Nano Lett. 2023, 23, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Weber, S.; Graumann, T.; Zebrowski, S.; Mainusch, N.; Dilger, N.; Cerdas, F.; Zellmer, S. An Environmental and Technical Evaluation of Vacuum-Based Thin Film Technologies: Lithium Niobate Coated Cathode Active Material for Use in All-Solid-State Battery Cells. Energies 2023, 16, 1278. [Google Scholar] [CrossRef]
- Kravchyk, K.; Okur, F.; Kovalenko, M. Break-Even Analysis of All-Solid-State Batteries with Li-Garnet Solid Electrolytes. ACS Energy Lett. 2021, 6, 2202–2207. [Google Scholar] [CrossRef]
- Gunduz, D.C.; Schierholz, R.; Yu, S.; Tempel, H.; Kungl, H.; Eichel, R. Combined quantitative microscopy on the microstructure and phase evolution in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J. Adv. Ceram. 2020, 9, 149–161. [Google Scholar] [CrossRef]
- Feng, W.; Yang, P.; Dong, X.; Xia, Y. A Low Temperature Soldered All Ceramic Lithium Battery. ACS Appl. Mater. Interfaces 2022, 14, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.K.; McGhie, A.R.; Farrington, G.C. Studies of the stability of poly(ethylene oxide) and PEO-based solid electrolytes using thermogravimetry-mass spectrometry. Macromolecules 1991, 24, 3285–3290. [Google Scholar] [CrossRef]
- Yu, G. High Performance Photonic Devices Made with Semiconducting Polymers. Synth. Met. 1996, 80, 143–150. [Google Scholar] [CrossRef]
- Gurunathan, K.; Murugan, A.V.; Marimuthu, R.; Mulik, U.P.; Amalnerkar, D.P. Electrochemically Synthesised Conducting Polymeric Materials for Applications Towards Technology in Electronics, Optoelectronics and Energy Storage Devices. Mater. Chem. Phys. 1999, 61, 173–191. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Hammond, P.T. Highly Ion Conductive Poly(ethylene oxide)-Based Solid Polymer Electrolytes from Hydrogen Bonding Layer-by-Layer Assembly. Langmuir 2004, 20, 5403–5411. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and Challenges Facing Rechargeable Thin Film Lithium Batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Xue, M.-Z.; Fu, Z.-W. Nanostructured Thin Film Electrodes for Lithium Storage and All-Solid-State Thin-Film Lithium Batteries. J. Power Sources 2013, 234, 310–332. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, T.P.; Kavvada, O.; Shah, N.; Sathre, R.; Scown, C.D. Life-Cycle Implications and Supply Chain Logistics of Electric Vehicle Battery Recycling in California. Environ. Res. Lett. 2015, 10, 014011. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The Role of the Solid Electrolyte Interphase Layer in Preventing Li Dendrite Growth in Solid-State Batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From Nanoscale Interface Characterization to Sustainable Energy Storage Using All-Solid-State Batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing Thin but Robust Electrolytes for Solid-State Batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Urgoiti-Rodriguez, M.; Vaquero-Vílchez, S.; Mirandona-Olaeta, A.; Fernández de Luis, R.; Goikolea, E.; Costa, C.M.; Lanceros-Mendez, S.; Fidalgo-Marijuan, A.; Ruiz de Larramendi, I. Exploring Ionic Liquid-Laden Metal-Organic Framework Composite Materials as Hybrid Electrolytes in Metal (Ion) Batteries. Front. Chem. 2022, 10, 995063. [Google Scholar] [CrossRef]



