Study of the Effects of Er Doping on the Physical Properties of CdSe Thin Films
Abstract
1. Introduction
2. Experimental
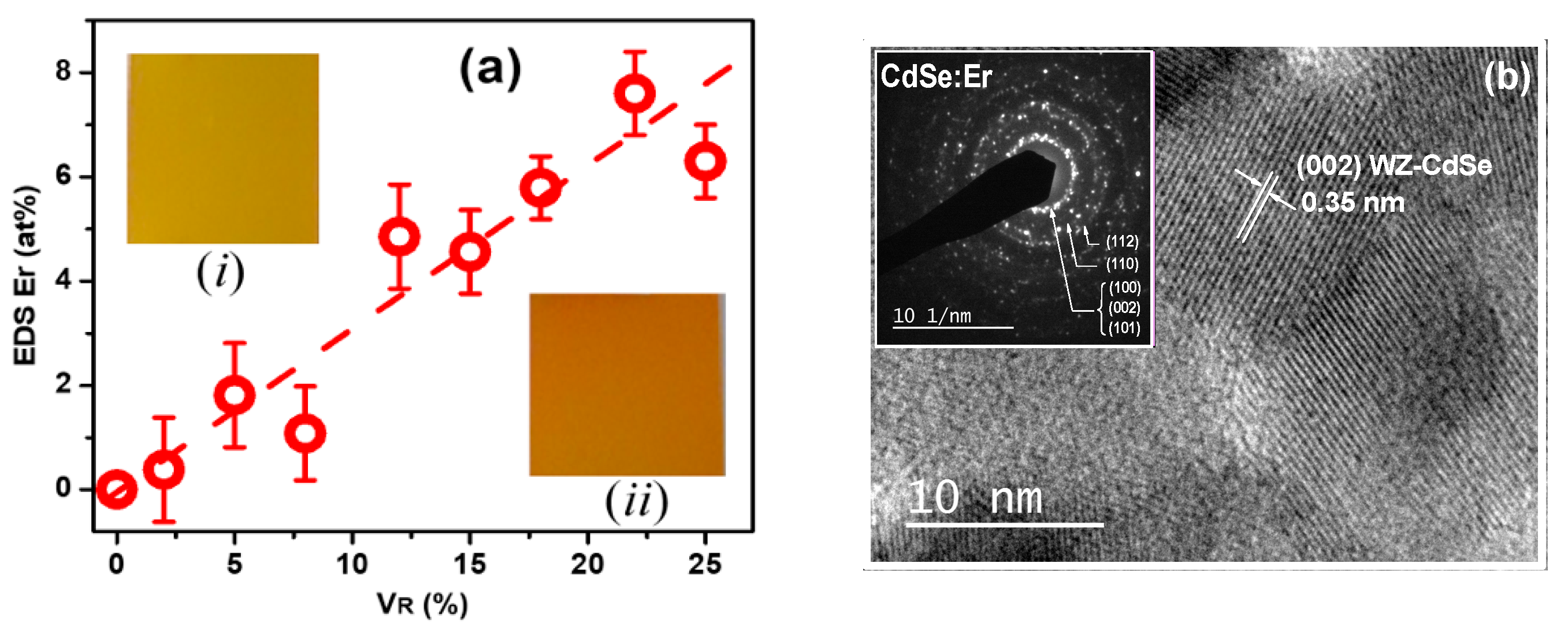
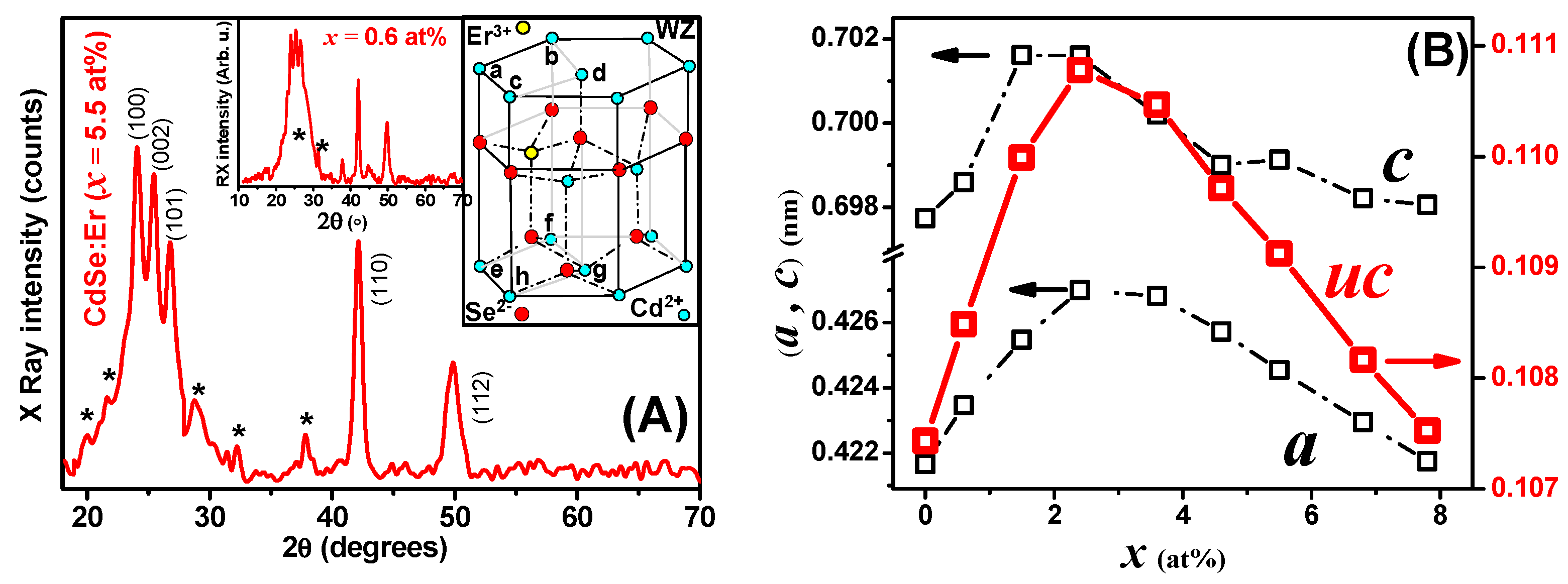
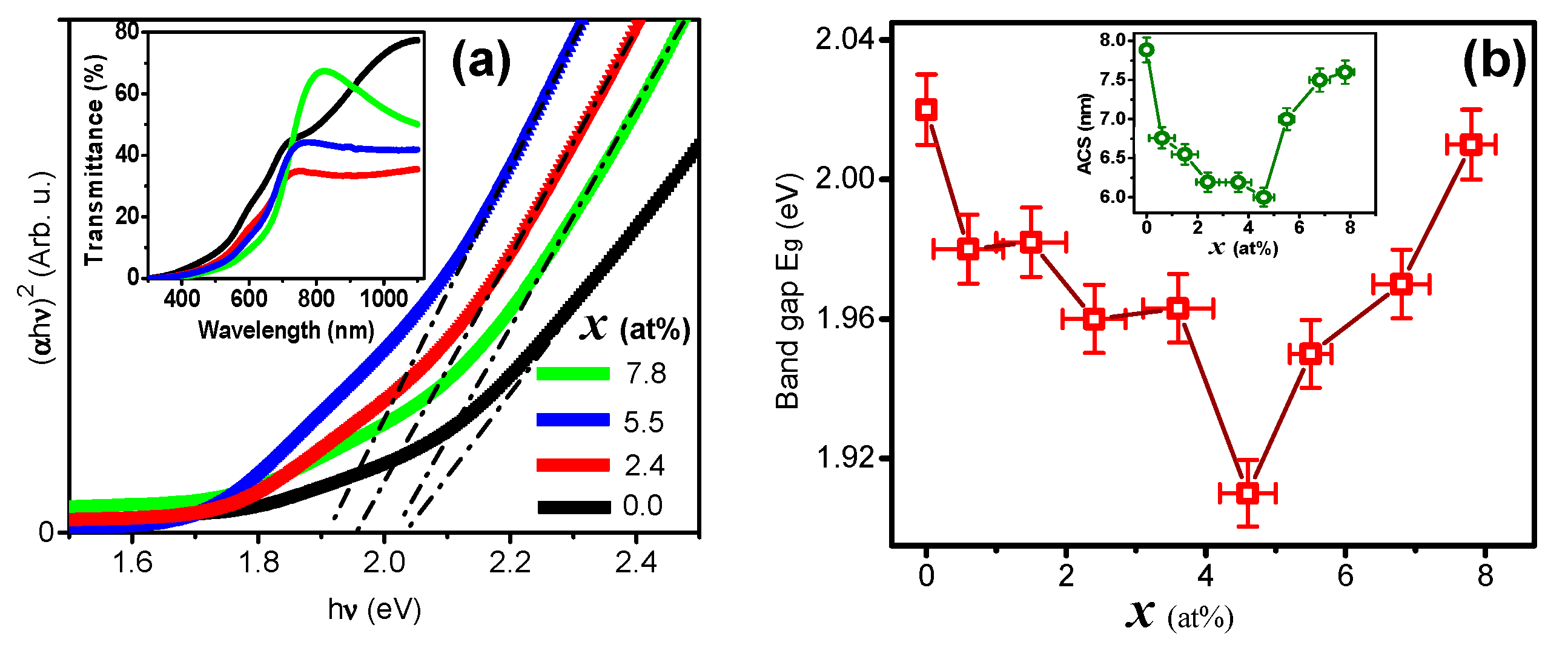
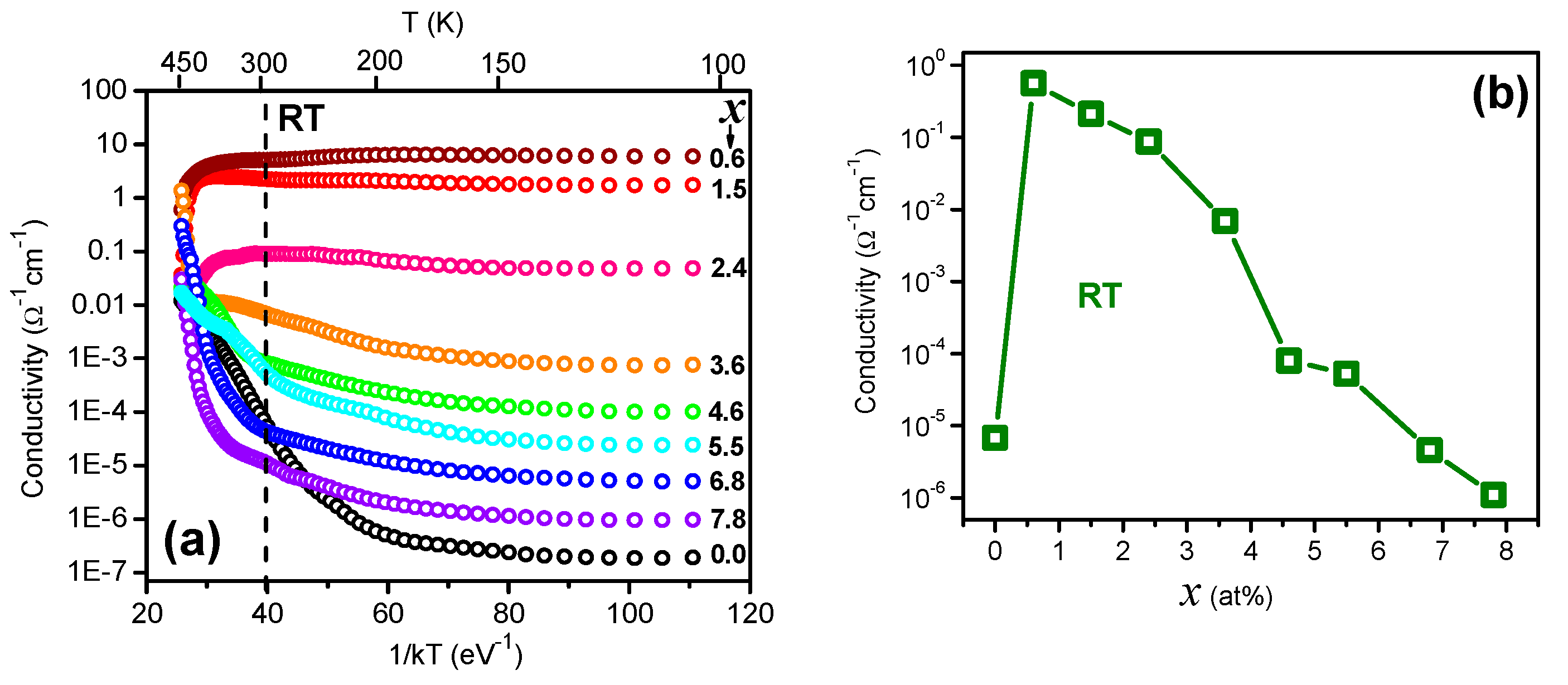
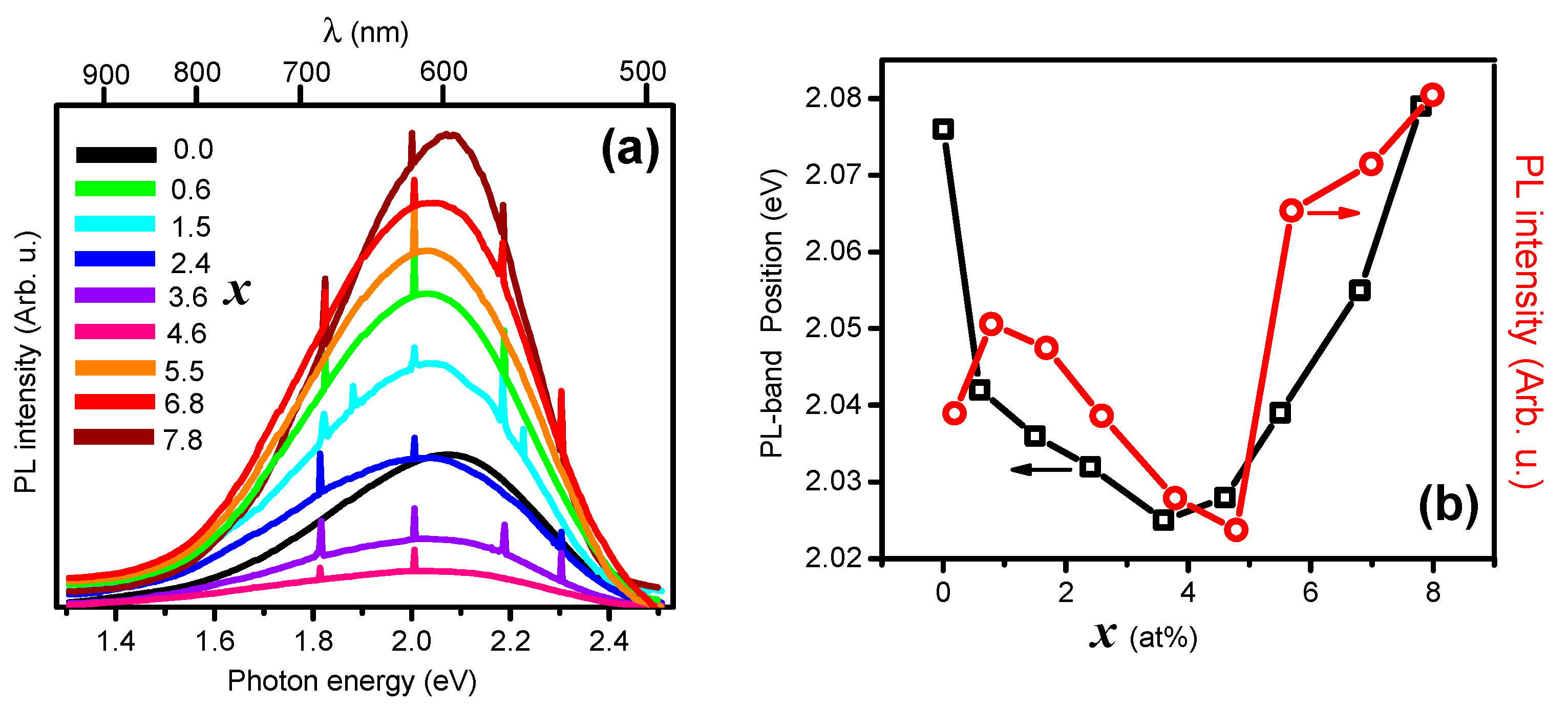
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babu, N.S.; Khadar, M.A. Electrical properties of grain size tuned CdSe nanocrystal films for practical applications. Sol. Energy Mater. Sol. Cells 2018, 178, 106–114. [Google Scholar] [CrossRef]
- Hassen, M.; Riahi, R.; Laatar, F.; Ezzaouia, H. Optical and surface properties of CdSe thin films prepared by sol-gel spin coating method. Surf. Interfaces 2019, 18, 100408. [Google Scholar] [CrossRef]
- Ghobadi, N.; Sohrabi, P.; Hatami, H.R. Correlation between the photocatalytic activity of CdSe nanostructured thin films with optical band gap and Urbach energy. Chem. Phys. 2020, 538, 110911. [Google Scholar] [CrossRef]
- Dai, G.; Chen, Y.; Wan, Q.; Zhang, Q.; Pan, A.; Zou, B. Fabrication and optical waveguide of Sn-catalyzed CdSe microstructures. Solid State Commun. 2013, 167, 31–35. [Google Scholar] [CrossRef]
- Shyju, T.; Anandhi, S.; Indirajith, R.; Gopalakrishnan, R. Solvothermal synthesis, deposition and characterization of cadmium selenide (CdSe) thin films by thermal evaporation technique. J. Cryst. Growth 2011, 337, 38–45. [Google Scholar] [CrossRef]
- Bayramoglu, H.; Peksoz, A. Electronic energy levels and electrochemical properties of co-electrodeposited CdSe thin films. Mater. Sci. Semicond. Process. 2019, 90, 13–19. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Sahu, A.; Kang, M.S.; Kompch, A.; Notthoff, C.; Wills, A.W.; Deng, D.; Winterer, M.; Frisbie, C.D.; Norris, D.J. Electronic Impurity Doping in CdSe Nanocrystals. Nano Lett. 2012, 12, 2587–2594. [Google Scholar] [CrossRef]
- Tolbert, S.H.; Alivisatos, A.P. Size Dependence of a First Order Solid-Solid Phase Transition: The Wurtzite to Rock Salt Transformation in CdSe Nanocrystals. Science 1994, 265, 373–376. [Google Scholar] [CrossRef]
- Trallero-Giner, C.; Debernardi, A.; Cardona, M.; Proupin, E.M.; Ekimov, A.I. Optical vibrons in CdSe dots and dispersion relation of the bulk material. Phys. Rev. B 1998, 57, 4664–4669. [Google Scholar] [CrossRef]
- Delikanli, S.; Yu, G.; Yeltik, A.; Bose, S.; Erdem, T.; Yu, J.; Erdem, O.; Sharma, M.; Sharma, V.; Quliyeva, U.; et al. Ultrathin Highly Luminescent Two-Monolayer Colloidal CdSe Nanoplatelets. Adv. Funct. Mater. 2019, 29, 1901028. [Google Scholar] [CrossRef]
- Alasvand, A.; Kafashan, H. Comprehensive physical studies on nanostructured Zn-doped CdSe thin films. J. Alloys Compd. 2019, 789, 108–118. [Google Scholar] [CrossRef]
