Recent Advances in Magnetic Nanoparticles and Nanocomposites for the Remediation of Water Resources
Abstract
1. Introduction
- The adsorption of the contaminant on the surface or internal structure of the adsorbent [17,18]. This process does not chemically alter the nature of the contaminant and provides the potential for the subsequent desorption and concentration of the contaminant, a process which can be used to isolate in an economical way select contaminants [19].
- Catalytic degradation. This is a term for a whole series of different chemical processes initiated or mediated by the presence of the catalyst. There are many different classes of catalytic activity which have been reported for nanomaterials in relation to removal of contaminants from water such as photocatalysis [20], reduction [21] and catalysis of the activation of oxidant species [22].
- Strong magnetic response. This will enable the rapid recovery of the nanomaterial from suspension and prevent loss of the nanomaterial to the environment.
- High rate of removal of contaminant. This may be due to effective adsorption of the contaminant or strong catalytic activity against the contaminant species.
- Rapid reuse. After recovery it would be important that the nanomaterial could be readily reused with the minimum of processing. In the case of catalytic nanomaterials, they may be re-utilizable immediately while adsorbent nanomaterials may require adsorbed contaminants be removed or desorbed through elution or other processes.
- Stability. It would be required that the nanomaterials do not degrade and maintain good performance through multiple cycles of use. This is necessary to ensure that both the target contaminant is removed and the degradation products from the nanomaterial do not contaminate the water resource.
- Single nanoparticle materials: These consist of a single type of nanomaterial. In this section, nanomaterials which have been modified through ligand exchange or through chemical modification of their ligand sphere are included;
- Magnetic nanocomposite materials: these consist of more than one class of nanomaterial in which one of these materials would be magnetic. This class of material can combine the properties of its various components acting synergistically to perform their function;
- Magnetic biologic composites: these are specific composite materials where a magnetic nanoparticle is mixed with either cells or with other material of biologic origin. These composites can mix the magnetic activity of these nanomaterials with the processes initiated by the biologic material in order to perform their function.
2. Single Magnetic Nanoparticle Materials
2.1. Control of Magnetic Nanoparticle Formation and Behavior in Suspension
2.2. Surface Modification of Magnetic Nanoparticles
2.3. Metal Ferrite Magnetic Nanoparticles
2.4. Zerovalent Iron Nanoparticles
2.5. Conclusions
3. Magnetic Nanocomposites
3.1. Clay/Magnetic Nanoparticle Composite Materials
| Core NMP | Clay Component | Surface Area (m2 g−1) | Contaminant | Method of Removal | Ref |
|---|---|---|---|---|---|
| Fe3O4 | Attapulgite/CuO/CeO2 | 150.8 | Methylene blue | Fenton catalysis | [82] |
| S–nZVIs | Montmorillonite | N/A | Trichloroethylene | Reduction | [83] |
| Fe3O4 | Schwertmannite | 59.7 | Ciprofloxacin | Fenton reaction | [84] |
| CoFe2O4 | Diatomite | 137.8 | Bisphenol A | PMS activation | [85] |
| CoMn2O4 | Fibrous phosphosilicate | 124 | 4-nitrophenol 2-nitroaniline | Catalytic reduction | [86] |
| Fe2O3 | Zeolite | 264 | Methylene blue | Fenton reaction | [87] |
| Fe3O4 | Halloysite ZnO | N/A | Various bacteria | Inhibition of growth | [88] |
3.2. Carbon/Magnetic Nanomaterial-Based Nanocomposites
3.3. Polymer/Magnetic Nanomaterial Composites
3.4. Metallic/Metal Oxide/Magnetic Nanoparticle-Based Nanocomposites
3.5. Conclusions
4. Magnetic Biological Nanocomposites
4.1. Biologic Polymer/Magnetic Nanocomposites
4.2. Biochar/Magnetic Nanocomposites
4.3. Microbial/Magnetic Nanocomposites
4.4. Conclusions
5. Key Concepts for the Future
- (1)
- The use of nanomaterials enables a high degree of economy for the use of materials for the removal of various contaminants from water. Within the course of the review, we have highlighted examples of near-to- or above-unity-adsorption of contaminants such as have been reported for layered double hydroxides [75] and layered double oxides [74] containing composites, nickel, magnesium-and-zinc-ferrite nanoparticles [40], and chitosan with tin oxide [154] all of which can adsorb a large quantity of contaminants in a short period of time. Linked with the ability to be retained with a magnetic field and then reused over multiple cycles, it appears that only a small quantity of this class of nanomaterial would be necessary for remediating a large volume of water. This would be beneficial both environmentally and economically, as less material would be necessarily dispersed into water sources in order to remove contamination, and less raw material would be needed to perform contaminant removal. A similar observation may be made for the catalytic nanomaterials reported that could serve to degrade contaminants either singly or in the presence of oxidizing agents such as hydrogen peroxide [37] and oxygen [105] or reducing agents such as borohydride [139,141].
- (2)
- We see an increasing usage of biological materials, both processed and unprocessed, in the generation of novel nanocomposite materials. The use of biologic materials has certain benefits, as they may be designed for the specific application to which the composite has been produced, for example the transfer of electrons between nZVIs and substrate molecules [165]. Enlisting biological materials in the formation of nanocomposites enables us to use the evolution-perfected properties of these materials in our desired materials.
- (3)
- We also observe the formation of nanocomposites using various forms of waste material (Table 11). Through the use of waste materials, it is possible to not only to reduce the effect of contamination on water resources, but also to reduce the amount of waste being generated by the industrial source of the feedstock. This can be an important contribution to the long-term goal of bringing about a zero-waste economy.
- Enabling synthesis methods for the reliable generation of magnetic nanomaterials. This review demonstrates many different types of magnetic nanomaterial which have been prepared using many different methods. Most these reports are preliminary and there will be a long time required to fully optimize the synthesis protocols for the use of these new technologies on the industrial scale;
- While promising, figures for the reuse of the reported nanomaterials still indicates a loss of material through repeated use. To better fulfill the promise of these new class of material it will be necessary to ensure complete retention of the nanomaterial and its full regeneration in a rapid and economical way;
- Finally, as stated in the introduction, nanomaterials can be contaminants either as full particles on their own, or as a source of breakdown products such as metal ions or organic molecules that can be hazardous to human health. This means that if there is a loss during use, then this potential method for remediating water can instead be a source of contamination. As such, before the widespread use of these novel materials for the remediation of water protocols must be developed to limit the potential risk to human health and the environment and to prevent accidental release.
Funding
Conflicts of Interest
References
- Glieck, P.H. Water in Crisis, A. Guide to the World’s Fresh Water Resources; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater reuse and risk: Definition of key objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Zhang, W.; Fan, Y.; Jaio, W. Wastewater reclamation and reuse in China: Opportunities and challenges. J. Environ. Sci. 2016, 39, 86–96. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci. Total Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef]
- Flanagan, P.J. Parameters of Water Quality Interpretation and Standards; The Environmental Protection Agency: Dublin, Ireland, 2001.
- Xu, J.-Z.; Dai, L.; Wu, B.; Ding, T.; Zhu, J.-J.; Lin, H.; Chen, H.-L.; Shen, C.-Y.; Jiang, Y. Determination of methylene blue residues in aquatic products by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2009, 32, 4193–4199. [Google Scholar] [CrossRef]
- Diagnostics, P.-L. Crystal Violet MSDS. 2017. Available online: https://www.pro-lab.com/wp-content/uploads/2016/11/Crystal-Violet-SDS780-EN.pdf (accessed on 4 September 2020).
- Compound Summary 2,3-Dichlorophenol. In PubChem. 2005. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/dichlorophenol (accessed on 4 September 2020).
