Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
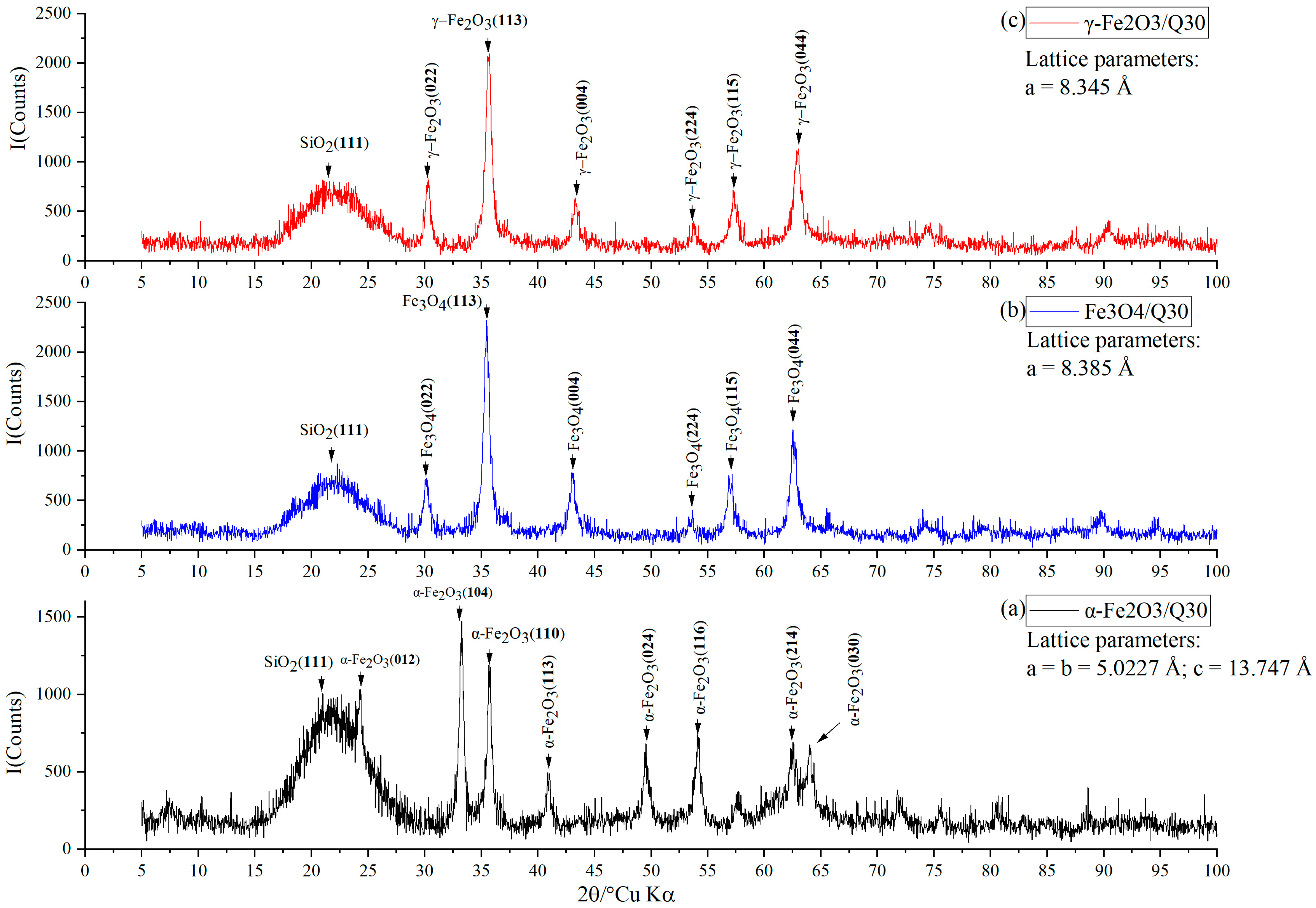
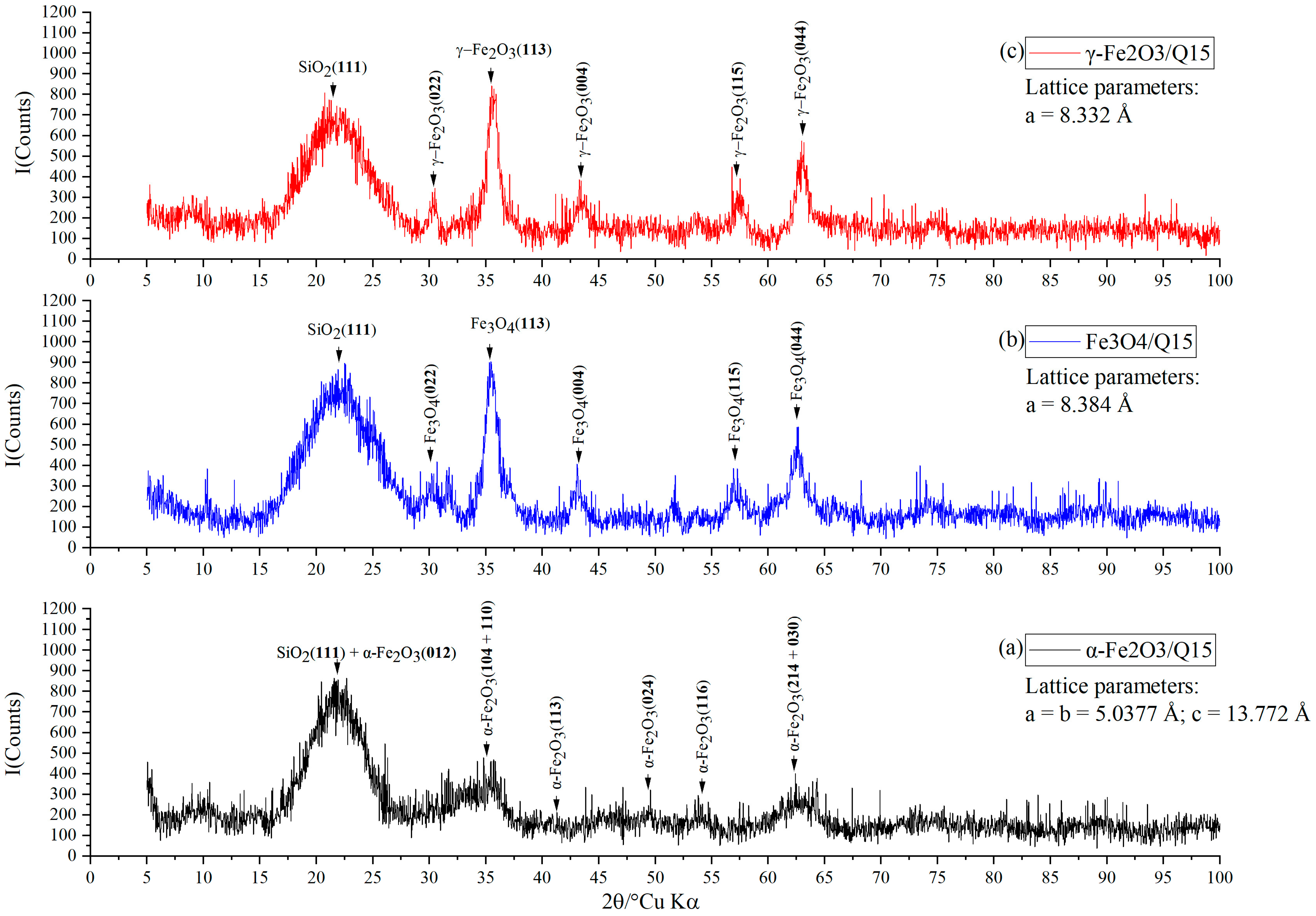
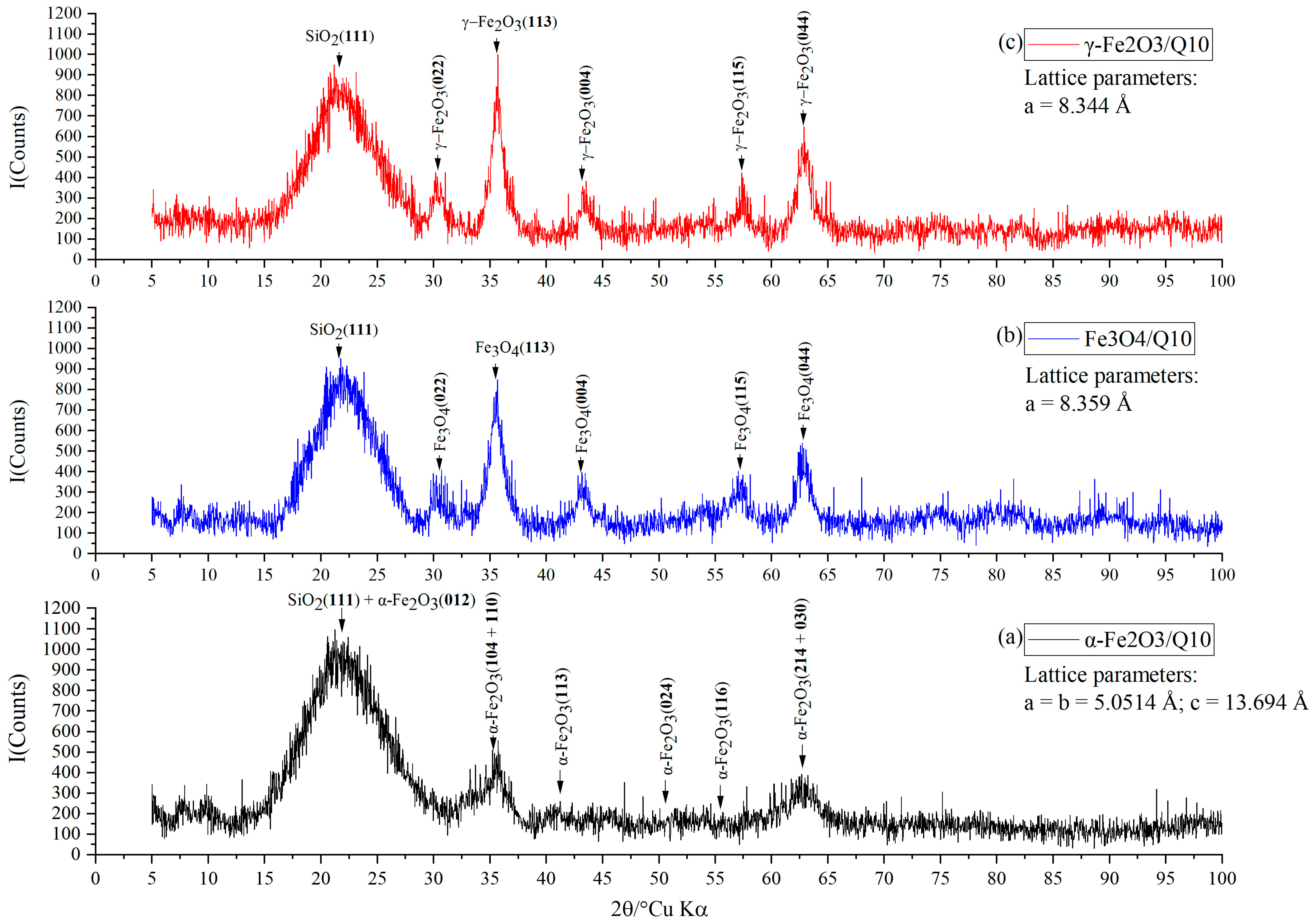
2.2. X-ray Diffraction Study
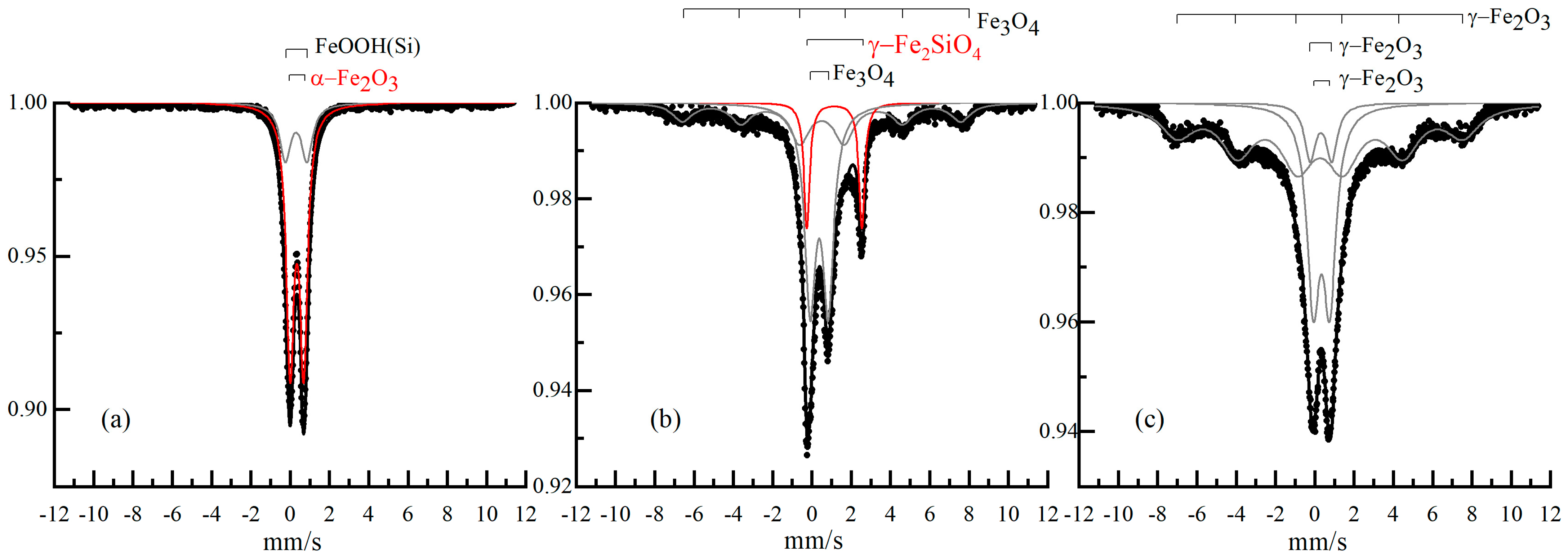
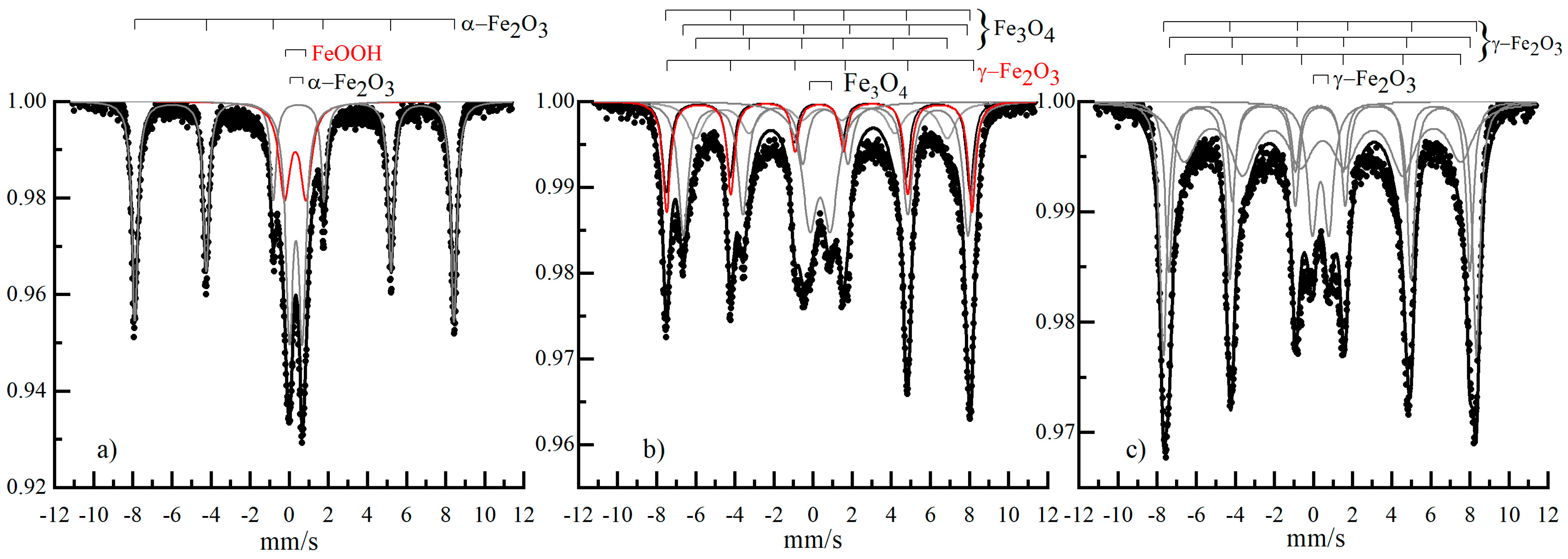
2.3. Mossbauer Spectroscopy
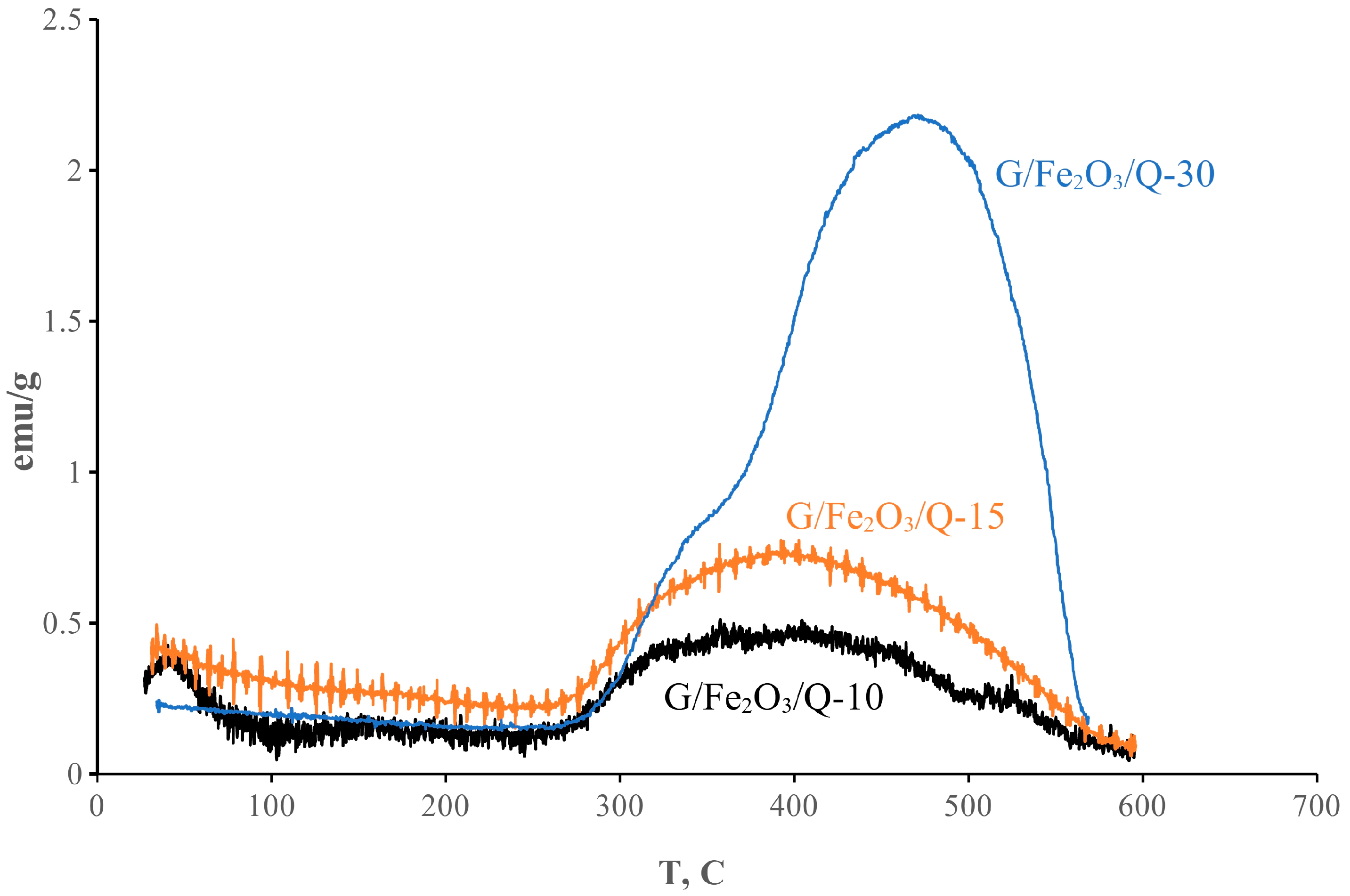
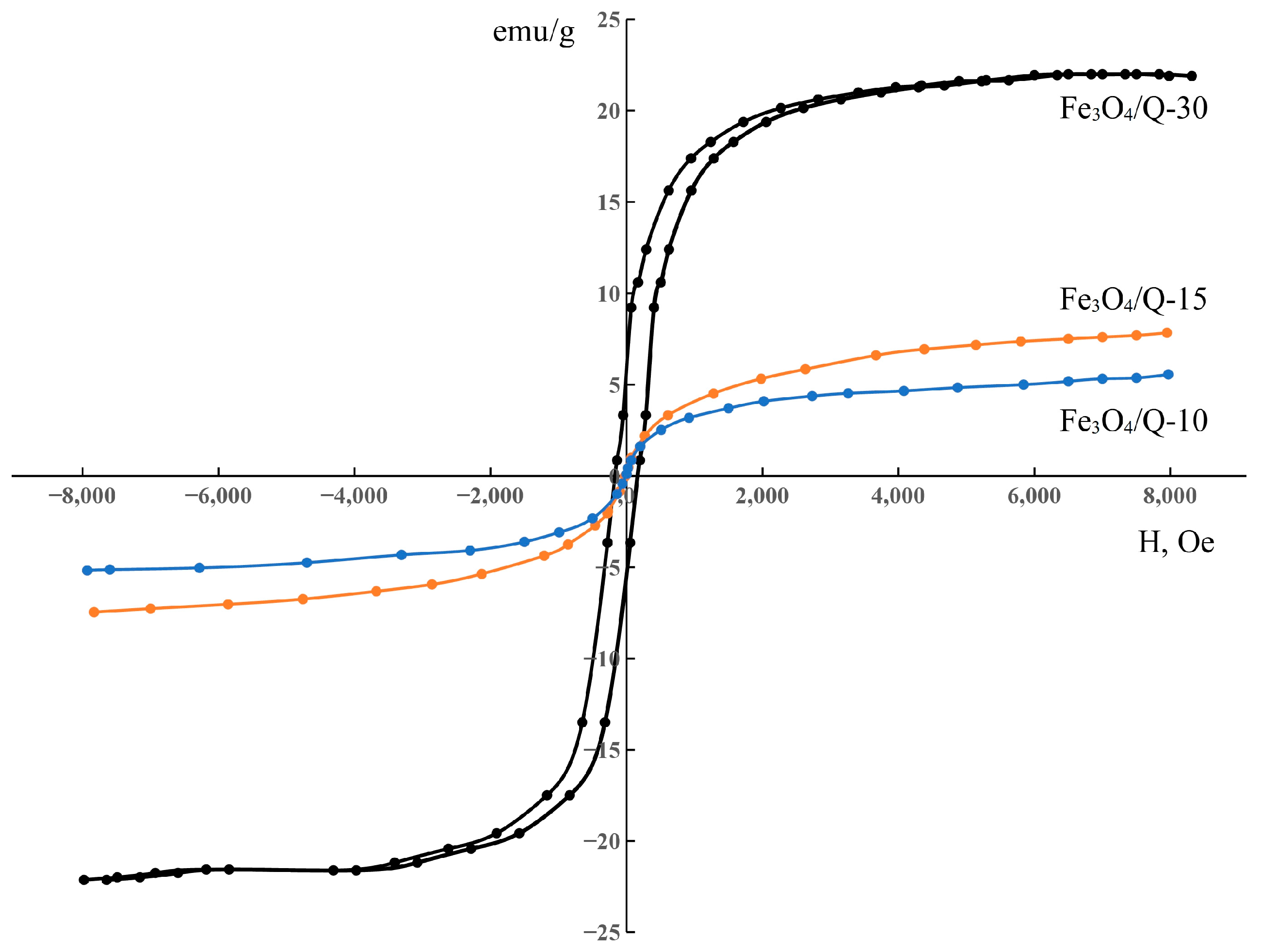
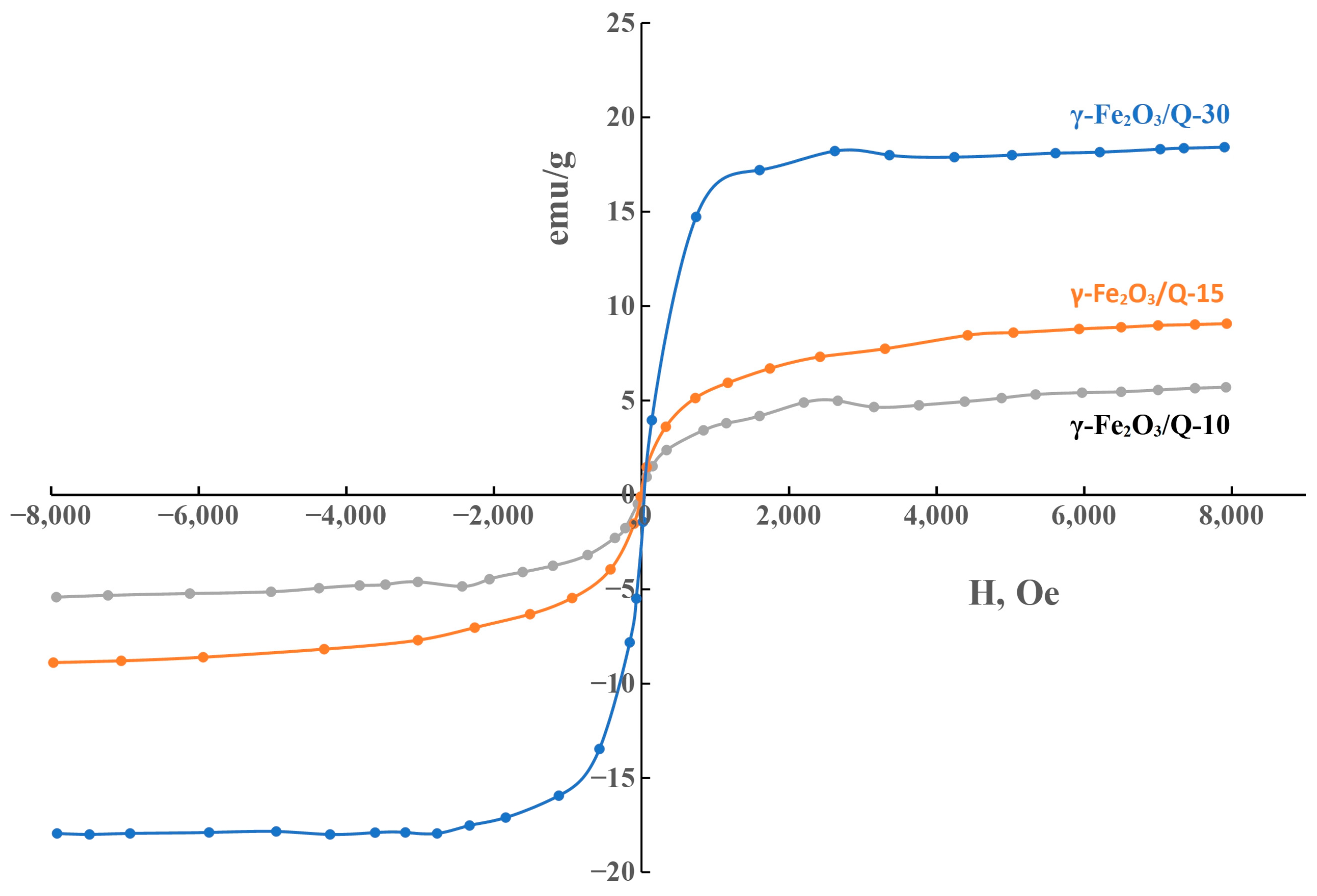
2.4. Magnetic Studies
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Souce, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol. Pharm. 2005, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064. [Google Scholar] [CrossRef]
- Batlle, X.; Perez, N.; Guardia, P.; Iglesias, O.; Labarta, A.; Bartolome, F.; Garcia, L.M.; Bartolome, J.; Roca, A.G.; Morales, M.P.; et al. Magnetic nanoparticles with bulklike properties (invited). J. Appl. Phys. 2011, 109, 07B524. [Google Scholar] [CrossRef]
- Yuen, S.; Chen, Y.; Kubsh, J.E.; Dumeslc, J.A. Metal Oxide-Support Interactions in Silica-Supported Iron Oxide Catalysts Probed by Nitric Oxide Adsorption. J. Phys. Chem. 1982, 86, 3022. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Heathcock, A.M.; Rebodos, R.L.; Makus, K.E. Particle Size and Aggregation Effects on Magnetite Reactivity toward Carbon Tetrachloride. Environ. Sci. Technol. 2007, 41, 5277. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Furukawa, K.; Takasuga, M.; Watanabe, K. A Magnetic Carbon Sorbent for Radioactive Material from the Fukushima Nuclear Accident. Sci. Rep. 2014, 4, 6053. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Materialstoday. 2019, 31, 86. [Google Scholar] [CrossRef]
- Ang, B.C.; Yaacob, I.I. Preparation of Maghemite-Silica Nanocomposites Using Sol-Gel Technique. Adv. Mater. Res. 2010, 97–101, 2140. [Google Scholar] [CrossRef]
- Heng, Z.; Schenk, J.; Spreitzer, D.; Wolfinger, T.; Daghagheleh, O. Review on the Oxidation Behaviors and Kinetics of Magnetite in Particle Scale. Steel Res. Int. 2021, 92, 2000687. [Google Scholar] [CrossRef]
- Dragomir, B.B.; Lang, X.; Mukesh, D.; Zimmerman, W.H.; Rosynek, M.P.; Li, C. Binder/support effects on the activity and selectivity of iron catalysts in the Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 1990, 29, 1588. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Shuvalova, E.V.; Redina, E.A.; Kirichenko, O.A.; Tkachenko, O.P.; Mishin, I.V.; Kustov, L.M. Silica-supported iron oxide nanoparticles: Unexpected catalytic activity in hydrogenation of phenylacetylene. Mendeleev Commun. 2017, 27, 512. [Google Scholar] [CrossRef]
- Martínez, F.; Calleja, G.; Melero, J.A.; Molina, R. Iron species incorporated over different silica supports for the heterogeneous photo-Fenton oxidation of phenol. Appl. Catal. B Environ. 2007, 70, 452. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Kazak, O.; Pankina, G.V.; Ordomsky, V.V.; Khodakov, A.Y. Mechanistic Aspects of the Activation of Silica-Supported Iron Catalysts for Fischer–Tropsch Synthesis in Carbon Monoxide and Syngas. ChemCatChem 2016, 8, 390. [Google Scholar] [CrossRef]
- de Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behavior. Chem. Soc. Rev. 2008, 37, 2758. [Google Scholar] [CrossRef]
- Chen, L.; Costa, E.; Kileti, P.; Tannenbaum, R.; Lindberg, J.; Devinder, M. Sonochemical Synthesis of Silica-Supported Iron Oxide Nanostructures and Their Application as Catalysts in Fischer–Tropsch. Synthesis. Micro 2022, 2, 632. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Dudchenko, N.O.; Brik, A.B. Reduction roasting of hematite to magnetite using carbohydrates. Int. J. Miner. Process. 2017, 164, 21. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, G.; Zeng, X.; Zhao, J.; Yao, G. Potential application of carbohydrate biomass in hydrometallurgy: One-pot reduction of metal oxides/salts under mild hydrothermal conditions. RSC Adv. 2022, 12, 20747. [Google Scholar] [CrossRef]
- Ikoma, S.; Ohki, K.; Yokoi, H. Preparation and Characterization of Silica-Supported Maghemite. J. Ceram. Soc. Jpn. 1992, 100, 864. [Google Scholar] [CrossRef][Green Version]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of Ultrasmall Superparamagnetic Iron Oxides Using Reduced Polysaccharides. Bioconjugate Chem. 2004, 15, 394. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Lunin, B.S.; Zakharyan, R.A.; Pankina, G.V.; Perov, N.S. Experimental setup for investigating topochemical transformations of ferromagnetic nanoparticles. Instr. Exp. Tech. 2014, 57, 78. [Google Scholar] [CrossRef]
- Barbier, A.; Hanif, A.; Dalmon, J.-A.; Martin, G.A. Preparation and characterization of well-dispersed and stable Co/SiO2 catalysts using the ammonia method. Appl. Catal. A Gen. 1998, 168, 333. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452. [Google Scholar] [CrossRef]