- Garg, N. A Brief Study on Characteristics, Properties, and Applications of CdSe. Proc. ICEMIT 2017, 2, 43–60. [Google Scholar]
- Yu, J.; Chen, R. Optical properties and applications of two-dimensional CdSe nanoplatelets. Infomat 2020, 2, 905–927. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, L.; Fang, X. Growth and Device Application of CdSe Nanostructures. Adv. Funct. Mater. 2012, 22, 1551–1566. [Google Scholar] [CrossRef]
- Trindade, T.; O’Brien, P.; Zhang, X.-M. Synthesis of CdS and CdSe Nanocrystallites Using a Novel Single-Molecule Precursors Approach. Chem. Mater. 1997, 9, 523–530. [Google Scholar] [CrossRef]
- Ahmed, M.; Guleria, A.; Rath, M.C.; Singh, A.K.; Adhikari, S.; Sarkar, S.K. Facile and green synthesis of CdSe quantum dots in protein matrix: Tuning of morphology and optical properties. J. Nanosci. Nanotechnol. 2014, 14, 5730–5742. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Lam, Y.M.; Xu, Y.P.; Li, L.-J. Synthesis and characterization of one-dimensional CdSe by a novel reverse micelle assisted hydrothermal method. J. Colloid Interface Sci. 2008, 320, 491–500. [Google Scholar] [CrossRef]
- Thomas, D.; Lee, H.O.; Santiago, K.C.; Pelzer, M.; Kuti, A.; Jenrette, E.; Bahoura, M. Rapid Microwave Synthesis of Tunable Cadmium Selenide (CdSe) Quantum Dots for Optoelectronic Applications. J. Nanomater. 2020, 2020, 5056875. [Google Scholar] [CrossRef]
- Skaff, H.; Emrick, T. Reversible Addition Fragmentation Chain Transfer (RAFT) Polymerization from Unprotected Cadmium Selenide Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 5383–5386. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Wang, J.; Cui, X.; Li, X.; Wei, S.; Lu, J. Layered Inorganic/Organic Hybrid (CdSe) n·Monoamine Nanobelts: Controllable Solvothermal Synthesis, Multiple Stage Amine De-Intercalation Transformation, and Two-Dimensional Exciton Quantum Confinement Effect. Inorg. Chem. 2018, 57, 10781–10790. [Google Scholar] [CrossRef]
- Miethe, J.F.; Schlosser, J.A.; Eckert, G.; Lübkemanna, F.; Bigall, N.C. Electronic transport in CdSe-polymer fibres. J. Mater. Chem. C 2018, 6, 10916–10923. [Google Scholar] [CrossRef]
- Yildiz, I.; Callan, B.; Cruickshank, S.F.; Callan, J.F. Biocompatible CdSe-ZnS Core-Shell Quantum Dots Coated with Hydrophilic Polythiols. Langmuir 2009, 25, 7090–7096. [Google Scholar] [CrossRef]