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Wang, H.; Le-Van, Q.; Aassime, A.; Roux, X.L.; Charra, F.; Chauvin, N.; Degiron, A. Electroluminescence of Colloidal Quantum Dots in Electrical Contact with Metallic Nanoparticles. Adv. Opt. Mater. 2018, 6, 1700658. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym. Plast. Technol. Eng. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Salem Alsaiari, N.S.; Mnif, W.; Ben Rebah, F. Effective Heavy Metals Removal from Water Using Nanomaterials: A Review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Xue, X.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for water pollution monitoring and remediation. Environ. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Qian, J.; Gao, X.; Pan, B. Nanoconfinement-Mediated Water Treatment: From Fundamental to Application. Environ. Sci. Technol. 2020, 54, 8509–8526. [Google Scholar] [CrossRef]
- Yin, Y.B.; Guo, S.; Heck, K.N.; Clark, C.A.; Coonrod, C.L.; Wong, M.S. Treating Water by Degrading Oxyanions Using Metallic Nanostructures. ACS Sustain. Chem. Eng. 2018, 6, 11160–11175. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- García Doménech, N.; Purcell-Milton, F.; Gun’ko, Y.K. Recent progress and future prospects in development of advanced materials for nanofiltration. Mater. Today Commun. 2020, 23, 100888. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Hansen, S.F.; Rygaard, M. Potential exposure and treatment efficiency of nanoparticles in water supplies based on wastewater reclamation. Environ. Sci. Nano 2015, 2, 191–202. [Google Scholar] [CrossRef]
- Beveridge, J.S.; Stephens, J.R.; Williams, M.E. The Use of Magnetic Nanoparticles in Analytical Chemistry. Annu. Rev. Anal. Chem. 2011, 4, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.; Gun’ko, Y.K. Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials 2014, 4, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lu, T.; Zeng, R.; Bi, Y. Preparation and highlighted applications of magnetic microparticles and nanoparticles: A review on recent advances. Microchim. Acta 2016, 183, 2655–2675. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Peralta, M.E.; Ocampo, S.; Funes, I.G.; Onaga Medina, F.; Parolo, M.E.; Carlos, L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics 2020, 8, 24. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Singhal, A. Chapter 29-Magnetic nanoparticle-based nanocontainers for water treatment. In Micro and Nano Technologies; Nguyen-Tri, P., Do, T.-O., Nguyen, T.-S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 487–498. [Google Scholar]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100305. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Rasika Dias, H.V.; Kharissova, O.V.; Manuel Jiménez-Pérez, V.; Olvera Pérez, B.; Muñoz Flores, B. Iron-containing nanomaterials: Synthesis, properties, and environmental applications. RSC Adv. 2012, 2, 9325–9358. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides–integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Fato, F.P.; Li, D.-W.; Zhao, L.-J.; Qiu, K.; Long, Y.-T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Shi, W.; Bao, J.; Yang, X. A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant. Materials 2020, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.E.; Lin, Y.; Peng, H.-H.; Yuan, B.; Dionysiou, D.D.; Huang, X.-D.; Zhang, D.-D.; Fu, M.-L. Novel magnetic rod-like Mn-Fe oxycarbide toward peroxymonosulfate activation for efficient oxidation of butyl paraben: Radical oxidation versus singlet oxygenation. Appl. Catal. B Environ. 2020, 268, 118549. [Google Scholar] [CrossRef]
- Ma, C.; Liu, F.-Y.; Wei, M.-B.; Zhao, J.-H.; Zhang, H.-Z. Synthesis of Novel Core-Shell Magnetic Fe3O4@C Nanoparticles with Carboxyl Function for Use as an Immobilisation Agent to Remediate Lead-Contaminated Soils. Pol. J. Environ. Stud. 2020, 29, 2273–2283. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Pan, S.; Huang, W.; Liu, R. Adsorption Mechanisms and Electrochemical Properties of Methyl Blue onto Magnetic NixMgyZn(1-x-y)Fe2O4 Nanoparticles Fabricated Via the Ethanol-Assisted Combustion Process. Water Air Soil Pollut. 2020, 231, 316. [Google Scholar] [CrossRef]
- Iqbal, A.; Jacob, J.; Mahmood, A.; Mehboob, K.; Mahmood, K.; Ali, A.; Bukhari, T.-H.; Adrees, M.; Adrees, M.; Ahmad, M. Synthesis and characterization of Zn–Mn–Fe nano oxide composites for the degradation of reactive yellow 15 dye. Phys. B Condens. Matter 2020, 588, 412210. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Zou, D. One-Step Synthesis of Metal-Modified Nanomagnetic Materials and Their Application in the Removal of Chlortetracycline. ACS Omega 2020, 5, 5116–5125. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Lyu, L.; Hu, C. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl. Catal. B Environ. 2020, 270, 118874. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Krstic, J.B.; Ribic, V.; Tadic, N.B.; Ognjanovic, M.; Auger, S.; Vidic, J.; Nikolic, M.V. Synthesis and antibacterial activity of iron manganite (FeMnO3) particles against the environmental bacterium Bacillus subtilis. RSC Adv. 2020, 10, 13879–13888. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; El-Khawaga, A.M.; Abd Elkodous, M.; Abokhadra, A.; Elsayed, M.A.; Gobara, M.; Soliman, L.I.; El-Bahnasawy, H.H.; Ashour, A.H. Nanostructured Mg substituted Mn-Zn ferrites: A magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazard. Mater. 2020, 399, 123000. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Hemdan, B.A.; El Nahrawy, A.M. Facile synthesis and potential application of Ni0.6Zn0.4Fe2O4 and Ni0.6Zn0.2Ce0.2Fe2O4 magnetic nanocubes as a new strategy in sewage treatment. J. Environ. Manag. 2020, 270, 110816. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, A.; Wang, W.; Li, R.; Zhang, W.-X. Feasibility of nanoscale zero-valent iron (nZVI) for enhanced biological treatment of organic dyes. Chemosphere 2019, 237, 124470. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Guo, Y.; Dong, H.; Guan, X.; Zhou, G.; Sun, Y. Activated peroxydisulfate by sulfidated zero-valent iron for enhanced organic micropollutants removal from water. Chem. Eng. J. 2020, 396, 125301. [Google Scholar] [CrossRef]
