Structural Parameters of the Interaction between Ciprofloxacin and Human Topoisomerase-II β Enzyme: Toward New 19F NMR Chemical Shift Probes
Abstract
1. Introduction
2. Methodology
2.1. Molecular Dynamics (MD) Simulations
2.2. 19F NMR Chemical Shift (δ) Calculations
3. Results and Discussion
3.1. MD Simulations
3.2. Spectroscopic Parameters: 19F- Chemical Shifts (δ)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pitman, S.K.; Hoang, U.T.P.; Wi, C.H.; Alsheikh, M.; Hiner, D.A.; Percival, K.M. Revisiting oral fluoroquinolone and multivalent cation drug-drug interactions: Are they still relevant? Antibiotics 2019, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Su, B.; Liu, R. Probing the binding of two fluoroquinolones to lysozyme: A combined spectroscopic and docking study. Mol. Biosyst. 2012, 8, 1222. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones structural and medicinal developments (2013–2018): Where are we now? Bioorganic Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Alminderej, F.M.; Messaoudi, S.; Saleh, S.M. Ratiometric ultrasensitive optical chemisensor film based antibiotic drug for Al(III) and Cu(II) detection. Talanta 2021, 221, 121412. [Google Scholar] [PubMed]
- Abdel-Aziz, M.; Park, S.E.; Abuo-Rahma, G.E.D.A.A.; Sayed, M.A.; Kwon, Y. Novel N-4-piperazinyl-ciprofloxacin-chalcone hybrids: Synthesis, physicochemical properties, anticancer and topoisomerase I and II inhibitory activity. Eur. J. Med. Chem. 2013, 69, 427–438. [Google Scholar] [CrossRef]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings or Curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- Jacob, D.A.; Mercer, S.L.; Osheroff, N.; Deweese, J.E. Etoposide Quinone Is a Redox-Dependent Topoisomerase II Poison. Biochemistry 2011, 50, 5660–5667. [Google Scholar] [CrossRef]
- Idowu, T.; Schweizer, F. Ubiquitous nature of fluoroquinolones: The oscillation between antibacterial and anticancer activities. Antibiotics 2017, 6, 26. [Google Scholar] [CrossRef]
- Bisacchi, G.; Hale, M. A “Double-Edged” Scaffold: Antitumor Power within the Antibacterial Quinolone. Curr. Med. Chem. 2016, 23, 520–577. [Google Scholar] [CrossRef]
- Heestand, G.M.; Schwaederle, M.; Gatalica, Z.; Arguello, D.; Kurzrock, R. Topoisomerase expression and amplification in solid tumours: Analysis of 24,262 patients. Eur. J. Cancer 2017, 83, 80–87. [Google Scholar] [CrossRef]
- Suresh, N.; Suresh, A.; Yerramsetty, S.; Bhadra, M.P.; Alvala, M.; Sekhar, K.V.G.C. Anti-proliferative activity, molecular modeling studies and interaction with calf thymus DNA of novel ciprofloxacin analogues. J. Chem. Sci. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Beberok, A.; Wrześniok, D.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDA-MB-231 cells via the p53/Bax/Bcl-2 signaling pathway. Int. J. Oncol. 