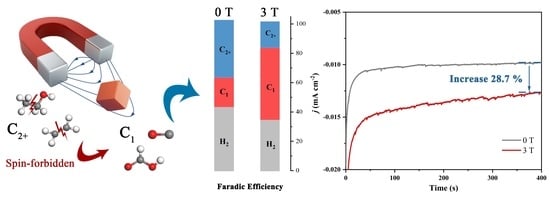
A Strong Magnetic Field Alters the Activity and Selectivity of the CO2RR by Restraining C–C Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalyst
2.2. Physical Characterizations
2.3. Electrochemical Measurements
2.4. Generation of a Magnetic Field
2.5. Analysis of Reaction Products
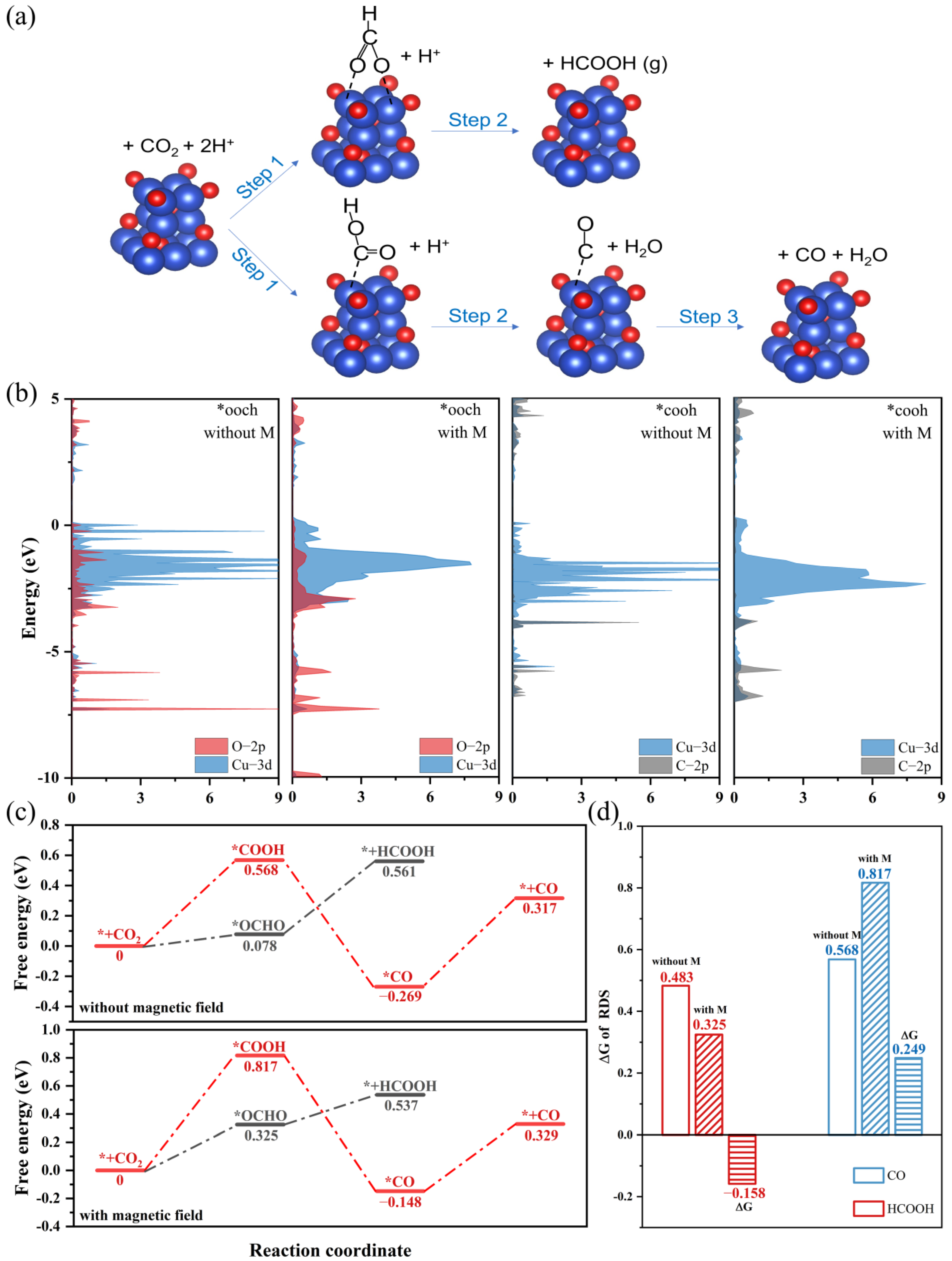
2.6. DFT Calculations
3. Results and Discussion
3.1. No Significant Structure Reconstruction during Testing
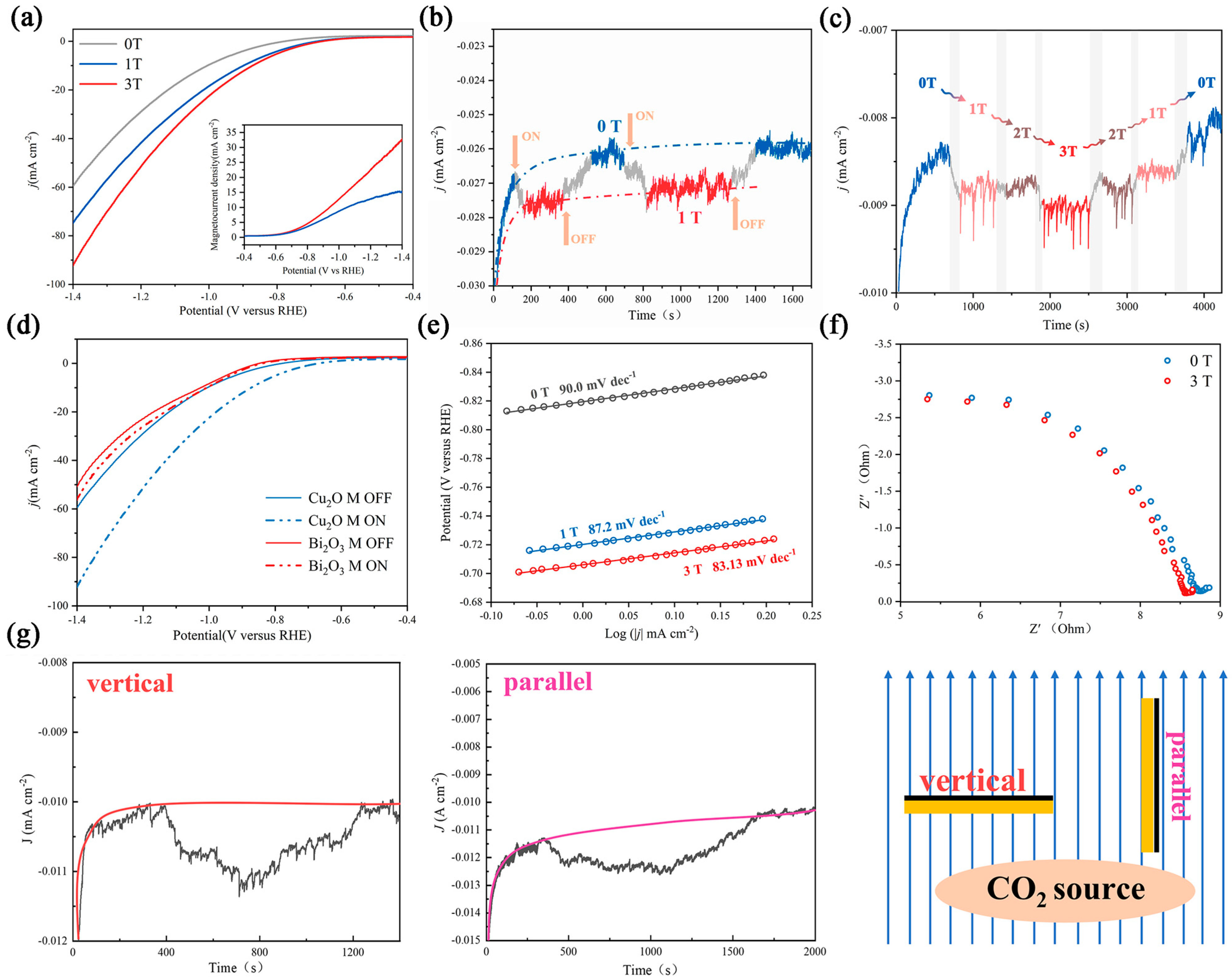
3.2. The Effect of a Magnetic Field on Electrocatalytic Performance
3.3. A hybrid Magnetic Effect in the CO2RR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Kotyk, J.K.; Sheehan, S.W. Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dinh, C.T.; Li, J.; Ozden, A.; Kibria, M.G.; Seifitokaldani, A.; Tan, C.; Gabardo, C.M.; Luo, M.; et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 2020, 3, 98–106. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Li, M.; Ma, Y.; Chen, J.; Lawrence, R.; Luo, W.; Sacchi, M.; Jiang, W.; Yang, J. Residual Chlorine Induced Cationic Active Species on a Porous Copper Electrocatalyst for Highly Stable Electrochemical CO2 Reduction to C2+. Angew. Chem. Int. Ed. 2021, 60, 2–9. [Google Scholar] [CrossRef]
- Jeon, H.S.; Timoshenko, J.; Scholten, F.; Sinev, I.; Herzog, A.; Haase, F.T.; Cuenya, B.R. Operando Insight into the Correlation between the Structure and Composition of CuZn Nanoparticles and Their Selectivity for the Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 19879–19887. [Google Scholar] [CrossRef]
- Zhang, F.; Co, A.C. Direct Evidence of Local pH Change and the Role of Alkali Cation during CO2 Electroreduction in Aqueous Media. Angew. Chem. Int. Ed. 2020, 59, 1674–1681. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, C.; Wu, J.; Liu, H.; Zhang, B.; Jiang, Z.; Li, S.; Xu, P. Recent Advances in Magnetic Field-Enhanced Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 10303–10316. [Google Scholar] [CrossRef]
- Elias, L.; Chitharanjan Hegde, A. Effect of Magnetic Field on HER of Water Electrolysis on Ni-W Alloy. Electrocatalysis 2017, 8, 375–382. [Google Scholar] [CrossRef]