- Guan, X.; Du, X.; Liu, M.; Qin, H.; Qiao, J.; Sun, Y. Enhanced trichloroethylene dechlorination by carbon-modified zero-valent iron: Revisiting the role of carbon additives. J. Hazard. Mater. 2020, 394, 122564. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhateria, R. Experimental and Modeling Process Optimization of Lead Adsorption on Magnetite Nanoparticles via Isothermal, Kinetics, and Thermodynamic Studies. ACS Omega 2020, 5, 10826–10837. [Google Scholar] [CrossRef] [PubMed]
- Amos-Tautua, B.M.; Fakayode, O.J.; Songca, S.P.; Oluwafemi, O.S. Effect of synthetic conditions on the crystallinity, porosity and magnetic properties of gluconic acid capped iron oxide nanoparticles. Nano-Struct. Nano-Objects 2020, 23, 100480. [Google Scholar] [CrossRef]
- Powell, C.D.; Atkinson, A.J.; Ma, Y.; Marcos-Hernandez, M.; Villagran, D.; Westerhoff, P.; Wong, M.S. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J. Nanopart. Res. 2020, 22, 48. [Google Scholar] [CrossRef]
- Di Iorio, E.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Nejad, S.B.; Mohammadi, A. Epoxy-Triazinetrione-Functionalized Magnetic Nanoparticles as an Efficient Magnetic Nanoadsorbent for the Removal of Malachite Green and Pb(II) from Aqueous Solutions. J. Chem. Eng. Data 2020, 65, 2731–2742. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, J.; Song, W.; Liu, J.; Hu, Z.; Bao, B. An environmental-friendly magnetic bio-adsorbent for high-efficiency Pb(Ⅱ) removal: Preparation, characterization and its adsorption performance. Ecotoxicol. Environ. Saf. 2020, 203, 111002. [Google Scholar] [CrossRef]
- H Kamel, A.; Hassan, A.A.; Amr, A.E.-G.E.; El-Shalakany, H.H.; Al-Omar, A.M. Synthesis and Characterization of CuFe2O4 Nanoparticles Modified with Polythiophene: Applications to Mercuric Ions Removal. Nanomaterials 2020, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.-M.; Yoon, I.-H.; Kim, I. Application of polyethylenimine-coated magnetic nanocomposites for the selective separation of Cs-enriched clay particles from radioactive soil. RSC Adv. 2020, 10, 21822–21829. [Google Scholar] [CrossRef]
- Villa, S.; Riani, P.; Soggia, F.; Magi, E.; Canepa, F. Thiol-functionalized magnetic nanoparticles for static and dynamic removal of Pb(II) ions from waters. J. Nanopart. Res. 2019, 21, 44. [Google Scholar] [CrossRef]
- Tavares, D.S.; Lopes, C.B.; Almeida, J.C.; Vale, C.; Pereira, E.; Trindade, T. Spinel-type ferrite nanoparticles for removal of arsenic(V) from water. Environ. Sci. Pollut. Res. 2020, 27, 22523–22534. [Google Scholar] [CrossRef]
- Cai, C.; Kang, S.; Xie, X.; Liao, C.; Duan, X.; Dionysiou, D.D. Efficient degradation of bisphenol A in water by heterogeneous activation of peroxymonosulfate using highly active cobalt ferrite nanoparticles. J. Hazard. Mater. 2020, 399, 122979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Wang, Z. Adsorption characteristics and electrochemical performance of reactive red onto magnetic MgFe2O4 nanoparticles prepared via a facile alcohol combustion process. J. Sol-Gel Sci. Technol. 2020, 93, 535–545. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, J.; Zhao, Z.; Cui, F. Copper substituted zinc ferrite with abundant oxygen vacancies for enhanced ciprofloxacin degradation via peroxymonosulfate activation. J. Hazard. Mater. 2020, 390, 121998. [Google Scholar] [CrossRef]
- Oh, D.; Lee, C.-S.; Kang, Y.-G.; Chang, Y.-S. Hydroxylamine-assisted peroxymonosulfate activation using cobalt ferrite for sulfamethoxazole degradation. Chem. Eng. J. 2020, 386, 123751. [Google Scholar] [CrossRef]
- Kaur, R.; Bakshi, M.S. Mechanistic Aspects of Simultaneous Extraction of Ag and Au Nanoparticles across Aqueous–Organic Interface by Surface Active Iron Oxide Nanoparticles. Langmuir 2020, 36, 7505–7516. [Google Scholar] [CrossRef]
- Tian, H.; Liang, Y.; Yang, D.; Sun, Y. Characteristics of PVP–stabilised NZVI and application to dechlorination of soil–sorbed TCE with ionic surfactant. Chemosphere 2020, 239, 124807. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced removal of sulfadiazine by sulfidated ZVI activated persulfate process: Performance, mechanisms and degradation pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Zheng, H.; Ren, X.; Zhang, X.; Song, G.; Chen, D.; Chen, C. Mutual effect of U(VI) and phosphate on the reactivity of nanoscale zero-valent iron (nZVI) for their co-removal. J. Mol. Liq. 2020, 297, 111853. [Google Scholar] [CrossRef]
- Wei, X.; Yin, H.; Peng, H.; Guo, Z.; Lu, G.; Dang, Z. Sulfidation enhanced reduction of polybrominated diphenyl ether and Pb(II) combined pollutants by nanoscale zerovalent iron: Competitive reaction between pollutants and electronic transmission mechanism. Chem. Eng. J. 2020, 395, 125085. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Darwish, N.; Shanableh, A. Phosphate removal using nanoscale zerovalent iron: Impact of chitosan and humic acid. J. Environ. Chem. Eng. 2020, 8, 104131. [Google Scholar] [CrossRef]
- Xu, W.; Hu, X.; Lou, Y.; Jiang, X.; Shi, K.; Tong, Y.; Xu, X.; Shen, C.; Hu, B.; Lou, L. Effects of environmental factors on the removal of heavy metals by sulfide-modified nanoscale zerovalent iron. Environ. Res. 2020, 187, 109662. [Google Scholar] [CrossRef]
