Process-Gas-Influenced Anti-Site Disorder and Its Effects on Magnetic and Electronic Properties of Half-Metallic Sr2FeMoO6 Thin Films
Abstract
1. Introduction
2. Experiment
3. Results and Discussion
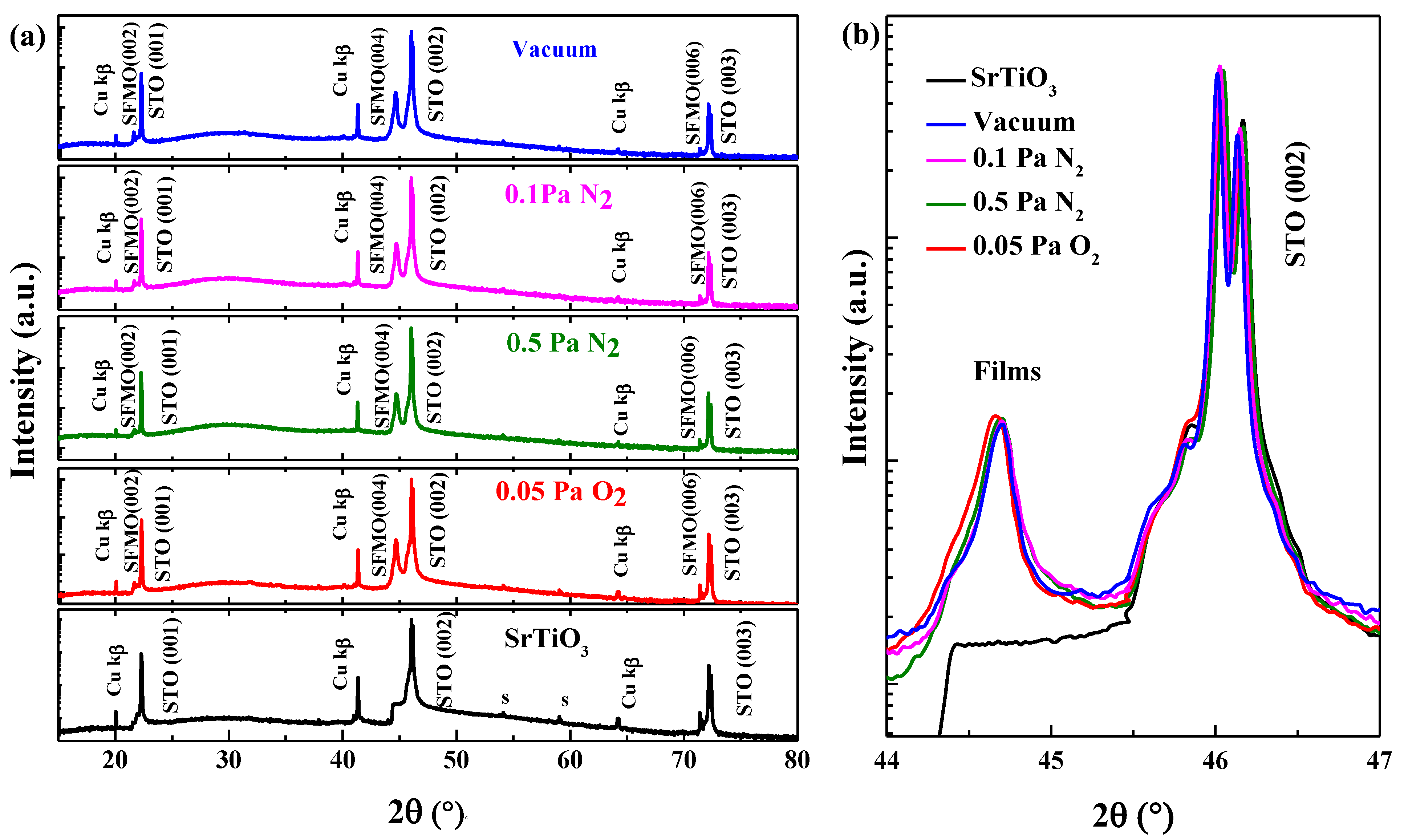
3.1. Structural Properties
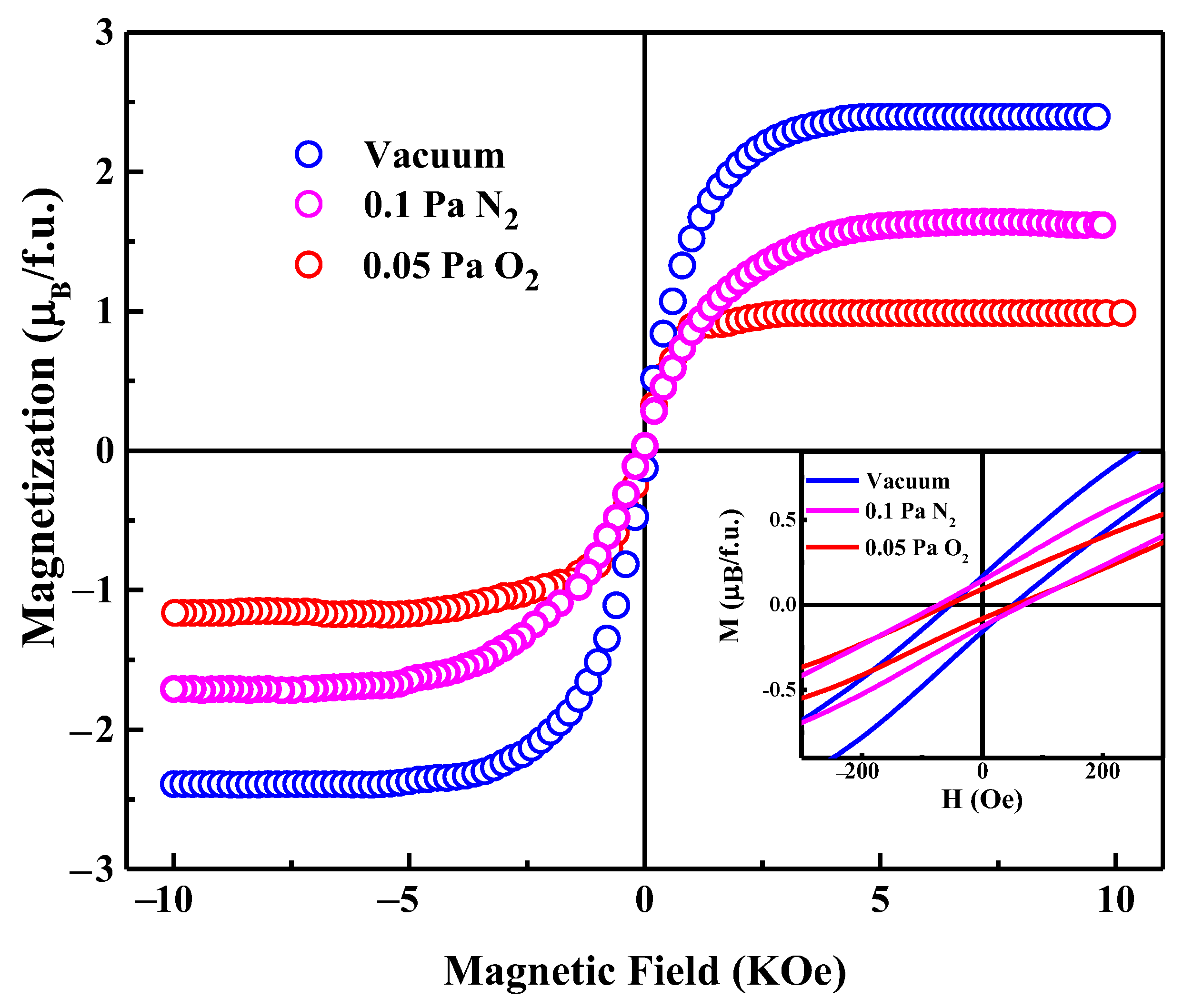
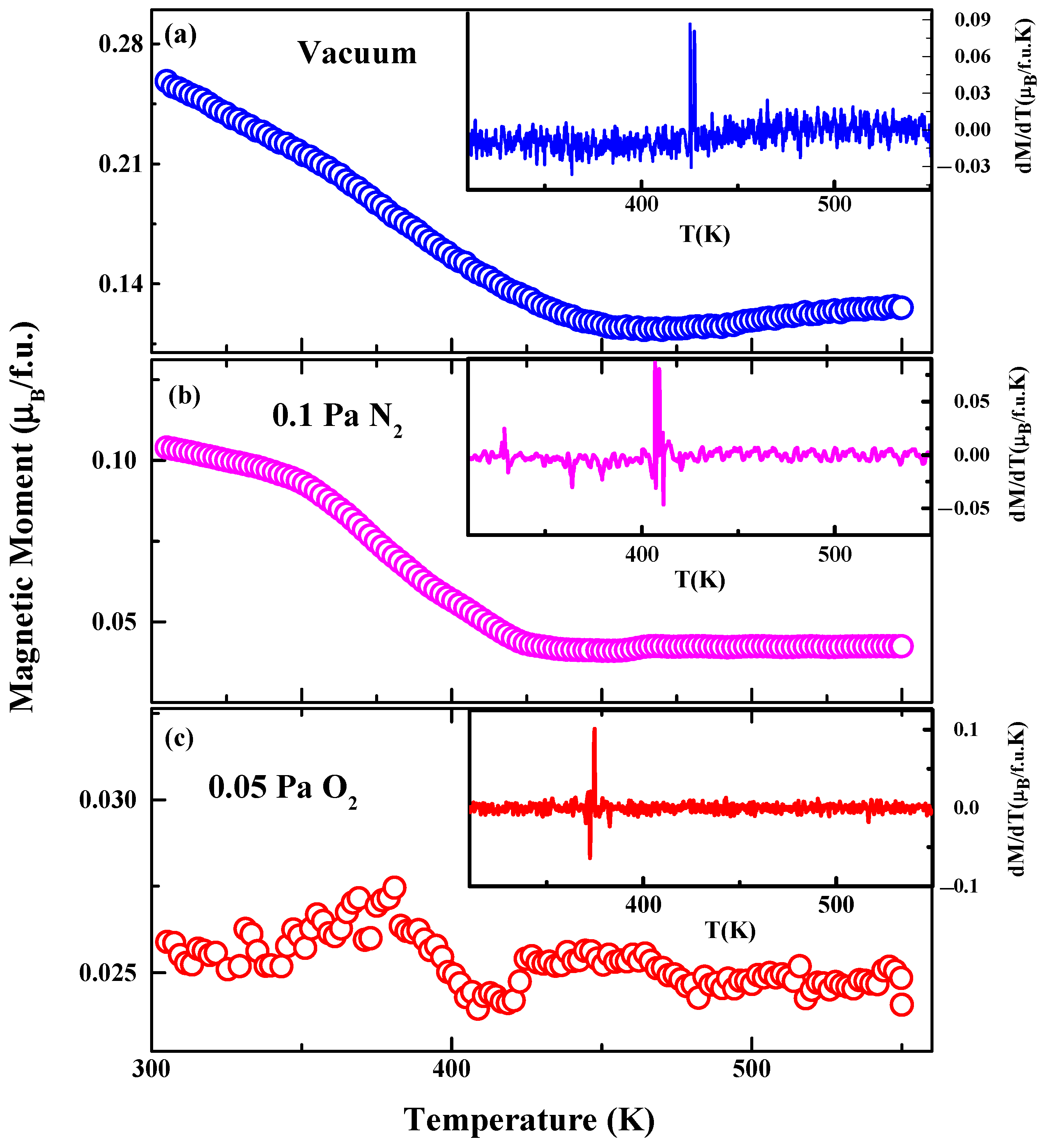
3.2. Magnetic Properties
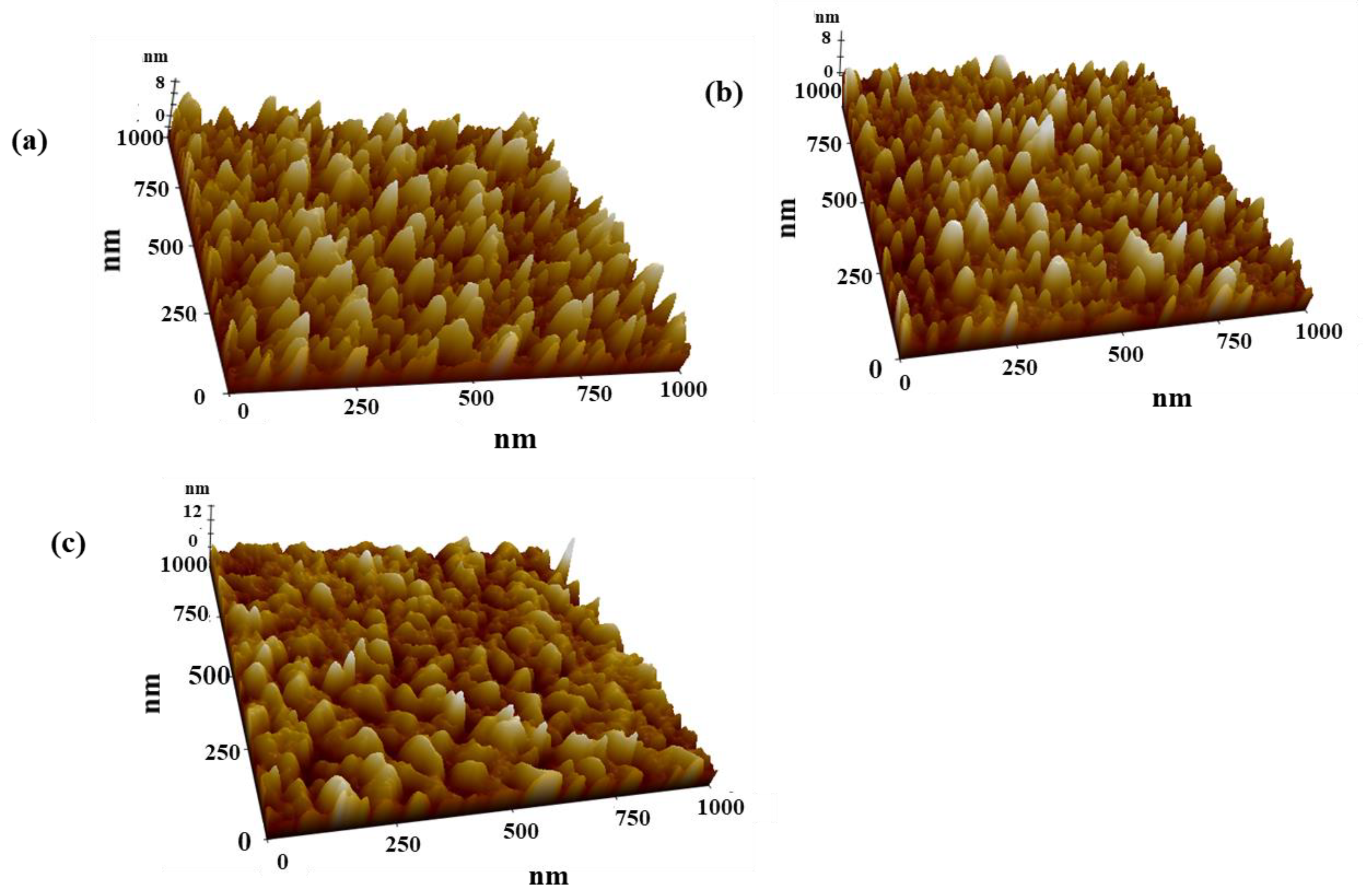
3.3. Surface Morphology
3.4. Electrical Properties
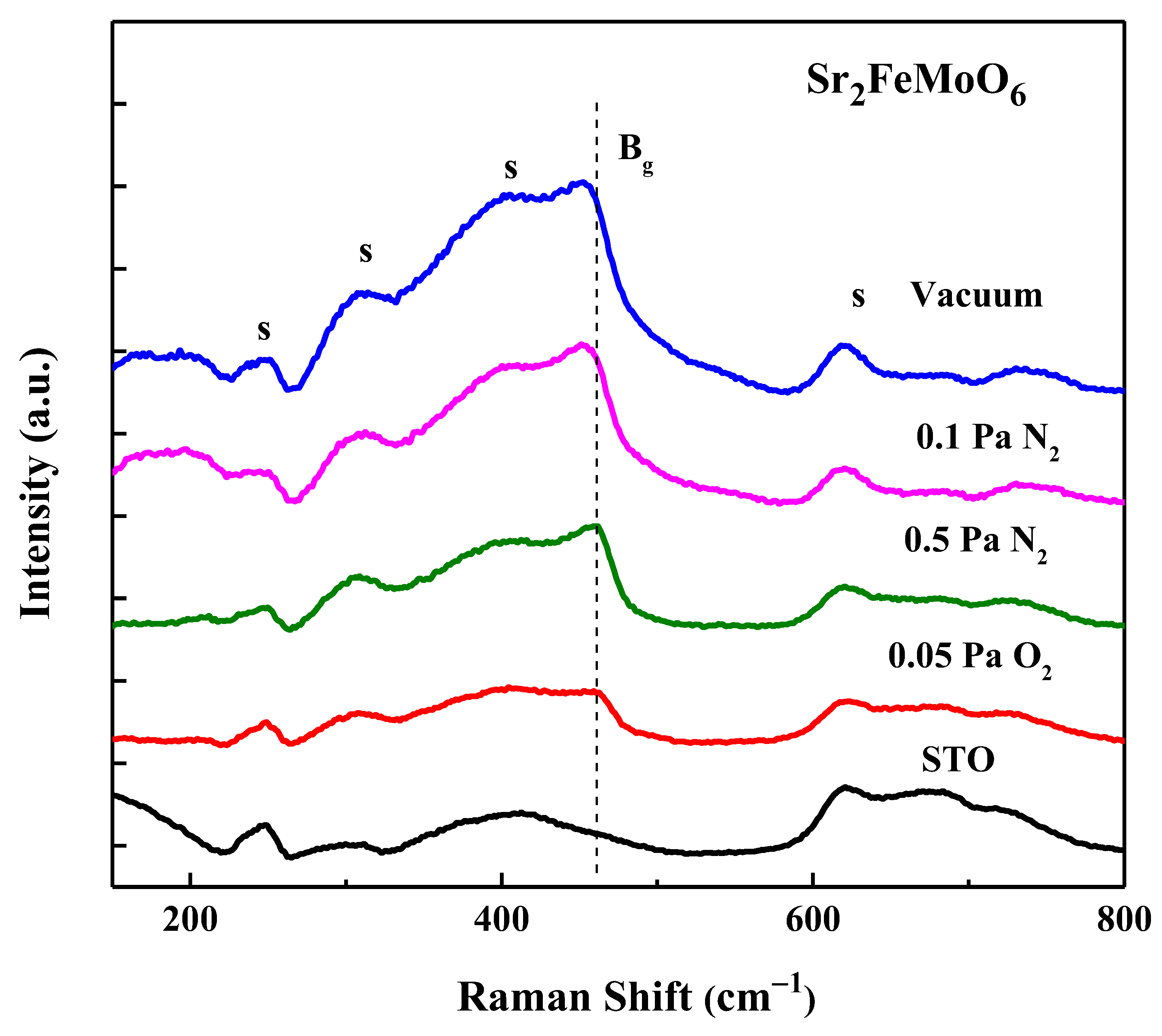
3.5. Vibrational Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prellier, W.; Smolyaninova, V.; Biswas, A.; Galley, C.; Greene, R.L.; Ramesha, K.; Gopalakrishnan, J. Properties of the ferrimagnetic double perovskites A2FeReO6 (A = Ba and Ca). J. Phys. Condens. Matter 2000, 12, 965–973. [Google Scholar] [CrossRef]
- Anderson, M.T.; Greenwood, K.B.; Taylor, G.A.; Poeppelmeier, K.R. B-cation arrangements in double perovskites. Prog. Solid State Chem. 1993, 22, 197–233. [Google Scholar] [CrossRef]
