Honey-like Odor Meets Single-Ion Magnet: Synthesis, Crystal Structure, and Magnetism of Cobalt(II) Complex with Aromatic Trans-Cinnamic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations and Instrumentation
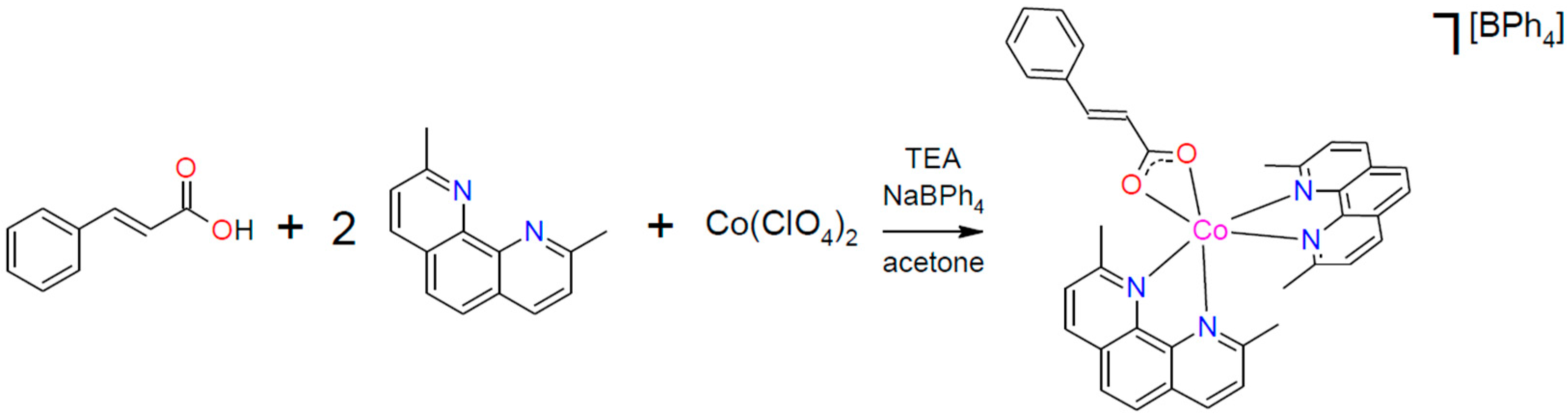
2.2. Synthesis of Compound 1·½Me2CO
2.3. X-ray Diffraction Analysis
2.4. Magnetic Measurements
2.5. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis and General Characterization
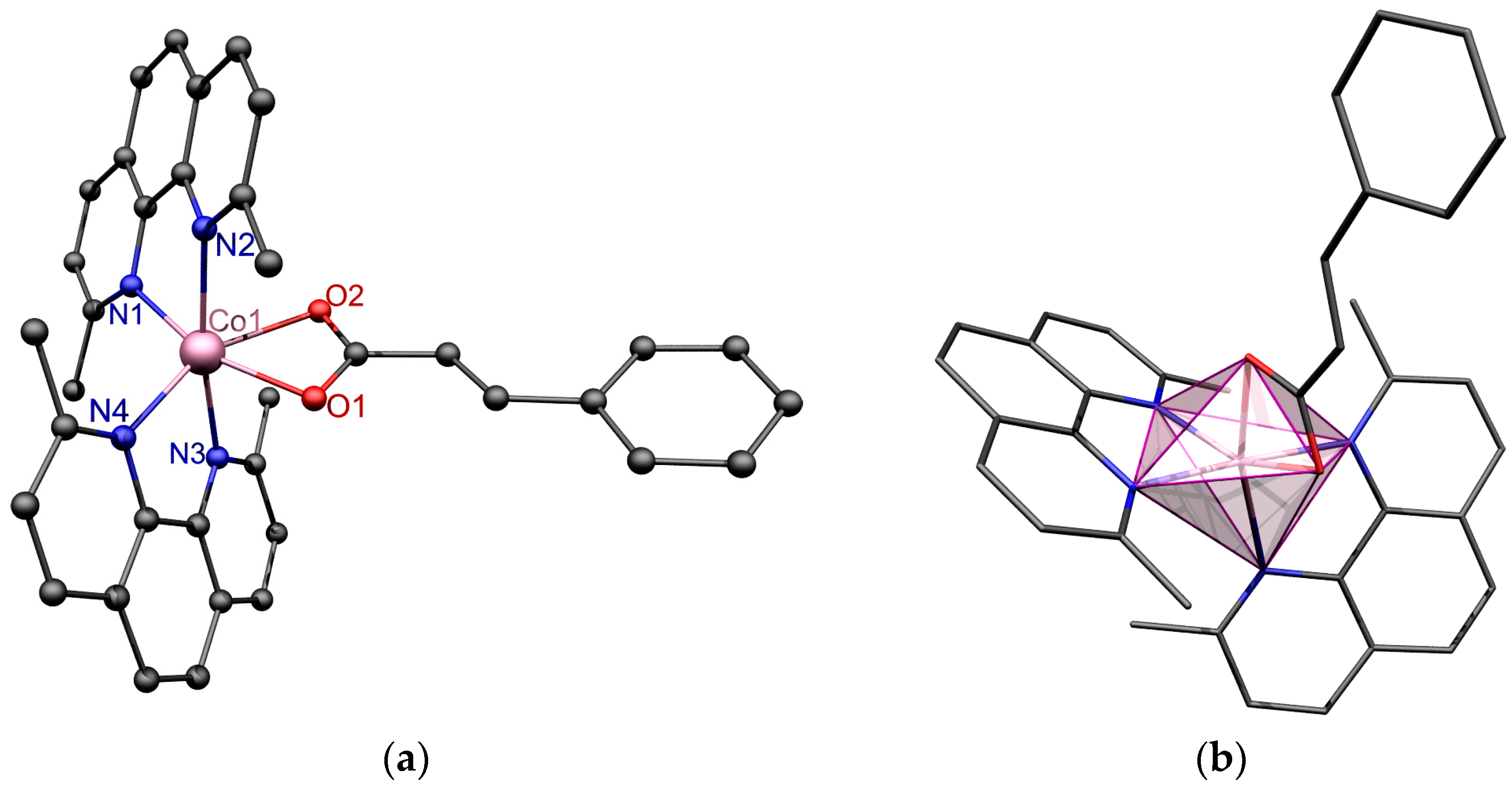
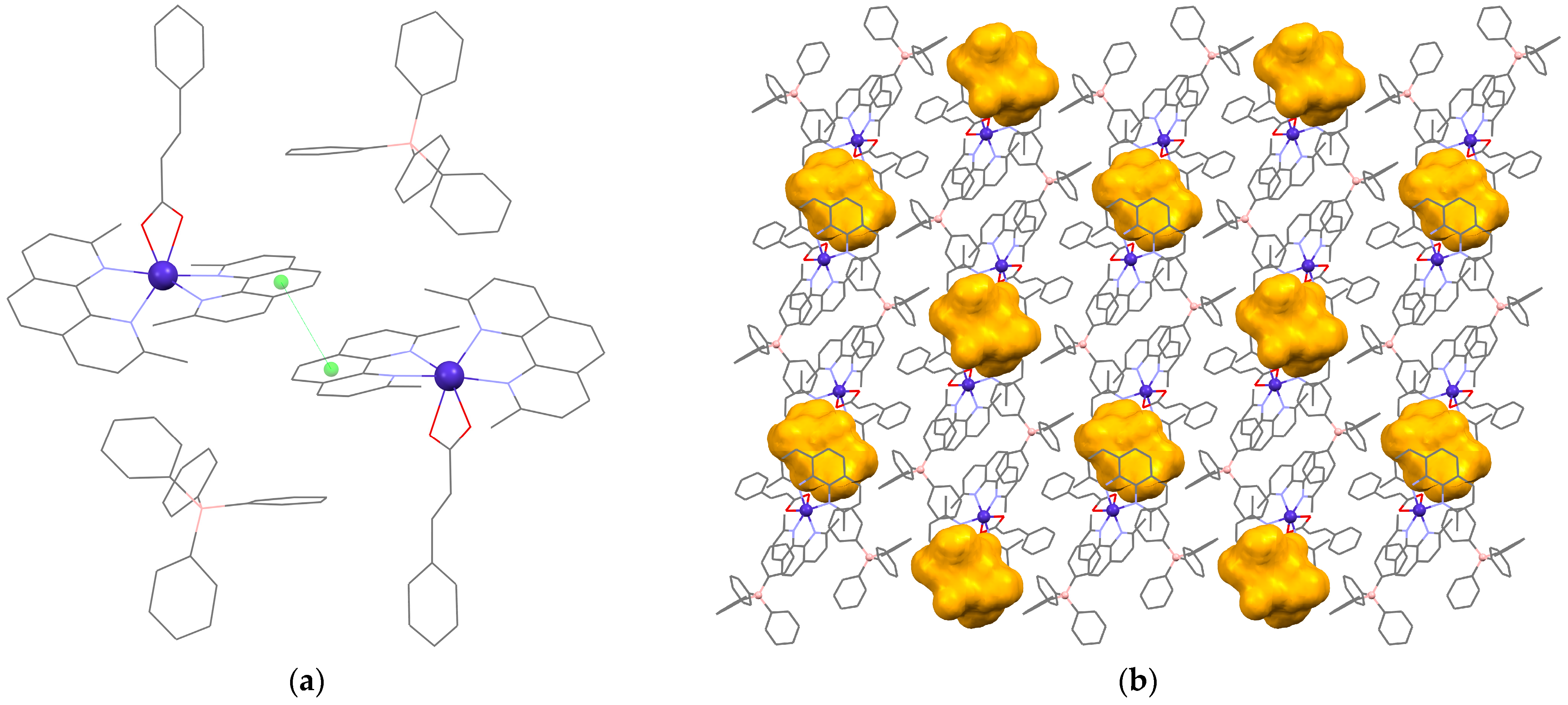
3.2. Description of the Crystal Structure
3.3. Magnetic Properties
3.3.1. Static Magnetic Properties
3.3.2. Dynamic Magnetic Properties
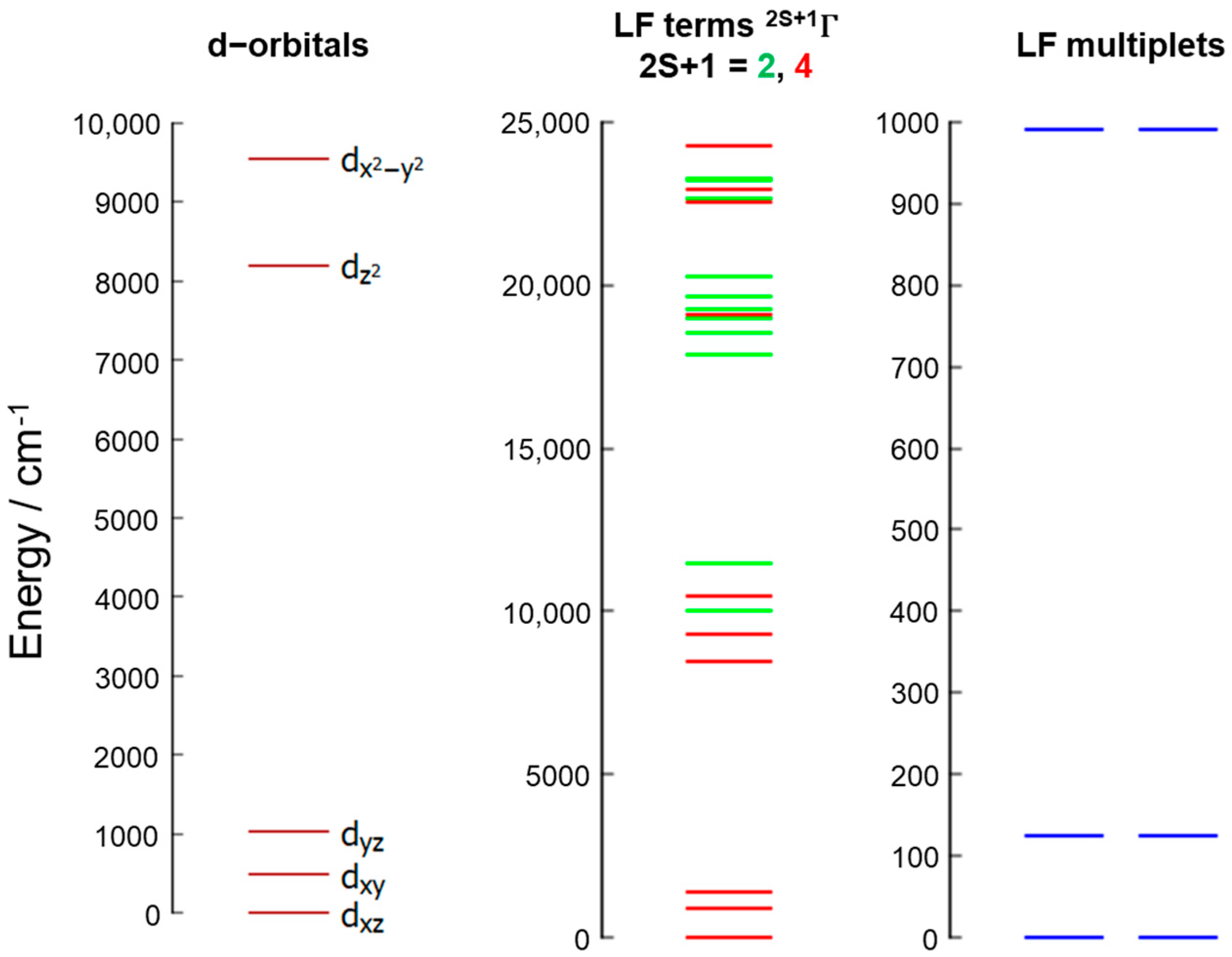
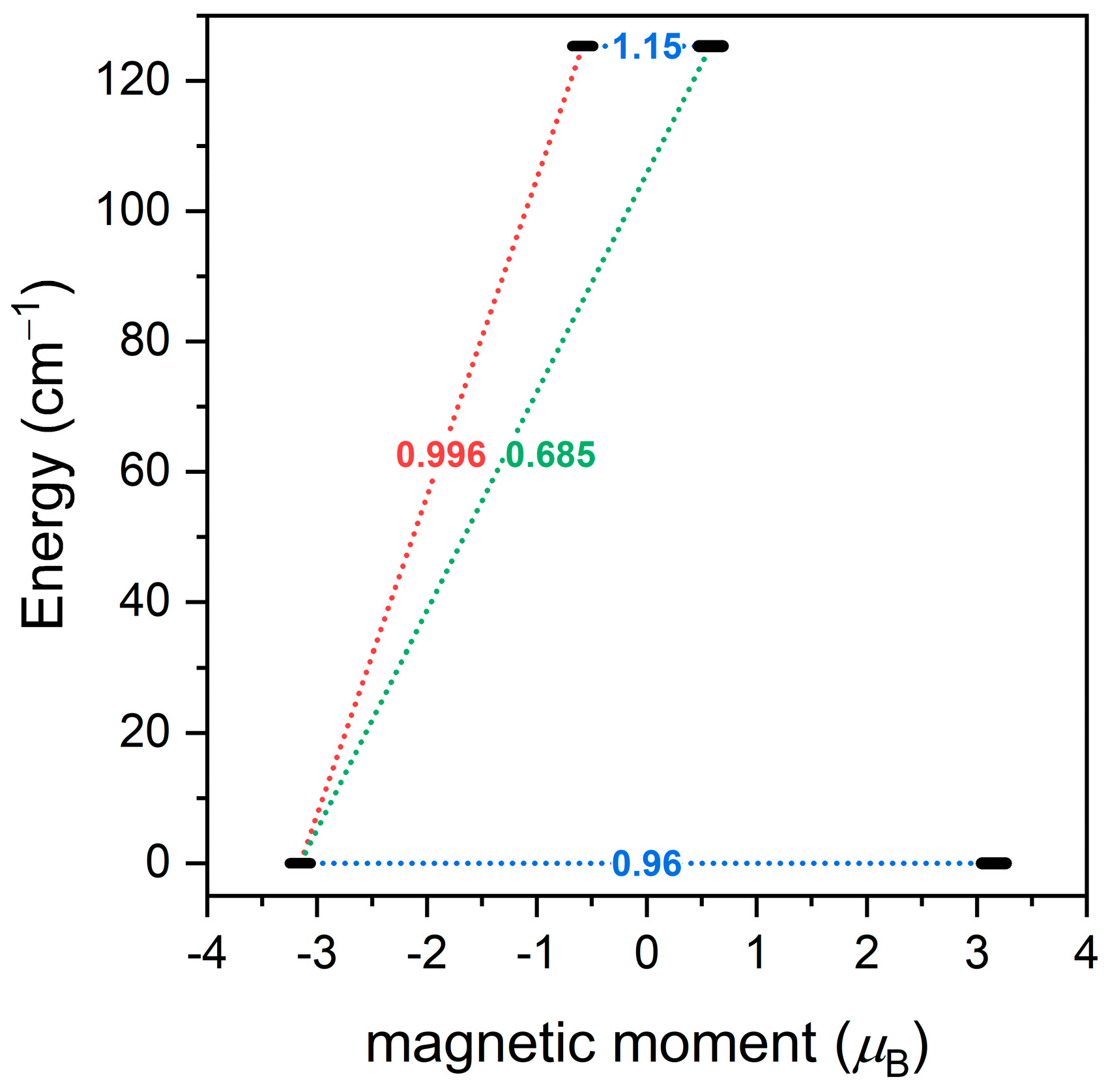
3.4. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwin, C.A.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. A dysprosium metallocene single-molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.C.; Liu, J.L.; Vieru, V.; Ungur, L.; Jia, J.H.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A stable pentagonal bipyramidal Dy (III) single-ion magnet with a record magnetization reversal barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef]
- Santana, F.S.; Perfetti, M.; Briganti, M.; Sacco, F.; Poneti, G.; Ravera, E.; Soares, J.F.; Sessoli, R. A dysprosium single molecule magnet outperforming current pseudocontact shift agents. Chem. Sci. 2022, 13, 5860–5871. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; Van Der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; Van Slageren, J. A four-coordinate cobalt (II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef]
- Herchel, R.; Váhovská, L.; Potočňák, I.; Travnicek, Z. Slow magnetic relaxation in octahedral cobalt (II) field-induced single-ion magnet with positive axial and large rhombic anisotropy. Inorg. Chem. 2014, 53, 5896–5898. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Long, J.R. Slow magnetic relaxation at zero field in the tetrahedral complex [Co(SPh)4]2−. J. Am. Chem. Soc. 2011, 133, 20732–20734. [Google Scholar] [CrossRef]
