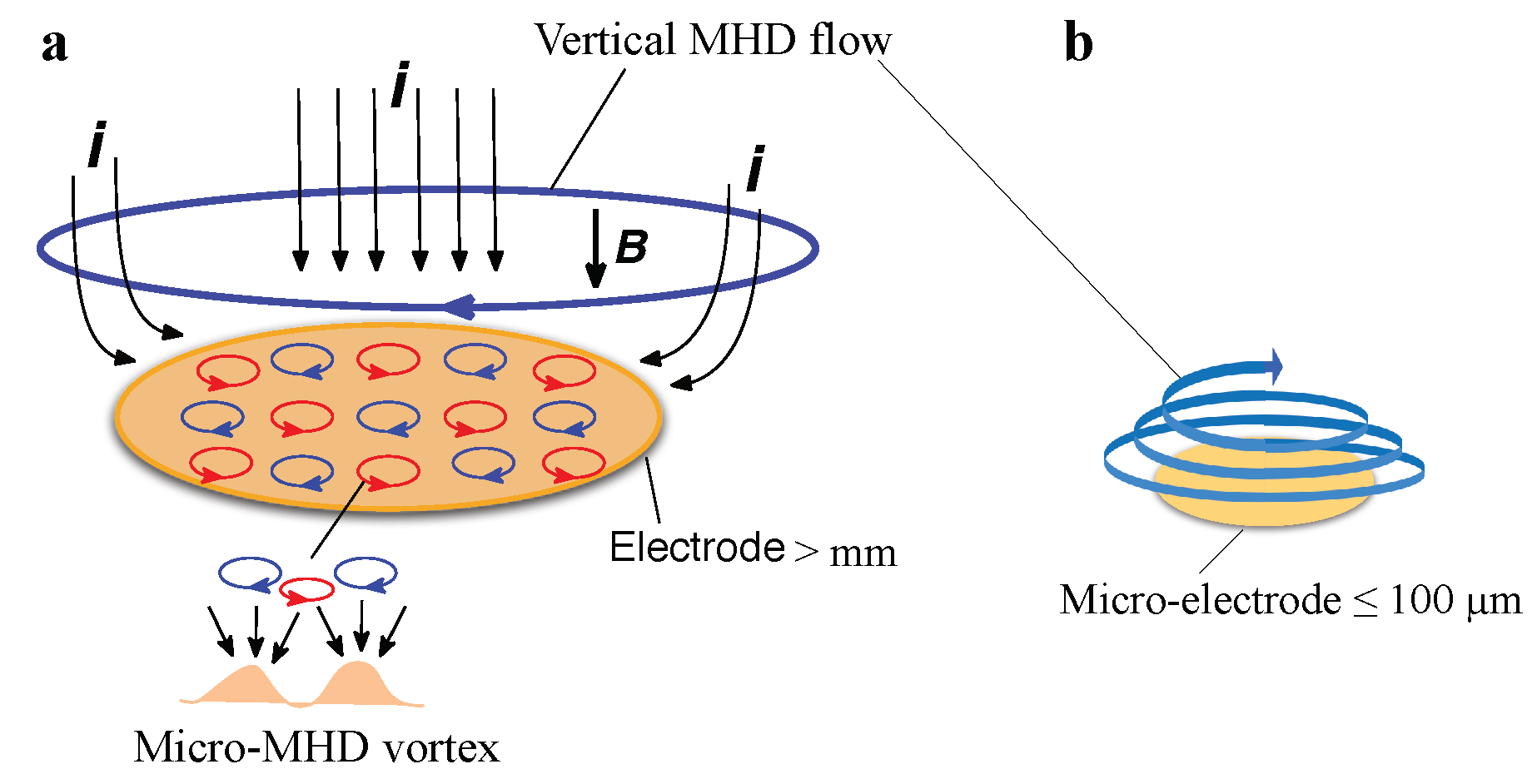
2.1. Chiral Behaviors of MED Films on a 100 µm-Electrode
The MED of copper films was conducted on a micro-disc electrode with 100 µm diameter in the magnetic fields of 1–5 T, which were parallel (+
B) or antiparallel (–
B) to the ionic currents. The film prepared in a magnetic field of +1 T, for example, is termed the +1 T-film. To estimate the surface chirality of MED films, the films were employed as electrodes, and the voltammograms of alanine enantiomers were measured on the MED film electrodes in a 0.1 M NaOH aqueous solution.