2018, 52, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Chekerov, R.; Klaman, I.; Zafrakas, M.; Könsgen, D.; Mustea, A.; Petschke, B.; Lichtenegger, W.; Sehouli, J.; Dahl, E. Altered Expression Pattern of Topoisomerase IIα in Ovarian Tumor Epithelial and Stromal Cells after Platinum-Based Chemotherapy. Neoplasia 2006, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Faggad, A.; Darb-Esfahani, S.; Wirtz, R.; Sinn, B.; Sehouli, J.; Könsgen, D.; Lage, H.; Weichert, W.; Noske, A.; Budczies, J.; et al. Topoisomerase IIα mRNA and protein expression in ovarian carcinoma: Correlation with clinicopathological factors and prognosis. Mod. Pathol. 2009, 22, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, L.D.; Li, J.; Lu, X. Targeting of topoisomerases for prognosis and drug resistance in ovarian cancer. J. Ovarian Res. 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, J.; Zhang, J.; Chen, B.; Kan, J.; Zhang, W.; Zhou, J.; Ma, H. A red lysosome-targeted fluorescent probe for carboxylesterase detection and bioimaging. J. Mater. Chem. B 2019, 7, 2989–2996. [Google Scholar] [CrossRef]
- Kirk, E.; Hevenern, T.A.; Verstak, K.E.L.; Daniel, L.; Riggsbee, J.W.M. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar]
- Cinelli, M.A. Topoisomerase 1B poisons: Over a half-century of drug leads, clinical candidates, and serendipitous discoveries. Med. Res. Rev. 2019, 39, 1294–1337. [Google Scholar] [CrossRef]
- Verma, A. Prions, prion-like prionoids, and neurodegenerative disorders. Ann. Indian Acad. Neurol. 2016, 19, 169–174. [Google Scholar] [CrossRef]
- Pilleron, S.; Soto-Perez-de-Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int. J. Cancer 2021, 148, 601–608. [Google Scholar] [CrossRef]
- Dan, N.; Setua, S.; Kashyap, V.; Khan, S.; Jaggi, M.; Yallapu, M.; Chauhan, S.; Dan, N.; Setua, S.; Kashyap, V.K.; et al. Antibody-Drug Conjugates for Cancer Therapy: Chemistry to Clinical Implications. Pharmaceuticals 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kassab, A.E.; Gedawy, E.M. Novel ciprofloxacin hybrids using biology oriented drug synthesis (BIODS) approach: Anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis, topoisomerase II inhibition, and antibacterial activity. Eur. J. Med. Chem. 2018, 150, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Pharoah, P.D.P. The challenge of early detection in cancer. Science 2020, 368, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Holtedahl, K. Challenges in early diagnosis of cancer: The fast track. Scand. J. Prim. Health Care 2020, 38, 251–252. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Module 3: Early Detection. Cancer Control: Knowledge into Action: WHO Guide for Effective Programmes 2007, 1–50. [Google Scholar] [PubMed]
- Unger-Saldaña, K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J. Clin. Oncol. 2014, 5, 465. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.; Kang, J.W.; Yu, C.-C.; Barman, I.; Dingari, N.C.; Feld, M.S.; Dasari, R.R.; Fitzmaurice, M. Portable Optical Fiber Probe-Based Spectroscopic Scanner for Rapid Cancer Diagnosis: A New Tool for Intraoperative Margin Assessment. PLoS ONE 2012, 7, e30887. [Google Scholar] [CrossRef]
- Pereira, B.T.L.L.; Gonçalves, M.A.; Mancini, D.T.; Kuca, K.; Ramalho, T.C. First attempts of the use of 195Pt NMR of phenylbenzothiazole complexes as spectroscopic technique for the cancer diagnosis. Molecules 2019, 24, 3970. [Google Scholar] [CrossRef]