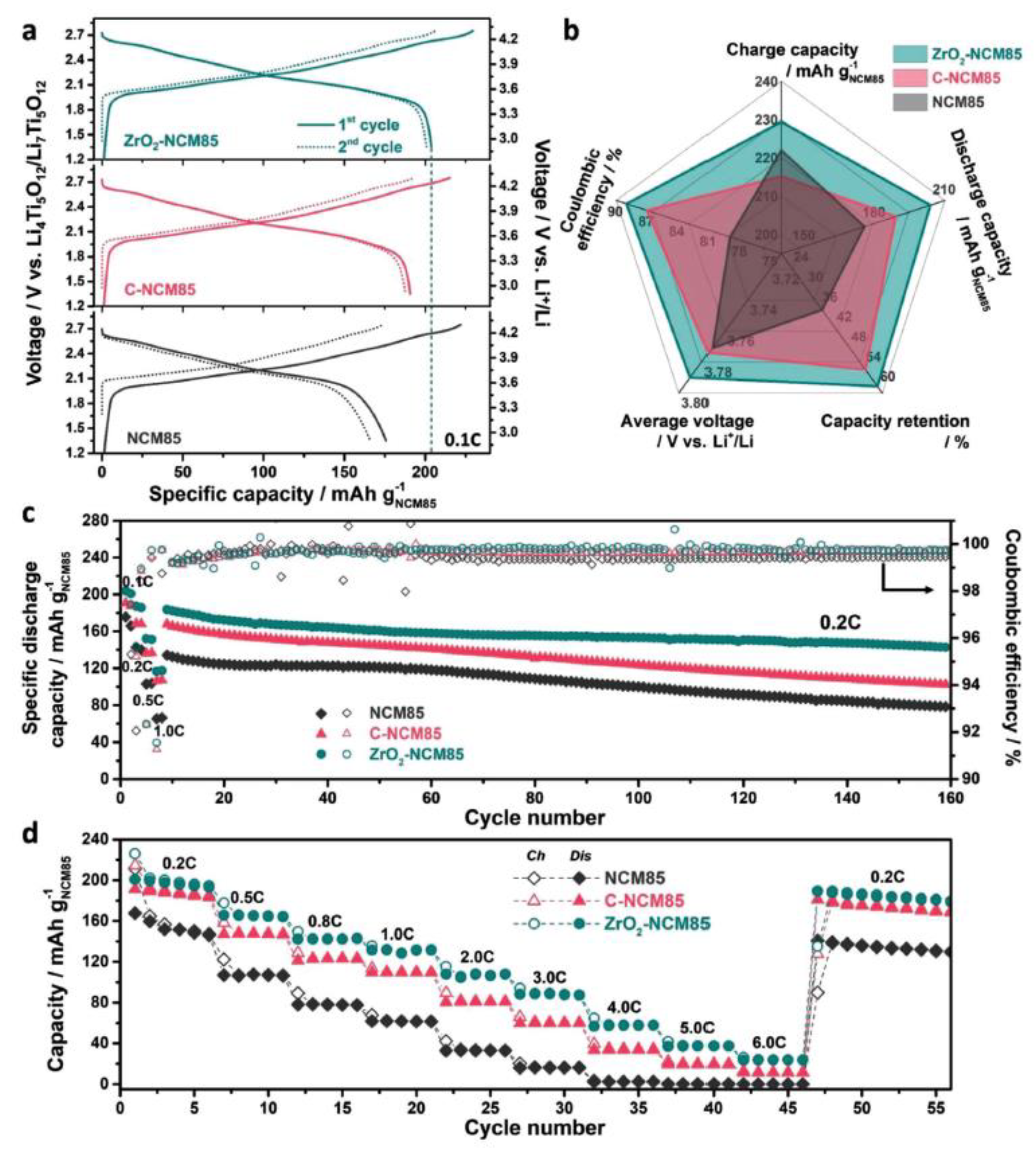

| Category | Aspect | Description and Performance Metrics | Reference |
|---|---|---|---|
| Advantages | |||
| High Energy Density | Emerging cathode materials in SSBs, like lithium nickel manganese cobalt oxide (NMC), offer higher energy density (up to 250–300 Wh/kg) compared to traditional lithium-ion batteries. This makes them suitable for longer-range electric vehicles. | [32] | |
| Enhanced Safety | Solid electrolytes in SSBs significantly reduce the risk of leakage and combustion. They are non-flammable and have a higher thermal stability, making them safer under extreme conditions. | [33] | |
| Longer Lifespan | Advanced cathode materials in SSBs show reduced degradation, with potential cycle lives exceeding 5000 cycles, significantly higher than conventional lithium-ion batteries, which typically last for about 1000–2000 cycles. | [34] | |
| Limitations | |||
| High Manufacturing Cost | The production of SSBs is more expensive due to complex manufacturing processes and the use of novel, sometimes scarce, materials. This cost is a significant barrier to widespread adoption and commercialization. | [35] | |
| Limited Power Density | Despite high energy density, SSBs currently have lower power density (around 1000–2000 W/kg) compared to traditional lithium-ion batteries. This limitation affects their ability to quickly charge or provide high power bursts. | [35] | |
| Challenges in Scalability | Scaling up production to meet large-scale demand is challenging due to the intricate manufacturing processes and the need for high precision and quality control. This issue is a key focus for research and development in the field. | [36] | |
| Operational Temperature | SSBs often have a narrower operational temperature range compared to liquid electrolyte batteries. This can limit their use in extreme weather conditions, though ongoing research aims to improve this aspect. | [37] |
| Year/Period | Development Milestone | Key Examples/Notes | Reference |
|---|---|---|---|
| Early 1990s | Initial exploration of solid electrolytes, focusing on stability and ionic conductivity. | Introduction of lithium phosphorus oxynitride (LiPON) and thio-LISICON as potential solid electrolytes. | [76] |
| Mid-1990s | Development of polymer-based solid electrolytes for enhanced flexibility and processability. | Advancements in poly(ethylene oxide) (PEO)-based electrolytes, demonstrating improved electrochemical stability. | [77] |
| Late 1990s | Emergence of oxide-based solid electrolytes, offering improved ionic conductivity and stability. | Lithium lanthanum titanate (LLTO) and other oxide ceramics explored for higher conductivity and stability. | [78] |
| Early 2000s | Introduction of sulfide-based solid electrolytes, significantly enhancing ionic conductivity. | LGPS (Li10GeP2S12) showing high ionic conductivities comparable to liquid electrolytes. | [79] |
| 2007 | Breakthrough in garnet-type solid electrolytes with the development of Li7La3Zr2O12 (LLZO). | LLZO noted for its high ionic conductivity, stability against lithium metal, and low grain boundary resistance. | [80] |
