Investigation of a Tetrathiafulvalene-Based Fe2+ Thermal Spin Crossover Assembled on Gold Surface
Abstract
:1. Introduction
2. Results and Discussion
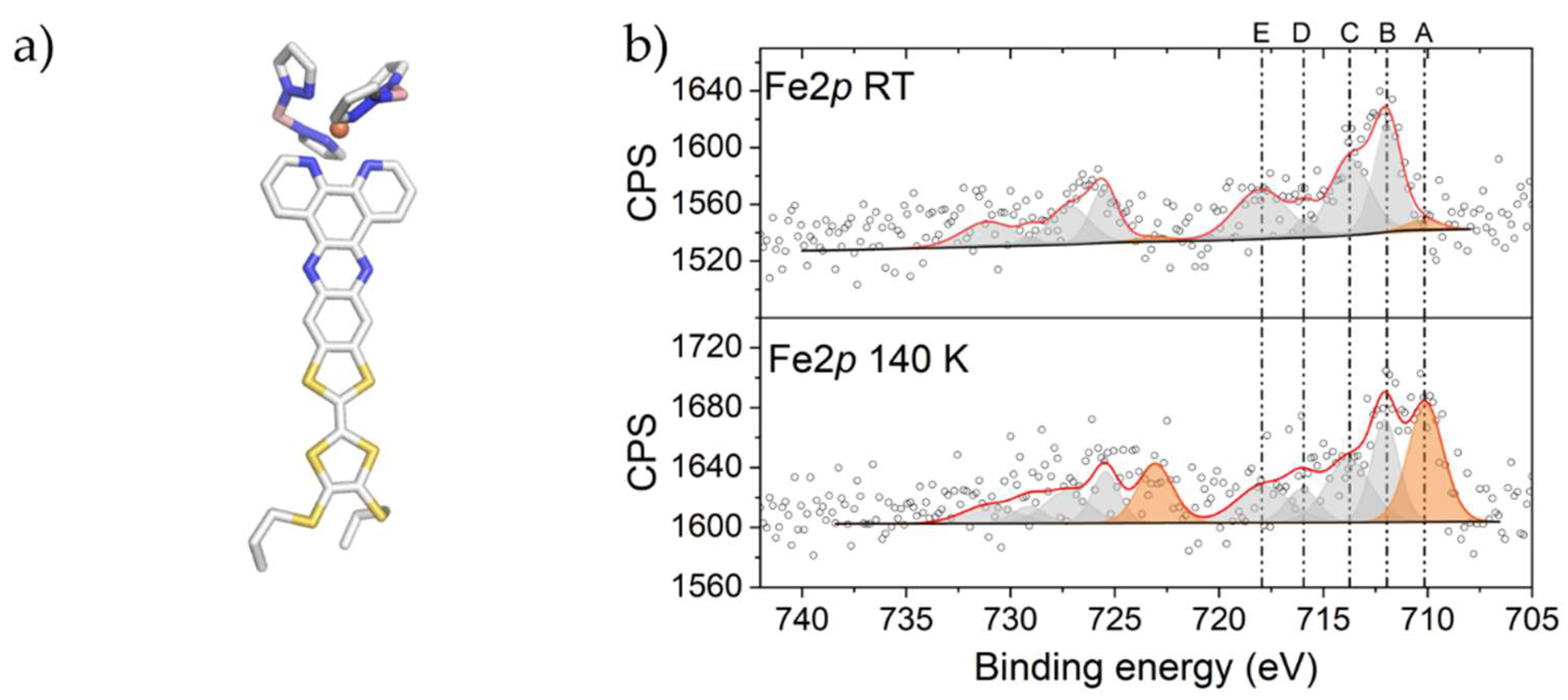
2.1. X-ray Photoelectron Spectroscopy (XPS) Characterization
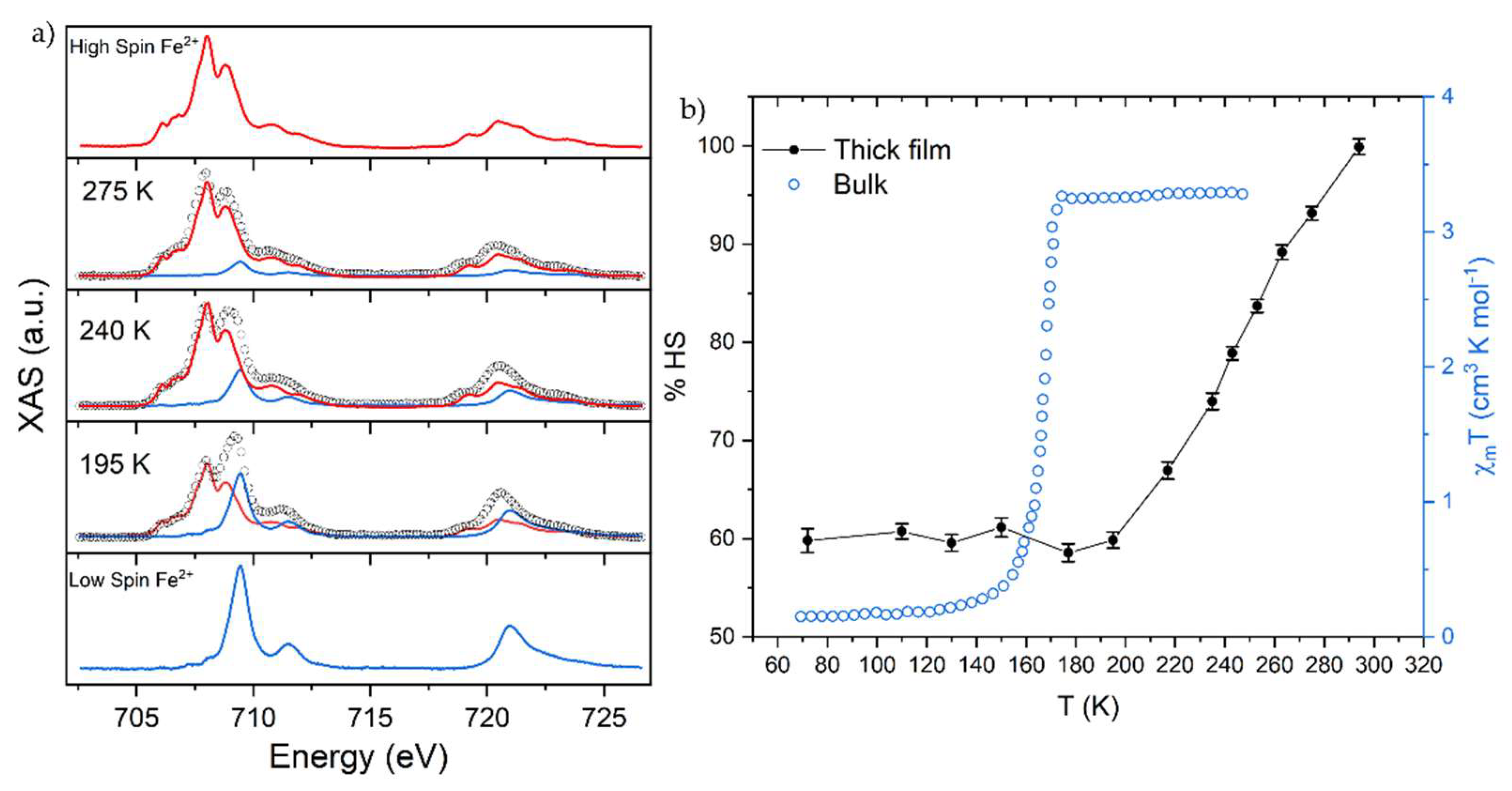
2.2. X-ray Absorption Spectroscopy (XAS) Characterization
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–47. [Google Scholar] [CrossRef]
- Poggini, L.; Gonidec, M.; Canjeevaram Balasubramanyam, R.K.; Squillantini, L.; Pecastaings, G.; Caneschi, A.; Rosa, P. Temperature-induced transport changes in molecular junctions based on a spin crossover complex. J. Mater. Chem. C 2019, 7, 5343–5347. [Google Scholar] [CrossRef] [Green Version]
- Poggini, L.; Londi, G.; Milek, M.; Naim, A.; Lanzilotto, V.; Cortigiani, B.; Bondino, F.; Magnano, E.; Otero, E.; Sainctavit, P.; et al. Surface effects on a photochromic spin-crossover iron(II) molecular switch adsorbed on highly oriented pyrolytic graphite. Nanoscale 2019, 11, 20006–20014. [Google Scholar] [CrossRef]
- Poggini, L.; Milek, M.; Londi, G.; Naim, A.; Poneti, G.; Squillantini, L.; Magnani, A.; Totti, F.; Rosa, P.; Khusniyarov, M.M.; et al. Room temperature control of spin states in a thin film of a photochromic iron(II) complex. Mater. Horizons 2018, 5, 506–513. [Google Scholar] [CrossRef]
- Gutlich, P.; Knenofontov, V.; Gaspar, A. Pressure effect studies on spin crossover systems. Coord. Chem. Rev. 2005, 249, 1811–1829. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalt. Trans. 2005, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Molnár, G.; Rat, S.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin Crossover Nanomaterials: From Fundamental Concepts to Devices. Adv. Mater. 2018, 30, 1703862. [Google Scholar] [CrossRef]
- Gütlich, P. Spin crossover in iron(II)-complexes. In Metal Complexes; Springer: Berlin/Heidelberg, Germany, 1981; pp. 83–195. [Google Scholar] [CrossRef]
- Cucinotta, G.; Poggini, L.; Giaconi, N.; Cini, A.; Gonidec, M.; Atzori, M.; Berretti, E.; Lavacchi, A.; Fittipaldi, M.; Chumakov, A.I.; et al. Space Charge-Limited Current Transport Mechanism in Crossbar Junction Embedding Molecular Spin Crossovers. ACS Appl. Mater. Interfaces 2020, 12, 31696–31705. [Google Scholar] [CrossRef]