- Saxena, M.; Tanwar, K.; Maiti, T. Environmental friendly Sr2TiMoO6 double perovskite for high temperature thermoelectric applications. Scr. Mater. 2017, 130, 205–209. [Google Scholar] [CrossRef]
- Azuma, M.; Takata, K.; Saito, T.; Ishiwata, S.; Shimakawa, Y.; Takano, M. Designed ferromagnetic, ferroelectric Bi2NiMnO6. J. Am. Chem. Soc. 2005, 127, 8889–8892. [Google Scholar] [CrossRef]
- Kobayashi, K.-I.; Kimura, T.; Sawada, H.; Terakura, K.; Tokura, Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Serrate, D.; Teresa, J.M.D.; Ibarra, M.R. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter 2006, 19, 023201. [Google Scholar] [CrossRef]
- Kim, T.H.; Uehara, M.; Cheong, S.-W.; Lee, S. Large room-temperature intergrain magnetoresistance in double perovskite SrFe1−x(MoorRe)xO3. Appl. Phys. Lett. 1999, 74, 1737–1739. [Google Scholar] [CrossRef]
- Tomioka, Y.; Okuda, T.; Okimoto, Y.; Kumai, R.; Kobayashi, K.-I.; Tokura, Y. Magnetic and electronic properties of a single crystal of ordered double perovskite Sr2FeMoO6. Phys. Rev. B 2000, 61, 422–427. [Google Scholar] [CrossRef]