- Huang, C.; Mao, J.; Chen, X.M.; Yang, J.; Du, X.W. Laser-activated gold catalysts for liquid-phase growth of cadmium selenide nanowires. Chem. Commun. 2014, 51, 2145–2148. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.I.; Vijaya, J.J.; Jesudoss, S.K.; Kaviyarasu, K.; Lee, S.-C.; Kennedy, L.J.; Jothiramalingam, R.; Al-Lohedan, H.A.; Abdullah, M.M. Investigation on preferably oriented abnormal growth of CdSe nanorods along (0002) plane synthesized by henna leaf extract-mediated green synthesis. R. Soc. Open Sci. 2018, 5, 171430. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.C.; Bozkurt, F.; Ansari, S.; Sozer, N.; Kokini, J.L. Applications of quantum dots in Food Science and biology. Trends Food Sci. Technol. 2016, 53, 75–89. [Google Scholar] [CrossRef]
- Almeida-Silva, A.C.; Peres-Freschi, A.P.; Mendonça-Rodrigues, C.; França-Matias, B.; Prado-Maia, L.; Goulart, L.R.; Oliveira-Dantas, N. Biological analysis and imaging applications of CdSe/CdSxSe1− x/CdS core–shell magic-sized quantum dot. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1421–1430. [Google Scholar] [CrossRef]
- Ung, T.D.T.; Tran, T.K.C.; Pham, T.N.; Nguyen, D.N.; Dinh, D.K.; Nguyen, Q.L. CdTe and CdSe quantum dots: Synthesis, characterizations and applications in agriculture. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 043001. [Google Scholar] [CrossRef]
- Dunpall, R.; Nejo, A.A.; Pullabhotla, V.S.R.; Opoku, A.R.; Revaprasadu, N.; Shonhai, A. An in Vitro Assessment of the Interaction of Cadmium Selenide Quantum Dots With DNA, Iron, and Blood Platelets. IUBMB Life 2012, 64, 995–1002. [Google Scholar] [CrossRef]
- Sathyalatha, K.; Uthanna, S.; Reddy, P. Electrical and photoconducting properties of vacuum evaporated pure and silver-doped CdSe films. Thin Solid Films 1989, 174, 233–238. [Google Scholar] [CrossRef]
- Walukiewicz, W. Intrinsic limitations to the doping of wide-gap semiconductors. Phys. B Condens. Matter 2001, 302–303, 123–134. [Google Scholar] [CrossRef]
- Majid, A.; Arshad, H.; Murtaza, S. Synthesis and characterization of Cr doped CdSe nanoparticles. Superlattices Microstruct. 2015, 85, 620–623. [Google Scholar] [CrossRef]
- Whitham, P.J.; Knowles, K.E.; Reid, P.J.; Gamelin, D.R. Photoluminescence Blinking and Reversible Electron Trapping in Copper-Doped CdSe Nanocrystals. Nano Lett. 2015, 15, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Proshchenko, V.; Dahnovsky, Y. Magnetic effects in Mn-doped CdSe nanocrystals. Phys. Status Solidi B 2015, 252, 2275–2279. [Google Scholar] [CrossRef]
- Al-Kabbi, A.S.; Sharma, K.; Saini, G.; Tripathi, S. Effect of doping on transport properties of nanocrystalline CdSe thin film. Thin Solid Films 2015, 586, 1–7. [Google Scholar] [CrossRef]
- Sharma, K.; Al-Kabbi, A.S.; Saini, G.; Tripathi, S. Influence of Zn doping on structural, optical and electrical properties of nanocrystalline CdSe thin films. J. Alloys Compd. 2015, 651, 42–48. [Google Scholar] [CrossRef]