- Reichel, V.; Kovács, A.; Kumari, M.; Bereczk-Tompa, É.; Schneck, E.; Diehle, P.; Pósfai, M.; Hirt, A.M.; Duchamp, M.; Dunin-Borkowski, R.E.; et al. Single crystalline superstructured stable single domain magnetite nanoparticles. Sci. Rep. 2017, 7, 45484. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios between the Chemicals. ACS Omega 2022, 7, 15996. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 32360. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, S.; Pollard, R.J.; Cashion, J.D. Dynamic magnetic phenomena in fine-particle goethite. Phys. Rev. 1992, B46, 11657. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; da Costa, G.M.; Van San, E.; De Grave, E. Mössbauer characterization of iron oxides and (oxy)hydroxides: The present state of the art. Hyperfine Interact. 2000, 126, 247. [Google Scholar] [CrossRef]
- Hanesch, M.; Stanjek, H.; Petersen, N. Thermomagnetic measurements of soil iron minerals: The role of organic carbon. Geophys. J. Int. 2006, 165, 53. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; McCammon, C.A.; Canil, D.; Rubie, D.C.; Ross, C.R., II; Seifert, F. Mössbauer spectroscopy of mantle transition zone phases and determination of minimum Fe3+ content. Am. Miner. 1993, 78, 456. [Google Scholar]


| Medium, nm | d (max) | d (min) | Catalysts |
|---|---|---|---|
| 5.7 | 7 nm | 4.5 nm | Fe3O4/Q-15 |
| 5.4 | 7 nm | 3.8 nm | Fe3O4/Q-10 |
| Particle Size, nm | Catalysts | ||
|---|---|---|---|
| γ-Fe2O3 | Fe3O4 | α-Fe2O3 | |
| 22 | α-Fe2O3/Q-30 | ||
| 13 | α-Fe2O3/Q-15 | ||
| 5 | α-Fe2O3/Q-10 | ||
| 24 | Fe3O4/Q-30 | ||
| 14 | Fe3O4/Q-15 | ||
| 7 | Fe3O4/Q-10 | ||
| 25 | γ-Fe2O3/Q-30 | ||
| 14 | γ-Fe2O3/Q-15 | ||
| 8 | γ-Fe2O3/Q-10 | ||
| S, % | Heff, kOe | Γexp, mm/s | Δ, mm/s | δ, mm/s | Phase | № | Sample |
|---|---|---|---|---|---|---|---|
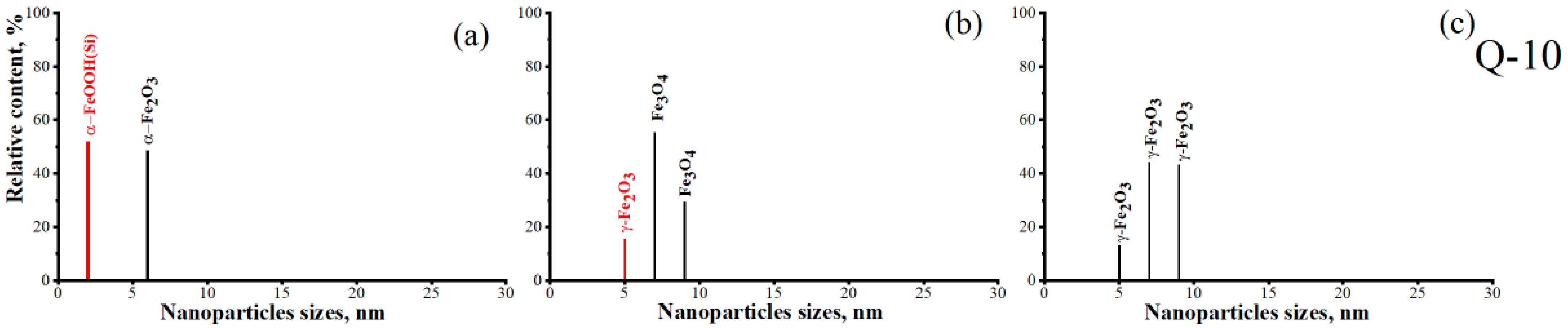
| 48 | 0.45 | 0.65 | 0.35 | α-Fe2O3 d~6 nm | 1 | α-Fe2O3/Q-10 | |
| 52 | 0.65 | 0.94 | 0.33 | α-FeOOH(Si) d~2 nm | 2 | ||
| 29 | 400 | 2 | 0 | 0.27 | Fe3O4 d~9 nm | 1 | Fe3O4/Q-10 |
| 55 | 0.62 | 0.76 | 0.35 | Fe3O4 d~7 nm | 2 | ||
| 15 | 0.68 | 1.14 | 0.31 | γ-Fe2O3 d~5 nm | 3 | ||
| 43 | 452 | 2 | 0 | 0.27 | γ-Fe2O3 d~9 nm | 1 | γ-Fe2O3/Q-10 |
| 44 | 0.65 | 0.76 | 0.34 | γ-Fe2O3 d~7 nm | 2 | ||