- Jurca, T.; Farghal, A.; Lin, P.H.; Korobkov, I.; Murugesu, M.; Richeson, D.S. Single-molecule magnet behavior with a single metal center enhanced through peripheral ligand modifications. J. Am. Chem. Soc. 2011, 133, 15814–15817. [Google Scholar] [CrossRef]
- Ma, X.; Chen, B.; Zhang, Y.Q.; Yang, J.; Shi, Q.; Ma, Y.; Liu, X. Enhancing single-molecule magnet behaviour through decorating terminal ligands in Dy2 compounds. Dalton Trans. 2019, 48, 12622–12631. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liedy, F.; Eng, J.; McNab, R.; Inglis, R.; Penfold, T.J.; Brechin, E.K.; Johansson, J.O. Vibrational coherences in manganese single-molecule magnets after ultrafast photoexcitation. Nat. Chem. 2020, 12, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Stupakiewicz, A.; Szerenos, K.; Afanasiev, D.; Kirilyuk, A.; Kimel, A.V. Ultrafast nonthermal photo-magnetic recording in a transparent medium. Nature 2017, 542, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Rogacz, K.; Brzozowska, M.; Baś, S.; Kurpiewska, K.; Pinkowicz, D. Low-coordinate erbium(III) single-molecule magnets with photochromic behavior. Inorg. Chem. 2022, 61, 16295–16306. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kume, S.; Nishihara, H. Reversible light-induced magnetization change in an azobenzene-attached pyridylbenzimidazole complex of iron(II) at room temperature. Dalton Trans. 2009, 2, 280–284. [Google Scholar] [CrossRef]
- Chastanet, G.; Lorenc, M.; Bertoni, R.; Desplanches, C. Light-induced spin crossover—Solution and solid-state processes. Comptes Rendus Chim. 2018, 21, 1075–1094. [Google Scholar] [CrossRef]