- Kodaimati, M.S.; Gao, R.; Root, S.E.; Whitesides, G.M. Magnetic fields enhance mass transport during electrocatalytic reduction of CO2. Chem Catal. 2022, 2, 797–815. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Coey, J.M.D. Magnetic fields in electrochemistry: The Lorentz force. A mini-review. Electrochem. Commun. 2014, 42, 38–41. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Li, J.; Du, Y.; Kong, X.; Liu, S.; Yi, D.; Takahashi, Y.K.; Hono, K.; Wang, X.; et al. Regulation of oxygen reduction reaction by the magnetic effect of L10-PtFe alloy. Appl. Catal. B 2020, 278, 119332. [Google Scholar] [CrossRef]
- Matsushima, H.; Iida, T.; Fukunaka, Y.; Bund, A. PEMFC Performance in a Magnetic Field. Fuel Cells 2008, 8, 33–36. [Google Scholar] [CrossRef]
- Dunne, P.; Coey, J.M.D. Influence of a Magnetic Field on the Electrochemical Double Layer. J. Phys. Chem. C 2019, 123, 24181–24192. [Google Scholar] [CrossRef]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. Phys. Chem. Chem. Phys. 2015, 17, 18924–18936. [Google Scholar] [CrossRef] [PubMed]
- Buchachenko, A.L.; Berdinsky, V.L. Electron Spin Catalysis. Chem. Rev. 2002, 102, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.E.; Ulrich, T. Magnetic Field Effects in Chemical Kinetics and Related Phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef]
- Pan, H.; Xiao, X.; Hu, B.; Shen, Y.; Wang, M. Generating Huge Magnetocurrent by Using Spin-Dependent Dehydrogenation Based on Electrochemical System. J. Phys. Chem. C 2017, 121, 28420–28424. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, X.; Wang, X.; Wang, Q.; Wang, M.; Shen, Y. Effective Magnetic Field Regulation of the Radical Pair Spin States in Electrocatalytic CO2 Reduction. J. Phys. Chem. Lett. 2020, 11, 48–53. [Google Scholar] [CrossRef]
- Garcés-Pineda, F.A.; Blasco-Ahicart, M.; Nieto-Castro, D.; López, N.; Galán-Mascarós, J.R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 2019, 4, 519–525. [Google Scholar] [CrossRef]