- Poggini, L.; Gonidec, M.; González-Estefan, J.H.; Pecastaings, G.; Gobaut, B.; Rosa, P. Vertical Tunnel Junction Embedding a Spin Crossover Molecular Film. Adv. Electron. Mater. 2018, 4, 1800204. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Sublimable Spin-Crossover Complexes: From Spin-State Switching to Molecular Devices. Angew. Chemie Int. Ed. 2021, 60, 7502–7521. [Google Scholar] [CrossRef] [Green Version]
- Kelai, M.; Repain, V.; Tauzin, A.; Li, W.; Girard, Y.; Lagoute, J.; Rousset, S.; Otero, E.; Sainctavit, P.; Arrio, M.-A.; et al. Thermal Bistability of an Ultrathin Film of Iron(II) Spin-Crossover Molecules Directly Adsorbed on a Metal Surface. J. Phys. Chem. Lett. 2021, 12, 6152–6158. [Google Scholar] [CrossRef] [PubMed]
- Ossinger, S.; Kipgen, L.; Naggert, H.; Bernien, M.; Britton, A.J.; Nickel, F.; Arruda, L.M.; Kumberg, I.; Engesser, T.A.; Golias, E.; et al. Effect of ligand methylation on the spin-switching properties of surface-supported spin-crossover molecules. J. Phys. Condens. Matter 2019, 32, 114003. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Poggini, L.; Chumakov, A.I.; Rüffer, R.; Spina, G.; Wattiaux, A.; Duttine, M.; Gonidec, M.; Fittipaldi, M.; Rosa, P.; et al. Synchrotron-based Mössbauer spectroscopy characterization of sublimated spin crossover molecules. Phys. Chem. Chem. Phys. 2020, 22, 6626–6637. [Google Scholar] [CrossRef] [PubMed]
- Konig, E.; Ritter, G.; Kulshreshtha, S.K. The nature of spin-state transitions in solid complexes of iron(II) and the interpretation of some associated phenomena. Chem. Rev. 1985, 85, 219–234. [Google Scholar] [CrossRef]
- Hauser, A.; Jeftić, J.; Romstedt, H.; Hinek, R.; Spiering, H. Cooperative phenomena and light-induced bistability in iron(II) spin-crossover compounds. Coord. Chem. Rev. 1999, 190–192, 471–491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tong, Y.; Kelai, M.; Bellec, A.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Boillot, M.; Rivière, E.; et al. Anomalous Light-Induced Spin-State Switching for Iron(II) Spin-Crossover Molecules in Direct Contact with Metal Surfaces. Angew. Chemie Int. Ed. 2020, 59, 13341–13346. [Google Scholar] [CrossRef]
- Rohlf, S.; Grunwald, J.; Jasper-Toennies, T.; Johannsen, S.; Diekmann, F.; Studniarek, M.; Berndt, R.; Tuczek, F.; Rossnagel, K.; Gruber, M. Influence of Substrate Electronic Properties on the Integrity and Functionality of an Adsorbed Fe(II) Spin-Crossover Compound. J. Phys. Chem. C 2019, 123, 17774–17780. [Google Scholar] [CrossRef]
- Zhang, X.; Costa, P.S.; Hooper, J.; Miller, D.P.; N’Diaye, A.T.; Beniwal, S.; Jiang, X.; Yin, Y.; Rosa, P.; Routaboul, L.; et al. Locking and Unlocking the Molecular Spin Crossover Transition. Adv. Mater. 2017, 29, 1702257. [Google Scholar] [CrossRef]
