Abstract
In this study, three types of hydrophobized alkyl-modified magnetic nanoparticles (MNPs) comprising direct alkylated-MNPs (A-MNPs), silica-mediated alkyl MNPs (A-SiMNPs), and arginine (Arg)-mediated alkyl MNPs (A-RMNPs) were synthesized successfully. For this purpose, the co-precipitation method was used to synthesize, and octadecyl trimethoxy silane (OTMS) was used as a functionalizing agent. Accordingly, the hydrophobic octadecyl moieties were connected to MNPs. The nanoparticles (NPs) were characterized by XRD, SEM, FTIR, CHN, DLS, and zeta potential analyses. The synthesized coated MNPs represented a decrease in surface charge and magnetization alongside increased surface hydrophobicity and size. It was revealed that the alkylation process was successfully performed to all three MNPs, but A-SiMNPs showed the highest hydrophobicity. Additionally, the novel A-RMNPs, as the most biocompatible type, and A-MNPs showed the highest magnetization among the synthesized MNPs. The results indicate that synthesized NPs can play an important role in bio applications. However, it was revealed that alkyl chains are easily connected to all three MNPs, and that A-MNPs contained the highest alkyl chains and could affect the re-folding and denaturation process of recombinant proteins.
1. Introduction
Magnetic nanoparticles (MNPs) have attracted increasing interest in recent years due to their higher specific surface area, lower mass transfer resistance for reacting substrates, nontoxicity and biocompatibility in most cases, and ease of separation from a solution mixture or targeting toward the application of a magnetic field. Accordingly, the applications of MNPs have shown further advancement in several specific areas, such as drug targeting, diagnostics, imaging, and genetic manipulation, that offer emerging strategies in the diagnosis and therapy of cancer, diabetes, cardiovascular diseases, and Alzheimer’s disease (AD) as theranostic agents [1,2,3]. The importance of MNPs in these fields can be evaluated from the number of papers published so far [4]. However, the surface modification of newly developed nanomaterials such as MNPs is still important for their applications [5,6,7].
The surface properties of a given nanoparticle impact its interaction with biomolecules or structures, and these properties determine its behavior and functionality [8,9]. Functionalization methods can influence the surface properties of different kinds of NPs. Core-shell NPs can combine characteristics of different materials by a variant range of properties, for example, magnetic properties of the core and optical properties of the shell, which were observed in the case of gold (Au) NPs in studies [10]. It is fascinating to study these features because of the availability of these materials in the production of new and particular applications. The dependence between the core and shell composition and properties such as super para-magnetism allow the planned synthesis of nanomaterials with well-controlled physicochemical properties for different subjects and aims [11]. The synthesis of layer-by-layer grown particles allows quality checking and estimation of the composition and features of MNPs by each consequent layer addition [12]. The surface layer is an important key feature of the modification of the magnetic properties of NPs [13]. However, modification is dependent on the elements used in their composition [14,15]. Amino acid-coated MNPs have recently received much interest and have been widely used in many areas because of their biodegradability, biocompatibility, and nontoxicity [16,17]. Among these NPs, L-Arginine modified magnetic NPs (RMNP) provide new surface properties for interactions with bio-structures such as proteins [18,19,20] and offer a novel opportunity for magnetic nanoparticle surface modification, which is reported in this paper.
Accurate knowledge of the average particle size and size distribution of each assembly of nanoparticles is crucially important for proper achievement of specific applications [21]. Moreover, shells prepared from materials such as polymers [22], lipids [23], proteins [24], and other biocompatible shells [25] do not allow agglomeration of the cores or improve the biocompatibility. Several approaches have been deployed to modify MNPs directly through Fe-OH groups on the surface of the hematite or magnetite core [26,27].
The functionalization of NPs is very important in bio applications. It is observed that the most uncoated NPs can induce oxidative stress, protein aggregation, liberation of toxic cations, membrane disruption and DNA damage in the human body [28,29,30].
Hydrophobic NPs can be used in various bio fields such as drug targeting and release, especially of hydrophobic drugs, enzyme immobilization [31], amyloidosis study [32], bio catalysis [33,34,35], DNA separation [36], and hyperthermia therapy [37]. Additionally, due to their hydrophobicity, magnetic characteristics and broad possibilities for surface modification, they are also applicable in chemical processes such as hydrometallurgy, metal recovery, recycling, petroleum oil spill collection, the control of chemical reactivity at solid−solution interfaces, etc. [36,38,39,40,41]. As hydrophobic NPs, Au NPs can be used as an interface to indirectly design specific oligomer antibodies [42] by mimicking Aβ oligomerization of the spherical amyloid oligomers on their surface. Hsieh et al. [43] observed that Au NPs affect insulin fibril formation, and Skaat et al. [44] concluded that fluorinated γ-Fe2O3 MNPs can inhibit insulin aggregation due to their strong hydrophobic character. In cosmetic product formulations, TiO2, ZnO and Fe2O3 NPs that are often coated with a hydrophobic polymer such as poly (methylvinylether)/maleic acid reduce direct contact with the human skin [45]. Alkyl groups (CnH2n+1) are prevalent in bio nanomaterials used for many bio and engineering applications. Hence, in this study, we alkylated the three synthesized MNPs by OTMS (C21H46O3Si).