- Ren, X.; Wu, T.; Sun, Y.; Li, Y.; Xian, G.; Liu, X.; Shen, C.; Gracia, J.; Gao, H.; Yang, H.; et al. Spin-polarized oxygen evolution reaction under magnetic field. Nat. Commun. 2021, 12, 2608. [Google Scholar] [CrossRef]
- Chen, C.; He, L.; Lai, L.; Zhang, H.; Lu, J.; Guo, L.; Li, Y. Magnetic properties of undoped Cu2O fine powders with magnetic impurities and/or cation vacancies. J. Phys. Condens. Matter 2009, 21, 145601. [Google Scholar] [CrossRef]
- Batsaikhan, E.; Lee, C.; Hsu, H.; Wu, C.; Peng, J.; Ma, M.; Deleg, S.; Li, W. Largely Enhanced Ferromagnetism in Bare CuO Nanoparticles by a Small Size Effect. ACS Omega 2020, 5, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Yao, Y.D.; Chen, J.W. Superparamagnetic Behavior of Antiferromagnetic CuO Nanoparticles. IEEE Trans. Magn. 2005, 41, 3409–3411. [Google Scholar] [CrossRef]
- Herzog, A.; Bergmann, A.; Jeon, H.S.; Timoshenko, J.; Kghl, S.; Rettenmaier, C.; Luna, M.L.; Haase, F.T.; Cuenya, B.R. Operando Investigation of Ag-Decorated Cu2O Nanocube Catalysts with Enhanced CO2 Electroreduction toward Liquid Products. Angew. Chem. Int. Ed. 2021, 60, 7426–7435. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within densityfunctional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, X.; Yang, D.; Ma, J.; Kang, X.; Zheng, L.; Zhang, J.; Wu, Z.; Han, B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.D.; Quintero-Bermudez, R.; Dinh, C.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Liu, X.; Zhang, Y.; Hu, J. RoomTemperature Ferromagnetism in CuO Sol-Gel Powders and Films. J. Magn. Magn. Mater. 2010, 322, 1994–1998. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Li, S.; Sun, J.; Wang, W.; Song, B.; Yang, X.; Wang, X.; Jiang, Z.; Wu, G.; et al. Magnetic field assisted electrocatalytic oxygen evolution reaction of nickel-based materials. J. Mater. Chem. A 2022, 10, 1760–1767. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, F.; Zhu, L.; Yang, Z.; Lin, Y.; Xu, Q.; Wang, Y. Insight into the Catalytic Activity of Amorphous Multimetallic Catalysts under a Magnetic Field toward the Oxygen Evolution Reaction. ACS Appl. Mater. 