- Zhang, K.-C.; Li, Y.-F.; Liu, Y.; Chi, F. Density-functional study on the robust ferromagnetism in rare-earth element Yb-doped SnO 2. J. Magn. Magn. Mater. 2014, 360, 165–168. [Google Scholar] [CrossRef]
- Lee, W.; Kwak, W.-C.; Min, S.K.; Lee, J.-C.; Chae, W.-S.; Sung, Y.-M.; Han, S.-H. Spectral broadening in quantum dots-sensitized photoelectrochemical solar cells based on CdSe and Mg-doped CdSe nanocrystals. Electrochem. Commun. 2008, 10, 1699–1702. [Google Scholar] [CrossRef]
- Martín-Rodríguez, R.; Geitenbeek, R.; Meijerink, A. Incorporation and Luminescence of Yb3+ in CdSe Nanocrystals. J. Am. Chem. Soc. 2013, 135, 13668–13671. [Google Scholar] [CrossRef]
- Li, I.-F.; Yeh, C.-S. Synthesis of Gd doped CdSe nanoparticles for potential optical and MR imaging applications. J. Mater. Chem. 2010, 20, 2079–2081. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Joo, S.W. Synthesis, characterization and sonophotocatalytic degradation of an azo dye on Europium doped cadmium selenide nanoparticles. Nanochemistry Res. 2018, 3, 178–188. [Google Scholar]
- Tomás, S.A.; Lozada-Morales, R.; Portillo, O.; Lima-Lima, H.; Palomino-Merino, R.; Zelaya, O. Characterization of chemical bath deposited CdS thin films doped with methylene blue and Er3+. Eur. Phys. J. Spéc. Top. 2008, 153, 299–302. [Google Scholar] [CrossRef]
- Moreno, O.P.; Pérez, R.G.; Portillo, M.C.; Téllez, G.H.; Rosas, E.R.; Cruz, S.C.A. Moreno Rodríguez, Synthesis, morphological, optical and structural properties of PbSSe2−nanocrystals. Optik 2016, 127, 8341–8349. [Google Scholar] [CrossRef]
- Dammak, M.; Zhang, D.-L. Spectra and energy levels of Er3+ in Er2O3 powder. J. Alloys Compd. 2006, 407, 8–15. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A. Optical properties of erbium oxide thin films deposited by electron beam evaporation. Thin Solid Films 2007, 515, 2885–2890. [Google Scholar] [CrossRef]
- Ionescu, A.M.; Munteanu, D.; Chovet, A.; Rusu, A.; Steriu, D. The intrinsic pseudo-MOSFET technique. In Proceedings of the 1997 International Semiconductor Conference 20th Edition, Sinaia, Romania, 7–11 October 1997; Volume 1, pp. 217–220. [Google Scholar]
- Bakhsh, A.; Maqsood, A. Sintering effects on structure, morphology, and electrical properties of sol-gel synthesized, nano-crystalline erbium oxide. Electron. Mater. Lett. 2012, 8, 605–608. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N. Erbium-doped nanoparticles/films for enhancing percentage photodegradation of direct red-31 dye. J. Mater. Sci. Mater. Electron. 2018, 29, 14960–14970. [Google Scholar] [CrossRef]
- Hegazy, M.A.; El-Hameed, A.M. A Characterization of CdSe-nanocrystals used in semiconductors for aerospace applications: Production and optical properties. NRIAG J. Astron. Geophys. 2014, 3, 82–87. [Google Scholar] [CrossRef]