- Qin, H.; Yin, D.; Bandstra, J.Z.; Sun, Y.; Cao, G.; Guan, X. Ferrous ion mitigates the negative effects of humic acid on removal of 4-nitrophenol by zerovalent iron. J. Hazard. Mater. 2020, 383, 121218. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Zhang, X.; Guan, X. Weak magnetic field enables high selectivity of zerovalent iron toward metalloid oxyanions under aerobic conditions. J. Hazard. Mater. 2020, 400, 123330. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Sun, Y.; Qiao, J.; Xu, C.; Guan, X. Selenate removal by Fe0 coupled with ferrous iron, hydrogen peroxide, sulfidation, and weak magnetic field: A comparative study. Water Res. 2019, 159, 375–384. [Google Scholar] [CrossRef]
- Palza, H.; Delgado, K.; Govan, J. Novel magnetic CoFe2O4/layered double hydroxide nanocomposites for recoverable anionic adsorbents for water treatment. Appl. Clay Sci. 2019, 183, 105350. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Wu, H.; Li, Y.; Ren, X.; Li, M.; Liu, J.; Sun, J.; Yue, T.; Wang, J. In Situ Cascade Derivation toward a Hierarchical Layered Double Hydroxide Magnetic Absorbent for High-Performance Protein Separation. ACS Sustain. Chem. Eng. 2020, 8, 4966–4974. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Low cost and efficient synthesis of magnetic iron oxide/activated sericite nanocomposites for rapid removal of methylene blue and crystal violet dyes. Mater. Charact. 2020, 163, 110275. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wu, L.; Shi, Y.; He, Q.; Chen, J.; Wan, D. Magnetic spent bleaching earth carbon (Mag-SBE@C) for efficient adsorption of tetracycline hydrochloride: Response surface methodology for optimization and mechanism of action. Sci. Total Environ. 2020, 722, 137817. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Dhar, S.S. Removal of cadmium(II) from aqueous solution by hydroxyapatite-encapsulated zinc ferrite (HAP/ZnFe2O4) nanocomposite: Kinetics and isotherm study. Environ. Sci. Pollut. Res. 2020, 27, 37977–37988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem. Eng. J. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials 2020, 10, 1361. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, L.; Du, J.; Qian, C.; Wang, Y. CuO and CeO2 assisted Fe2O3/attapulgite catalyst for heterogeneous Fenton-like oxidation of methylene blue. RSC Adv. 2020, 10, 23431–23439. [Google Scholar] [CrossRef]
- Xu, B.-D.; Li, D.-C.; Qian, T.-T.; Jiang, H. Boosting the activity and environmental stability of nanoscale zero-valent iron by montmorillonite supporting and sulfidation treatment. Chem. Eng. J. 2020, 387, 124063. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Chen, Y.; Liang, J.; Zhou, L. Producing, O.H.; SO4− and O2− in heterogeneous Fenton reaction induced by Fe3O4-modified schwertmannite. Chem. Eng. J. 2020, 393, 124735. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Sun, Z.; Bian, R.; Dong, X.; Zhang, X.; Zheng, S. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate. Chem. Eng. J. 2020, 388, 124386. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, L.; Sadeghzadeh, S.M. Reduction of 4-nitrophenol and 2-nitroaniline using immobilized CoMn2O4 NPs on lignin supported on FPS. RSC Adv. 2020, 10, 19553–19561. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Miteva, S.; Marinov, I.; Behunová, D.M.; Trendafilova, I.; Popova, M.; Václaviková, M. Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery. Processes 2020, 8, 778. [Google Scholar] [CrossRef]
- Jee, S.-C.; Kim, M.; Shinde, S.K.; Ghodake, G.S.; Sung, J.-S.; Kadam, A.A. Assembling ZnO and Fe3O4 nanostructures on halloysite nanotubes for anti-bacterial assessments. Appl. Surf. Sci. 2020, 509, 145358. [Google Scholar] [CrossRef]
- Rashid, M.H.; Mandal, T.K. Synthesis and Catalytic Application of Nanostructured Silver Dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Li, X.; Zhang, W.; Dong, C.; Ma, J. Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Appl. Catal. B Environ. 2014, 158–159, 129–135. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Meng, X. Carbon Spheres with Controllable Silver Nanoparticle Doping. J. Phys. Chem. C 2010, 114, 977–982. [Google Scholar] [CrossRef]
- Sahiner, N.; Ozay, H.; Ozay, O.; Aktas, N. A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl. Catal. B Environ. 2010, 101, 137–143. [Google Scholar] [CrossRef]
- Luciano, A.J.R.; de Sousa Soletti, L.; Ferreira, M.E.C.; Cusioli, L.F.; de Andrade, M.B.; Bergamasco, R.; Yamaguchi, N.U. Manganese ferrite dispersed over graphene sand composite for methylene blue photocatalytic degradation. J. Environ. Chem. Eng. 2020, 8, 104191. [Google Scholar] [CrossRef]
- Gonçalves, A.H.A.; Siciliano, P.H.C.; Alves, O.C.; Cesar, D.V.; Henriques, C.A.; Gaspar, A.B. Synthesis of a Magnetic Fe3O4/RGO Composite for the Rapid Photo-Fenton Discoloration of Indigo Carmine Dye. Top. Catal. 2020. [Google Scholar] [CrossRef]