2022, 14, 10227–10236. [Google Scholar] [CrossRef]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, Y.; Tang, J.; Wang, J.; Liu, B.; Steinrück, H.; Lim, K.; Li, Y.; Toney, M.F.; Chan, K.; et al. Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat. Catal. 2019, 2, 55–61. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, H.; Cai, W.; Wen, Z.; Jia, B.; Wang, L.; Jin, W.; Ma, T. Engineering Bi-Sn Interface in Bimetallic Aerogel with 3D Porous Structure for Highly Selective Electrocatalytic CO2 Reduction to HCOOH. Angew. Chem. Int. Ed. 2021, 60, 12554–12559. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.; Wang, X.; Gao, C.; Chen, Z.; Wu, J.; Meng, H.; Song, Z.; Du, S.; Ren, Z. Copper-triggered delocalization of bismuth p-orbital favours high-throughput CO2 electroreduction. Appl. Catal. B 2022, 301, 120781. [Google Scholar] [CrossRef]
- Gao, L.; Wang, C.; Li, R.; Li, R.; Chen, Q. The effect of external magnetic fields on the catalytic activity of Pd nanoparticles in Suzuki cross-coupling reactions. Nanoscale 2016, 8, 8355–8362. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Qu, Y.; Meng, X.; Tu, J.; Zheng, W.; Hu, L.; Chen, Q. A Strong Magnetic Field Alters the Activity and Selectivity of the CO2RR by Restraining C–C Coupling. Magnetochemistry 2023, 9, 65. https://doi.org/10.3390/magnetochemistry9030065
Wang P, Qu Y, Meng X, Tu J, Zheng W, Hu L, Chen Q. A Strong Magnetic Field Alters the Activity and Selectivity of the CO2RR by Restraining C–C Coupling. Magnetochemistry. 2023; 9(3):65. https://doi.org/10.3390/magnetochemistry9030065
Chicago/Turabian StyleWang, Peichen, Yafei Qu, Xiangfu Meng, Jinwei Tu, Wei Zheng, Lin Hu, and Qianwang Chen. 2023. "A Strong Magnetic Field Alters the Activity and Selectivity of the CO2RR by Restraining C–C Coupling" Magnetochemistry 9, no. 3: 65. https://doi.org/10.3390/magnetochemistry9030065
APA StyleWang, P., Qu, Y., Meng, X., Tu, J., Zheng, W., Hu, L., & Chen, Q. (2023). A Strong Magnetic Field Alters the Activity and Selectivity of the CO2RR by Restraining C–C Coupling. Magnetochemistry, 9(3), 65. https://doi.org/10.3390/magnetochemistry9030065