- Cernea, M.; Vasiliu, F.; Plapcianu, C.; Bartha, C.; Mercioniu, I.; Pasuk, I.; Lowndes, R.; Trusca, R.; Aldica, G.V.; Pintilie, L. Preparation by sol–gel and solid state reaction methods and properties investigation of double perovskite Sr2FeMoO6. J. Eur. Ceram. Soc. 2013, 33, 2483–2490. [Google Scholar] [CrossRef]
- Shinde, S.R.; Ogale, S.B.; Greene, R.L.; Venkatesan, T.; Tsoi, K.; Cheong, S.-W.; Millis, A.J. Thin films of double perovskite Sr2FeMoO6: Growth, optimization, and study of the physical and magnetotransport properties of films grown on single-crystalline and polycrystalline SrTiO3 substrates. J. Appl. Phys. 2003, 93, 1605–1612. [Google Scholar] [CrossRef]
- Santosh, M.; Lacotte, M.; David, A.; Boullay, P.; Grygiel, C.; Pravarthana, D.; Rohrer, G.S.; Salvador, P.A.; Padhan, P.; Lüders, U.; et al. Pulsed laser deposition of Sr2FeMoO6 thin films grown on spark plasma sintered Sr2MgWO6 substrates. J. Phys. Appl. Phys. 2017, 50, 235301. [Google Scholar] [CrossRef]
- Jalili, H.; Heinig, N.F.; Leung, K.T. X-ray photoemission study of Sr2FeMoO6 and SrMoO4 films epitaxially grown on MgO(001): Near-surface chemical-state composition analysis. Phys. Rev. B 2009, 79, 174427. [Google Scholar] [CrossRef]