| 13 | 0.68 | 1.1 | 0.31 | γ-Fe2O3 d~5 nm | 3 | ||
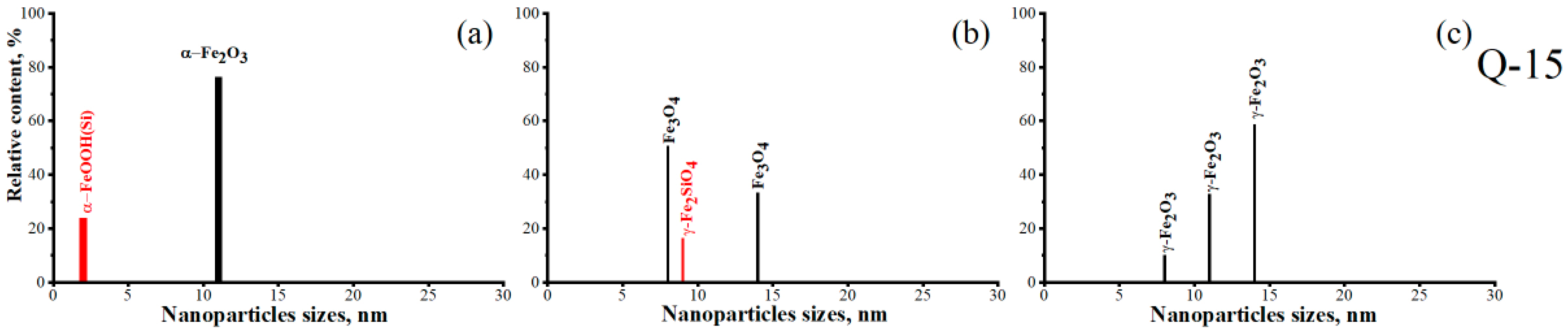
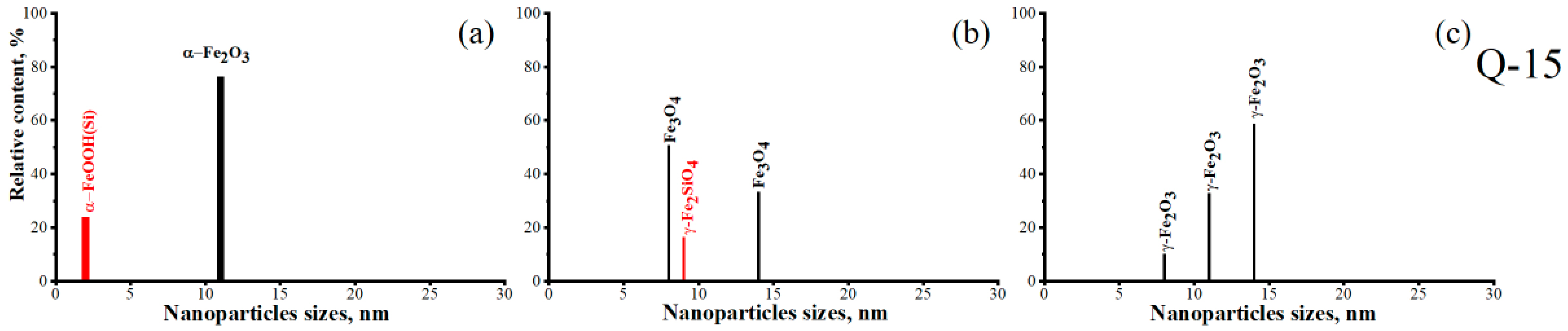
| 76 | 0.47 | 0.69 | 0.34 | α-Fe2O3 d~11 nm | 1 | α-Fe2O3/Q-15 | |
| 24 | 0.67 | 1.1 | 0.3 | α-FeOOH(Si) d~2 nm | 2 | ||
| 33 | 440 | 1.2 | 0.5 | 0.35 | Fe3O4 d~14 nm | 1 | Fe3O4/Q-15 |
| 51 | 0.76 | 0.92 | 0.37 | Fe3O4 d~8 nm | 2 | ||
| 16 | 0.6 | 2.8 | 1.14 | γ-Fe2SiO4 d~9 nm | 3 | ||
| 59 | 451 | 2 | 0 | 0.27 | γ-Fe2O3 d~14 nm | 1 | γ-Fe2O3/Q-15 |
| 33 | 0.78 | 0.84 | 0.34 | γ-Fe2O3 d~11 nm | 2 | ||
| 8 | 0.68 | 1.1 | 0.31 | γ-Fe2O3 d~8 nm | 3 | ||
| 53 | 507 | 0.36 | −0.22 | 0.37 | α-Fe2O3 d~17 nm | 1 | α-Fe2O3/Q-30 |
| 29 | 0.45 | 0.66 | 0.34 | α-Fe2O3 d~12 nm | 2 | ||
| 18 | 0.68 | 1.1 | 0.31 | α-FeOOH(Si) d~2 nm | 3 | ||
| 49 | 452 | 0.6 | 0.02 | 0.64 | Fe3O4 d~29 nm | 1 | Fe3O4/Q-30 |
| 483 | 0.46 | 0 | 0.26 | Fe3O4 d~29 nm | |||
| 13 | 420 | 1 | −0.03 | 0.45 | Fe3O4 d~15 nm | 2 | |
| 17 | 0.91 | 1.06 | 0.36 | Fe3O4 d~8 nm | 3 | ||
| 21 | 485 | 0.36 | 0 | 0.32 | γ-Fe2O3 d~27 nm | 4 | |
| 33 | 497 | 0.45 | −0.02 | 0.34 | γ-Fe2O3 d~29 nm | 1 | γ-Fe2O3/Q-30 |
| 21 | 478 | 0.45 | 0 | 0.3 | γ-Fe2O3 d~20 nm | 2 | |