- da Rocha, E.P.; Rodrigues, H.A.; da Cunha, E.F.F.; Ramalho, T.C. Probing kinetic and thermodynamic parameters as well as solvent and substituent effects on spectroscopic probes of 2-amino-1,4-naphthoquinone derivatives. Comput. Theor. Chem. 2016, 1096, 17–26. [Google Scholar] [CrossRef]
- Wan, X.; Wang, H.; Shi, B.; Guo, Y.; Liu, S.Y.; Wang, X. An enzyme activated fluorescent probe for LTA4H activity sensing and its application in cancer screening. Talanta 2023, 253, 123887. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, D.; Yu, H.; Fu, Y.; Song, L.; Wu, Y.; Chen, K.; Li, J.; Yang, Y.; Chen, H.; et al. Detection of rare CTCs by electrochemical biosensor built on quaternary PdPtCuRu nanospheres with mesoporous architectures. Talanta 2023, 253, 123955. [Google Scholar] [CrossRef] [PubMed]
- Gab, M.; Allah, A.; Sarhan, M.A.; Elshennawy, M.N. Edge U-Net: Brain tumor segmentation using MRI based on deep U-Net model with boundary information. Expert Syst. Appl. 2023, 213, 118833. [Google Scholar]
- Kim, J.H.; An, Y.J.; Kim, T.M.; Kim, J.E.; Park, S.; Choi, S.H. Ex vivo NMR metabolomics approach using cerebrospinal fluid for the diagnosis of primary CNS lymphoma: Correlation with MR imaging characteristics. Cancer Med. 2022, 00, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Maryam, A.; Bokhari, S.A.; Ashiq, A.; Rauf, S.A.; Khalid, R.R.; Qureshi, F.A.; Siddiqi, A.R. Design, synthesis, characterization and computational docking studies of novel sulfonamide derivatives. EXCLI J. 2018, 17, 169–180. [Google Scholar]
- Larkin, J.R.; Anthony, S.; Johanssen, V.A.; Yeo, T.; Sealey, M.; Yates, A.G.; Smith, C.F.; Claridge, T.D.W.; Nicholson, B.D.; Moreland, J.A.; et al. Metabolomic Biomarkers in Blood Samples Identify Cancers in a Mixed Population of Patients with Nonspecific Symptoms. Clin. Cancer Res. 2022, 28, 1651–1661. [Google Scholar] [CrossRef]
- Derveauex, E.; Thomeer, M.; Mesotten, L.; Reekmans, G.; Adriaensens, P. Detection of Lung Cancer via Blood Plasma and 1H-NMR Metabolomics: Validation by a Semi-Targeted and Quantitative Approach Using a Protein-Binding Competitor. Metabolites 2021, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.N.; Lee, H.; Park, J.W.; Kim, Y.H.; Park, S.; Kim, J.J. Screening for Early Gastric Cancer Using a Noninvasive Urine Metabolomics Approach. Cancers 2020, 12, 2904. [Google Scholar] [CrossRef]
- Michálková, L.; Horník, Š.; Sýkora, J.; Habartová, L.; Setnička, V. Diagnosis of pancreatic cancer via1H NMR metabolomics of human plasma. Analyst 2018, 143, 5974–5978. [Google Scholar] [CrossRef]
- Mikkonen, J.J.W.; Singh, S.P.; Akhi, R.; Salo, T.; Lappalainen, R.; González-Arriagada, W.A.; Lopes, M.A.; Kullaa, A.M.; Myllymaa, S. Potential role of nuclear magnetic resonance spectroscopy to identify salivary metabolite alterations in patients with head and neck cancer. Oncol. Lett. 2018, 16, 6795–6800. [Google Scholar] [CrossRef]