- Du, C.; Adur, R.; Wang, H.; Hauser, A.J.; Yang, F.; Hammel, P.C. Control of Magnetocrystalline Anisotropy by Epitaxial Strain in Double Perovskite Sr2FeMoO6 Films. Phys. Rev. Lett. 2013, 110, 147204. [Google Scholar] [CrossRef]
- Suchaneck, G.; Kalanda, N.; Artsiukh, E.; Gerlach, G. Challenges in Sr2FeMoO6−δ Thin Film Deposition. Phys. Status Solidi B 2020, 257, 1900312. [Google Scholar] [CrossRef]
- Song, J.H.; Park, B.-G.; Park, J.-H.; Jeong, Y.H. Double-Perovskite Sr2FeMoO6 Thin Films Prepared by Using Pulsed Laser Deposition: Growth and Crystal, Electronic and Magnetic Structures. J. Korean Phys. Soc. 2008, 53, 1084–1088. [Google Scholar] [CrossRef]
- Kumar, D.; Kaur, D. Substrate-dependent structural and magnetic properties of Sr2FeMoO6 nanostructured double perovskite thin films. Phys. B Condens. Matter Phys. B 2010, 405, 3259–3266. [Google Scholar] [CrossRef]
- Sanchez, D.; Auth, N.; Jakob, G.; Martínez, J.L.; García-Hernández, M. Pulsed laser deposition of Sr2FeMoO6 thin films. J. Magn. Magn. Mater. 2005, 294, e119–e122. [Google Scholar] [CrossRef]
- Borges, R.P.; Lhostis, S.; Bari, M.A.; Versluijs, J.J.; Lunney, J.G.; Coey, J.M.D.; Besse, M.; Contour, J.-P. Thin films of the double perovskite Sr2FeMoO6 deposited by pulsed laser deposition. Thin Solid Films 2003, 429, 5–12. [Google Scholar] [CrossRef]
- Kim, K.-W.; Ghosh, S.; Buvaev, S.; Mhin, S.; Jones, J.L.; Hebard, A.F.; Nortan, D.P. The effects of oxygen pressure on disordering and magneto-transport properties of Ba2FeMoO6 thin films grown via pulsed laser deposition. J. Appl. Phys. 2015, 118, 033903. [Google Scholar] [CrossRef]
- Qian, Y.; Wu, H.; Lu, R.; Tan, W.; Xiao, C.; Deng, K. Effect of High-Pressure on the Electronic and Magnetic Properties in Double Perovskite Oxide Sr2FeMoO6. J. Appl. Phys. 2012, 112, 103712. [Google Scholar] [CrossRef]
- Zhang, J.S.; Yu, R.C.; Li, F.Y.; Li, X.D.; Liu, J.; Feng, C.G.; Jin, C.Q. Strucutral stability and electrical properties of Sr2Fe1+xMo1-yO6-δ under high pressure. J. Ally. Compds. 2004, 3482, 67. [Google Scholar]