Nanoparticle surface hydrophobization can be achieved by using amphiphiles, such as oleic acid [46], detergents (e.g., sodium dodecyl sulfate [47]), or silane derivatives [48]. Among them, silane derivatives have several advantages, including better structural stability through surface chemical grafting by silanization; effective and efficient tuning of interfacial hydrophilicity and hydrophobicity for different applications by the selection of silane precursors; feasible functionalization after presenting various reactive groups such as -NH2, -SH, and COOH on the nanoparticle surface [49], and protection of magnetic particles from oxidation by coating chemically inert silica without affecting their magnetic properties [50,51]. According to many results with silica-coated MNP applications in the fields both of bio and engineering, it was observed that silica-coated MNPs are a good sorbent for the extraction of Au (III), Pd (II) and Ag (I) from water samples, thus showing the potential of synthesized A-SiMNPs in this work [52]. In addition, amino acid-coated MNPs have recently received much interest due to their biodegradability, biocompatibility, and nontoxicity and have been widely used in many fields of study. Green synthesis, as an environmentally friendly method, aims to eliminate toxic waste and reduce energy consumption [53,54,55,56]. Various factors, such as pH, temperature and reaction time, control the properties of NPs synthesized by biological entities [57,58,59].
In this paper, due to the importance of synthesizing various hydrophobic surfaces and their broad bio and industrial benefits, we green-synthesized new Fe3O4-based MNPs with capping agents of materials such as L-Arginine, SiO2, and OTMS. Layer-by-layer growth was realized during the synthesis procedure for these NPs. The aim was to synthesize three hydrophobic MNPs for detailed characterization and comparison. Their properties, strengths and weaknesses were discussed. Further, two new alkylated NPs, A-SiMNP and A-RMNP, are novel, with a two-coating layer that could be applied in the bio and separation engineering fields.
2. Experimental
2.1. Materials and Instrumentation
Iron (II) chloride tetrahydrate (FeCl2·4H2O, Sigma-Aldrich, Washington, DC, USA) and iron (III) chloride hexahydrate (FeCl3·6H2O, Sigma-Aldrich) were used as the precursors for the synthesis of magnetite (Fe3O4) as the core. L-Arginine (Merck, Kenilworth, NJ, USA) and ammonia solution (NH4OH, 25%, Merck, Kenilworth, NJ, USA) were used as coating and precipitating agents, respectively. Octadecyl trimethoxy silane (OTMS, 95%) was used to functionalize the surface of MNPs. Tetraethyl orthosilicate (TEOS, 95%, Merck, Kenilworth, NJ, USA) was also applied to process a silica coating around the Fe3O4 core.
X-ray diffraction analysis (XRD, Rigaku-Dmax 2500) was performed to identify the crystalline structure of the particles using Cu Kα radiation (λ = 1.5406 nm) and 2 theta range from 5° to 90°, in which the rate of warming-up was adjusted at 0.02° min−1. Scanning electron microscopy (SEM, CamScan MV2300) was used to monitor the size and morphology of all NPs. Fourier transform infrared (FTIR, Rayleigh WQF-510A) spectra were recorded using a KBr pellet spectrophotometer. A vibrating sample magnetometer (VSM, MagKav Co.) was used at room temperature to measure sample magnetization. CHN analysis (Thermo Finnigan Co., Waltham MA, USA, FlashEA1112series) was performed for precise demonstration of carbon chains as proof of alkylation. CHN, as a destructive method of elemental analysis, measures carbon, hydrogen, sulfur, oxygen and nitrogen elemental content with accuracy and precision. The sample needs to be very pure to pass this test, as the accuracy of this method is ±0.3%. Dynamic light scattering (DLS) and zeta potential of MNPs were measured by Malvern ZS-Nano series 1001767.
2.2. Synthesis of Magnetic NPs
2.2.1. Synthesis of Fe3O4 NPs
Synthesis of Fe3O4 NPs was performed by the co-precipitation method. In brief, 2 mmol FeCl3·6H2O and 1 mmol FeCl2·4H2O salts were dissolved at a molar ratio of 2:1 in 20 mL deionized water under 250 rpm stirring and 50 °C for 30 min. To achieve the complete reaction of compounds in solution, 7 mL ammonia solution was added to the solution by syringe. Eventually, as a sign of completion of the reaction, it was observed that the color of the mixture became black. After 30 min, MNPs were prepared. Finally, to eliminate the other impurities in the solution, we washed it with acetone and deionized water three times and separated the MNPs from the liquid phase with a permanent magnet. Finally, the MNP powder was dried at room temperature.
2.2.2. Synthesis of Fe3O4@Arg NPs (RMNP)
For the synthesis of RMNP based on the co-precipitation method, at the primary step, 2 mmol FeCl3·6H2O and 1 mmol FeCl2·4H2O salts were mixed in a ratio of 2:1 (M). Then, the mixture was dissolved in 20 mL of deionized water. The solution was stirred under 250 rpm stirring rate and temperature of 80 °C for 30 min. A 0.147 g (10 µM) amount of powdered L-Arginin amino acid (Arg) was dissolved in 10 mL deionized water and placed on a magneto stirrer at 50 °C. After preparation of Arg and NPs, Arg solution was added droplet-wise to the suspension by syringe. To achieve the complete reaction of compounds in solution, 7 mL ammonia solution was added by syringe to the solution. Eventually, as a sign of completion of the reaction, it was observed that the color of the mixture became black. After 30 min, RMNPs were prepared. Finally, to eliminate the other impurities in the solution, it was washed using acetone and deionized water three times. The RMNP was separated from the liquid phase with a permanent magnet. Finally, the RMNP powder was dried at room temperature.
2.2.3. Synthesis of Fe3O4@SiO2 NPs (SiMNP)
For the synthesis of Fe3O4@SiO2 NPs (SiMNP), in the first setup, 0.3 g MNP, 30 mL deionized water, 120 mL ethanol, and 1 mL ammonia solution were mixed, respectively. In the second setup, 1 mL TEOS was dissolved and mixed using a magneto stirrer in 20 mL ethanol until it became a single-phase suspension. In the next step, the solvent of setup 2 was added to the solvent of setup 1 by syringe in droplets. The final solution was purged by nitrogen injection for 5 min and stirred at a temperature of 60 °C for 10 h vigorously. Finally, to eliminate other impurities in the solution, it was washed with ethanol and deionized water three times, and the SiMNPs were separated from the liquid phase with a permanent magnet. Finally, the SiMNP powder was dried at room temperature overnight.