- Kume, S.; Kurihara, M.; Nishihara, H. Reversible trans–cis photoisomerization of azobenzene-attached bipyridine ligands coordinated to cobalt using a single UV light source and the Co(III)/Co(II) redox change. Chem. Commun. 2001, 17, 1656–1657. [Google Scholar] [CrossRef]
- Yutaka, T.; Mori, I.; Kurihara, M.; Tamai, N.; Nishihara, H. Photochemical behavior of azobenzene-conjugated CoII, CoIII, and FeII bis(terpyridine) complexes. Inorg. Chem. 2003, 42, 6306–6313. [Google Scholar] [CrossRef]
- Chen, L.; Tan, Y.; Xu, H.; Wang, K.; Chen, Z.H.; Zheng, N.; Li, Y.Q.; Lin, L.R. Enhanced E/Z-photoisomerization and luminescence of stilbene derivative co-coordinated in di-β-diketonate lanthanide complexes. Dalton Trans. 2020, 49, 16745–16761. [Google Scholar] [CrossRef]
- Pal, P.; Mukherjee, S.; Maity, D.; Baitalik, S. Synthesis, Structural Characterization, and Luminescence Switching of Diarylethene-Conjugated Ru(II)-Terpyridine Complexes by trans–cis Photoisomerization: Experimental and DFT/TD-DFT Investigation. Inorg. Chem. 2018, 57, 5743–5753. [Google Scholar] [CrossRef]
- Guerchais, V.; Le Bozec, H. Metal complexes featuring photochromic ligands. In Molecular Organometallic Materials for Optics. Topics in Organometallic Chemistry; Bozec, H., Guerchais, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 28, pp. 171–225. [Google Scholar] [CrossRef]
- Lin, L.R.; Wang, X.; Wei, G.N.; Tang, H.H.; Zhang, H.; Ma, L.H. Azobenzene-derived tris-β-diketonate lanthanide complexes: Reversible trans-to-cis photoisomerization in solution and solid state. Dalton Trans. 2016, 45, 14954–14964. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Umemura, Y.; Einaga, Y. Structure-distortion-induced photomagnetic effect in azobenzene/polyoxometalate Langmuir–Blodgett films. Dalton Trans. 2013, 42, 16014–16020. [Google Scholar] [CrossRef] [PubMed]
- Arn, H.; Acree, T.E. Flavornet: A database of aroma compounds based on odor potency in natural products. In Food Flavors: Formation, Analysis and Packaging Influences; Contis, E.T., Ho, C.-T., Mussinan, C.J., Parliment, T.H., Shahidi, F., Spanier, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 40, p. 27. ISBN 0167-4501. [Google Scholar]