- Alajlouni, S.; Sun, Z.Y.; Li, J.; Zavada, J.M.; Lin, J.Y.; Jiang, H.X. Refractive index of erbium doped GaN thin films. Appl. Phys. Lett. 2014, 105, 081104. [Google Scholar] [CrossRef]
- Kenyon, A.J. Erbium in silicon. Semicond. Sci. Technol. 2005, 20, R65–R84. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Pang, G.; Sun, X.W.; Chen, R. Effect of Lateral Size and Surface Passivation on the Near-Band-Edge Excitonic Emission from Quasi-Two-Dimensional CdSe Nanoplatelets. ACS Appl. Mater. Interfaces 2019, 11, 41821–41827. [Google Scholar] [CrossRef]
- Nasiri, S.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Janusas, G. The effect of calcination temperature on the photophysical and mechanical properties of copper iodide (5 mol%)–doped hydroxyapatite. Opt. Mater. 2021, 121, 111559. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, C.-W.; Huang, J.Y.; Pan, C.-L.; Zhang, J.-Y.; Chang, C.-S. Erbium doped GaSe crystal for mid-IR applications. Opt. Express 2006, 14, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shangguan, W. Hydrogen production from water splitting on CdS-based photocatalysts using solar light. Front. Energy 2013, 7, 111–118. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Li, Y.; Chen, Y.; Liu, M. Photocatalytic Activities of Copper Doped Cadmium Sulfide Microspheres Prepared by a Facile Ultrasonic Spray-Pyrolysis Method. Molecules 2016, 21, 735. [Google Scholar] [CrossRef]
- Liu, M.; Du, Y.; Ma, L.; Jing, D.; Guo, L. Manganese doped cadmium sulfide nanocrystal for hydrogen production from water under visible light. Int. J. Hydrogen Energy 2012, 37, 730–736. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Zhang, J.; Huang, C.; Mao, L. Nickel nanoparticles modified CdS—A potential photocatalyst for hydrogen production through water splitting under visible light irradiation. Int. J. Hydrogen Energy 2015, 40, 340–345. [Google Scholar] [CrossRef]
- Portillo-Moreno, O.; Gutiérrez-Pérez, R.; Palomino-Merino, R.; Chávez-Portillo, M.; Márquez-Specia, M.N.; Hernández-Torres, M.E.; Gracia-Jiménez, M.; Cerna, J.R.; Zamora-Tototzintle, M. Near-infrared-to-visible upconverting luminescence of Er3+-doped CdSe nanocrystals grown by chemical bath. Optik 2017, 138, 229–239. [Google Scholar] [CrossRef]
- Yan, D.; Wu, P.; Zhang, S.P.; Liang, L.; Yang, F.; Pei, Y.L.; Chen, S. Assignments of the Raman modes of monoclinic erbium oxide. J. Appl. Phys. 2013, 114, 193502. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Lin, C.; Kelley, D.F.; Rico, M.; Kelley, A.M. The “Surface Optical” Phonon in CdSe Nanocrystals. ACS Nano 2014, 8, 3928–3938. [Google Scholar] [CrossRef] [PubMed]