2.3. Surface Modification of NPs by OTMS
2.3.1. Alkylated Fe3O4 NPs (A-MNP)
In the primary step, 0.15 g MNP was dissolved and dispersed in 15 mL ethanol by a sonication device. As a secondary step, 75 mL OTMS with 100 µL deionized water were added to the first solution by micro pump at a 2 mL h−1 rate. The solution was deoxidized by nitrogen injection for 10 min. The solution was vigorously mixed at 30 °C for 6–10 h. Finally, it was washed with ethanol, then with deionized water three times, and A-MNP was separated from the liquid phase with a permanent magnet. The A-MNP powder was then dried at room temperature for 12–24 h.
2.3.2. Alkylated Fe3O4@Arg NPs (A-RMNP) and Fe3O4@SiO2 NPs (A-SiMNP)
All steps were carried out using the same procedure as described for the synthesis of A-MNP, but instead of functionalizing the MNP, the RMNPs and SiMNP were inserted as the cores and alkylated by OTMS. Figure 1 shows the explained processes.
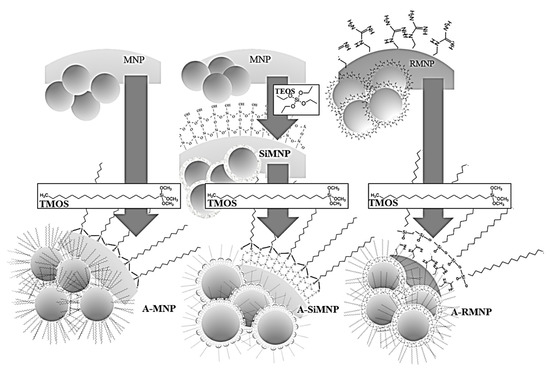
Figure 1.
Schematic representation of the flow of preparation of the magnetic NPs and their alkylated counterparts, as discussed in the current work, including MNP/A-MNP, RMNP/A-RMNP, and SiMNP/A-SiMNP.
3. Results and Discussion
Figure 2 shows the XRD patterns of the samples. The peak positions at the corresponding values were indexed as 022, 113, 004, 224, 115, 044, 026, and 335, showing the successful synthesis of Fe3O4 NPs according to JCPDS card no. 19-629 and other studies [60,61]. Besides the major peaks that verified the existence of the magnetite core, peaks of some complexes that had the key elements of modifying caps were observed. This proved the addition of new phases to the core substrate that did not exist in the bare uncapped magnetite. Additionally, most diffraction patterns showed the same peaks as those of the unmodified MNPs, indicating that the functionalization did not degrade the Fe3O4 core. The Williamson–Hall calculations were performed to measure the average crystallite size of MNPs (more details in supplementary materials). According to the calculations, all of the MNPs apparently had an average size of 8.0–8.5 nm, which make sense because they all were synthesized in the same way. According to size measurements with SEM, the size of the coated MNPs was larger due to the particle coating. Then, the size of the coated MNPs > uncoated MNPs > average crystallite size. Average crystallite sizes of 8.6, 8.8, 5.9, 8, 10.9 and 8.4 nm were obtained for MNP, A-MNP, RMNP, A-RMNP, SiMNP and A-SiMNP, respectively. It was obvious that the sizes of the MNPs obtained from XRD were smaller than those obtained with SEM analysis.
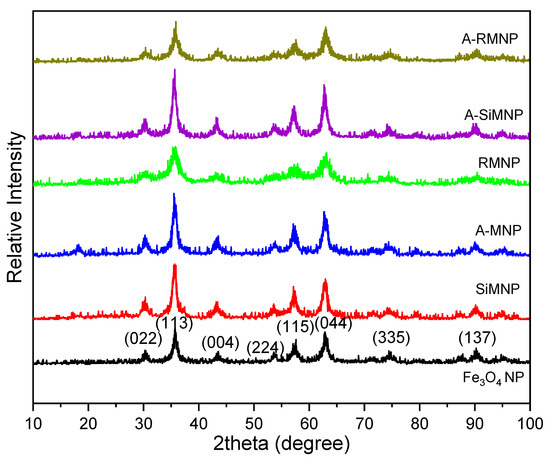
Figure 2.
XRD patterns of MNP, SiMNP, A-MNP, RMNP, A-SiMNP, and A-RMNP obtained at room temperature.
The SEM and TEM images of amyloid fibrils were examined to monitor the size and morphology of all NPs. According to the TEM images (Figure S1), the Fe3O4 NPs were successfully coated. The SEM images of NPs are shown in Figure 3.
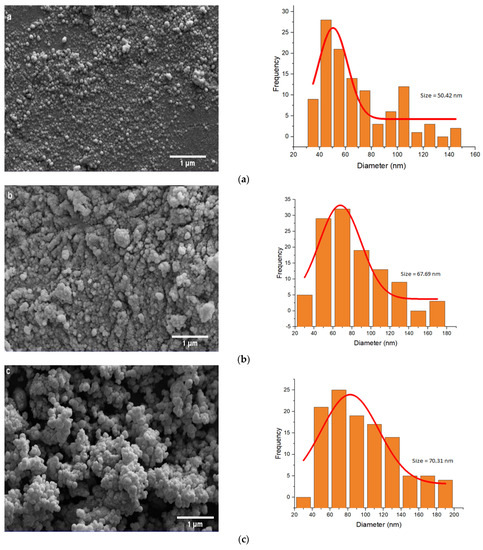
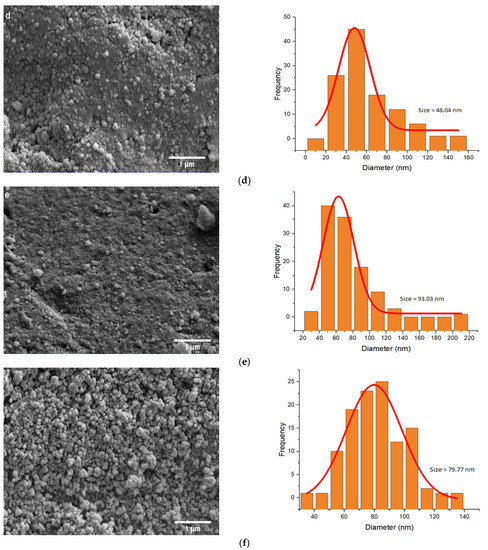
Figure 3.