- Stoermer, R. Über die Umlagerung stabiler stereoisomerer äthylenkörper in labile durch ultraviolettes Licht (I). Ber. Dtsch. Chem. Ges. 1909, 42, 4865–4871. [Google Scholar] [CrossRef]
- Stoermer, R. Über die Umlagerung der stabilen Stereoisomeren in labile durch ultraviolettes Licht. Ber. Dtsch. Chem. Ges. 1911, 44, 637–668. [Google Scholar] [CrossRef]
- Vaidya, B.K. Geometrical inversion in light. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1930, 129, 299–313. [Google Scholar] [CrossRef]
- Hocking, M.B. Photochemical and thermal isomerizations of cis-and trans-cinnamic acids, and their photostationary state. Can. J. Chem. 1969, 47, 4567–4576. [Google Scholar] [CrossRef]
- Salum, M.L.; Robles, C.J.; Erra-Balsells, R. Photoisomerization of ionic liquid ammonium cinnamates: One-pot synthesis−isolation of Z-cinnamic acids. Org. Lett. 2010, 12, 4808–4811. [Google Scholar] [CrossRef]
- Zhu, H.L.; Shao, S.C.; Qiu, X.Y.; Sun, L.; Yang, S.; Ma, J.L. Crystal structure of diaqua-trans-dicinnamato-dihexamethylene-tetraminecobalt(II)dihydrate, Co(C6H12N4)2(C9H7O2)2(H2O)2·2H2O. Z. Krist. New Cryst. Struct. 2003, 218, 545–546. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, Z.R.; Li, X.; Han, S.; Wang, J.J.; Li, W.F.; Liu, C.L.; Li, C.B. A novel Co(II) based multifunctional metal-organic framework: Synthesis, fluorescence sensing and magnetic analysis. Inorg. Chim. Acta 2019, 486, 750–756. [Google Scholar] [CrossRef]
- Fan, X.Y.; Li, K.; Huang, X.; Sun, T.; Yun, R.; Wu, H. Crystal structure of 4-hydroxycinnamato-[tris(1-methylbenzimidazol-2-ylmethyl)amine]cobalt(II) perchlorate–dimethylformamide–ethanol–methanol (1:1:1:1:0.5), Co(C27H27N7)(C9H7O3)](ClO4)·(CH3)2NCHO·C2H5OH·0.5CH3OH. Z. Krist. New Cryst. Struct. 2009, 224, 59–61. [Google Scholar] [CrossRef][Green Version]