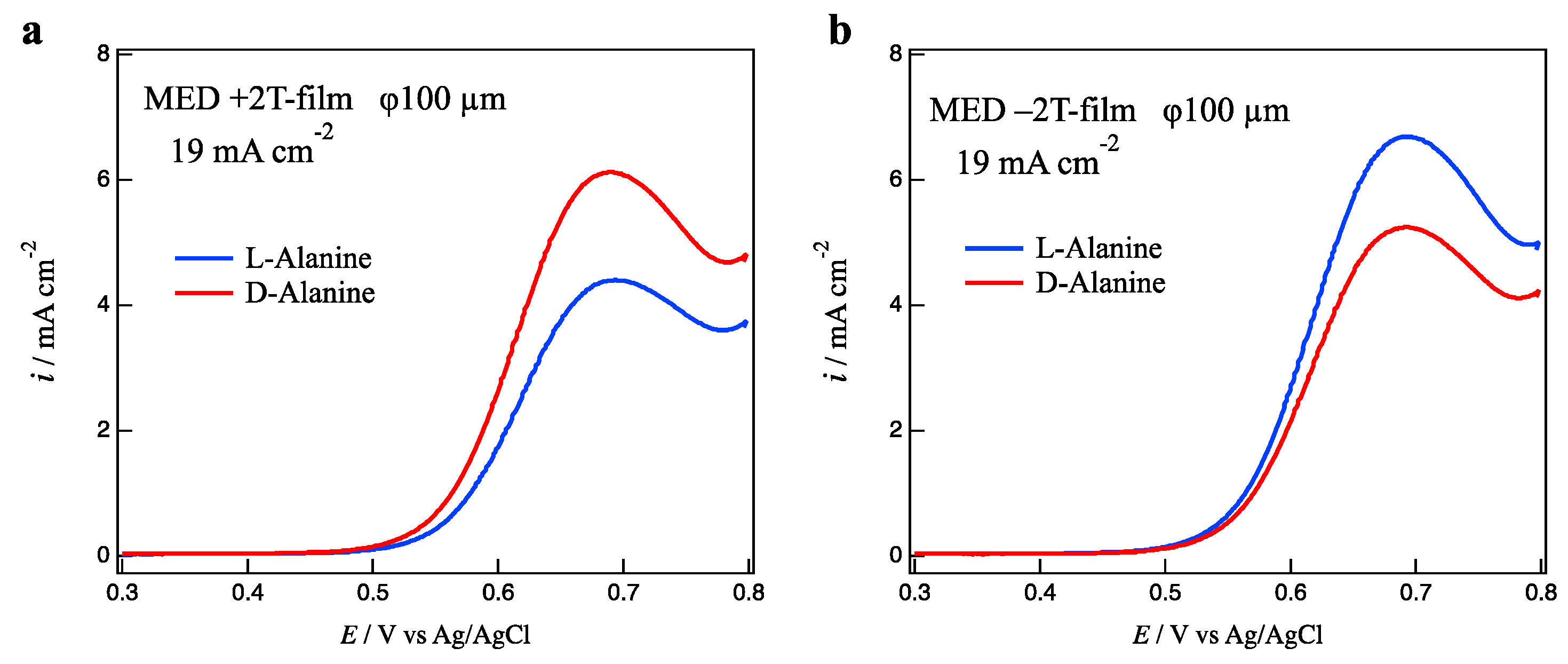
Figure 2a shows voltammograms of L- and D-alanines on the +2 T-film electrode prepared at a deposition current of 19 mA cm
−2. Alanine molecules are oxidized around 0.7 V on a copper electrode [
16], resulting in the current peaks in
Figure 2a. The surface chirality of MED films can be reflected in the peak currents of enantiomers. The peak current of D-alanine is greater than that of L-alanine. This fact represents that the +2 T-film electrode has D-activity. On the contrary, the −2 T-film electrode exhibits L-activity as shown in
Figure 2b.
The chirality in voltammograms was evaluated by an enantiomeric excess (
ee) ratio defined as
where
ipL and
ipD represent the peak currents of L- and D-alanines, respectively. The positive and negative signs of
ee ratios stand for L- and D-activity, respectively. As reported in our previous papers [
13], the surface chirality depends on the deposition current, thus the MED films were prepared at various deposition currents.