SEM images and size distribution of (a) MNP, (b) SiMNP, (c) A-MNP, (d) RMNP, (e) A-SiMNP, and (f) A-RMNP.
As shown in Figure 3, by increasingly decorating and modifying the NPs, the average size of the NPs became larger. The results of image analysis indicate that in the first step, the mean size of bare Fe3O4 NPs was about 50.42 nm, and when they were capped by SiO2 and OTMS, their mean size increased to 67.69 and 70.31 nm, respectively. The size order of NPs based on the SEM images was: A-SiMNP > A-RMNP > A-MNP > SiMNP > MNP > RMNP. According to the size order of the NPs, the two-layer capped NPs (A-SiMNP and A-RMNP) were larger than the other four NPs because of their binary capping nature. The third position was assigned to the third alkylated NP (A-MNP). This size order sequence indicated that the alkylation process’s carbon chains had the highest impact on the size increment of NPs among all six NPs. After the three hydrophobic NPs, the next one was SiMNP, and it was according to our expectation because the silica coating of the magnetite core by TEOS formed a highly branched and mesoporous siloxane polymer on its surface, and this mesoporous nanosphere shape was also seen for other cores such as gold [62]. However, the size of RMNP became lower than that of MNP, and it was incomplete, in accordance with our previous results [18].
Figure 4 displays the results of the VSM analysis that was carried out to study the magnetic hysteresis loops of the dried samples. Accordingly, all six MNPs exhibited no hysteresis loop with remanence and coercivity of relatively zero, indicating that all samples were superparamagnetic due to their small size (smaller than the critical size) [63]. The saturation magnetization values obtained at room temperature were recorded at about 80, 38, 33, 28, 15, and 8 emu g−1 for MNP, SiMNP, A-MNP, RMNP, A-SiMNP, and A-RMNP, respectively. As observed, the saturation magnetization of MNPs was decreased by the modifying layers and increased diameters, maybe due to the compounds of diamagnetic materials (SiO2, Arg, OTMS) surrounding the bare MNPs.
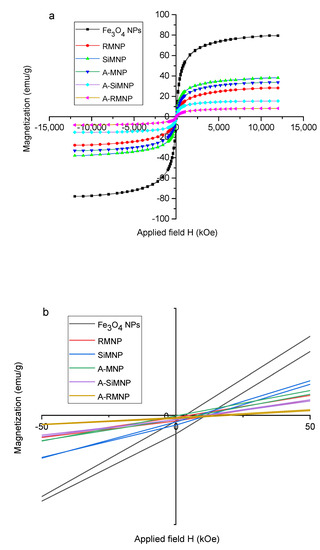
Figure 4.
(a) Hysteresis loops for A-SiMNP, A-RMNP, A-MNP, RMNP, MNP, and SiMNP, (b) the magnification of the graph (a).
Figure 4b shows the magnification of Figure 4a for better investigation of coercivity. As can be seen in Figure 4b, with the increased coating around the core, the magnetic moments decreased. On the other hand, as the magnetization was reduced, the conductivity also decreased. In the particles with two coatings, the magnetization decreased due to the presence of carbon chains on the surface, and in the case of SiMNP, it should be noted that due to the porous nature of the silica coating [62,64], the magnetic moments had the possibility of leaving the first layer, and, therefore, the magnetization was higher than that of the other coated particles.
To verify the binding of silanes on the surface of NPs, FTIR spectra of the MNPs before and after alkyl silane coating were collected, and the results are shown in Figure 5. Peaks at 2974, 2922, and 2850 cm−1 originated from the stretching vibrations of C-H bonds in alkyl chains, and peaks at 1455 and 1395 cm−1 were due to the in-plane bending vibrations of -CH3 and -CH2-. The intensity and position of these bands were slightly different because of the length differences of the alkyl chains. The strong absorption peak at 583 cm−1 was the characteristic band of the Fe-O stretching vibrations of Fe3O4 NPs. The bulk magnetite’s Fe-O stretching vibration band is usually at 570 cm−1. Compared with the uncoated Fe3O4 NPs, the Fe-O absorption bands of Fe3O4 NPs coated with alkyl silanes were shifted to a higher wavenumber of 585 cm−1 due to the formation of Fe-O-Si bonds on the surfaces of the coated NPs. Since -Si(O-)3- is more electronegative than H, it increases the force constant of Fe-O bonds and the consequent shift of the peak position [65]. The weak bands at 1050 cm−1 corresponded to Fe-O-Si stretching vibrations, showing that alkyl silanes were successfully crossed on Fe3O4 NPs. Two peaks at 3400 and 1630 cm−1 were attributed to the stretching and bending vibrations of the O-H band from residual water in the samples.