- Lehleh, A.; Boutebdja, M.; Beghidja, C.; Beghidja, A. Synthesis, crystal structure and Hirshfeld surface analysis of aqua(3-methoxycinnamato-κO)bis(1,10-phenanthroline-κ2N,N′)cobalt(II) nitrate. Acta Crystallogr. E Crystallogr. Commun. 2022, 78, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Viciano-Chumillas, M.; Lloret, F.; Julve, M.; Castro, I.; Krzystek, J.; Ozerov, M.; Armentano, D.; De Munno, G.; Cano, J. Coligand effects on the field-induced double slow magnetic relaxation in six-coordinate cobalt (II) single-ion magnets (SIMs) with positive magnetic anisotropy. Inorg. Chem. 2019, 58, 15726–15740. [Google Scholar] [CrossRef] [PubMed]
- Nemec, I.; Herchel, R.; Trávníček, Z. Two polymorphic Co(II) field-induced single-ion magnets with enormous angular distortion from the ideal octahedron. Dalton Trans. 2018, 47, 1614–1623. [Google Scholar] [CrossRef]
- Smolko, L.; Černák, J.; Kuchár, J.; Rajnák, C.; Titiš, J.; Boča, R. Field-Induced Slow Magnetic Relaxation in Mononuclear Tetracoordinate Cobalt(II) Complexes Containing a Neocuproine Ligand. Eur. J. Inorg. Chem. 2017, 2017, 3080–3086. [Google Scholar] [CrossRef]
- Nemec, I.; Fellner, O.F.; Indruchová, B.; Herchel, R. Trigonally Distorted Hexacoordinate Co(II) Single-Ion Magnets. Materials 2022, 15, 1064. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 13, 2209–2233. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Diamond 4.6.8-Crystal and Molecular Structure Visualization, Crystal Impact-Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 2 April 2022).
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment–Olex2 dissected. Acta Crystallogr. A 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.40.82a; Rigaku Oxford Diffraction: Oxford, UK, 2020.