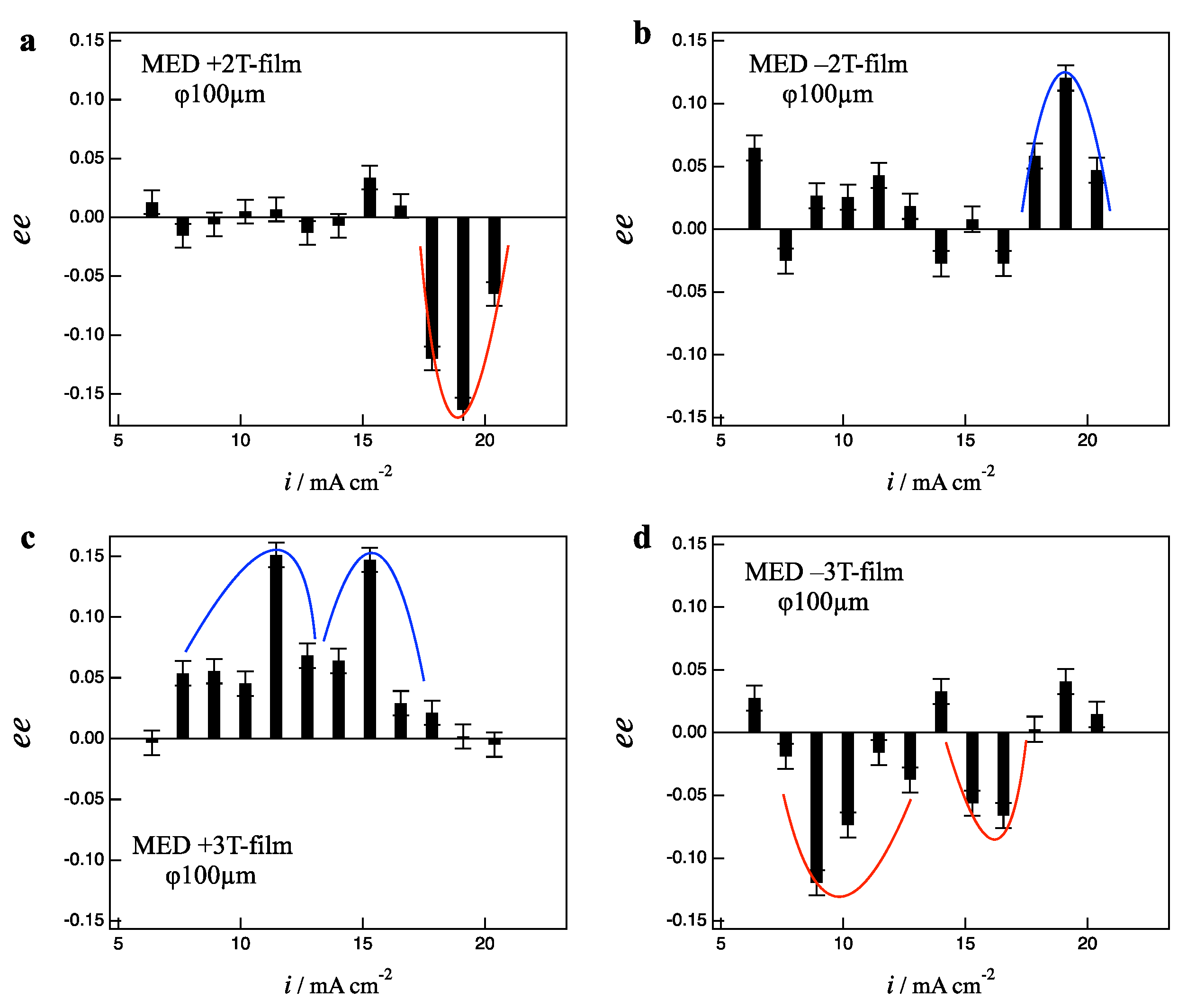
Figure 3a,b show the deposition current dependence of
ee ratios (
ee ratio profile) for the +2 T-film and −2 T-film electrodes, respectively. The +2 T-film shows D-activity in the current region around 19 mA cm
−2, on the contrary, the −2 T-film shows the L-activity at the same current region. This result can be described using the following diagrams:
Such a relation represents the odd chirality for the magnetic field polarity. The odd chirality is easily understood by the self-organized MHD model in
Figure 1a, as described above.
Similar odd chirality can be seen in the 3 T-films.
Figure 3c,d show the
ee ratio profiles for the +3 T- and −3 T-film electrodes, respectively. The +3 T-films exhibit L-activity in the wide current region of 7–17 mA cm
−2, and the −3 T-films exhibit D-activity in the same region. The results in
Figure 3 indicate that the self-organized MHD state is formed in the MED at 2 and 3 T even on the 100 µm-electrodes.
When the MED is conducted in the higher magnetic fields, the influence of vertical magnetic field is expected to be more substantial.