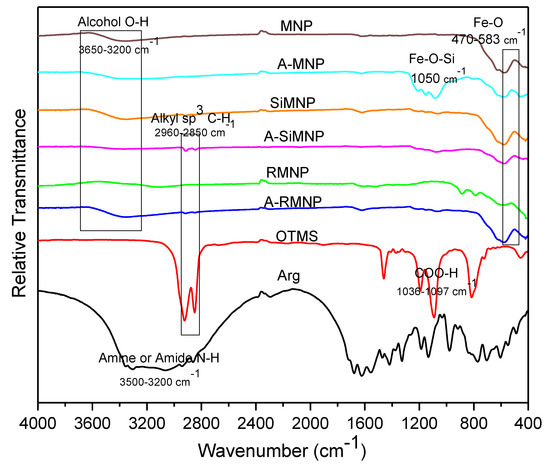
Figure 5.
FTIR spectra of all the MNPs.
On the other hand, the absorption band of the Fe-O-Si group, which was reported to appear at around 584 cm−1, was probably overlapped with the absorption bands of the Fe-O groups [9]. Characteristic absorption bands of the -OH stretching vibration mode were located at 3414 and 3742 cm−1. In addition, the absorption band at 1624 cm−1 was assigned to the H-O-H bending mode. The bands at 800 and 1080 cm−1 were related to the asymmetrical and symmetrical vibrations of the Si-O-Si, respectively. In addition, a band at 937 cm−1 was allocated to the Si-OH vibrations [10]. These results suggest that silica was chemisorbed onto the MNPs. FTIR spectroscopy was carried out on the sample after washing with deionized water to remove free -OH groups. Hence, the remaining-OH groups (with a band at 3414 cm−1) were probably due to silanol groups in the system.
OTMS showed strong peaks at 2920 cm−1 and 2860 cm−1, the absorption peaks of C-H stretching vibrations. These peaks were not observed in the FTIR spectra of MNPs, while after immobilization of OTMS on the surface, these peaks could again be identified. The results suggest that OTMS was successfully immobilized on the surface of the NPs.
Elemental analysis (CHN analysis) was performed to verify the amount of element content in alkylated NPs. The results showed 7.42% carbon content for A-SiMNP and 2.48 and 1.76% for A-MNP and A-RMNP, respectively. Since OTMS has 21 carbon atoms, this amount showed the amount of adsorbed alkyl chains on the NPs (Table 1).

Table 1.
CHN analysis of magnetite and alkylated NPs.
As shown in Table 1, the carbon content in A-MNP increased from 0.81% (just MNP) to 2.58%, which indicates that OTMS was successfully immobilized on the surface of MNP. However, for A-RMNP and A-SiMNP, the carbon content increased from 0.81 to 1.76 and 7.42%, respectively. We found that the increment in carbon content was about 2, 3 and 9% for A-RMNP, A-MNP, and A-SiMNP, respectively. It is obvious that among all the alkylated NPs, the alkylated SiMNP was the best in the sense of decoration by OTMS. This is due to the porous structure of SiMNP [66], the presence of Si in the SiMNP, the linkage of Si-O-Si and Si-Fe, and consequently the ease of covalent bonds between C and Si [67].
SEM images and histograms showed that these surface modifications did not lead to any significant aggregation of the NPs. The hydrodynamic particle sizes of these six NPs were also determined by DLS, the results of which are shown in Figure 6. DLS provides statistical representative data about the hydrodynamic size of nanomaterials (hydrodynamic radius on an ensemble average). However, the interpretation of DLS data involves the interplay of a few parameters, such as the size, concentration, shape, polydispersity, and surface properties of the nanoparticles [68]. The hydrodynamic diameters were overall larger than the values determined by SEM. Such large size differences indicate the formation of aggregates of these NPs in an aqueous solution. It is interesting to note that all particles had their peak sizes around 130 nm. However, Figure 3 shows a small but clear increase in aggregate size with the chain length of surface modification, an effect arising from increasing surface hydrophobicity. The hydrodynamic size order was: A-RMNP > A-SiMNP > SiMNP > RMNP > A-MNP > MNP.
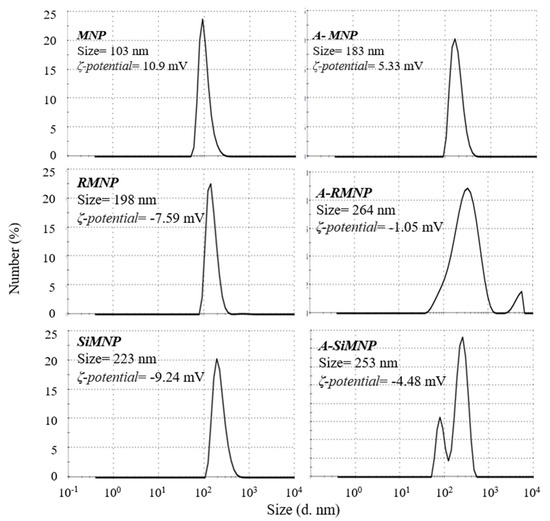
Figure 6.
DLS analysis of MNP, SiMNP, A-MNP, RMNP, A-SiMNP, and A-RMNP.
Zeta potential (ζ) is significant for biomedical applications to achieve expected and consistent outcomes. A low value of zeta potential implies that the particle may show poor stability in aqueous solutions. Low zeta potential values (0 to ±5 mV) will improve van der Waals interparticle attractions and causes rapid coagulation and flocculation of particles. On the other hand, higher values of zeta potential imply that the particle may show good stability in aqueous solutions. There is a specific zeta potential value (≈±30 mV) that determines the stability of particles. At this value, high electrostatic repulsive forces between the particles occur [69]. The zeta potentials of the magnetite particles (indicated in Figure 6) significantly decreased after coating with alkyl silane compounds, showing the different extent of shielding of the bare oxide surface. The magnetite surface is amphoteric [70], and the surface charges originate from the protonation or deprotonation [71] of surface Fe-OH according to the pH environment. Changes in zeta potential indicated that the Fe3O4 NPs were coated with silane groups, consistent with the FTIR and CHN results. Because of the steric hindrance of alkyl silanes [72], not all hydroxyl groups on the particle surface reacted with alkyl trimethoxy silanes. However, suppose the alkyl chains are very long. In that case, they can provide the NPs with a strong hydrophobic layer to prevent proton diffusion to the MNP surface, resulting in the almost lower zeta potential of A-MNP, A-SiMNP, and A-RMNP. Hence, the non-zero zeta potentials in A-RMNP and A-MNP arose from a partial silane neutralization and weak alkyl chain coverage, leaving surface defects for surface proton diffusion. According to Figure 6, the zeta potentials of the alkylated NPs were lower than those of the other three NPs.