- Erben, V.; Bhardwaj, M.; Schrotz-King, P.; Brenner, H. Metabolomics Biomarkers for Detection of Colorectal Neoplasms: A Systematic Review. Cancers 2018, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liao, G.; Wen, X.; Chen, W.; Cheng, S.; Stolzenburg, J.U.; Ganzer, R.; Neuhaus, J. Nuclear magnetic resonance spectroscopy as a new approach for improvement of early diagnosis and risk stratification of prostate cancer. J. Zhejiang Univ. B 2017, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-Y.; Zhang, Y.; Chen, Y.-T.; Yu, Q.; An, L.-K. The first small fluorescent probe as Tyrosyl-DNA phosphodiesterase 1 (TDP1) substrate. Dye. Pigment. 2019, 169, 45–50. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, H. Design principles of spectroscopic probes for biological applications. Chem. Sci. 2016, 7, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.D. Principles and Topical Applications of 19F NMR Spectrometry. In Organofluorines. The Handbook of Environmental Chemistry; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 3N, pp. 1–61. [Google Scholar]
- .Marsh, E.N.G.; Suzuki, Y. Using 19F NMR to Probe Biological Interactions of Proteins and Peptides. ACS Chem. Biol. 2014, 9, 1242–1250. [Google Scholar] [CrossRef]
- Gimenez, D.; Phelan, A.; Murphy, C.D.; Cobb, S.L. 19F NMR as a tool in chemical biology. Beilstein J. Org. Chem. 2021, 17, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Jawaria, R.; Hussain, M.; Khalid, M.; Khan, M.U.; Tahir, M.N.; Naseer, M.M.; Braga, A.A.C.; Shafiq, Z. Synthesis, crystal structure analysis, spectral characterization and nonlinear optical exploration of potent thiosemicarbazones based compounds: A DFT refine experimental study. Inorg. Chim. Acta 2019, 486, 162–171. [Google Scholar] [CrossRef]
- Koch, A.; Stamboliyska, B.; Mikhova, B.; Breznica-Selmani, P.; Mladenovska, K.; Popovski, E. Calculations of 13C NMR chemical shifts and F–C coupling constants of ciprofloxacin. Magn. Reson. Chem. 2019, 57, S75–S84. [Google Scholar] [CrossRef]
- Ghosh, D.; Rhodes, S.; Winder, D.; Atkinson, A.; Gibson, J.; Ming, W.; Padgett, C.; Landge, S.; Aiken, K. Spectroscopic investigation of bis-appended 1,2,3-triazole probe for the detection of Cu(II) ion. J. Mol. Struct. 2017, 1134, 638–648. [Google Scholar] [CrossRef]
- Ramalho, T.C.; Taft, C.A. Thermal and solvent effects on the NMR and UV parameters of some bioreductive drugs. J. Chem. Phys. 2005, 123, 054319. [Google Scholar] [CrossRef]
- Pudipeddi, A.; Vasudevan, S.; Shanmugam, K.; Mohan, S.S.; Vairaprakash, P.; Neelakantan, P.; Balraj, A.S.; Solomon, A.P. Design, dynamic docking, synthesis, and in vitro validation of a novel DNA gyrase B inhibitor. J. Biomol. Struct. Dyn. 2022, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 2018, 103, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated force field Topology Builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Christen, M.; Hünenberger, P.H.; Bakowies, D.; Baron, R.; Bürgi, R.; Geerke, D.P.; Heinz, T.N.; Kastenholz, M.A.; Kräutler, V.; Oostenbrink, C.; et al. The GROMOS software for biomolecular simulation: GROMOS05. J. Comput. Chem. 2005, 26, 1719–1751. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Santos, L.S.; Prata, D.M.; Peixoto, F.C.; da Cunha, E.F.F.; Ramalho, T.C. Optimal wavelet signal compression as an efficient alternative to investigate molecular dynamics simulations: Application to thermal and solvent effects of MRI probes. Theor. Chem. Acc. 2017, 136, 15. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1998, 90, 1007. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1998, 96, 6796. [Google Scholar] [CrossRef]