- Ferreira, F.N.; Benevides, A.P.; Cesar, D.V.; Luna, A.S.; de Gois, J.S. Magnetic solid-phase extraction and pre-concentration of 17β-estradiol and 17α-ethinylestradiol in tap water using maghemite-graphene oxide nanoparticles and determination via HPLC with a fluorescence detector. Microchem. J. 2020, 157, 104947. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, S.; Yang, Z.; Zhang, X.; Ge, J. Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation. Materials 2019, 12, 4106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Liu, Z.; Lyu, C.; Niu, S.; Dong, Z.; Lyu, C. Enhanced catalytic oxidation of benzotriazole via peroxymonosulfate activated by CoFe2O4 supported onto nitrogen-doped three-dimensional graphene aerogels. Chem. Eng. J. 2020, 400, 125897. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Xu, L.; Hu, F. Polypyrrole modified magnetic reduced graphene oxide composites: Synthesis, characterization and application for selective lead adsorption. RSC Adv. 2020, 10, 17524–17533. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, J.; Wang, Y.; Tang, Q.; Kang, C. Fabrication of a magnetic ternary ZnFe2O4/TiO2/RGO Z-scheme system with efficient photocatalytic activity and easy recyclability. RSC Adv. 2020, 10, 17293–17301. [Google Scholar] [CrossRef]
- Rong, Y.; Huang, Y.; Jin, P.; Yang, C.; Zhong, Z.; Dong, C.; Liang, W. Highly efficient removal of cationic, anionic and neutral dyes by hierarchically porous structured three-dimensional magnetic sulfur/nitrogen co-doped reduced graphene oxide nanohybrid. J. Water Process Eng. 2020, 37, 101345. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, M.; Zhou, Y.; Ma, C.; Ning, F.; Qiu, Z. Facile synthesis of polyethyleneimine modified magnetic graphite: An effective adsorbent for the removal of humic acid from aqueous solution. Mater. Chem. Phys. 2020, 255, 123549. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, C.; Wang, Y.; Chang, J.; Ma, D.; Wang, J. An efficient method for removal of pentachlorophenol using adsorption and microwave regeneration with different magnetic carbon nanotubes. Water Sci. Technol. 2020, 81, 585–595. [Google Scholar] [CrossRef]
- Wu, X.; Xu, G.; Wang, J. Ultrasound-assisted coagulation for Microcystis aeruginosa removal using Fe3O4-loaded carbon nanotubes. RSC Adv. 2020, 10, 13525–13531. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Liu, Y.; Liu, Y. Fenton-like degradation of sulfamerazine at nearly neutral pH using Fe-Cu-CNTs and Al0-CNTs for in-situ generation of H2O2/OH/O2−. Chem. Eng. J. 2020, 396, 125329. [Google Scholar] [CrossRef]
- Manickam-Periyaraman, P.; Espinosa, J.C.; Ferrer, B.; Subramanian, S.; Álvaro, M.; García, H.; Navalón, S. Bimetallic iron-copper oxide nanoparticles supported on nanometric diamond as efficient and stable sunlight-assisted Fenton photocatalyst. Chem. Eng. J. 2020, 393, 124770. [Google Scholar] [CrossRef]
- Zenobio, J.E.; Modiri-Gharehveran, M.; de Perre, C.; Vecitis, C.D.; Lee, L.S. Reductive transformation of perfluorooctanesulfonate by nNiFe0-Activated carbon. J. Hazard. Mater. 2020, 397, 122782. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Cao, Z.; Bai, W.; Shi, Q.; Yang, Y. Fast degradation, large capacity, and high electron efficiency of chloramphenicol removal by different carbon-supported nanoscale zerovalent iron. J. Hazard. Mater. 2020, 384, 121253. [Google Scholar] [CrossRef] [PubMed]
- EPA. Drinking Water Health Advisories for PFOA and PFOS. Gr. Water Drink. Water. 2020. Available online: https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos#:~:text=To provide Americans%2C including the,at70parts per trillion (accessed on 9 September 2020).
- WHO. Fluoride in Drinking-water. In Backgr. Doc. Dev. WHO Guidel. Drink. Qual. 2004. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/fluoride.pdf?ua=1 (accessed on 9 September 2020).
- WHO. Sulfate in Drinking-water. In Backgr. Doc. Dev. WHO Guidel. Drink. Qual. 2004. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/sulfate.pdf (accessed on 9 September 2020).
- Nkinahamira, F.; Alsbaiee, A.; Zeng, Q.; Li, Y.; Zhang, Y.; Feng, M.; Yu, C.-P.; Sun, Q. Selective and fast recovery of rare earth elements from industrial wastewater by porous β-cyclodextrin and magnetic β-cyclodextrin polymers. Water Res. 2020, 181, 115857. [Google Scholar] [CrossRef]
- Chu, F.-J.; Wan, T.-J.; Chen, H.; Wu, C.-H.; Kao, P.-M. Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads. Water 2020, 12, 236. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Ociński, D.; Mazur, P.; Stanisławska, E.; Kociołek-Balawejder, E. Cu(II)-Fe(III) oxide doped anion exchangers–Multifunctional composites for arsenite removal from water via As(III) adsorption and oxidation. J. Hazard. Mater. 2020, 394, 122527. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Shahat, A.; Al-Bogami, A.S.; Wijesiri, B.; Goonetilleke, A. The synergistic effect of ultrasound power and magnetite incorporation on the sorption/desorption behavior of Cr(VI) and As(V) oxoanions in an aqueous system. J. Colloid Interface Sci. 2020, 569, 76–88. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Liu, J.; Ji, X.; Ma, J.; Tian, G. Preparation and excellent adsorption of water pollution dyes over magnetic Fe3O4/C nanoparticles with hollow grape cluster morphology. J. Nanopart. Res. 2020, 22, 196. [Google Scholar] [CrossRef]
- Aliahmadipoor, P.; Ghazanfari, D.; Gohari, R.J.; Akhgar, M.R. Preparation of PVDF/FMBO composite electrospun nanofiber for effective arsenate removal from water. RSC Adv. 2020, 10, 24653–24662. [Google Scholar] [CrossRef]
- Ahmad, M.; Wu, F.; Cui, Y.; Zhang, Q.; Zhang, B. Preparation of Novel Bifunctional Magnetic Tubular Nanofibers and Their Application in Efficient and Irreversible Uranium Trap from Aqueous Solution. ACS Sustain. Chem. Eng. 2020, 8, 7825–7838. [Google Scholar] [CrossRef]