- Gomonnai, A.V.; Azhniuk, Y.M.; Yukhymchuk, V.O.; Kranjčec, M.; Lopushansky, V.V. Confinement-, surface- and disorder-related effects in the resonant Raman spectra of nanometric CdS1−xSex crystals. Phys. Status Solidi B 2003, 239, 490–499. [Google Scholar] [CrossRef]
- Dzhagan, V.; Lokteva, I.; Himcinschi, C.; Jin, X.; Kolny-Olesiak, J.; Zahn, D.R.T. Phonon Raman spectra of colloidal CdTe nanocrystals: Effect of size, non-stoichiometry and ligand exchange. Nanoscale Res. Lett. 2011, 6, 79. [Google Scholar] [CrossRef]
- Dıaz-Reyes, J.; Galvan-Arellano, M.; Mendoza-Alvarez, J.G.; Arias-Ceron, J.S.; Herrera-Perez, J.L.; Lopez-Cruz, E. Characterization of highly doped Ga0.86In0.14As0.13Sb0.87 grown by liquid phase epitaxy. Rev. Mex. Física 2017, 63, 55–64. [Google Scholar]
- Dzhagan, V.M.; Azhniuk, Y.M.; Milekhin, A.G.; Zahn, D.R.T. Vibrational spectroscopy of compound semiconductor nanocrystals. J. Phys. D Appl. Phys. 2018, 51, 503001. [Google Scholar] [CrossRef]
- Nasiri, S.; Dashti, A.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Doustmohammadi, A.; Janusas, G. Mechanochromic and thermally activated delayed fluorescence dyes obtained from D–A–D′ type, consisted of xanthen and carbazole derivatives as an emitter layer in organic light emitting diodes. Chem. Eng. J. 2021, 430, 131877. [Google Scholar] [CrossRef]
- Venkatram, N.; Sathyavathi, R.; Rao, D.N. Size dependent multiphoton absorption and refraction of CdSe nanoparticles. Opt. Express 2007, 15, 12258–12263. [Google Scholar] [CrossRef]
- Gautam, S.K.; Gautam, N.; Singh, R.G.; Ojha, S.; Shukla, D.K.; Singh, F. Anomalous behavior of B1g mode in highly transparent anatase nano-crystalline Nb-doped Titanium Dioxide (NTO) thin films. AIP Adv. 2015, 5, 127212. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Lattice distortion and corresponding changes in optical properties of CeO2 nanoparticles on Nd doping. Curr. Appl. Phys. 2013, 13, 217–223. [Google Scholar] [CrossRef]
- Wang, T.; Layek, A.; Hosein, I.D.; Chirmanov, V.; Radovanovic, P.V. Correlation between native defects and dopants in colloidal lanthanide-doped Ga2O3nanocrystals: A path to enhance functionality and control optical properties. J. Mater. Chem. C 2013, 2, 3212–3222. [Google Scholar] [CrossRef]
- Sercel, P.C.; Shabaev, A.; Efros, A.L. Photoluminescence Enhancement through Symmetry Breaking Induced by Defects in Nanocrystals. Nano Lett. 2017, 17, 4820–4830. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ren, G.; Xu, C.; Yang, Q. Modifying crystal phase, shape, size, optical and magnetic properties of monodispersed multifunctional NaYbF4nanocrystals through lanthanide doping. CrystEngComm 2011, 13, 4276–4281. [Google Scholar] [CrossRef]