- Sánchez, D.; García-Hernández, M.; Auth, N.; Jakob, G. Structural, magnetic, and transport properties of high-quality epitaxial Sr2FeMoO6 thin films prepared by pulsed laser deposition. J. Appl. Phys. 2004, 96, 2736–2742. [Google Scholar] [CrossRef]
- Paul, D.K.; Mitra, S.S. Evaluation of Mott’s parameters for hopping conduction in amorphous Ge, Si, and Se-Si. Phys. Rev. Lett. 1973, 31, 1000–1003. [Google Scholar] [CrossRef]
- Zhai, Y.; Qiao, J.; Huo, G.; Han, S. Synthesis, magnetic and electrical transport properties of magnetoresistance material Sr2FeMoO6 by microwave sintering. J. Magn. Magn. Mater. 2012, 324, 2006–2010. [Google Scholar] [CrossRef]
- Weber, M.C.; Kreisel, J.; Thomas, P.A.; Newton, M.; Sardar, K.; Walton, R.I. Phonon Raman scattering of RCrO3 perovskites (R = Y, La, Pr, Sm, Gd, Dy, Ho, Yb, Lu). Phys. Rev. B 2012, 85, 054303. [Google Scholar] [CrossRef]
- Yadav, E.; Harisankar, S.; Soni, K.; Mavani, K.R. Effects of Cu-doping on the vibrational and electronic properties of epitaxial PrNiO3 thin films. Vib. Spectrosc. 2021, 112, 103185. [Google Scholar] [CrossRef]