- Woon, D.E., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1998, 98, 1358. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 2002, 112, 8251–8260. [Google Scholar] [CrossRef]
- Mejía-Urueta, R.; Mestre-Quintero, K.; Vivas-Reyes, R. DFT-GIAO Calculation of Properties of 19 F NMR and Stability Study of Environmentally Relevant Perfluoroalkylsulfonamides (PFASAmide). Artic. J. Braz. Chem. Soc. 2011, 22, 2268–2274. [Google Scholar]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. Theochem. 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Trefi, S.; Gilard, V.; Malet-Martino, M.; Martino, R. Generic ciprofloxacin tablets contain the stated amount of drug and different impurity profiles: A 19F, 1H and DOSY NMR analysis. J. Pharm. Biomed. Anal. 2007, 44, 743–754. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Jiang, M.-H.; Sun, L.-L.; Zheng, F.; Dong, L.; Shah, V.; Shen, W.-B.; Ding, Y. Quantitative analysis of sitagliptin using the 19F-NMR method: A universal technique for fluorinated compound detection. Analyst 2015, 140, 280–286. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.D.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. 2018, 351, 1800141. [Google Scholar] [CrossRef]
- Fief, C.A.; Hoang, K.G.; Phipps, S.D.; Wallace, J.L.; Deweese, J.E. Examining the Impact of Antimicrobial Fluoroquinolones on Human DNA Topoisomerase IIα and IIβ. ACS Omega 2019, 4, 4049–4055. [Google Scholar] [CrossRef]
- Beberok, A.; Wrześniok, D.; Minecka, A.; Rok, J.; Delijewski, M.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin-mediated induction of S-phase cell cycle arrest and apoptosis in COLO829 melanoma cells. Pharmacol. Rep. 2018, 70, 6–13. [Google Scholar] [CrossRef]
- Perrone, C.E.; Takahashi, K.C.; Williams, G.M. Inhibition of human topoisomerase IIα by fluoroquinolones and ultraviolet A irradiation. Toxicol. Sci. 2002, 69, 16–22. [Google Scholar] [CrossRef][Green Version]
- Swedan, H.K.; Kassab, A.E.; Gedawy, E.M.; Elmeligie, S.E. Design, synthesis, and biological evaluation of novel ciprofloxacin derivatives as potential anticancer agents targeting topoisomerase II enzyme. J. Enzym. Inhib. Med. Chem. 2023, 38, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Mazandaran, K.E.; Mirshokraee, S.A.; Didehban, K.; Houshdar Tehrani, M.H. Design, Synthesis and Biological Evaluation of Ciprofloxacin- Peptide Conjugates as Anticancer Agents. Iran. J. Pharm. Res. IJPR 2019, 18, 1823. [Google Scholar]
- Mohammed, H.H.H.; Abd El-Hafeez, A.A.; Ebeid, K.; Mekkawy, A.I.; Abourehab, M.A.S.; Wafa, E.I.; Alhaj-Suliman, S.O.; Salem, A.K.; Ghosh, P.; Abuo-Rahma, G.E.D.A.; et al. New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization. J. Enzym. Inhib. Med. Chem. 2022, 37, 1346–1363. [Google Scholar]