- Sun, F.; He, J.; Wu, P.; Zeng, Q.; Liu, C.; Jiang, W. Magnetic photocatalyst CoFe2O4-Ag2O with magnetic aggregation bed photocatalytic reactor for continuous photodegradation of methyl orange. Chem. Eng. J. 2020, 397, 125397. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; El Nahwary, A.M.; Hemdan, B.A.; Abia, A.L.K. Nanoceramics and novel functionalized silicate-based magnetic nanocomposites as substitutional disinfectants for water and wastewater purification. Environ. Sci. Pollut. Res. 2020, 27, 26668–26680. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.; Anantharaju, K.S.; Vidya, Y.S.; Renuka, L.; Malini, S.; Sharma, S.C.; Nagabhushana, H. MnFe2O4/ZrO2 nanocomposite as an efficient magnetically separable photocatalyst with good response to sunlight: Preparation, characterization and catalytic mechanism. SN Appl. Sci. 2020, 2, 328. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Huang, G.; Liu, Y.; Yang, G.; Lin, C.; Xiao, G.; Wang, Y.; Liu, M. Remediation of organic arsenic contaminants with heterogeneous Fenton process mediated by SiO2-coated nano zero-valent iron. Environ. Sci. Pollut. Res. 2020, 27, 12017–12029. [Google Scholar] [CrossRef]
- Wan, H.; Islam, M.S.; Qian, D.; Ormsbee, L.; Bhattacharyya, D. Reductive degradation of CCl4 by sulfidized Fe and Pd-Fe nanoparticles: Kinetics, longevity, and morphology aspects. Chem. Eng. J. 2020, 394, 125013. [Google Scholar] [CrossRef]
- Rahdar, A.; Rahdar, S.; Labuto, G. Environmentally friendly synthesis of Fe2O3@SiO2 nanocomposite: Characterization and application as an adsorbent to aniline removal from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 9181–9191. [Google Scholar] [CrossRef]
- Khodadadi, M.; Al-Musawi, T.J.; Kamani, H.; Silva, M.F.; Panahi, A.H. The practical utility of the synthesis FeNi3@SiO2@TiO2 magnetic nanoparticles as an efficient photocatalyst for the humic acid degradation. Chemosphere 2020, 239, 124723. [Google Scholar] [CrossRef]
- Nasseh, N.; Hossein Panahi, A.; Esmati, M.; Daglioglu, N.; Asadi, A.; Rajati, H.; Khodadoost, F. Enhanced photocatalytic degradation of tetracycline from aqueous solution by a novel magnetically separable FeNi3/SiO2/ZnO nano-composite under simulated sunlight: Efficiency, stability, and kinetic studies. J. Mol. Liq. 2020, 301, 112434. [Google Scholar] [CrossRef]
- Khodadadi, M.; Hossein Panahi, A.; Al-Musawi, T.J.; Ehrampoush, M.H.; Mahvi, A.H. The catalytic activity of FeNi3@SiO2 magnetic nanoparticles for the degradation of tetracycline in the heterogeneous Fenton-like treatment method. J. Water Process Eng. 2019, 32, 100943. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A.; Allahresani, A. FeNi3/SiO2 magnetic nanocomposite as an efficient and recyclable heterogeneous fenton-like catalyst for the oxidation of metronidazole in neutral environments: Adsorption and degradation studies. Compos. Part B Eng. 2019, 166, 328–340. [Google Scholar] [CrossRef]
- Tao, Q.; Huang, X.; Bi, J.; Wei, R.; Xie, C.; Zhou, Y.; Yu, L.; Hao, H.; Wang, J. Aerobic Oil-Phase Cyclic Magnetic Adsorption to Synthesize 1D Fe2O3@TiO2 Nanotube Composites for Enhanced Visible-Light Photocatalytic Degradation. Nanomaterials 2020, 10, 1345. [Google Scholar]
- Boruah, P.K.; Yadav, A.; Das, M.R. Magnetic mixed metal oxide nanomaterials derived from industrial waste and its photocatalytic applications in environmental remediation. J. Environ. Chem. Eng. 2020, 8, 104297. [Google Scholar] [CrossRef]
- Mohan, H.; Lim, J.-M.; Lee, S.-W.; Jang, J.S.; Park, Y.-J.; Seralathan, K.-K.; Oh, B.-T. Enhanced visible light photocatalysis with E-waste-based V2O5/zinc–ferrite: BTEX degradation and mechanism. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.A.; Isakovski, M.K.; Tubić, A.; Šolić, M.; Kordić, B.; Agbaba, J. Synthesis, characterization and application of magnetic nanoparticles modified with Fe-Mn binary oxide for enhanced removal of As(III) and As(V). Environ. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Krishna, S.; Sathishkumar, P.; Pugazhenthiran, N.; Guesh, K.; Mangalaraja, R.V.; Kumaran, S.; Gracia-Pinilla, M.A.; Anandan, S. Magnetically recyclable CoFe2O4/ZnO nanocatalysts for the efficient catalytic degradation of Acid Blue 113 under ambient conditions. RSC Adv. 2020, 10, 16473–16480. [Google Scholar] [CrossRef]
- Massoud-Sharifi, A.; Kara, G.K.; Rabbani, M. CuFe2O4@CuO: A Magnetic Composite Synthesized by Ultrasound Irradiation and Degradation of Methylene Blue on Its Surface in the Presence of Sunlight. Proceedings 2020, 48, 17. [Google Scholar]
- Tian, C.; Li, J.; Li, Q.; Nie, Y.; Tian, X.; Dai, C.; Yang, C.; Zhou, Z.; Wang, Y. Surface weak acid-base pair of FeOOH/Al2O3 for enhanced peroxymonosulfate activation in degradation of humic substances from water. Chem. Eng. J. 2020, 387, 124064. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolešová, A.; Bouša, D.; Sofer, Z.; Friess, K. Co0·5Ni0·5FeCrO4 spinel nanoparticles decorated with UiO-66-based metal-organic frameworks grafted onto GO and O-SWCNT for gas adsorption and water purification. Chemosphere 2020, 255, 126966. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Khedkar, C.V.; Khupse, N.D.; Thombare, B.R.; Dusane, P.R.; Lole, G.; Devan, R.S.; Deshpande, A.S.; Patil, S.I. Magnetically separable Ag-Fe3O4 catalyst for the reduction of organic dyes. Chem. Phys. Lett. 2020, 742, 137131. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Lemus, J.; Palomares, F.J.; Morales, M.P.; Mazarío, E. Superparamagnetic nanosorbent for water purification: Assessment of the adsorptive removal of lead and methyl orange from aqueous solutions. Sci. Total Environ. 2020, 711, 134644. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Bai, X.-H.; Gong, Y.; Peng, M.-L.; Zhang, Y.-Y.; Ma, X.-R.; Zhang, Y. Enhanced catalytic properties of Fe3O4/Ag magnetic microspheres synthesized by a novel thermal co-reduction method. J. Magn. Magn. Mater. 2020, 510, 166951. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Sulaimon, A.O. Plant-Mediated Iron Nanoparticles and their Applications as Adsorbents for Water Treatment–A Review. J. Chem. Rev. 2020, 2, 103–113. [Google Scholar] [CrossRef]