- Pointillart, F.; Liu, X.; Kepenekian, M.; Le Guennic, B.; Golhen, S.; Dorcet, V.; Roisnel, T.; Cador, O.; You, Z.; Hauser, J.; et al. Thermal and near-infrared light induced spin crossover in a mononuclear iron(II) complex with a tetrathiafulvalene-fused dipyridophenazine ligand. Dalt. Trans. 2016, 45, 11267–11271. [Google Scholar] [CrossRef] [Green Version]
- Totaro, P.; Poggini, L.; Favre, A.; Mannini, M.; Sainctavit, P.; Cornia, A.; Magnani, A.; Sessoli, R. Tetrairon(III) Single-Molecule Magnet Monolayers on Gold: Insights from ToF-SIMS and Isotopic Labeling. Langmuir 2014, 30, 8645–8649. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, S.; Chastanet, G.; Daro, N.; Palamarciuc, T.; Rosa, P.; Létard, J.-F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Complexities in the Molecular Spin Crossover Transition. J. Phys. Chem. C 2015, 119, 16293–16302. [Google Scholar] [CrossRef]
- Atzori, M.; Poggini, L.; Squillantini, L.; Cortigiani, B.; Gonidec, M.; Bencok, P.; Sessoli, R.; Mannini, M. Thermal and light-induced spin transition in a nanometric film of a new high-vacuum processable spin crossover complex. J. Mater. Chem. C 2018, 6, 8885–8889. [Google Scholar] [CrossRef]
- Chandesris, D.; Lecante, J.; Petroff, Y. Two-electron resonances in transition metals. Phys. Rev. B 1983, 27, 2630–2635. [Google Scholar] [CrossRef]
- Beniwal, S.; Zhang, X.; Mu, S.; Naim, A.; Rosa, P.; Chastanet, G.; Létard, J.-F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Surface-induced spin state locking of the [Fe(H2B(pz)2)2(bipy)] spin crossover complex. J. Phys. Condens. Matter 2016, 28, 206002. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of multiplet structure of core p-vacancy levels. II. Phys. Rev. B 1975, 12, 15. [Google Scholar] [CrossRef]
- Ellingsworth, E.C.; Turner, B.; Szulczewski, G. Thermal conversion of [Fe(phen)3](SCN)2 thin films into the spin crossover complex Fe(phen)2(NCS)2. RSC Adv. 2013, 3, 3745. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Rosa, P.; Létard, J.-F.O.L.; Doudin, B.; Zhang, Z.; Wang, J.; Dowben, P.A. Electronic Structure of a Spin Crossover Molecular Adsorbate. J. Phys. Chem. 2012, 116, 23291–23296. [Google Scholar] [CrossRef]
- Singhbabu, Y.N.; Kumari, P.; Parida, S.; Sahu, R.K. Conversion of pyrazoline to pyrazole in hydrazine treated N-substituted reduced graphene oxide films obtained by ion bombardment and their electrical properties. Carbon N. Y. 2014, 74, 32–43. [Google Scholar] [CrossRef]