| Late 2000s | Progress in thin-film solid-state batteries, focusing on integration into electronic devices and miniaturization. | Development of micro-batteries for electronic devices, leveraging thin-film technology for compact design. | [81] |
| Early 2010s | Advances in interface engineering between solid electrolytes and electrodes to enhance performance and durability. | Exploration of various coating and interface modification techniques to reduce interfacial resistance and improve cycling stability. | [82] |
| 2012 | Development and adoption of composite solid electrolytes combining polymers and ceramics for improved properties. | Composite electrolytes exhibiting enhanced mechanical properties and ionic conductivity, suitable for practical applications. | [83] |
| Mid-2010s | Scale-up and design improvements for larger applications, particularly in the electric vehicle sector. | Transition from laboratory-scale research to prototypes demonstrating feasibility for electric vehicle applications. | [84] |
| 2017 | Surge in commercial interest and investments for the development of solid-state batteries in electric vehicles. | Major automotive and battery manufacturers announcing projects and collaborations for ASSB development. | [85] |
| 2018 | Addressing lithium dendrite formation in solid-state lithium metal batteries. | Innovations in electrolyte and anode design to suppress dendrite growth, enhancing safety and cycle life. | [86] |
| 2020 | Advancements in scalable manufacturing techniques for solid-state batteries, focusing on cost reduction and mass production. | Development of novel manufacturing processes, including roll-to-roll and sputtering techniques for large-scale production. | [87] |
| 2021 | Enhanced understanding and mitigation of interfacial challenges to improve cycle life and performance. | Research on interfacial phenomena, leading to strategies for stabilizing the electrode/electrolyte interface. | [88] |
| 2022 | Introduction of novel materials and hybrid systems, pushing the boundaries of energy density and safety. | Exploration of new solid electrolyte materials and innovative hybrid solid–liquid electrolyte systems for higher performance batteries. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Márquez, F. The Next Frontier in Energy Storage: A Game-Changing Guide to Advances in Solid-State Battery Cathodes. Batteries 2024, 10, 13. https://doi.org/10.3390/batteries10010013
Machín A, Márquez F. The Next Frontier in Energy Storage: A Game-Changing Guide to Advances in Solid-State Battery Cathodes. Batteries. 2024; 10(1):13. https://doi.org/10.3390/batteries10010013
Chicago/Turabian StyleMachín, Abniel, and Francisco Márquez. 2024. "The Next Frontier in Energy Storage: A Game-Changing Guide to Advances in Solid-State Battery Cathodes" Batteries 10, no. 1: 13. https://doi.org/10.3390/batteries10010013
APA StyleMachín, A., & Márquez, F. (2024). The Next Frontier in Energy Storage: A Game-Changing Guide to Advances in Solid-State Battery Cathodes. Batteries, 10(1), 13. https://doi.org/10.3390/batteries10010013