- Bezkrovnyi, O.; Małecka, M.A.; Lisiecki, R.; Ostroushko, V.; Thomas, A.G.; Gorantlad, S.; Kepinski, L. The effect of Eu doping on the growth, structure and red-ox activity of ceria nanocubes. CrystEngComm 2018, 20, 1698–1704. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Miyauchi, M. Ce-doped ZnO (CexZn1−xO) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu2+) grafting. Phys. Chem. Chem. Phys. 2011, 13, 14937–14945. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Fei, J.; Lu, J. Sm-doping effect on optical and electrical properties of ZnO films. J. Nanostructure Chem. 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Q.; Chan, S.W. Ceria nanoparticles: Size, size distribution, and shape. J. Appl. Phys. 2004, 95, 4319–4326. [Google Scholar] [CrossRef]
- Lang, J.; Wang, J.; Zhang, Q.; Xu, S.; Han, D.; Yang, J.; Han, Q.; Yang, L.; Sui, Y.; Li, X.; et al. Synthesis and photoluminescence characterizations of the Er3+-doped ZnO nanosheets with irregular porous microstructure. Mater. Sci. Semicond. Process. 2016, 41, 32–37. [Google Scholar] [CrossRef]
- Chen, W.-B.; Liu, X.-C.; Li, F.; Chen, H.-M.; Zhou, R.-W.; Shi, E.-W. Influence of oxygen partial pressure on the microstructural and magnetic properties of Er-doped ZnO thin films. AIP Adv. 2015, 5, 067105. [Google Scholar] [CrossRef]
- Dakhel, A.A.; El-Hilo, M. Ferromagnetic nanocrystalline Gd-doped ZnO powder synthesized by coprecipitation. J. Appl. Phys. 2010, 107, 123905. [Google Scholar] [CrossRef]
- Shi, J.; Shen, L.; Meng, F.; Liu, Z. Structural, electrical and optical properties of highly crystalline indium tin oxide films fabricated by RPD at room temperature. Mater. Lett. 2016, 182, 32–35. [Google Scholar] [CrossRef]
- Greenberg, B.L.; Robinson, Z.L.; Ayino, Y.; Held, J.T.; Peterson, T.A.; Mkhoyan, K.A.; Pribiag, V.S.; Aydil, E.S.; Kortshagen, U.R. Metal-insulator transition in a semiconductor nanocrystal network. Sci. Adv. 2019, 5, eaaw1462. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.M.; Marques, A.C.; Cabeça, R.; Zampedri, L.; Chiasera, A.; Ferrari, M. Photoluminescence of Erbium-Doped Silicate Sol-Gel Planar Waveguides. J. Sol-Gel Sci. Technol. 2004, 31, 317–322. [Google Scholar] [CrossRef]
- Sethi, S.; Bhattacharya, P.K. Characteristics and Device Applications of Erbium Doped IIl-V Semiconductors Grown by Molecular Beam Epitaxy. J. Electron. Mater. 1996, 25, 467–477. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Douglas, L.; Mustafa, H.; Mundle, R.; Hunter, D.; Bonner, C.E. Pulsed-laser deposited Er:ZnO films for 1.54 μm emission. Appl. Phys. Lett. 2007, 90, 072108. [Google Scholar] [CrossRef]
- Popa, M.; Schmerber, G.; Toloman, D.; Gabor, M.S.; Mesaros, A.; Petrişor, T. Magnetic and Electrical Properties of Undoped and Holmium Doped ZnO Thin Films Grown by Sol-Gel Method. Adv. Eng. Forum 2013, 8–9, 301–308. [Google Scholar] [CrossRef]