- Mohammed, H.H.H.; Abbas, S.H.; Hayallah, A.M.; Abuo-Rahma, G.E.D.A.; Mostafa, Y.A. Novel urea linked ciprofloxacin-chalcone hybrids having antiproliferative topoisomerases I/II inhibitory activities and caspases-mediated apoptosis. Bioorg. Chem. 2021, 106, 104422. [Google Scholar] [CrossRef]
- Sales, T.A.; Ramalho, T.C. Ciprofloxacin/Topoisomerase-II complex as a promising dual UV–Vis/fluorescent probe: Accomplishments and opportunities for the cancer diagnosis. Theor. Chem. Acc. 2022, 141, 1–10. [Google Scholar] [CrossRef]
- Cowen, T.; Karim, K.; Piletsky, S. No Title. Anal. Chim. Acta 2016, 936, 62–74. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Molecular docking studies on thirteen fluoroquinolines with human topoisomerase II a and b. Silico Pharmacol. 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Joshi, H.V.; Shah, U.A.; Patel, J.K. A Review on Computational Software Tools for Drug Design and Discovery. Indo. Glob. J. Pharm. Sci. 2022, 12, 53–81. [Google Scholar] [CrossRef]
- Pajeva, I.; Tsakovska, I.; Pencheva, T.; Alov, P.; Al Sharif, M.; Lessigiarska, I.; Jereva, D.; Diukendjieva, A. In silico Studies of Biologically Active Molecules. Stud. Comput. Intell. 2021, 934, 421–451. [Google Scholar]
- Caballero, J. The latest automated docking technologies for novel drug discovery. Expert Opin. Drug Discov. 2020, 16, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Frye, L.; Bhat, S.; Akinsanya, K.; Abel, R. From computer-aided drug discovery to computer-driven drug discovery. Drug Discov. Today Technol. 2021, 39, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Tripathi, T. Molecular Dynamics Simulation in Drug Discovery: Opportunities and Challenges. In Innovations and Implementations of Computer Aided Drug Discovery Strategies in Rational Drug Design; Singh, S.K.., Ed.; Springer: Singapore, 2021; pp. 295–316. [Google Scholar]
- McClendon, A.K.; Osheroff, N. DNA Topoisomerase II, Genotoxicity, and Cancer. Mutat. Res. Mol. Mech. Mutagen. 2007, 623, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Sales, T.A.; Marcussi, S.; da Cunha, E.F.F.; Kuca, K.; Ramalho, T.C. Can inhibitors of snake venom phospholipases A2 lead to new insights into anti-inflammatory therapy in humans? A theoretical study. Toxins 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, W.; Karch, R.; Knapp, B.; Ilieva, N. Relaxation estimation of RMSD in molecular dynamics immunosimulations. Comput. Math. Methods Med. 2012, 2012, 173521. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Biswas, G.P.; Mahata, N.; Ghanta, S.; Bhunia, B. Exploration of binding mechanism of triclosan towards cancer markers using molecular docking and molecular dynamics. Chemosphere 2022, 293, 133550. [Google Scholar] [CrossRef]