- Gautam, R.K.; Tiwari, I. Humic acid functionalized magnetic nanomaterials for remediation of dye wastewater under ultrasonication: Application in real water samples, recycling and reuse of nanosorbents. Chemosphere 2020, 245, 125553. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.F.; Minella, M.; Fabbri, D.; Calza, P.; Malitesta, C.; Mazzotta, E.; Bianco Prevot, A. Humic acid coated magnetic particles as highly efficient heterogeneous photo-Fenton materials for wastewater treatments. Chem. Eng. J. 2020, 390, 124619. [Google Scholar] [CrossRef]
- Hu, L.; Guang, C.; Liu, Y.; Su, Z.; Gong, S.; Yao, Y.; Wang, Y. Adsorption behavior of dyes from an aqueous solution onto composite magnetic lignin adsorbent. Chemosphere 2020, 246, 125757. [Google Scholar] [CrossRef]
- Chen, H.; Xu, F.; Chen, Z.; Jiang, O.; Gustave, W.; Tang, X. Arsenic and cadmium removal from water by a calcium-modified and starch-stabilized ferromanganese binary oxide. J. Environ. Sci. 2020, 96, 186–193. [Google Scholar] [CrossRef]
- Patil, S.A.; Kumbhar, P.D.; Patil, S.K.; Vadiyar, M.M.; Suryawanshi, U.P.; Jambhale, C.L.; Anuse, M.A.; Kim, J.H.; Kolekar, S.S. Dynamic adsorption of toxic indigo carmine dye on bio-inspired synthesised Fe3O4 nanoparticles: Kinetic and thermodynamic study. Int. J. Environ. Anal. Chem. 2020, 1–23. [Google Scholar] [CrossRef]
- Egorin, A.; Tokar, E.; Matskevich, A.; Ivanov, N.; Tkachenko, I.; Sokolnitskaya, T.; Zemskova, L. Composite Magnetic Sorbents Based on Iron Oxides in Different Polymer Matrices: Comparison and Application for Removal of Strontium. Biomimetics 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, P.; Li, G.; Li, L.; Yi, J.; Wang, S.; Lu, S.; Ding, P.; Chen, C.; Pan, H. Optimization on preparation of Fe3O4/chitosan as potential matrix material for the removal of microcystin-LR and its evaluation of adsorption properties. Int. J. Biol. Macromol. 2020, 156, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.; Castellari, J.; Colman Lerner, J.; Bertola, N.; Zaritzky, N. Functional iron chitosan microspheres synthesized by ionotropic gelation for the removal of arsenic (V) from water. Int. J. Biol. Macromol. 2020, 164, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, C.; Chen, W.; He, M.; Huang, B. Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: Removal efficacy and mechanisms. Sci. Total Environ. 2020, 733, 139316. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Cui, J. Preparation of a novel chitosan-based magnetic adsorbent CTS@SnO2@Fe3O4 for effective treatment of dye wastewater. Int. J. Biol. Macromol. 2020, 156, 1474–1482. [Google Scholar] [CrossRef]
- Silva, M.A.; Rocha, C.V.; Gallo, J.; Felgueiras, H.P.; Pessoa de Amorim, M.T. Porous composites based on cellulose acetate and alfa-hematite with optical and antimicrobial properties. Carbohydr. Polym. 2020, 241, 116362. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, R.; Chen, Y.; Tu, S.; Yin, Y.; Ye, L. Immobilization of nano-zero-valent irons by carboxylated cellulose nanocrystals for wastewater remediation. Front. Chem. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Muniz, E.P.; de Assunção, L.S.D.; de Souza, L.M.; Ribeiro, J.J.K.; Marques, W.P.; Pereira, R.D.; Porto, P.S.S.; Proveti, J.R.C.; Passamani, E.C. On cobalt ferrite production by sol-gel from orange fruit residue by three related procedures and its application in oil removal. J. Clean. Prod. 2020, 265, 121712. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, P.; Liu, P.; Li, H.; Han, X.; Liu, L.; Zou, W. Green synthesis of stable Fe,Cu oxide nanocomposites from loquat leaf extracts for removal of Norfloxacin and Ciprofloxacin. Water Sci. Technol. 2020, 81, 694–708. [Google Scholar] [CrossRef]
- Din, M.I.; Jabbar, S.; Najeeb, J.; Khalid, R.; Ghaffar, T.; Arshad, M.; Khan, S.A.; Ali, S. Green synthesis of zinc ferrite nanoparticles for photocatalysis of methylene blue. Int. J. Phytoremediat. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, L.M.; Shukla, N.; Baranwal, K.; Gupta, S.; Siddique, S.; Singh, V. Gum Acacia Modified Ni Doped CuO Nanoparticles: An Excellent Antibacterial Material. J. Clust. Sci. 2020. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Hegazy, A.K.; Moustafa, Y.M.; Mohamed, R.R.; Safwat, G.; Diab, A.A. Green Technology for Remediation of Water Polluted with Petroleum Crude Oil: Using of Eichhornia crassipes (Mart.) Solms Combined with Magnetic Nanoparticles Capped with Myrrh Resources of Saudi Arabia. Nanomaterials 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Nikić, J.; Tubić, A.; Watson, M.; Maletić, S.; Šolić, M.; Majkić, T.; Agbaba, J. Arsenic Removal from Water by Green Synthesized Magnetic Nanoparticles. Water 2019, 11, 2520. [Google Scholar] [CrossRef]
- Afridi, M.N.; Lee, W.-H.; Kim, J.-O. Application of synthesized bovine serum albumin-magnetic iron oxide for phosphate recovery. J. Ind. Eng. Chem. 2020, 86, 113–122. [Google Scholar] [CrossRef]
- Zhou, L.; Li, A.; Ma, F.; Zhao, H.; Deng, F.; Pi, S.; Tang, A.; Yang, J. Combining high electron transfer efficiency and oxidation resistance in nZVI with coatings of microbial extracellular polymeric substances to enhance Sb(V) reduction and adsorption. Chem. Eng. J. 2020, 395, 125168. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Sun, A.; Tong, S.; Jiang, X.; Mu, Y.; Li, J.; Han, W.; Sun, X.; Wang, L.; et al. Optimization ofS/Fe ratio for enhanced nitrobenzene biological removal in anaerobicSystem amended withSulfide-modified nanoscale zerovalent iron. Chemosphere 2020, 247, 125832. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ye, X.; Lv, Y.; Pei, R.; Wu, M.; Liu, M. Lignin-based magnetic activated carbon for p-arsanilic acid removal: Applications and absorption mechanisms. Chemosphere 2020, 258, 127276. [Google Scholar] [CrossRef]