| 36 | 439 | 1.5 | 0 | 0.46 | γ-Fe2O3 d~15 nm | 3 | |
| 10 | 0.6 | 0.85 | 0.36 | γ-Fe2O3 d~9 nm | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernavskiy, P.A.; Novakova, A.A.; Pankina, G.V.; Pankratov, D.A.; Panfilov, S.I.; Petrovskaya, G.A. Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel. Magnetochemistry 2023, 9, 228. https://doi.org/10.3390/magnetochemistry9110228
Chernavskiy PA, Novakova AA, Pankina GV, Pankratov DA, Panfilov SI, Petrovskaya GA. Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel. Magnetochemistry. 2023; 9(11):228. https://doi.org/10.3390/magnetochemistry9110228
Chicago/Turabian StyleChernavskiy, P. A., A. A. Novakova, G. V. Pankina, D. A. Pankratov, S. I. Panfilov, and G. A. Petrovskaya. 2023. "Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel" Magnetochemistry 9, no. 11: 228. https://doi.org/10.3390/magnetochemistry9110228
APA StyleChernavskiy, P. A., Novakova, A. A., Pankina, G. V., Pankratov, D. A., Panfilov, S. I., & Petrovskaya, G. A. (2023). Synthesis and Characterization of Hematite, Magnetite and Maghemite Supported on Silica Gel. Magnetochemistry, 9(11), 228. https://doi.org/10.3390/magnetochemistry9110228