- Chekmenev, E.Y.; Chow, S.K.; Tofan, D.; Weitekamp, D.P.; Ross, B.D.; Bhattacharya, P. Fluorine-19 NMR chemical shift probes molecular binding to lipid membranes. J. Phys. Chem. B 2008, 112, 6285–6287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maxwell, R.J.; Workman, P.; Griffiths, J.R. Demonstration of tumor-selective retention of fluorinated nitroimidazole probes by 19F magnetic resonance spectroscopy in vivo. Int. J. Radiat. Oncol. 1989, 16, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated paramagnetic complexes: Sensitive and responsive probes for magnetic resonance spectroscopy and imaging. Front. Chem. 2018, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Ojugo, A.S.E.; Mcsheehy, P.M.J.; Mcintyre, D.J.O.; Mccoy, C.; Stubbs, M.; Leach, M.O.; Judson, I.R.; Grifths, J.R. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: A comparison of exogenous 19 F and 31 P probes. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 1999, 12, 495–504. [Google Scholar]
- Ye, Y.; Liu, X.; Zhang, Z.; Wu, Q.; Jiang, B.; Jiang, L.; Zhang, X.; Liu, M.; Pielak, G.J.; Li, C. 19FNMR Spectroscopy as a Probe of Cytoplasmic Viscosity and Weak Protein Interactions in Living Cells. Chem. A Eur. J. 2013, 19, 12705–12710. [Google Scholar] [CrossRef]
- Ulrich, A.S. Solid state 19F NMR methods for studying biomembranes. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 46, 1–21. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions—United States; CDC: Atlanta, GA, USA, 2017.
- Crump, B.; Wise, R.; Dent, J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob. Agents Chemother. 1983, 24, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Staß, H.; Möller, J.G.; Schrolnberger, C.; Erovic, B.; Hollenstein, U.; Zeitlinger, M.; Georg Eichler, H.; Müller, M. Target Site Concentrations of Ciprofloxacin after Single Intravenous and Oral Doses. Antimicrob. Agents Chemother. 2002, 46, 3724. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hu, C.; Hu, X.; Wei, D.; Chen, Y.; Qu, J. Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J. Hazard. Mater. 2011, 185, 1256–1263. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Mucke, H.A. The case of galantamine: Repurposing and late blooming of a cholinergic drug. Futur. Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Mullard, A. Drug repurposing programmes get lift off. Nat. Rev. Drug Discov. 2012, 11, 505–506. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E.; Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef]
- Edmond, L.Y.; Gerig, J.T. Origins of Fluorine NMR Chemical Shifts in Fluorine-Containing Proteins. J. Am. Chem. Soc. 2000, 122, 4408–4417. [Google Scholar]
- Modo, M. 19F Magnetic Resonance Imaging and Spectroscopy in Neuroscience. Neuroscience 2021, 474, 37–50. [Google Scholar] [CrossRef]
- Evanics, F.; Kitevski, J.L.; Bezsonova, I.; Forman-Kay, J.; Prosser, R.S. 19F NMR studies of solvent exposure and peptide binding to an SH3 domain. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.A.; Gonçalves, A.S.; Franca, T.C.C.; Santana, M.S.; da Cunha, E.F.F.; Ramalho, T.C. Improved Protocol for the Selection of Structures from Molecular Dynamics of Organic Systems in Solution: The Value of Investigating Different Wavelet Families. J. Chem. Theory Comput. 2022, 18, 5810–5818. [Google Scholar] [CrossRef] [PubMed]
- Rayene, K.; Imane, D.; Abdelaziz, B.; Leila, N.; Fatiha, M.; Abdelkrim, G.; Bouzid, G.; Ismahan, L.; Brahim, H.; Rabah, O. Molecular modeling study of structures, Hirschfield surface, NBO, AIM, RDG, IGM and 1HNMR of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex from QM calculations. J. Mol. Struct. 2022, 1249, 131565. [Google Scholar] [CrossRef]