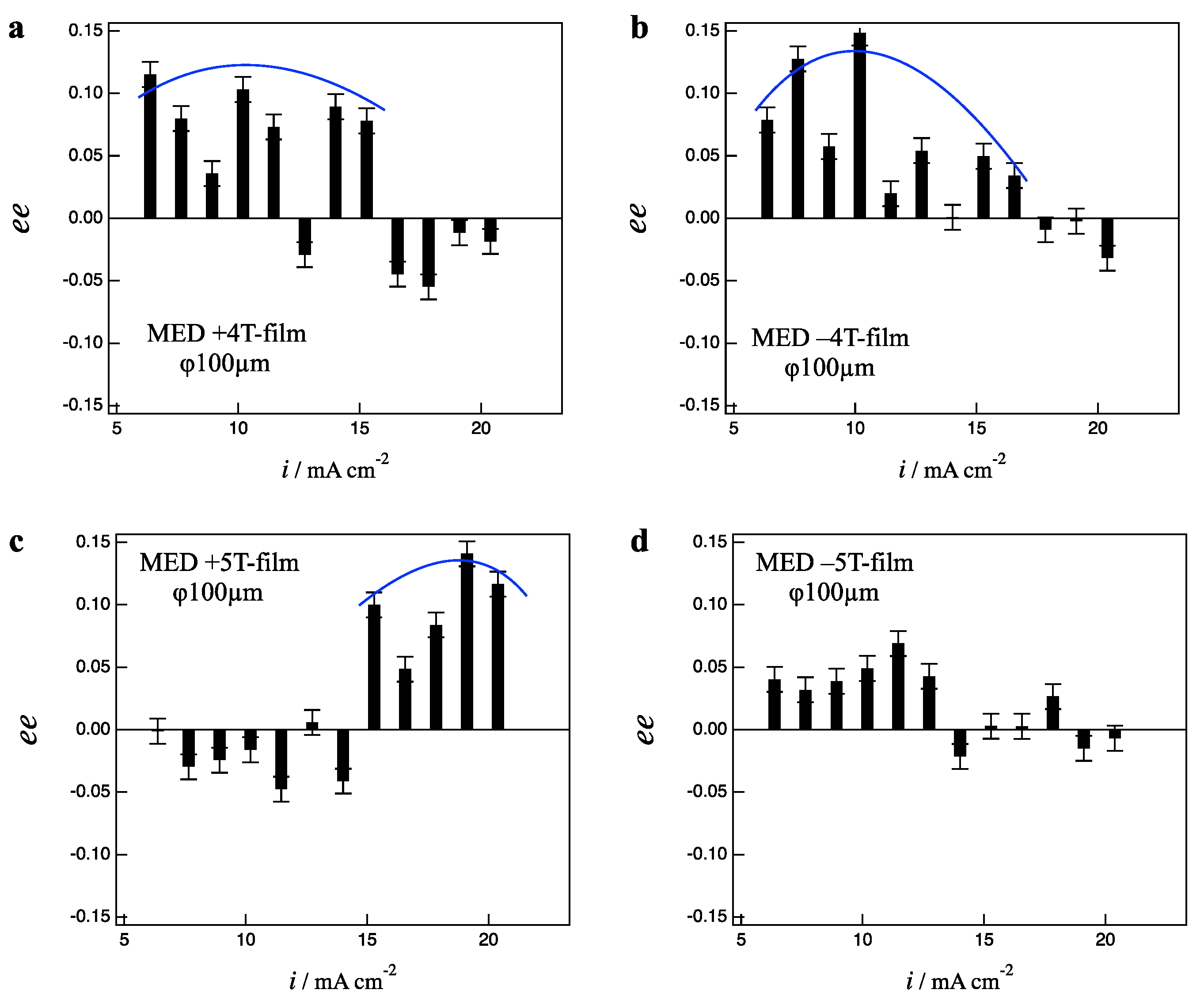
Figure 4a,b show the
ee ratio profiles for the +4 T- and −4 T-film electrodes, respectively. The +4 T-film exhibits L-activity in the current region of 6–15 mA cm
−2, and the −4 T-film also exhibits L-activity in the same current region. Such behaviors represent the clear breaking of odd chirality, rather even chirality for the magnetic field polarity.
Figure 4c,d show the
ee ratio profiles for the +5 T- and −5 T-film electrodes, respectively. The +5 T-film exhibits L-activity in the current region of 15–21 mA cm
−2, on the other hand, the −5 T-film exhibits achirality in the same current region. These facts also represent the breaking of odd chirality. The results in
Figure 4 indicate that the strong influence of vertical MHD flows causes the fluctuation of micro-MHD vortices, leading to the breaking of odd chirality. When the influence of vertical MHD flows is strong enough, the micro-MHD vortices have random fluctuation, leading to the disappearance of surface chirality as observed in the −5 T-film (
Figure 4d). When the influence is not so strong, the micro-MHD vortices have “ordered fluctuation”. Such ordered fluctuation could lead to the even chirality as observed in 4 T-films.
The ordered and random fluctuations of micro-MHD vortices were proposed in our previous paper [
14], where the effects of low magnetic fields on the surface chirality were studied with and without the specific adsorption of chloride ions. The low magnetic fields induce ordered fluctuation, leading to the even chirality for the magnetic field polarity. The even chirality is forbidden symmetry on the basis of the simple MHD model. A number of MED results [
13,
14,
15] suggest that the ordered fluctuation is crucial for the forbidden symmetry. On the other hand, the superimposed effects of low magnetic fields and specific adsorption induce random fluctuation, leading to the disappearance of surface chirality.
When the MED was conducted in the magnetic field lower than 2 T, the effects of low magnetic fields can be expected even in the micro-electrodes.