- Deleuze, M.S. Valence one-electron and shake-up ionization bands of polycyclic aromatic hydrocarbons. II. Azulene, phenanthrene, pyrene, chrysene, triphenylene, and perylene. J. Chem. Phys. 2002, 116, 7012–7026. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for X-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 13204. [Google Scholar] [CrossRef]
- Gruber, M.; Berndt, R. Spin-Crossover Complexes in Direct Contact with Surfaces. Magnetochemistry 2020, 6, 35. [Google Scholar] [CrossRef]
- Kipgen, L.; Bernien, M.; Ossinger, S.; Nickel, F.; Britton, A.J.; Arruda, L.M.; Naggert, H.; Luo, C.; Lotze, C.; Ryll, H.; et al. Evolution of cooperativity in the spin transition of an iron(II) complex on a graphite surface. Nat. Commun. 2018, 9, 2984. [Google Scholar] [CrossRef]
- Poneti, G.; Poggini, L.; Mannini, M.; Cortigiani, B.; Sorace, L.; Otero, E.; Sainctavit, P.; Magnani, A.; Sessoli, R.; Dei, A. Thermal and optical control of electronic states in a single layer of switchable paramagnetic molecules. Chem. Sci. 2015, 6, 2268–2274. [Google Scholar] [CrossRef] [Green Version]
- Ossinger, S.; Näther, C.; Buchholz, A.; Schmidtmann, M.; Mangelsen, S.; Beckhaus, R.; Plass, W.; Tuczek, F. Spin Transition of an Iron(II) Organoborate Complex in Different Polymorphs and in Vacuum-Deposited Thin Films: Influence of Cooperativity. Inorg. Chem. 2020, 59, 7966–7979. [Google Scholar] [CrossRef]
- Warner, B.; Oberg, J.C.; Gill, T.G.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Arrio, M.-A.; Sainctavit, P.; Mannini, M.; et al. Temperature- and Light-Induced Spin Crossover Observed by X-ray Spectroscopy on Isolated Fe(II) Complexes on Gold. J. Phys. Chem. Lett. 2013, 4, 1546–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Joly, L.; Scheurer, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust spin crossover and memristance across a single molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Poggini, L.; Briganti, M.; Sorrentino, A.L.; Cucinotta, G.; Malavolti, L.; Cortigiani, B.; Otero, E.; Sainctavit, P.; Loth, S.; et al. Quantum dynamics of a single molecule magnet on superconducting Pb(111). Nat. Mater. 2020, 19, 546–551. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Bernien, M.; Naggert, H.; Matino, F.; Hermanns, C.F.; Bannwarth, A.; Mühlenberend, S.; Krüger, A.; Krüger, D.; Nickel, F.; et al. Spin-Crossover Complex on Au(111): Structural and Electronic Differences between Mono- and Multilayers. Chem.—Eur. J. 2013, 19, 15702–15709. [Google Scholar] [CrossRef] [PubMed]