- Das, P.; Bathula, S.; Gollapudi, S. Evaluating the effect of grain size distribution on thermal conductivity of thermoelectric materials. Nano Express 2020, 1, 020036. [Google Scholar] [CrossRef]
- Morais, E.A.; Scalvi, L.V.A.; Ribeiro, S.J.L.; Geraldo, V. Poole-Frenkel effect in Er doped SnO2thin films deposited by sol-gel-dip-coating. Phys. Status Solidi A 2005, 202, 301–308. [Google Scholar] [CrossRef]
- Kityk, I.V.; Halyan, V.V.; Yukhymchuk, V.O.; Strelchuk, V.V.; Ivashchenko, I.A.; Zhydachevskyy, Y.; Suchocki, A.; Olekseyuk, I.D.; Kevshyn, A.H.; Piasecki, M. NIR and visible luminescence features of erbium doped Ga2S3–La2S3 glasses. J. Non-Crystalline Solids 2018, 498, 380–385. [Google Scholar] [CrossRef]
- Yang, J.-H.; Gao, J.-F.; Yong, S.-L.; Ma, X.-L.; Liu, L.-J. Synthesis, characterization and optical studies on Sm2+-doped CdSe nanocrystals: A blueshift and fixed emission with high quantum yields. Rare Met. 2019, 38, 1097–1104. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wong, S.S. Observation of Photoinduced Charge Transfer in Novel Luminescent CdSe Quantum Dot-CePO4:Tb Metal Oxide Nanowire Composite Heterostructures. J. Phys. Chem. C 2014, 118, 5671–5682. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Yao, Q.; Ng, M.; Lin, M.; Li, X.; Bhakoo, K.K.; Chang, A.Y.; Rosei, F.; Vetrone, F. Morphology Control of Lanthanide Doped NaGdF4 Nanocrystals via One-Step Thermolysis. Chem. Mater. 2019, 31, 5160–5171. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Silva, Y.d.J.; Godínez, L.A.; Toledano-Ayala, M.; Lozada-Morales, R.; Zelaya-Angel, O.; Méndez-López, A. Study of the Effects of Er Doping on the Physical Properties of CdSe Thin Films. Magnetochemistry 2023, 9, 107. https://doi.org/10.3390/magnetochemistry9040107
Acosta-Silva YdJ, Godínez LA, Toledano-Ayala M, Lozada-Morales R, Zelaya-Angel O, Méndez-López A. Study of the Effects of Er Doping on the Physical Properties of CdSe Thin Films. Magnetochemistry. 2023; 9(4):107. https://doi.org/10.3390/magnetochemistry9040107
Chicago/Turabian StyleAcosta-Silva, Yuliana de Jesús, Luis A. Godínez, Manuel Toledano-Ayala, Rosendo Lozada-Morales, Orlando Zelaya-Angel, and Arturo Méndez-López. 2023. "Study of the Effects of Er Doping on the Physical Properties of CdSe Thin Films" Magnetochemistry 9, no. 4: 107. https://doi.org/10.3390/magnetochemistry9040107
APA StyleAcosta-Silva, Y. d. J., Godínez, L. A., Toledano-Ayala, M., Lozada-Morales, R., Zelaya-Angel, O., & Méndez-López, A. (2023). Study of the Effects of Er Doping on the Physical Properties of CdSe Thin Films. Magnetochemistry, 9(4), 107. https://doi.org/10.3390/magnetochemistry9040107