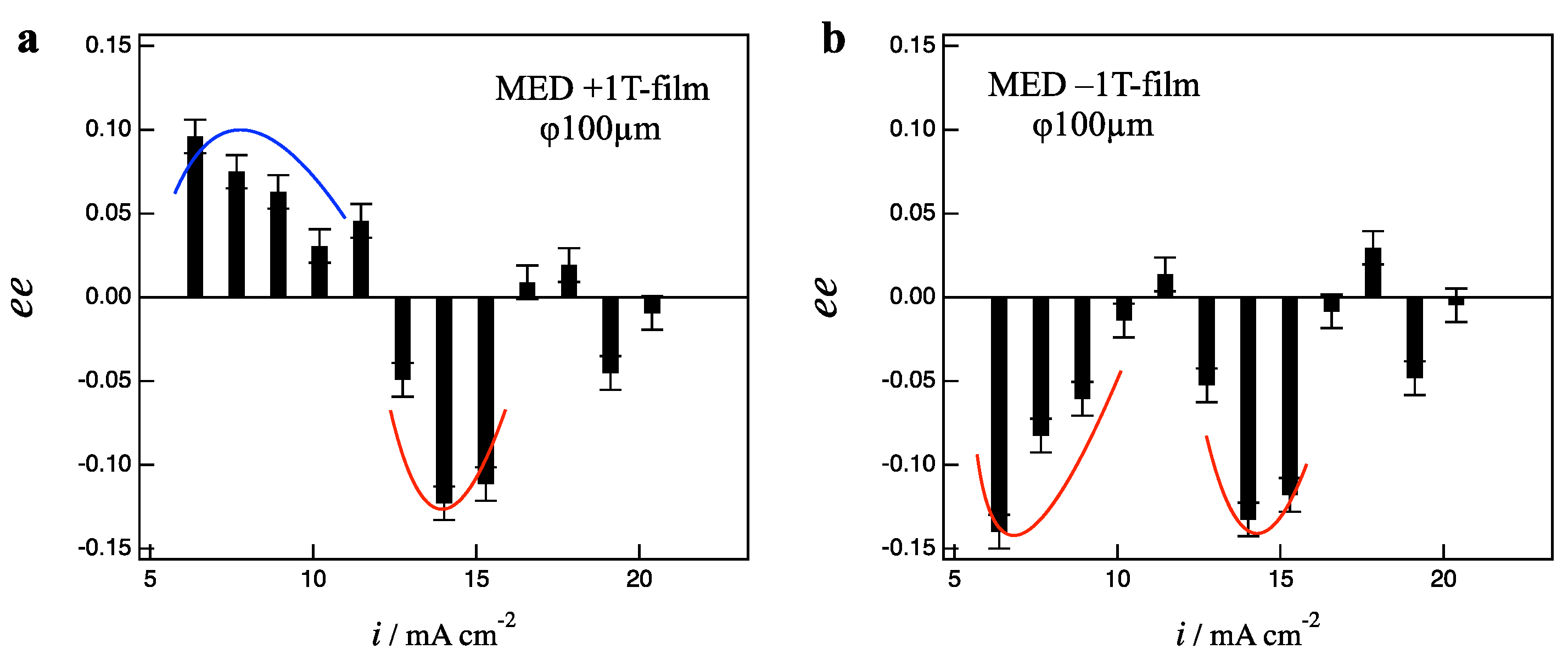
Figure 5a,b show the
ee ratio profiles for the +1 T- and −1 T-film electrodes, respectively. In the low current region of 6–10 mA cm
−2, the +1 T-film shows L-activity, and the −1 T-film shows D-activity, representing the odd chirality. On the contrary, in the middle current region of 12–16 mA cm
−2, both +1 T- and −1 T-films show D-activity, representing the even chirality. The
ee ratio profiles of 1 T-films exhibit the coexistence of the odd and even chirality. In the low magnetic field of 1T, the vertical MHD flows have tiny influence, instead, the low magnetic field causes the fluctuation of micro-MHD vortices, leading to the breaking of odd chirality.
2.2. Chiral Behaviors of MED Films on a 25 µm-Electrode
The influence of vertical MHD flows on the micro-MHD vortices depends on the electrode diameter. As the electrode is smaller, the influence would spread over the whole electrode surface, and it becomes more remarkable. To examine the effects of smaller electrode, the MED of copper films was conducted on a micro-disc electrode with 25 µm diameter.