4. Conclusions
Synthesis of six NPs was performed by adding OTMS, Arg, and TEOS as the precipitators in the solution. Coating the surface of the NPs with various types of functionalization agents can induce different hydrophobic degrees of MNPs. XRD analysis confirmed the core of the MNP in the six synthesized NPs. The results of the VSM analysis showed decreasing magnetization with an increase in the density of capped agents. The alkylation of NPs was demonstrated by C and Si, determined by CHN analysis and the FTIR method. Zeta potential measurements of the samples exhibited a large negative charge that could attract positively charged targets in the aqueous environments that are so important and useful in bio-applications. Because of the remarkable linkage of carbon chains to alkylated NPs and their significant hydrophobicity, in addition to industrial applications, bioseparation will be an interesting subject for further studies. Our study demonstrated the successful synthesis of MNP and functionalized magnetic NPs composed of MNP capped by silane derivatives (SiMNP) and MNPs capped by L-Arginine (RMNP) as core, which then was modified with 18 carbon chains of alkyl groups that were prepared by the co-precipitation method. It was revealed that alkyl chains easily connected to all three MNPs, while their different properties were characterized and compared. It was observed that the SiMNP had a high amount of alkyl chains between the three alkylated NPs. The impact of alkylated NPs on the refolding, denaturation, and aggregation process of recombinant proteins using lysozyme as a model protein could be a subject for future study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/magnetochemistry8110143/s1, Figure S1. TEM images of (a) Fe3O4 NPs and (b) alkylated Fe3O4 NPs (A-MNPs).
Author Contributions
Conceptualization, S.A.S.E. and M.H.-R.; Data curation, F.K. and Z.L.; Investigation, V.A.; Methodology, V.A.; Project administration, S.A.S.E. and M.H.-R.; Resources, S.A.S.E.; Supervision, S.A.S.E.; Validation, V.A.; Visualization, V.A.; Writing—original draft, V.A. and Z.L.; Writing—review and editing, S.A.S.E., M.H.-R. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
The support from the University of Tehran and National Institute for Medical Research Development (NIMAD) are gratefully acknowledged.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s disease: Pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [PubMed]
- Raouf, I.; Khalid, S.; Khan, A.; Lee, J.; Kim, H.S.; Kim, M.H. A review on numerical modeling for magnetic nanoparticle hyperthermia: Progress and challenges. J. Therm. Biol. 2020, 91, 102644. [Google Scholar] [CrossRef] [PubMed]
- Lemmerman, L.R.; Das, D.; Higuita-Castro, N.; Mirmira, R.G.; Gallego-Perez, D. Nanomedicine-based strategies for diabetes: Diagnostics, monitoring, and treatment. Trends Endocrinol. Metab. 2020, 31, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Ale Ebrahim, S.; Ashtari, A.; Zamani Pedram, M.; Ale Ebrahim, N. Publication trends in drug delivery and magnetic nanoparticles. Nanoscale Res. Lett. 2019, 14, 164. [Google Scholar] [CrossRef]
- Liu, G.; Swierczewska, M.; Lee, S.; Chen, X. Functional nanoparticles for molecular imaging guided gene delivery. Nano Today 2010, 5, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Da, H.; Yang, X.; Hu, Y. Preparation and characterization of core-shell monodispersed magnetic silica microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2003, 231, 123–129. [Google Scholar] [CrossRef]
- Liu, H.; Hou, P.; Zhang, W.; Wu, J. Synthesis of monosized core–shell Fe3O4/Au multifunctional nanoparticles by PVP-assisted nanoemulsion process. Colloids Surf. A Physicochem. Eng. Asp. 2010, 356, 21–27. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-nano interactions: Binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater. Res. 2021, 25, 12. [Google Scholar] [CrossRef]
- Dutz, S.; Weidner, A.; von der Lühe, M.; Gräfe, C.; Biehl, P.; Demut, J.; Warncke, P.; Jungmann, S.; Fischer, D.; Schacher, F.H.; et al. Hybrid nanomaterials of biomolecule corona coated magnetic nanoparticles and their interaction with biological systems. Phys. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Hekmatian, E.; Sadeghi, M.; Kennedy, K. Synthesis and characterization of core-shell Fe3O4-gold-chitosan nanostructure. J. Nanobiotechnol. 2012, 10, 3. [Google Scholar] [CrossRef]
- Pana, O.; Teodorescu, C.M.; Chauvet, O.; Payen, C.; Macovei, D.; Turcu, R.; Soran, M.L.; Aldea, N.; Barbu, L. Structure, morphology and magnetic properties of Fe–Au core-shell nanoparticles. Surf. Sci. 2007, 601, 4352–4357. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, K.; An, J.; Sun, Y.; Sun, C. Synthesis and layer-by-layer self-assembly of silver nanoparticles capped by mercaptosulfonic acid. Mater. Lett. 2006, 60, 1215–1218. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Core–shell and multilayered magnetite nanoparticles—Structural and Mössbauer studies. Appl. Surf. Sci. 2014, 306, 7–15. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Kropiewnicka, K. The influence of the transition metal substitution on chemically prepared ferrite nanoparticles–Mössbauer studies. Curr. Appl. Phys. 2012, 12, 896–902. [Google Scholar] [CrossRef]