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Boča, R. Theoretical Foundation of Molecular Magnetism; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Malmqvist, P.Å.; Roos, B.O. The CASSCF state interaction method. Chem. Phys. Lett. 1989, 155, 189–194. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.P. Introduction of n-Electron Valence States for Multireference Perturbation Theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. n-Electron Valence State Perturbation Theory: A Fast Implementation of the Strongly Contracted Variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Hanai, K.; Kuwae, A.; Takai, T.; Senda, H.; Kunimoto, K.-K. A Comparative Vibrational and NMR Study of Cis-Cinnamic Acid Polymorphs and Trans-Cinnamic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 513–519. [Google Scholar] [CrossRef]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Courier Dover Publications: Mineola, NY, USA, 2021. [Google Scholar]
- Shrivastava, K.N. Theory of Spin–Lattice Relaxation. Phys. Status Solidi B 1983, 117, 437–458. [Google Scholar] [CrossRef]
- Singh, A.; Shrivastava, K.N. Optical-acoustic two-phonon relaxation in spin systems. Phys. Status Solidi B 1979, 95, 273. [Google Scholar] [CrossRef]
- Van Slageren, J.; Vongtragool, S.; Gorshunov, B.; Mukhin, A.A.; Karl, N.; Krzystek, J.; Telser, J.; Müller, A.; Sangregorio, C.; Gatteschi, D.; et al. Frequency domain magnetic resonance spectroscopy of molecular magnetic materials. Phys. Chem. Chem. Phys. 2003, 5, 3837. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Aleshin, D.Y.; Savkina, S.A.; Belov, A.S.; Efimov, N.N.; Nehrkorn, J.; Ozerov, M.; Voloshin, Y.Z.; Nelyubina, Y.V.; Novikov, V.V. A Trigonal Prismatic Cobalt(II) Complex as a Single Molecule Magnet with a Reduced Contribution from Quantum Tunneling. ChemPhysChem 2019, 20, 1001. [Google Scholar] [CrossRef]