- Dishner, M.H.; Ivey, M.M.; Gorer, S.; Hemminger, J.C.; Feher, F.J. Preparation of gold thin films by epitaxial growth on mica and the effect of flame annealing. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 16, 3295–3300. [Google Scholar] [CrossRef]
- Ohresser, P.; Otero, E.; Choueikani, F.; Chen, K.; Stanescu, S.; Deschamps, F.; Moreno, T.; Polack, F.; Lagarde, B.; Daguerre, J.-P.; et al. DEIMOS: A beamline dedicated to dichroism measurements in the 350–2500 eV energy range. Rev. Sci. Instrum. 2014, 85, 013106. [Google Scholar] [CrossRef] [Green Version]
- Henke, B.L.; Liesegang, J.; Smith, S.D. Soft-x-ray-induced secondary-electron emission from semiconductors and insulators: Models and measurements. Phys. Rev. B 1979, 19, 3004–3021. [Google Scholar] [CrossRef]
- Heinrich, A.J.; Oliver, W.D.; Vandersypen, L.M.K.; Ardavan, A.; Sessoli, R.; Loss, D.; Jayich, A.B.; Fernandez-Rossier, J.; Laucht, A.; Morello, A. Quantum-coherent nanoscience. Nat. Nanotechnol. 2021, 16, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaconi, N.; Sorrentino, A.L.; Poggini, L.; Serrano, G.; Cucinotta, G.; Otero, E.; Longo, D.; Douib, H.; Pointillart, F.; Caneschi, A.; et al. Investigation of a Tetrathiafulvalene-Based Fe2+ Thermal Spin Crossover Assembled on Gold Surface. Magnetochemistry 2022, 8, 14. https://doi.org/10.3390/magnetochemistry8020014
Giaconi N, Sorrentino AL, Poggini L, Serrano G, Cucinotta G, Otero E, Longo D, Douib H, Pointillart F, Caneschi A, et al. Investigation of a Tetrathiafulvalene-Based Fe2+ Thermal Spin Crossover Assembled on Gold Surface. Magnetochemistry. 2022; 8(2):14. https://doi.org/10.3390/magnetochemistry8020014
Chicago/Turabian StyleGiaconi, Niccolò, Andrea Luigi Sorrentino, Lorenzo Poggini, Giulia Serrano, Giuseppe Cucinotta, Edwige Otero, Danilo Longo, Haiet Douib, Fabrice Pointillart, Andrea Caneschi, and et al. 2022. "Investigation of a Tetrathiafulvalene-Based Fe2+ Thermal Spin Crossover Assembled on Gold Surface" Magnetochemistry 8, no. 2: 14. https://doi.org/10.3390/magnetochemistry8020014
APA StyleGiaconi, N., Sorrentino, A. L., Poggini, L., Serrano, G., Cucinotta, G., Otero, E., Longo, D., Douib, H., Pointillart, F., Caneschi, A., Sessoli, R., & Mannini, M. (2022). Investigation of a Tetrathiafulvalene-Based Fe2+ Thermal Spin Crossover Assembled on Gold Surface. Magnetochemistry, 8(2), 14. https://doi.org/10.3390/magnetochemistry8020014