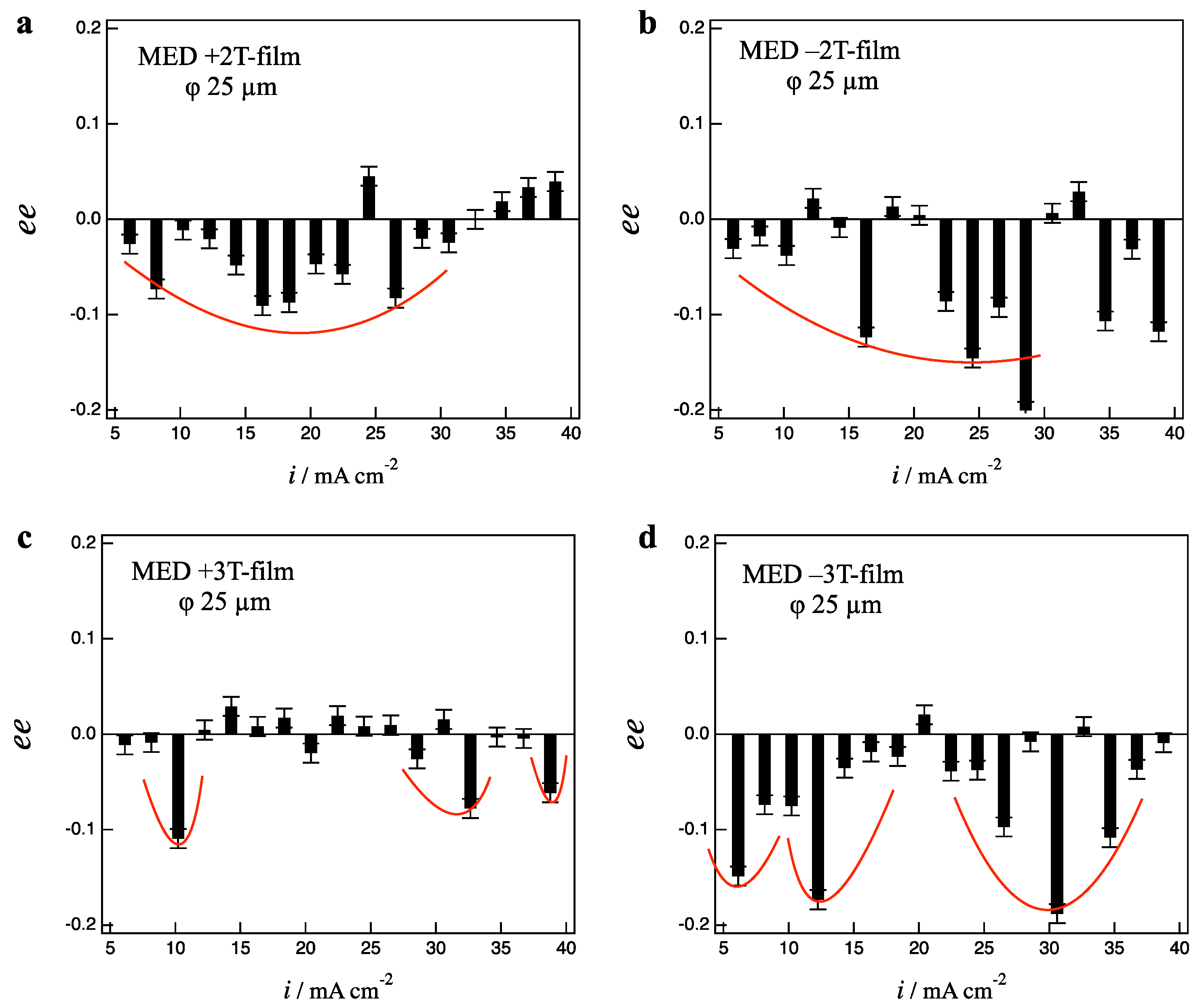
Figure 6a,b show the
ee ratio profiles for the +2 T- and −2 T-film electrodes, respectively. Both +2 T- and −2 T-films show D-activity in the wide current region of 6–30 mA cm
−2, representing even chirality. While the 2 T-films exhibit clear odd chirality on the 100 µm-electrodes (
Figure 4), they exhibit the breaking of odd chirality on the 25 µm-electrodes. This indicates that the fluctuation of micro-MHD vortices is ascribed to the vertical MHD flows.
The breaking of odd chirality can be seen in the 3 T-film as shown in
Figure 6c,d, where the −3 T-film show clear D-activity around 10 and 30 mA cm
−2, on the other hand, the +3 T-film show almost achirality except tiny D-activity around 10 and 30–40 mA cm
−2. As reported in our previous paper [
17], both +5 T- and −5 T-films showed achirality on the 25 µm-electrodes. The random fluctuation of micro-MHD vortices is dominant in the MED processes of the +3 T- and 5 T-films.
When the MED was conducted in the lower magnetic fields, the influence of vertical MHD flows could be suppressed even in the 25 µm-electrodes.