- Son, L.H.; Phuc, N.X.; Phuc, P.V.; Hong, N.M.; Hong, L.V. Observation of phase decomposition of Sr2FeMoO6 by Raman spectroscopy. J. Raman Spectrosc. 2001, 32, 817–820. [Google Scholar] [CrossRef]
- Zhang, T.; Branford, W.R.; Trodahl, H.J.; Sharma, A.; Rager, J.; MacManus-Driscoll, J.L.; Cohen, L.F. Raman spectroscopy of highly aligned thin films of Sr2FeMoO6. J. Raman Spectrosc. 2004, 35, 1081–1085. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Postorino, P.; Di Castro, D.; Arcangeletti, E.; Dore, P.; Cestelli Guidi, M.; Ray, S.; Sarma, D.D. Pressure and temperature dependence of the Fano resonance in the Raman spectrum of A2FeMoO6 systems (A = Sr, Ca). Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 172405. [Google Scholar] [CrossRef]

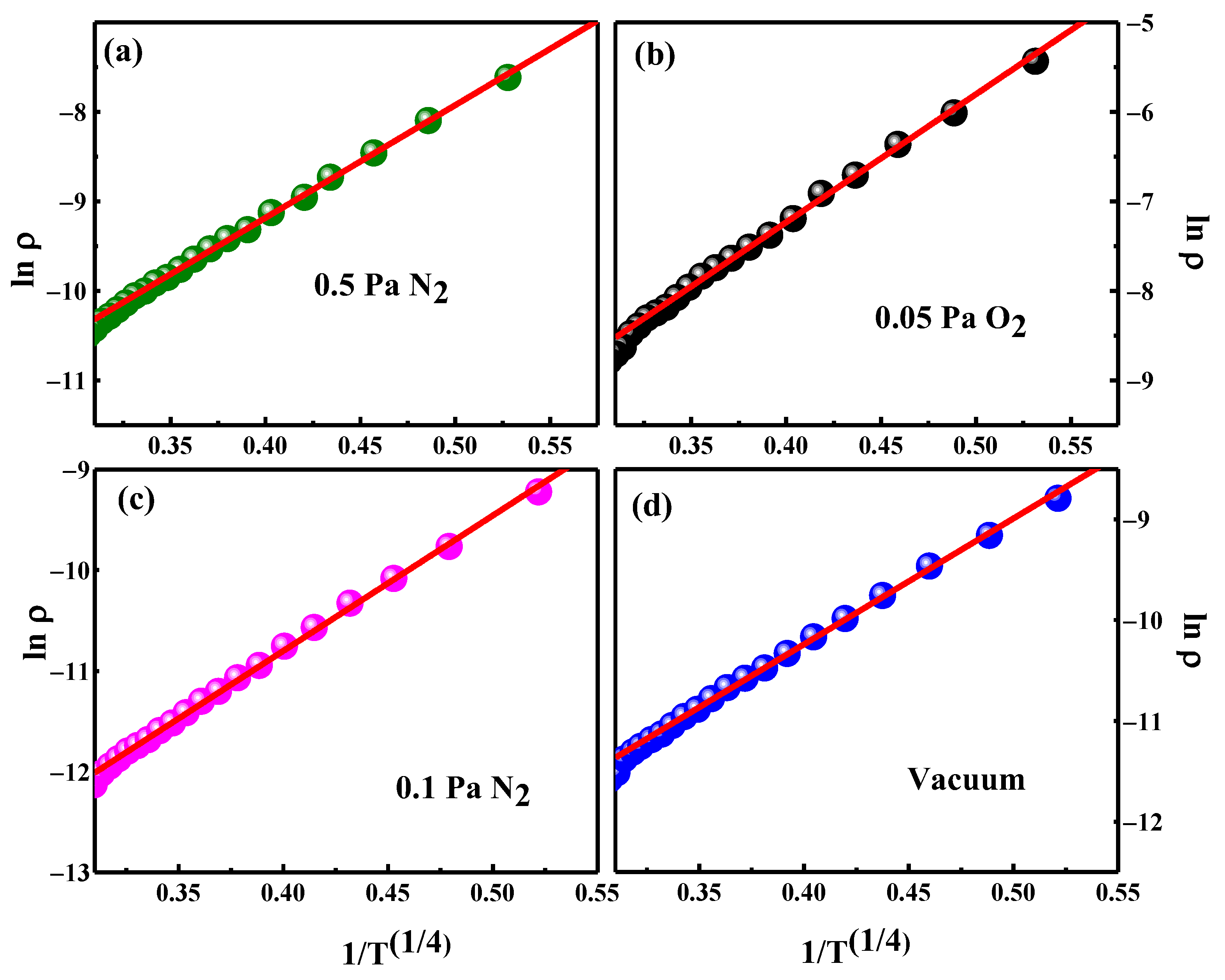
| Background Pressure (Pa) | Mott Temperature (104 K) |
|---|---|
| Vacuum (10−4) 0.1 N2 0.5 N2 0.05 O2 | 2.20 2.75 3.45 3.