- Jaber, D.F.; Jallad, M.A.N.; Abdelnoor, A.M. The effect of ciprofloxacin on the growth of B16F10 melanoma cells. J. Cancer Res. Ther. 2017, 13, 956–960. [Google Scholar] [PubMed]
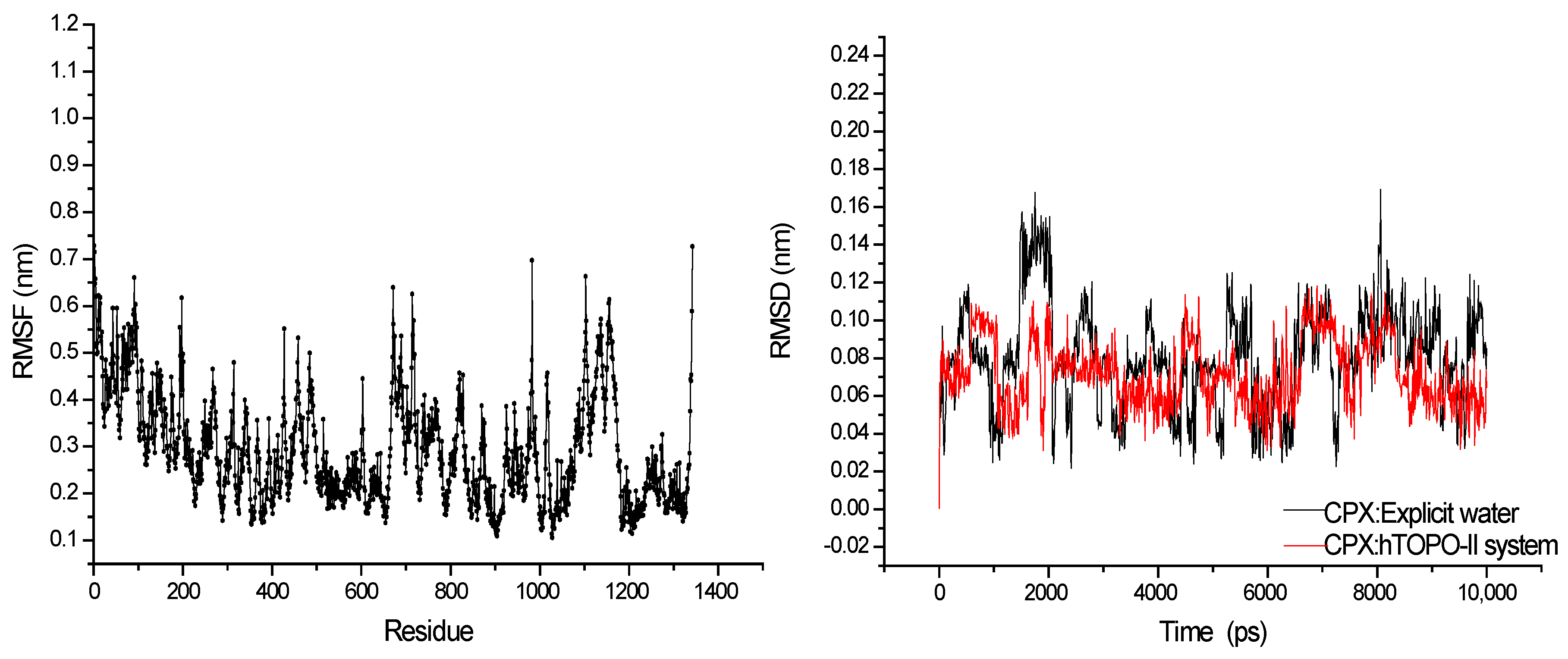
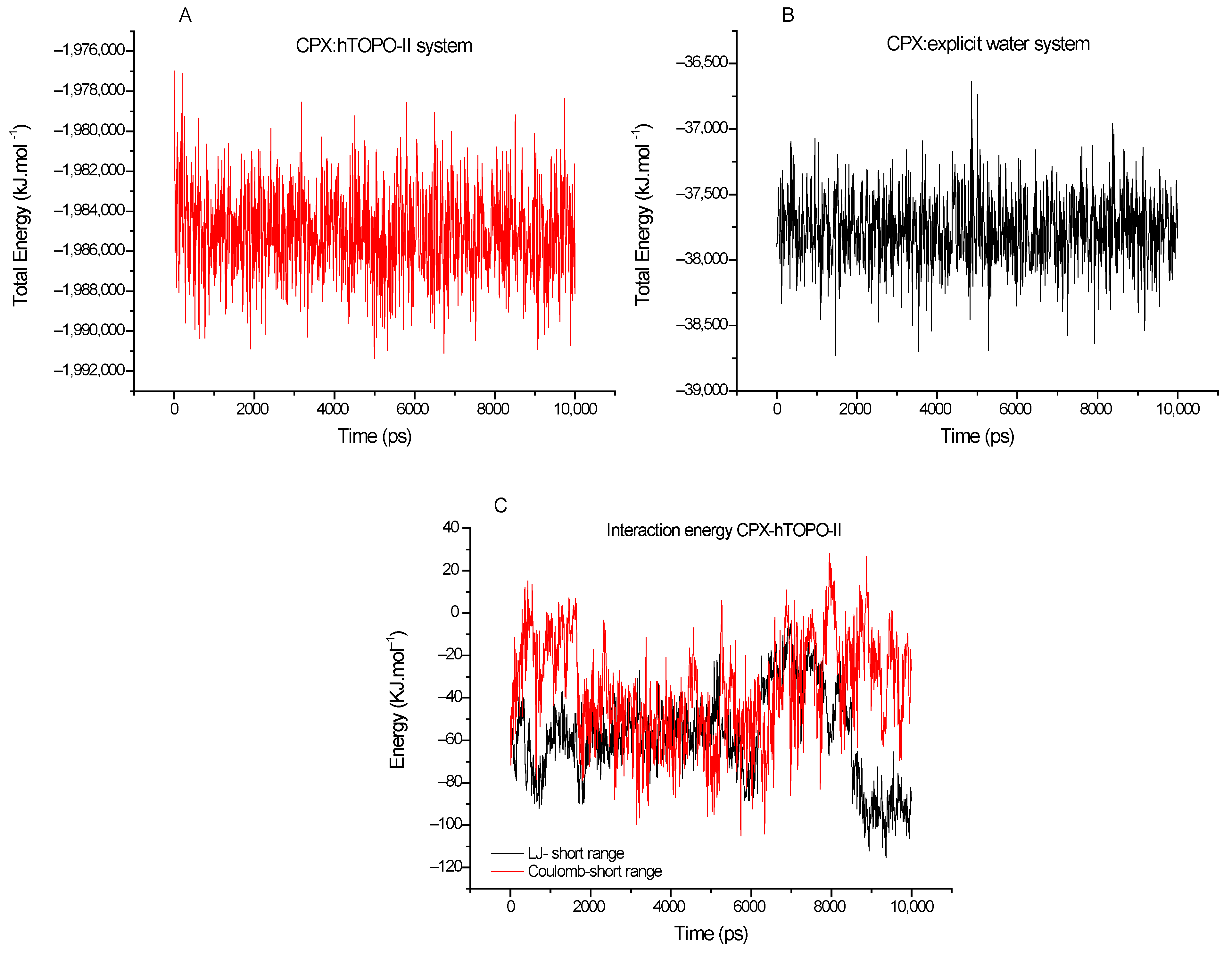
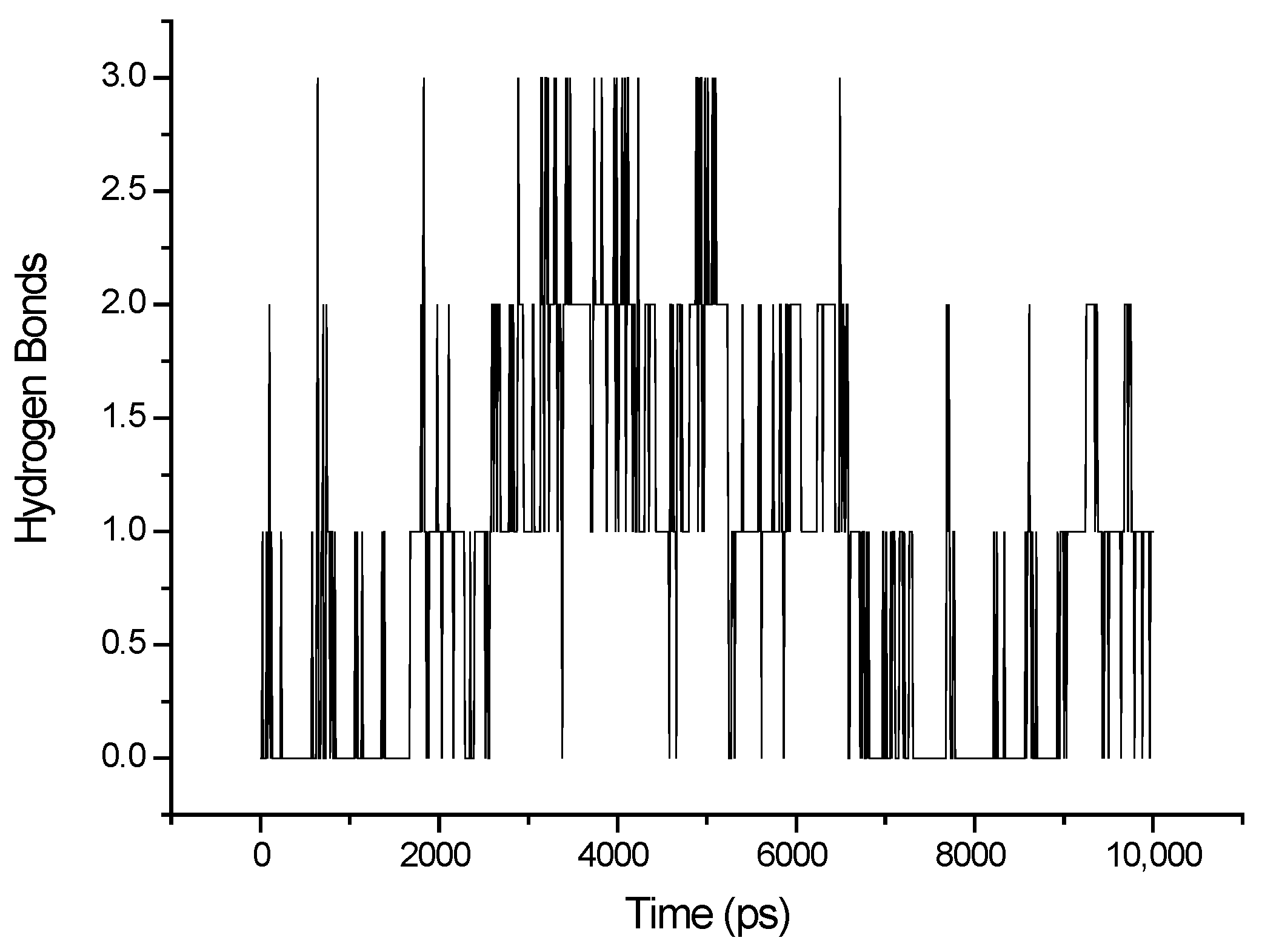
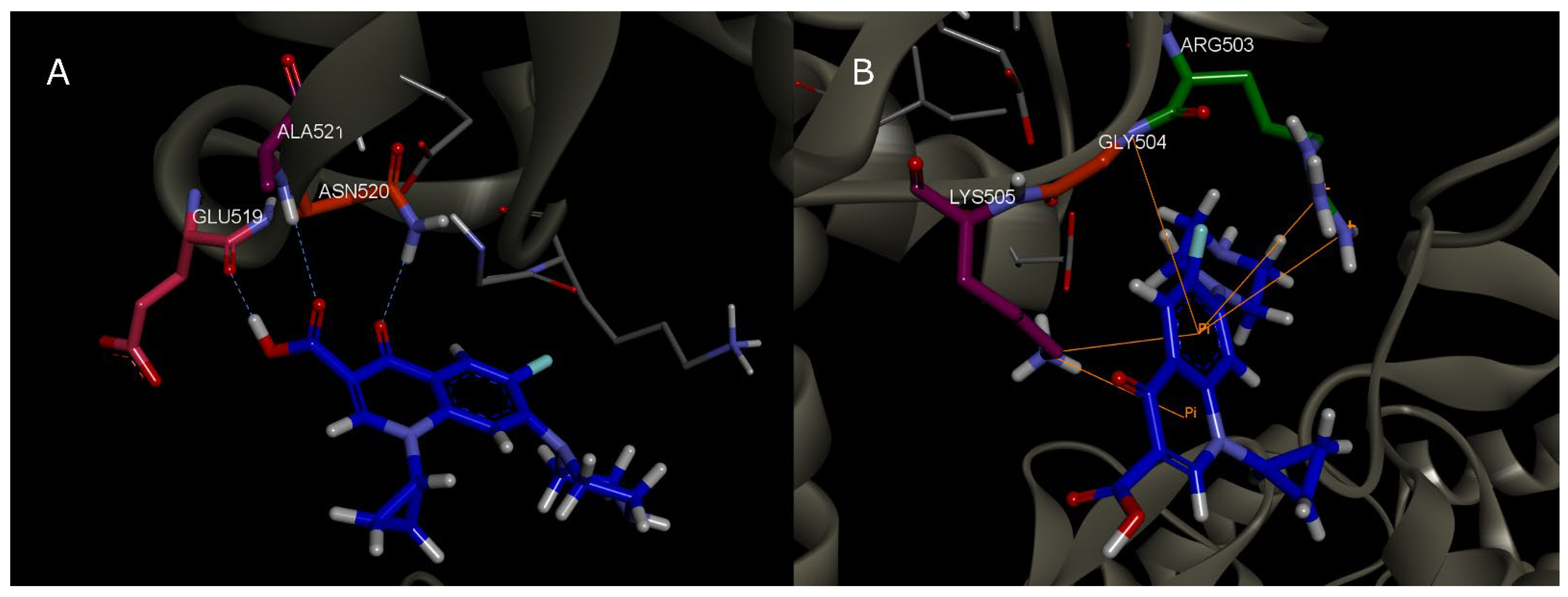
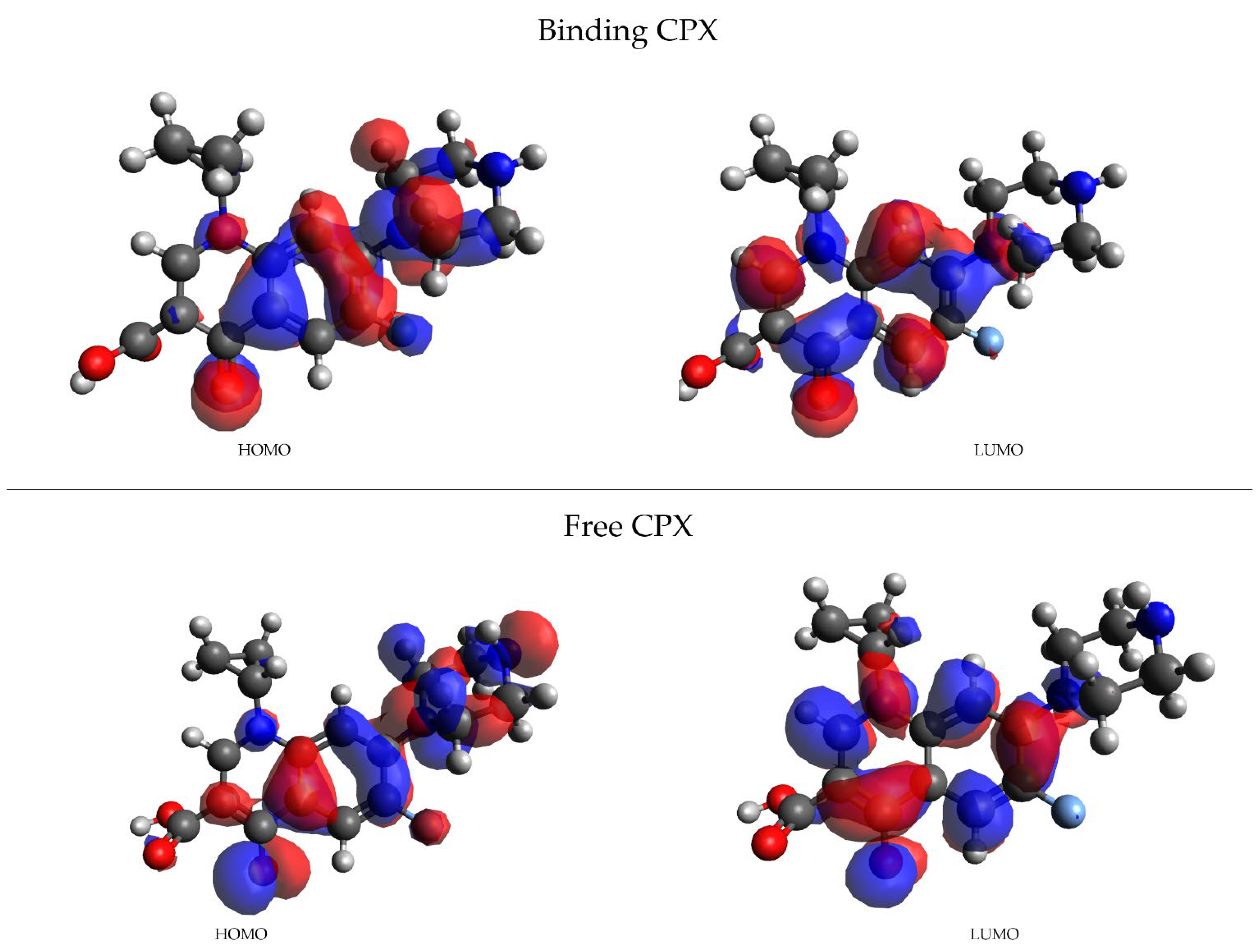
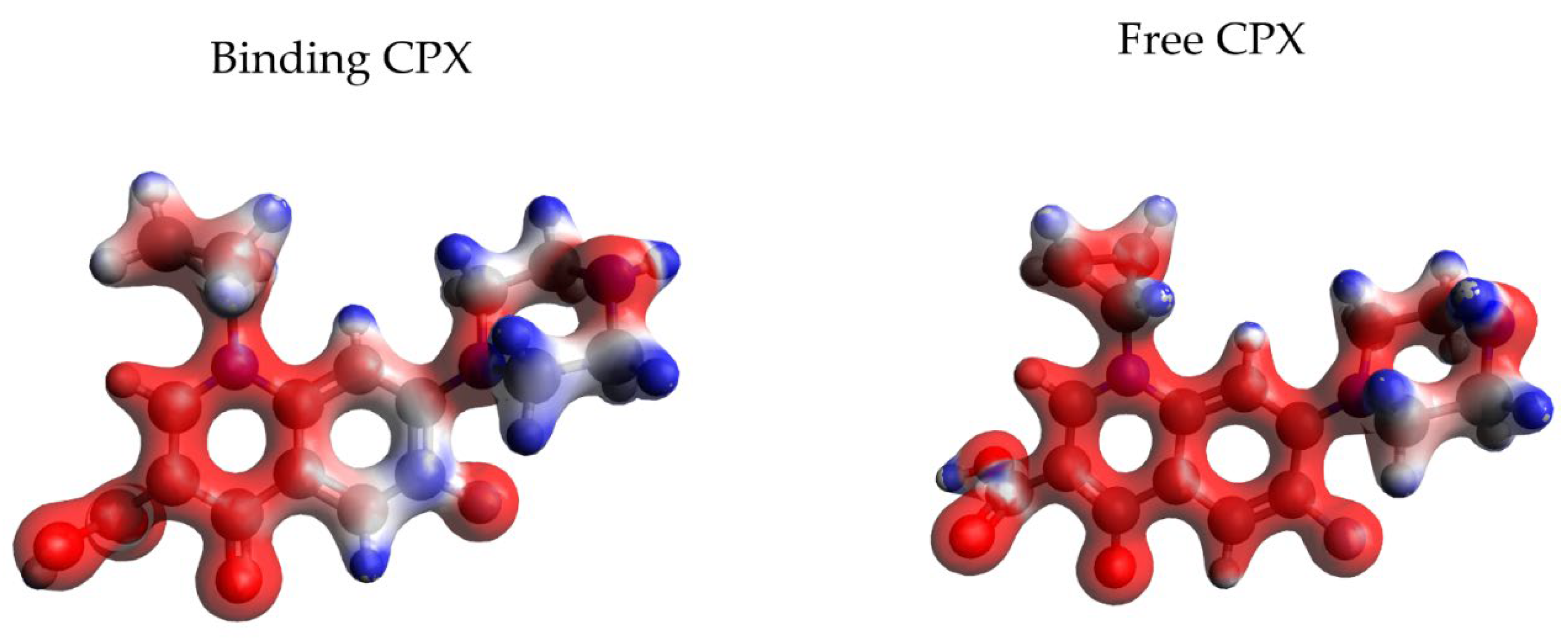
| Frame | Time (ps) | Residue | Interaction Type |
|---|---|---|---|
| 1 | 2000 | Asn 520 | HBond |
| 2 | 2200 | Asn 520 | HBond |
| 3 | 2300 | Leu 507 | HBond |
| 4 | 2400 | Asn 520 | HBond |
| 5 | 2600 | Asn 520; Gln 516 | HBond |
| 6 | 3000 | Asn 520 | HBond |
| 7 | 3100 | Asn 520 | HBond |
| 8 | 3200 | Glu 519; Asn 520; Ala 521 | HBond |
| 9 | 3700 | Asn 520; Ala 521 | HBond |
| 10 | 3900 | Asn 520; Ala 521 | HBond |
| 11 | 4200 | Asn 520 | HBond |
| 12 | 4400 | Asn 520; Ala 521 | HBond |
| 13 | 4700 | Asn 520; Ala 521 | HBond |
| 14 | 5100 | Asn 520; Ala 521 | HBond |
| 15 | 5500 | Ala 521 | HBond |
| 16 | 7000 | - | - |
| 17 | 7300 | Lys 505 | π-π; Electrostatic HBond |
| 18 | 7500 | - | - |
| 19 | 7700 | Arg 503 | HBond |
| 20 | 7900 | Arg 503; Lis 505; Gly 504 | π-π |
| 21 | 8000 | - | - |
| 22 | 8250 | Lys 505 | HBond; π-π |
| 23 | 8800 | - | - |
| 24 | 9000 | Ile 506 | HBond |
| 25 | 9200 | Ile 506 | HBond |
| 26 | 9400 | Ile 506 | HBond |
| 27 | 9500 | - | |
| 28 | 9800 | Ile 506 | HBond |
| 29 | 10,000 | Ile 506 | HBond |
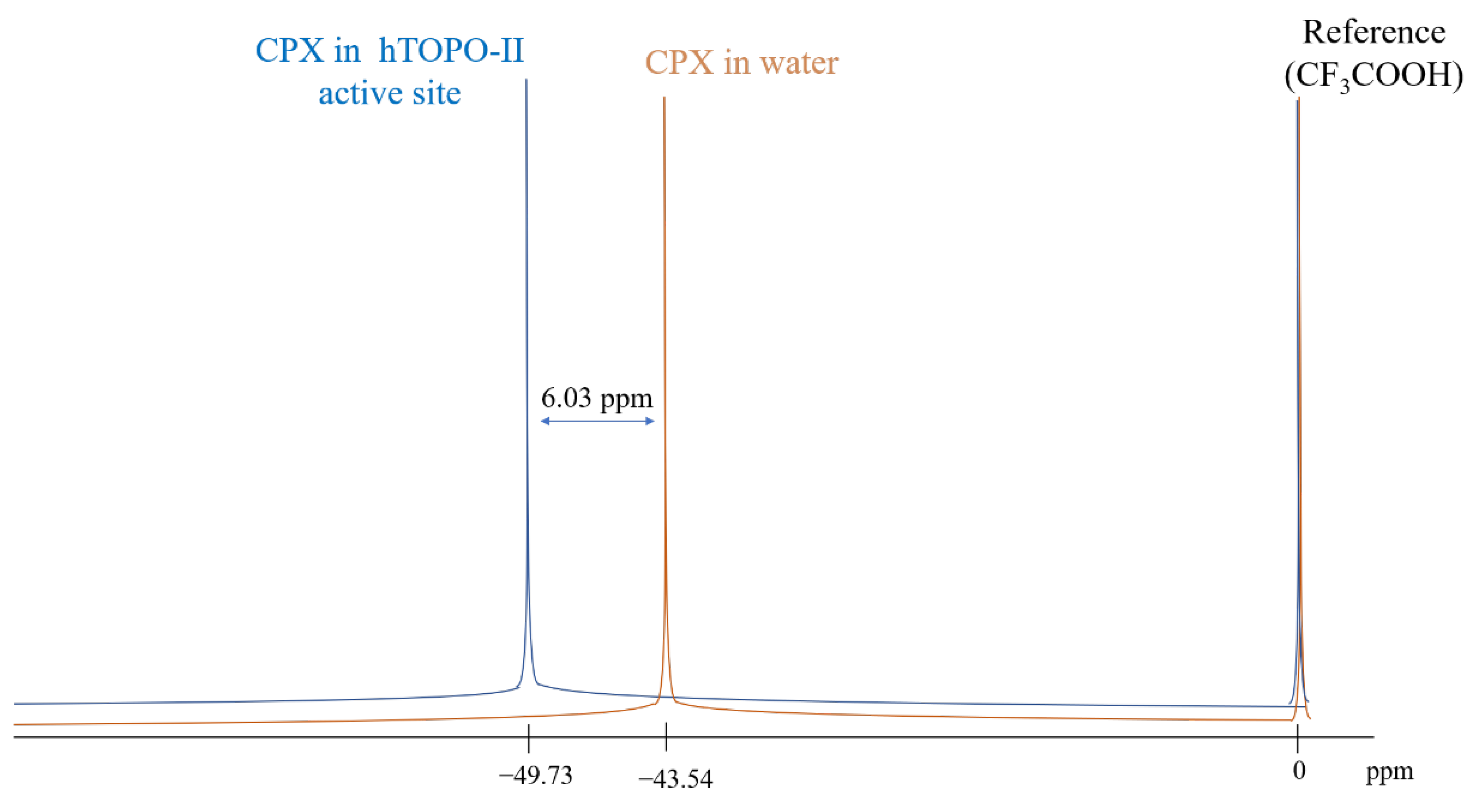
| System | 19F δppm | Δδppm |
|---|---|---|
| CPX:aqueous solution (experimental) | −43.70 | 0.00 |
| CPX:explicit water | −43.54 | −0.16 |
| CPX:hTOPO-II | −49.73 | 6.03 |
| CPX:vacuum | −55.11 | 11.41 |
| CPX:implicit water | −56.20 | 12.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, T.A.; Gonçalves, M.A.; Ramalho, T.C. Structural Parameters of the Interaction between Ciprofloxacin and Human Topoisomerase-II β Enzyme: Toward New 19F NMR Chemical Shift Probes. Magnetochemistry 2022, 8, 181. https://doi.org/10.3390/magnetochemistry8120181
Sales TA, Gonçalves MA, Ramalho TC. Structural Parameters of the Interaction between Ciprofloxacin and Human Topoisomerase-II β Enzyme: Toward New 19F NMR Chemical Shift Probes. Magnetochemistry. 2022; 8(12):181. https://doi.org/10.3390/magnetochemistry8120181
Chicago/Turabian StyleSales, Thais Aparecida, Mateus Aquino Gonçalves, and Teodorico Castro Ramalho. 2022. "Structural Parameters of the Interaction between Ciprofloxacin and Human Topoisomerase-II β Enzyme: Toward New 19F NMR Chemical Shift Probes" Magnetochemistry 8, no. 12: 181. https://doi.org/10.3390/magnetochemistry8120181
APA StyleSales, T. A., Gonçalves, M. A., & Ramalho, T. C. (2022). Structural Parameters of the Interaction between Ciprofloxacin and Human Topoisomerase-II β Enzyme: Toward New 19F NMR Chemical Shift Probes. Magnetochemistry, 8(12), 181. https://doi.org/10.3390/magnetochemistry8120181