- Kalska, B.; Paggel, J.J.; Fumagalli, P.; Rybczyński, J.; Satula, D.; Hilgendorff, M.; Giersig, M. Magnetite particles studied by Mössbauer and magneto-optical Kerr effect. J. Appl. Phys. 2004, 95, 1343–1350. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Rehana, D.; Haleel, A.K.; Rahiman, A.K. Hydroxy, carboxylic and amino acid functionalized superparamagnetic iron oxide nanoparticles: Synthesis, characterization and in vitro anti-cancer studies. J. Chem. Sci. 2015, 127, 1155–1166. [Google Scholar] [CrossRef]
- Bagherpour, A.R.; Kashanian, F.; Ebrahimi, S.S.; Habibi-Rezaei, M. L-arginine modified magnetic nanoparticles: Green synthesis and characterization. Nanotechnology 2018, 29, 075706. [Google Scholar] [CrossRef]
- Kashanian, F.; Habibi-Rezaei, M.; Bagherpour, A.R.; Seyedarabi, A.; Moosavi-Movahedi, A.A. Magnetic nanoparticles as double-edged swords: Concentration-dependent ordering or disordering effects on lysozyme. RSC Adv. 2017, 7, 54813–54822. [Google Scholar] [CrossRef]
- Lajmorak, A.; Seyyed Ebrahimi, S.A.; Yazdian, F.; Lalegani, Z.; Hamawandi, B. The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme. Int. J. Mol. Sci. 2022, 23, 9657. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.Y.; Stephanie, I.; Lim, I.; Schadt, M.J.; Mott, D.; Luo, J.; Wang, X.; Zhong, C.J. Core@ shell nanomaterials: Gold-coated magnetic oxide nanoparticles. J. Mater. Chem. 2008, 18, 2629–2635. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.; Vitiello, G. Understanding the nano-bio interfaces: Lipid-coatings for inorganic nanoparticles as promising strategy for biomedical applications. Front. Chem. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Zaloga, J.; Pöttler, M.; Dürr, S.; Eberbeck, D.; Tietze, R.; Lyer, S.; Alexiou, C. Strategies to optimize the biocompatibility of iron oxide nanoparticles–“SPIONs safe by design”. J. Magn. Magn. Mater. 2017, 431, 281–284. [Google Scholar] [CrossRef]
- El-Gendy, A.A. Core/shell magnetic nanoparticles for biomedical applications. In Magnetic Nanostructured Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–58. [Google Scholar]
- Ishikawa, T.; Cai, W.Y.; Kandori, K. Adsorption of molecules onto microporous hematite. Langmuir 1993, 9, 1125–1128. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Grassian, V.H. Surface reactions of carbon dioxide at the adsorbed water–iron oxide interface. J. Phys. Chem. B 2005, 109, 12227–12230. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Guo, D.; Wu, C.; Li, X.; Jiang, H.; Wang, X.; Chen, B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. J. Nanosci. Nanotechnol. 2008, 8, 2301–2307. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Pansieri, J.; Gerstenmayer, M.; Lux, F.; Mériaux, S.; Tillement, O.; Forge, V.; Larrat, B.; Marquette, C. Magnetic nanoparticles applications for amyloidosis study and detection: A review. Nanomaterials 2018, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Dencheva, N.; Oliveira, S.; Braz, J.; Getya, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Magnetically responsive PA6 microparticles with immobilized laccase show high catalytic efficiency in the enzymatic treatment of catechol. Catalysts 2021, 11, 239. [Google Scholar] [CrossRef]
- Dencheva, N.; Braz, J.; Scheibel, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Polymer-assisted biocatalysis: Polyamide 4 microparticles as promising carriers of enzymatic function. Catalysts 2020, 10, 767. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Adil, S.F.; Shaik, M.R.; Abdelgawad, A.; Hatshan, M.R.; Khan, M. Surface-coated magnetic nanostructured materials for robust bio-catalysis and biomedical applications-A review. J. Adv. Res. 2021, 38, 157–177. [Google Scholar] [CrossRef]
- Huang, G.; Lu, C.H.; Yang, H.H. Magnetic nanomaterials for magnetic bioanalysis. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–109. [Google Scholar]
- Wiedemann, G.J.; Robins, H.I.; Gutsche, S.; Mentzel, M.; Deeken, M.; Katschinski, D.M.; Eleftheriadis, S.; Crahe, R.; Weiss, C.; Storer, B.; et al. Ifosfamide, carboplatin and etoposide (ICE) combined with 41.8 C whole body hyperthermia in patients with refractory sarcoma. Eur. J. Cancer 1996, 32, 888–892. [Google Scholar] [CrossRef]
- Willner, I.; Katz, E. Controlling chemical reactivity at solid–solution interfaces by means of hydrophobic magnetic nanoparticles. Langmuir 2006, 22, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.; Atta, A.M.; Allohedan, H.A.; Alkhathlan, H.Z.; Khan, M.; Ezzat, A.O. Green synthesis of hydrophobic magnetite nanoparticles coated with plant extract and their application as petroleum oil spill collectors. Nanomaterials 2018, 8, 855. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of Palladium (II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef]
- Condomitti, U.; Zuin, A.; Silveira, A.T.; Araki, K.; Toma, H.E. Magnetic nanohydrometallurgy: A promising nanotechnological approach for metal production and recovery using functionalized superparamagnetic nanoparticles. Hydrometallurgy 2012, 125, 148–151. [Google Scholar] [CrossRef]
- Kayed, R.; Glabe, C.G. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol. 2006, 413, 326–344. [Google Scholar]