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, E.; Navale, K.S.; Prajapati, G.L.; Mavani, K.R. Process-Gas-Influenced Anti-Site Disorder and Its Effects on Magnetic and Electronic Properties of Half-Metallic Sr2FeMoO6 Thin Films. Magnetochemistry 2023, 9, 167. https://doi.org/10.3390/magnetochemistry9070167
Yadav E, Navale KS, Prajapati GL, Mavani KR. Process-Gas-Influenced Anti-Site Disorder and Its Effects on Magnetic and Electronic Properties of Half-Metallic Sr2FeMoO6 Thin Films. Magnetochemistry. 2023; 9(7):167. https://doi.org/10.3390/magnetochemistry9070167
Chicago/Turabian StyleYadav, Ekta, Ketan S. Navale, Gulloo L. Prajapati, and Krushna R. Mavani. 2023. "Process-Gas-Influenced Anti-Site Disorder and Its Effects on Magnetic and Electronic Properties of Half-Metallic Sr2FeMoO6 Thin Films" Magnetochemistry 9, no. 7: 167. https://doi.org/10.3390/magnetochemistry9070167
APA StyleYadav, E., Navale, K. S., Prajapati, G. L., & Mavani, K. R. (2023). Process-Gas-Influenced Anti-Site Disorder and Its Effects on Magnetic and Electronic Properties of Half-Metallic Sr2FeMoO6 Thin Films. Magnetochemistry, 9(7), 167. https://doi.org/10.3390/magnetochemistry9070167