- Misochko, E.Y.; Akimov, A.V.; Korchagin, D.V.; Nehrkorn, J.; Ozerov, M.; Palii, A.V.; Clemente-Juan, J.M.; Aldoshin, S.M. Purely Spectroscopic Determination of the Spin Hamiltonian Parameters in High-Spin Six-Coordinated Cobalt(II) Complexes with Large Zero-Field Splitting. Inorg. Chem. 2019, 58, 16434. [Google Scholar] [CrossRef] [PubMed]
- Landart-Gereka, A.; Quesada-Moreno, M.M.; Díaz-Ortega, I.F.; Nojiri, H.; Ozerov, M.; Krzystek, J.; Palacios, M.A.; Colacio, E. Large easy-axis magnetic anisotropy in a series of trigonal prismatic mononuclear cobalt(II) complexes with zero-field hidden single-molecule magnet behaviour: The important role of the distortion of the coordination sphere and intermolecular interactions in the slow relaxation. Inorg. Chem. Front. 2022, 9, 2810. [Google Scholar] [CrossRef]
- Atanasov, M.; Ganyushin, D.; Sivalingam, K.; Neese, F. Molecular Electronic Structures of Transition Metal Complexes II; Mingos, D.M.P., Day, P., Dahl, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 149–220. [Google Scholar]
- Singh, S.K.; Eng, J.; Atanasov, M.; Neese, F. Covalency and chemical bonding in transition metal complexes: An ab initio based ligand field perspective. Coord. Chem. Rev. 2017, 344, 2–25. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Kern, M.; Neugebauer, P.; Van Slageren, J.; Trávníček, Z. Magnetic anisotropy and field-induced slow relaxation of magnetization in tetracoordinate CoII compound [Co(CH3-im)2Cl2]. Materials 2017, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Chibotaru, L.F.; Ungur, L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 2012, 137, 064112. [Google Scholar] [CrossRef] [PubMed]



| Compound | 1@100K | 1@293K |
|---|---|---|
| Empirical formula | C61H51BCoN4O2 | C61H51BCoN4O2 |
| Formula weight | 941.79 | 941.79 |
| T/K | 100.0(1) | 293(2) |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n |
| Unit cell dimensions | ||
| a/Å | 19.2108(2) | 19.1252(2) |
| b/Å | 11.24860(10) | 11.49370(10) |
| c/Å | 23.3407(2) | 23.4892(2) |
| α (°) | 90 | 90 |