- Hsieh, S.; Chang, C.W.; Chou, H.H. Gold nanoparticles as amyloid-like fibrillogenesis inhibitors. Colloids Surf. B Biointerfaces 2013, 112, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhuo, Y.; Zhou, Y.; Wang, H.J.; Yuan, R.; Chai, Y.Q. Ceria doped zinc oxide nanoflowers enhanced luminol-based electrochemiluminescence immunosensor for amyloid-β detection. ACS Appl. Mater. Interfaces 2016, 8, 12968–12975. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; Pernodet, N.; Li, B.; Lin, C.H.; Hatchwell, E.; Rafailovich, M.H. Multicomponent polymer coating to block photocatalytic activity of TiO 2 nanoparticles. Chem. Commun. 2007, 45, 4815–4817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.Y.; Zhang, H.Q.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Petcu, C.; Purcar, V.; Spătaru, C.I.; Alexandrescu, E.; Şomoghi, R.; Trică, B.; Niţu, S.G.; Panaitescu, D.M.; Donescu, D.; Jecu, M.L. The influence of new hydrophobic silica nanoparticles on the surface properties of the films obtained from bilayer hybrids. Nanomaterials 2017, 7, 47. [Google Scholar] [CrossRef]
- Nguyen, K.C. Quantitative analysis of COOH-terminated alkanethiol SAMs on gold nanoparticle surfaces. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045008. [Google Scholar] [CrossRef]
- Can, K.; Ozmen, M.; Ersoz, M. Immobilization of albumin on aminosilane modified superparamagnetic magnetite nanoparticles and its characterization. Colloids Surf. B Biointerfaces 2009, 71, 154–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Chen, L.; Chen, L.; Wan, Q.H. Synthesis of novel porous magnetic silica microspheres as adsorbents for isolation of genomic DNA. Biotechnol. Prog. 2006, 22, 514–518. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Banaei, A.; Bahar, S.; Karimi, S.; Mohammadi Ziarani, G.; Ganjali, M.R. Extraction of gold, palladium and silver ions using organically modified silica-coated magnetic nanoparticles and silica gel as a sorbent. Microchim. Acta 2017, 184, 3859–3866. [Google Scholar] [CrossRef]
- Hamawandi, B.; Ballikaya, S.; Batili, H.; Roosmark, V.; Orlovska, M.; Yusuf, A.; Johnsson, M.; Szukiewicz, R.; Kuchowicz, M.; Toprak, M.S. Facile solution synthesis, processing and characterization of n-and p-type binary and ternary Bi–Sb tellurides. Appl. Sci. 2020, 10, 1178. [Google Scholar] [CrossRef]
- Bedlovičová, Z. Green synthesis of silver nanoparticles using actinomycetes. In Green Synthesis of Silver Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 547–569. [Google Scholar]
- Malhotra SP, K.; Alghuthaymi, M.A. Biomolecule-assisted biogenic synthesis of metallic nanoparticles. In Nanobiotechnology for Plant Protection; Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–163. [Google Scholar]
- Zhu, X.; Pathakoti, K.; Hwang, H.M. Green synthesis of titanium dioxide and zinc oxide nanoparticles and their usage for antimicrobial applications and environmental remediation. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–263. [Google Scholar]
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cao, X.; Liu, G.H.; Hong, R.Y.; Chen, Y.M.; Chen, X.F.; Li, H.Z.; Xu, B.; Wei, D.G. Synthesis of Fe3O4 magnetic fluid used for magnetic resonance imaging and hyperthermia. J. Magn. Magn. Mater. 2011, 323, 2953–2959. [Google Scholar] [CrossRef]
- Zijlstra, P.; Chon, J.W.; Gu, M. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Lalegani, Z.; Ebrahimi, S.S.; Hamawandi, B.; La Spada, L.; Batili, H.; Toprak, M.S. Targeted dielectric coating of silver nanoparticles with silica to manipulate optical properties for metasurface applications. Mater. Chem. Phys. 2022, 287, 126250. [Google Scholar] [CrossRef]
- Ilankoon, N.D.; Aldrich, C. Recovery of Gold from Thiosulfate Leaching Solutions with Magnetic Nanoparticles. In Application of Nanotechnology in Mining Processes: Beneficiation and Sustainability; Scrivener Publishing LLC: Beverly, MA, USA, 2022; pp. 153–196. [Google Scholar]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@ SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Yamada, T.; Inoue, T.; Yamada, K.; Takano, N.; Osaka, T.; Harada, H.; Nishiyama, K.; Taniguchi, I. Detection of C–Si Covalent Bond in CH3 Adsorbate Formed by Chemical Reaction of CH3MgBr and H: Si (111). J. Am. Chem. Soc. 2003, 125, 8039–8042. [Google Scholar] [CrossRef]
- Darwish, M.S.; Bakry, A.; Al-Harbi, L.M.; Khowdiary, M.M.; El-Henawy, A.A.; Yoon, J. Core/shell PA6@ Fe3O4 nanofibers: Magnetic and shielding behavior. J. Dispers. Sci. Technol. 2020, 41, 1711–1719. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Darwish, M.S. Functionalized iron oxide nanoparticles: Synthesis through ultrasonic-assisted co-precipitation and performance as hyperthermic agents for biomedical applications. Heliyon 2022, 8, e09654. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, M.; Demetriou, M.; Marinica, O.; Vekas, L.; Krasia-Christoforou, T. An innovative synthesis approach toward the preparation of structurally defined multiresponsive polymer (co) networks. Polym. Chem. 2014, 5, 4365–4374. [Google Scholar] [CrossRef]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Cartledge, F.K. Steric effects on reactivity in silicon chemistry. Organometallics 1983, 2, 425–430. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).