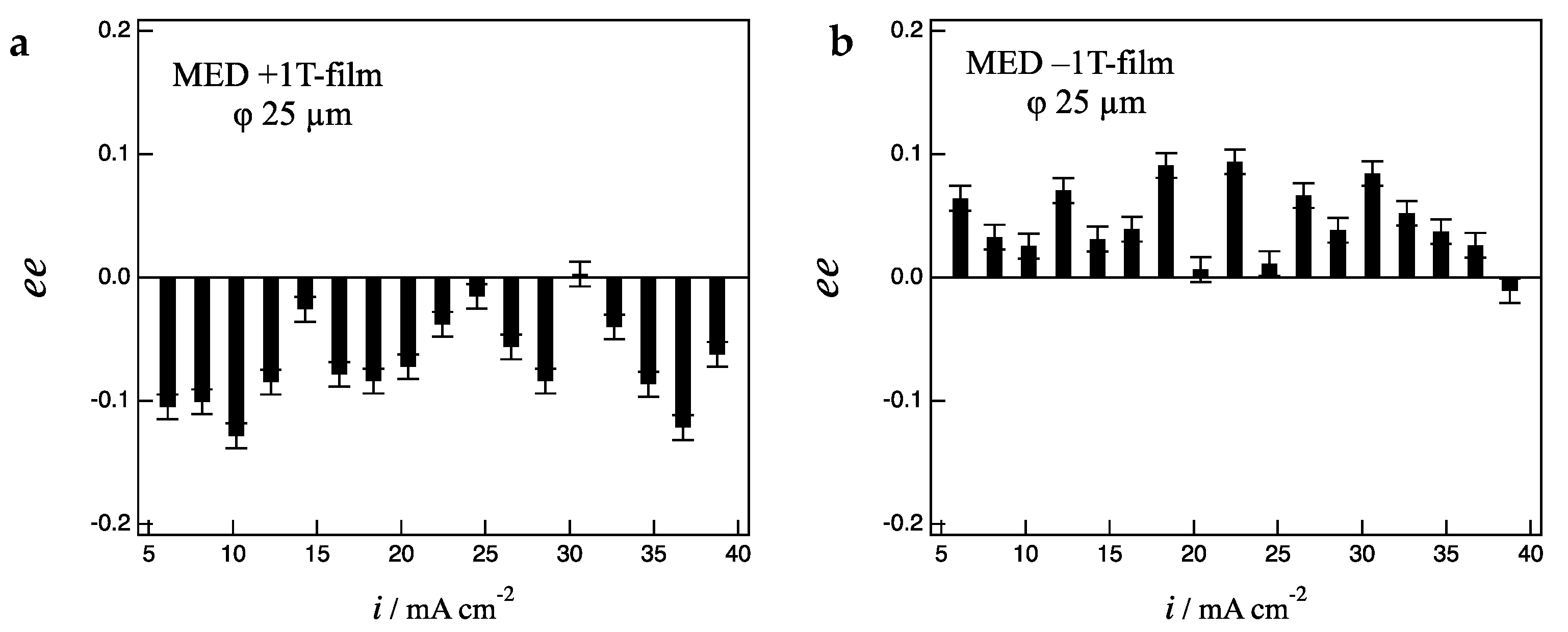
Figure 7a,b show the
ee ratio profiles for the +1 T- and −1 T-film electrodes, respectively. The +1 T-film shows D-activity in the whole current region, in contrast, the −1 T-film shows L-activity, representing the clear odd chirality. While the 1 T-films partially exhibits the breaking of odd chirality on the 100 µm-electrodes (
Figure 5), they exhibit only odd chirality. This result suggests that the fluctuation effects arising from low magnetic fields could be suppressed in the smaller-size electrode.
2.3. Mapping of Chiral Symmetry
We have shown the chiral symmetry behaviors of MED films in the magnetic fields of 1–5 T on the 100 µm- and 25 µm-electrodes.