| β (°) | 94.0510(10) | 93.9610(10) |
| γ (°) | 90 | 90 |
| V/Å3 | 5031.20(8) | 5151.05(8) |
| Z, Dc/g·cm−3 | 4, 1.243 | 4, 1.214 |
| Absorption coefficient (mm−1) | 3.042 | 2.971 |
| F(000) | 1972 | 1972 |
| Reflections collected/unique (Rint) | 32,069/9403 (0.0259) | 9354/7614 (0.0248) |
| Data/restraints/parameters | 9403/0/626 | 9354/0/626 |
| Goodness-of-fit on F2 | 1.045 | 1.048 |
| Final R indices [I > 2σ(I)] | R1 = 0.0359, wR2 = 0.0932 | R1 = 0.0608, wR2 = 0.1851 |
| R indices (all data) | R1 = 0.0401, wR2 = 0.0956 | R1 = 0.0718, wR2 = 0.1959 |
| CCDC | 2299689 | 2306545 |
| Co1-N1 | 2.119(1) |
| Co1-N2 | 2.161(1) |
| Co1-N3 | 2.137(1) |
| Co1-N4 | 2.126(1) |
| Co1-O1 | 2.134(1) |
| Co1-O2 | 2.193(1) |
| N1-Co1-N2 | 78.88(5) |
| N3-Co1-N4 | 79.60(5) |
| O1-Co1-O2 | 61.12(4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halaš, P.; Nemec, I.; Herchel, R. Honey-like Odor Meets Single-Ion Magnet: Synthesis, Crystal Structure, and Magnetism of Cobalt(II) Complex with Aromatic Trans-Cinnamic Acid. Magnetochemistry 2023, 9, 229. https://doi.org/10.3390/magnetochemistry9110229
Halaš P, Nemec I, Herchel R. Honey-like Odor Meets Single-Ion Magnet: Synthesis, Crystal Structure, and Magnetism of Cobalt(II) Complex with Aromatic Trans-Cinnamic Acid. Magnetochemistry. 2023; 9(11):229. https://doi.org/10.3390/magnetochemistry9110229
Chicago/Turabian StyleHalaš, Petr, Ivan Nemec, and Radovan Herchel. 2023. "Honey-like Odor Meets Single-Ion Magnet: Synthesis, Crystal Structure, and Magnetism of Cobalt(II) Complex with Aromatic Trans-Cinnamic Acid" Magnetochemistry 9, no. 11: 229. https://doi.org/10.3390/magnetochemistry9110229
APA StyleHalaš, P., Nemec, I., & Herchel, R. (2023). Honey-like Odor Meets Single-Ion Magnet: Synthesis, Crystal Structure, and Magnetism of Cobalt(II) Complex with Aromatic Trans-Cinnamic Acid. Magnetochemistry, 9(11), 229. https://doi.org/10.3390/magnetochemistry9110229