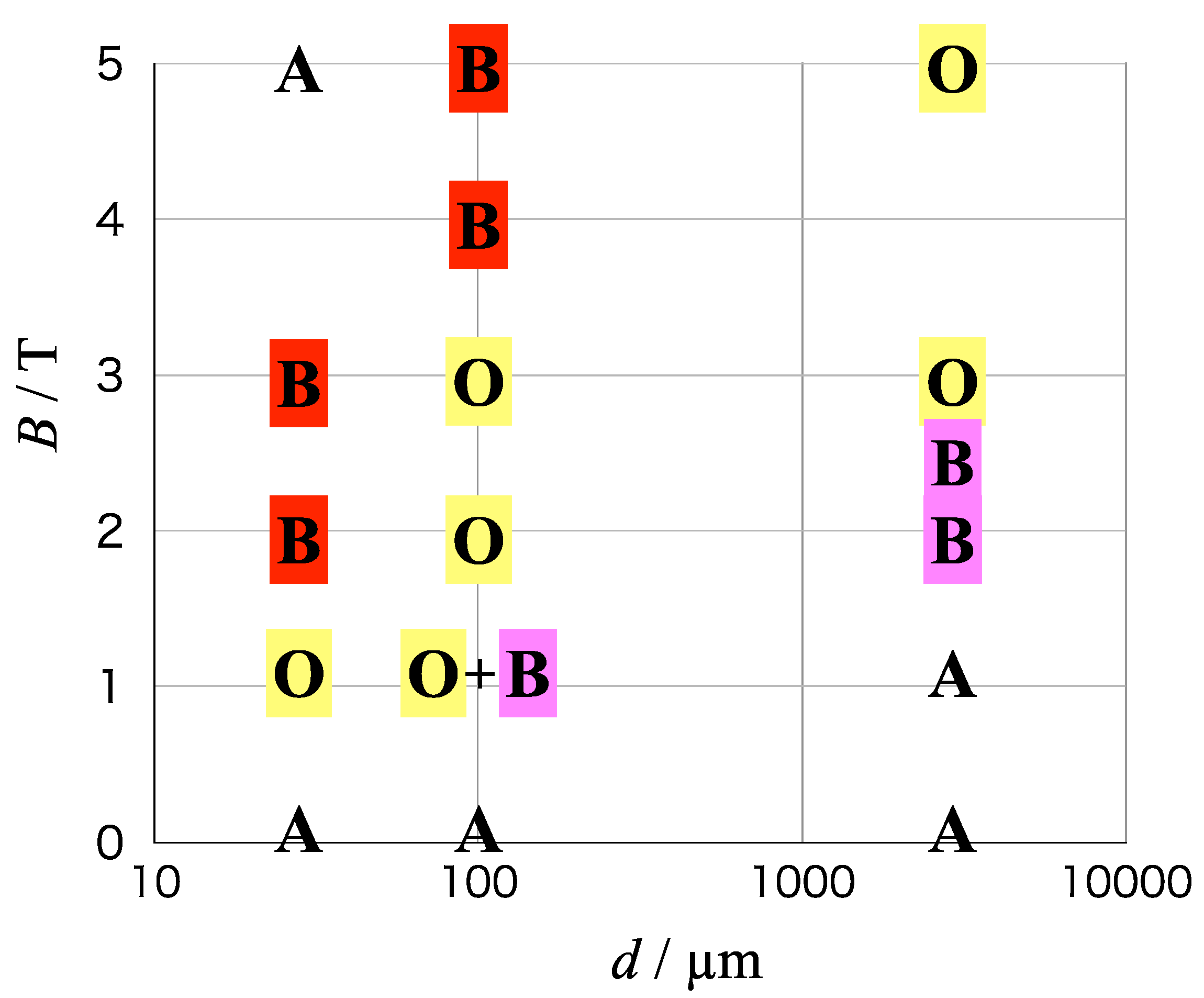
Figure 8 shows the mapping of chiral symmetry on the axes of magnetic field vs. electrode diameter, including the results of 3 mm-electrodes in our previous paper [
14]. The chiral symmetry is classified into three types: odd chirality (O) for the magnetic field polarity, breaking of odd chirality (B) and achirality (A).
The surface chirality on copper MED films was found in the 5 T-film on the 3 mm-electrode for the first time [
13,
14]. As the electrode size decreases, the influence of vertical MHD flows causes the ordered fluctuation of micro-MHD vortices, leading to the breaking of odd chirality (red-B) on the 100 µm-electrode. More drastic influence of vertical MHD flows causes the random fluctuation on the 25 µm-electrode, leading to the achirality. On the other hand, as the magnetic field decreases on the 3 mm-electrode, the ordered fluctuation of micro-MHD vortices emerges at 2–2.5 T, leading to the breaking of odd chirality (pink-B). The self-organized state of micro-MHD vortices cannot well develop in the lower magnetic field of 1 T, thereby the MED films show achirality.
The odd chirality appears at the lower magnetic fields of 1–3 T on the 100 µm-electrodes and at 1 T on the 25 µm-electrodes. The low magnetic fields reduce the influence of vertical MHD flows, thereby the self-organized state of micro-MHD vortices survives on the micro-electrodes. The breaking of odd chirality at the 1 T-film on the 100 µm-electrodes implies the fluctuation effect induced by the low magnetic fields.
Figure 8 shows that the chiral surfaces are formed in the wide areas of magnetic field and electrode diameter (the types O and B). It is remarkable that the chiral surface can be formed without chiral agents. There are two types of breaking of odd chirality: red-B and pink-B. The red-B is caused by the vertical MHD flows, and the pink-B is caused by the low magnetic fields. The area of type O is surrounded by those of type B, and the achirality is in the outer areas. Hence, the odd chirality exists in the confined areas of magnetic field and electrode diameter. This fact implies that the odd chirality could be easily broken by the fluctuation of micro-MHD vortices.