- Oladebeye, A.O. Potentials of Starch Nanoparticles Jack Bean (Canavalia ensiformis) Coprecipitated with Iron (II, III) Oxide. Int. Res. J. Pure Appl. Chem. 2020, 21, 17–24. [Google Scholar] [CrossRef][Green Version]
- Yildiz, A.; Vatansever Bayramol, D.; Atav, R.; Ağirgan, A.Ö.; Aydin Kurç, M.; Ergünay, U.; Mayer, C.; Hadimani, R.L. Synthesis and characterization of Fe3O4@Cs@Ag nanocomposite and its use in the production of magnetic and antibacterial nanofibrous membranes. Appl. Surf. Sci. 2020, 521, 146332. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, L.; Zhang, X.; Zhong, L.; Li, Y. Selective adsorption of Ag (Ⅰ) from aqueous solutions using Chitosan/polydopamine@C@magnetic fly ash adsorbent beads. J. Hazard. Mater. 2020, 381, 120943. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, X.; Liu, N.; Lv, J.; Yang, Y. Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar. Chemosphere 2020, 245, 125542. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Yang, L.; Ouyang, D.; Zhang, B.; Zhang, W.; Gu, M.; Li, J.; Chen, M.; Huang, L.; Qian, L. Enhanced removal of 1,2,4-trichlorobenzene by modified biochar supported nanoscale zero-valent iron and palladium. Chemosphere 2020, 249, 126518. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Qian, L.; Han, L.; Chen, M. Nano-magnetite supported by biochar pyrolyzed at different temperatures as hydrogen peroxide activator: Synthesis mechanism and the effects on ethylbenzene removal. Environ. Pollut. 2020, 261, 114020. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Luo, L.; Zheng, Y.; Huang, L.; Zhang, J.; Yang, Y.; Huang, H. Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: Indispensable role of reactive oxygen species and redox-active moieties. J. Hazard. Mater. 2020, 391, 122057. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Zhang, S.; Lyu, H.; Tang, J.; Song, B.; Zhen, M.; Liu, X. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 2019, 217, 686–694. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X.; et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Han, L.; Yan, J.; Qian, L.; Zhang, W.; Chen, M. Multifunctional Pd/Fe-biochar composites for the complete removal of trichlorobenzene and its degradation products. J. Environ. Manag. 2019, 245, 238–244. [Google Scholar] [CrossRef]
- Qian, L.; Liu, S.; Zhang, W.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2019, 533, 428–436. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, Y.; Zhu, Z.; Liu, Z.; Wang, W. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation. Chem. Eng. J. 2020, 384, 123298. [Google Scholar] [CrossRef]
- Chen, R.-H.; Cheng, Y.-Y.; Wang, P.; Liu, Z.-M.; Wang, Y.-G.; Wang, Y.-Y. High efficient removal and mineralization of Cr(VI) from water by functionalized magnetic fungus nanocomposites. J. Cent. South Univ. 2020, 27, 1503–1514. [Google Scholar] [CrossRef]
- Debs, K.B.; Cardona, D.S.; da Silva, H.D.T.; Nassar, N.N.; Carrilho, E.N.V.M.; Haddad, P.S.; Labuto, G. Oil spill cleanup employing magnetite nanoparticles and yeast-based magnetic bionanocomposite. J. Environ. Manag. 2019, 230, 405–412. [Google Scholar] [CrossRef]
- Cardona, D.S.; Debs, K.B.; Lemos, S.G.; Vitale, G.; Nassar, N.N.; Carrilho, E.N.V.M.; Semensatto, D.; Labuto, G. A comparison study of cleanup techniques for oil spill treatment using magnetic nanomaterials. J. Environ. Manag. 2019, 242, 362–371. [Google Scholar] [CrossRef]
- Ozdemir, S.; Kılınc, E.; Yalcin, M.S.; Soylak, M.; Sen, F. A new magnetized thermophilic bacteria to preconcentrate uranium and thorium from environmental samples through magnetic solid-phase extraction. J. Pharm. Biomed. Anal. 2020, 186, 113315. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Wu, J. One-pot synthesis of magnetic algal carbon/sulfidated nanoscale zerovalent iron composites for removal of bromated disinfection by-product. Chemosphere 2020, 250, 126257. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Ren, L.Y.; Zhou, L.Y.; Tan, X.J.; Zhu, Y.; Belver, C.; Bedia, J.; Yang, J. Biochar mediates activation of aged nanoscale ZVI by Shewanella putrefaciens CN32 to enhance the degradation of Pentachlorophenol. Chem. Eng. J. 2019, 368, 148–156. [Google Scholar] [CrossRef]
- Shi, Z.; Shen, W.; Yang, K.; Zheng, N.; Jiang, X.; Liu, L.; Yang, D.; Zhang, L.; Ai, Z.; Xie, B. Hexavalent chromium removal by a new composite system of dissimilatory iron reduction bacteria Aeromonas hydrophila and nanoscale zero-valent iron. Chem. Eng. J. 2019, 362, 63–70. [Google Scholar] [CrossRef]
- Özdemir, S.; Serkan Yalçın, M.; Kılınç, E. Preconcentrations of Ni(II) and Pb(II) from water and food samples by solid-phase extraction using Pleurotus ostreatus immobilized iron oxide nanoparticles. Food Chem. 2020, 336, 127675. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, H.; Li, H.; Tang, H.; Peng, H.; Xu, H. High-effectively degrade the di-(2-ethylhexyl) phthalate via biochemical system: Resistant bacterial flora and persulfate oxidation activated by BC@Fe3O4. Environ. Pollut. 2020, 262, 114100. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Huo, M.; Hao, P.; Zheng, J.; An, Y. Transport of nano zerovalent iron (nZVI) coupling with Alcaligenes sp. strain in porous media. RSC Adv. 2020, 10, 24265–24272. [Google Scholar] [CrossRef]
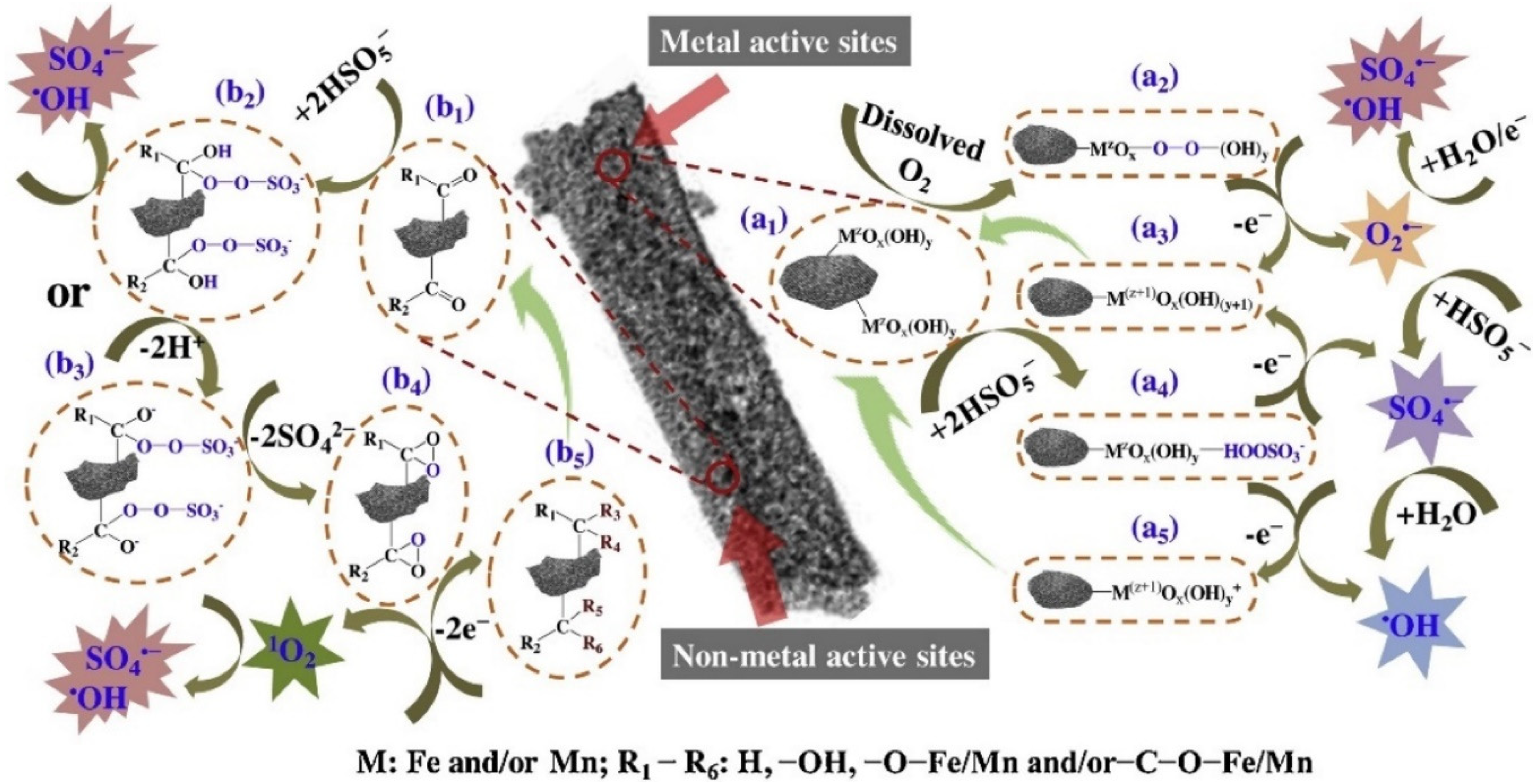
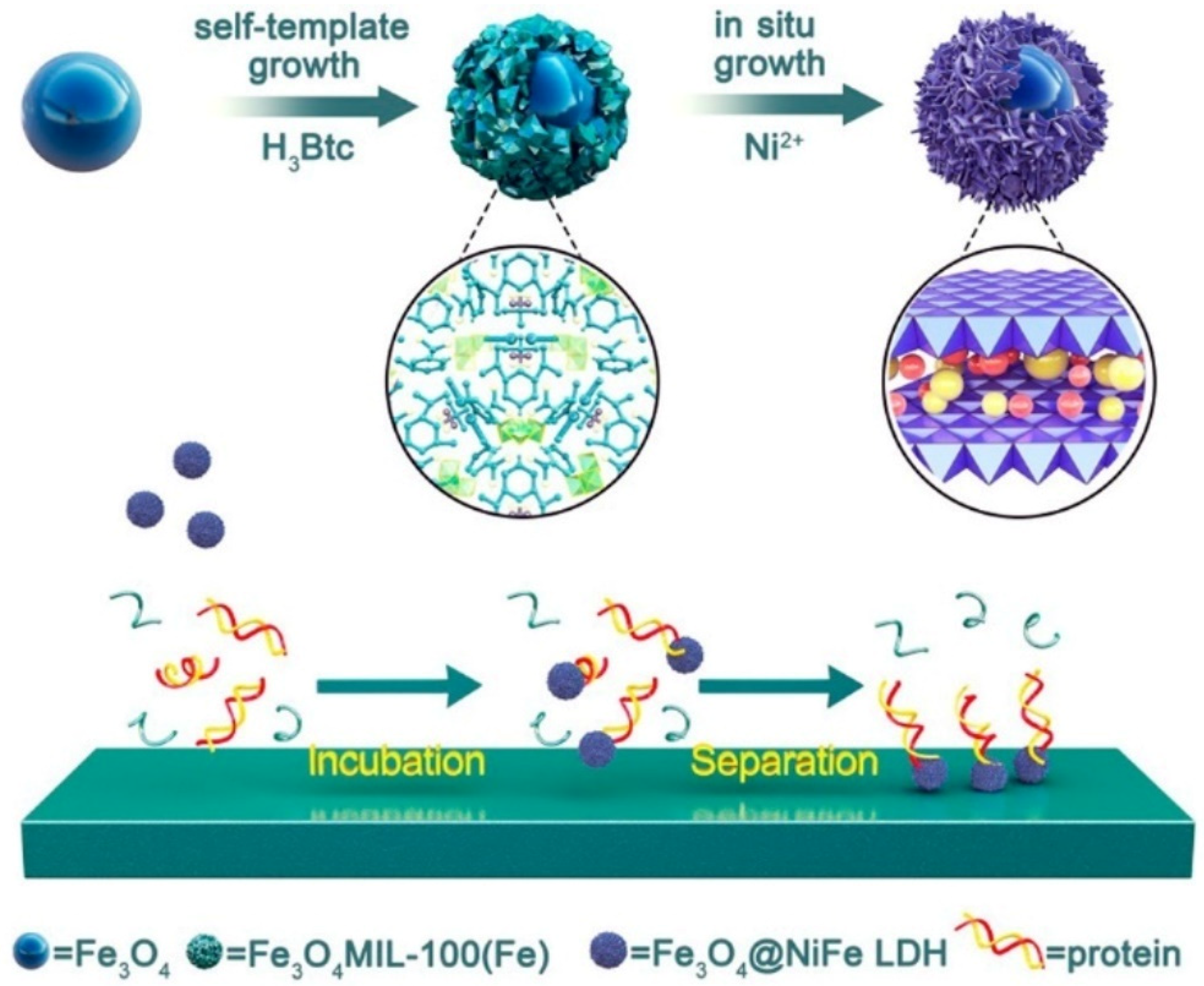
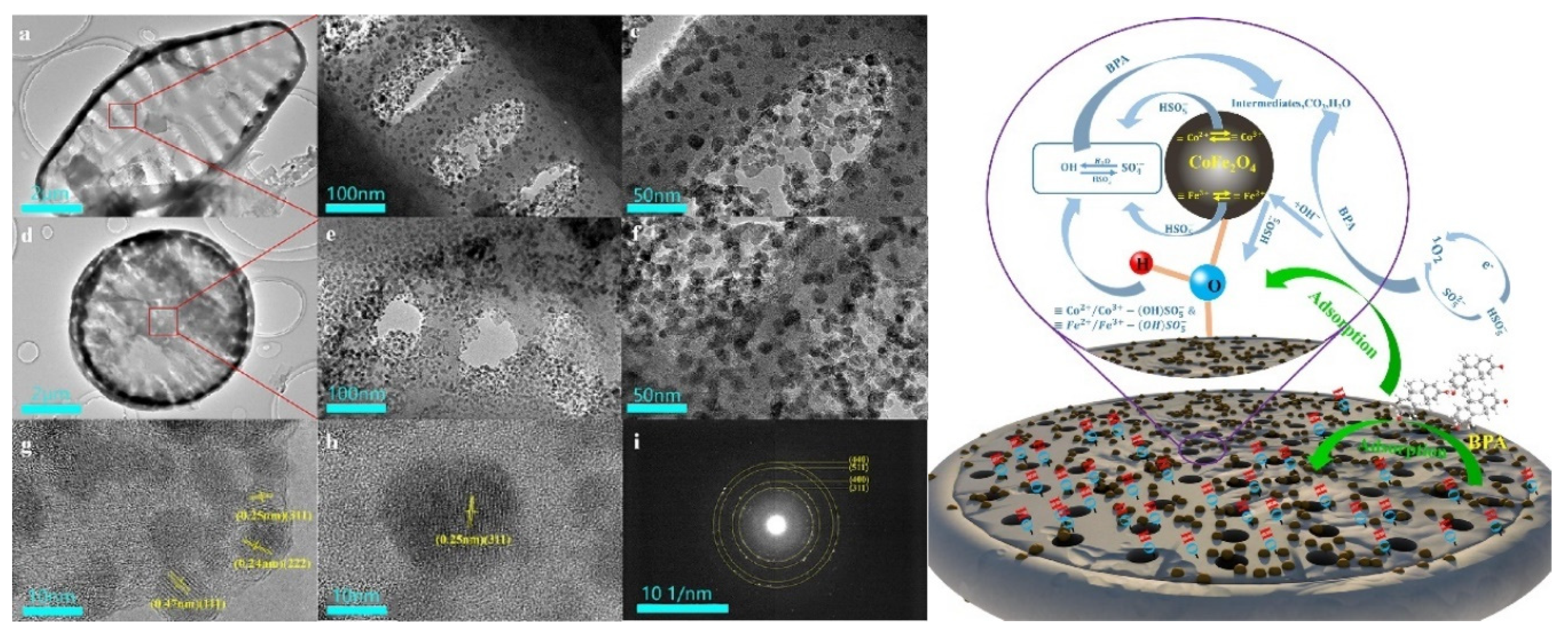

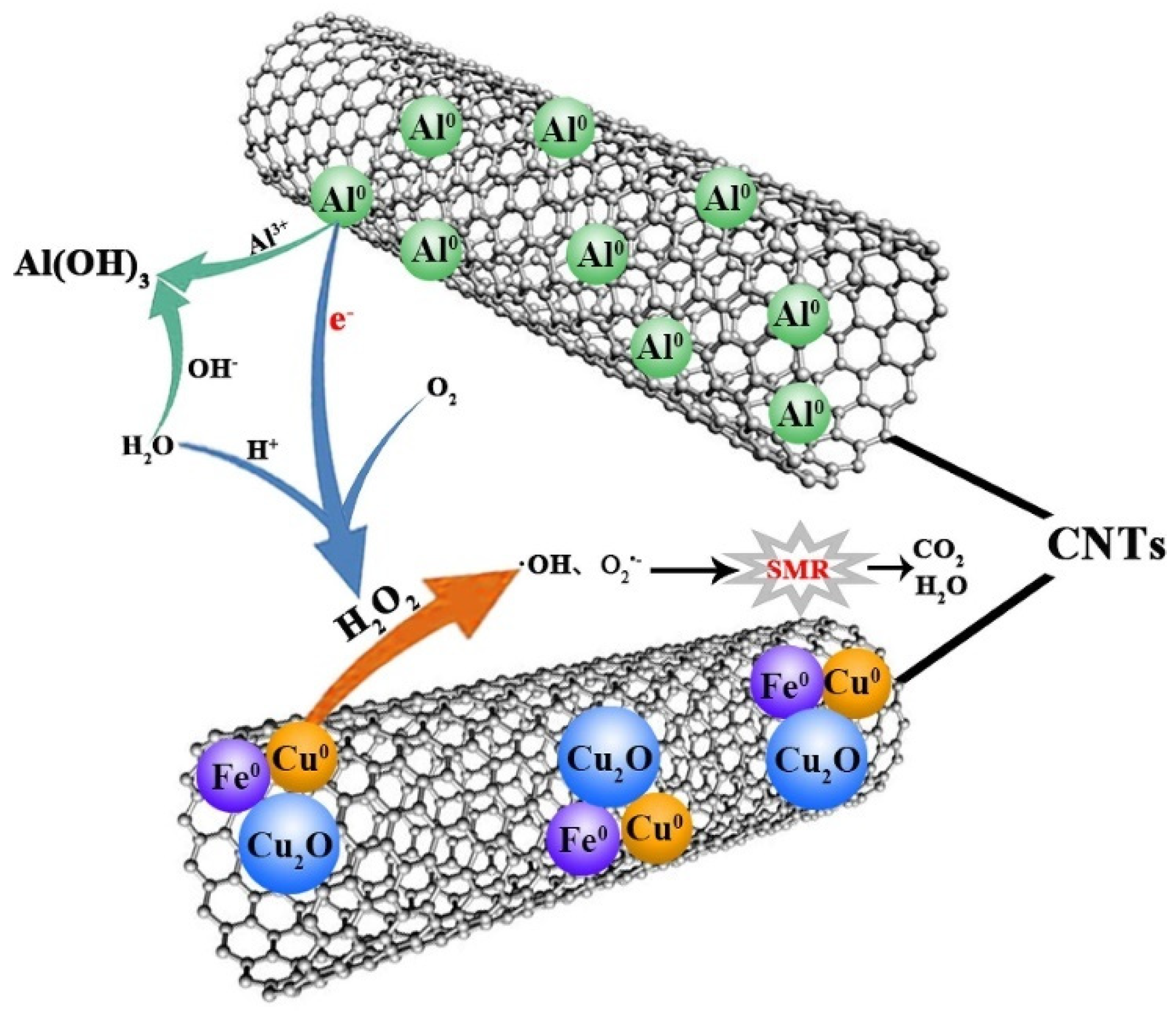
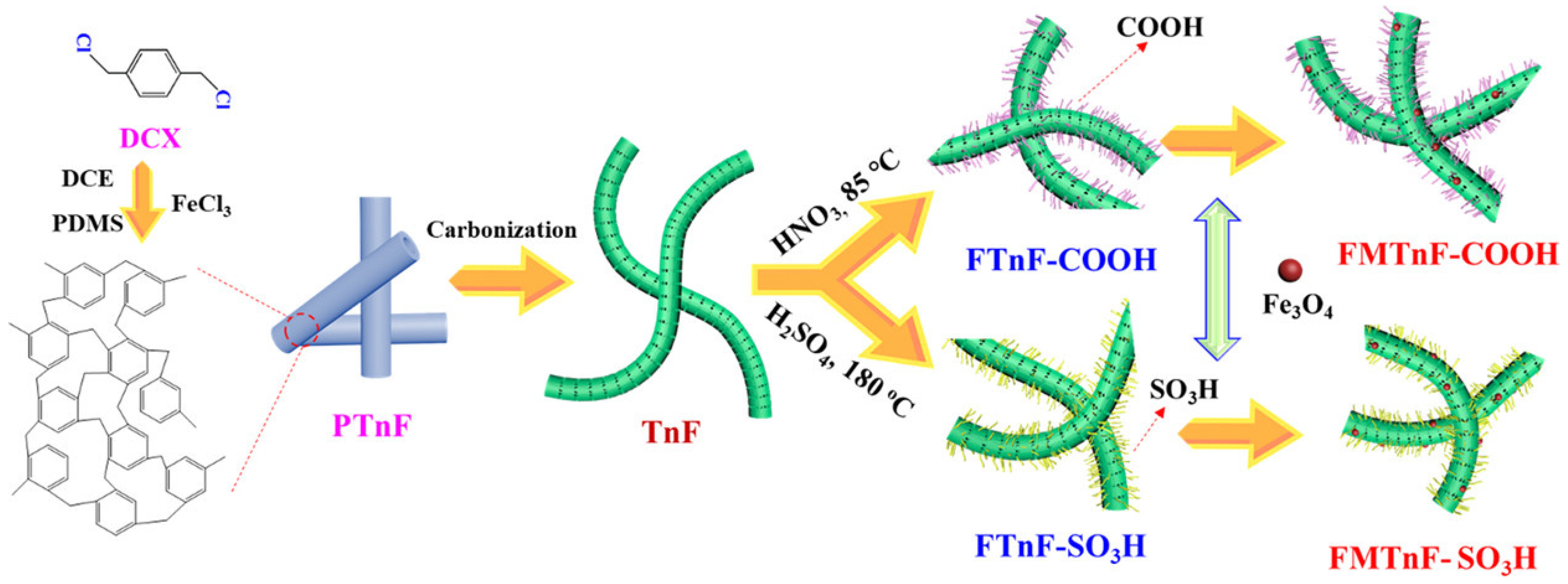


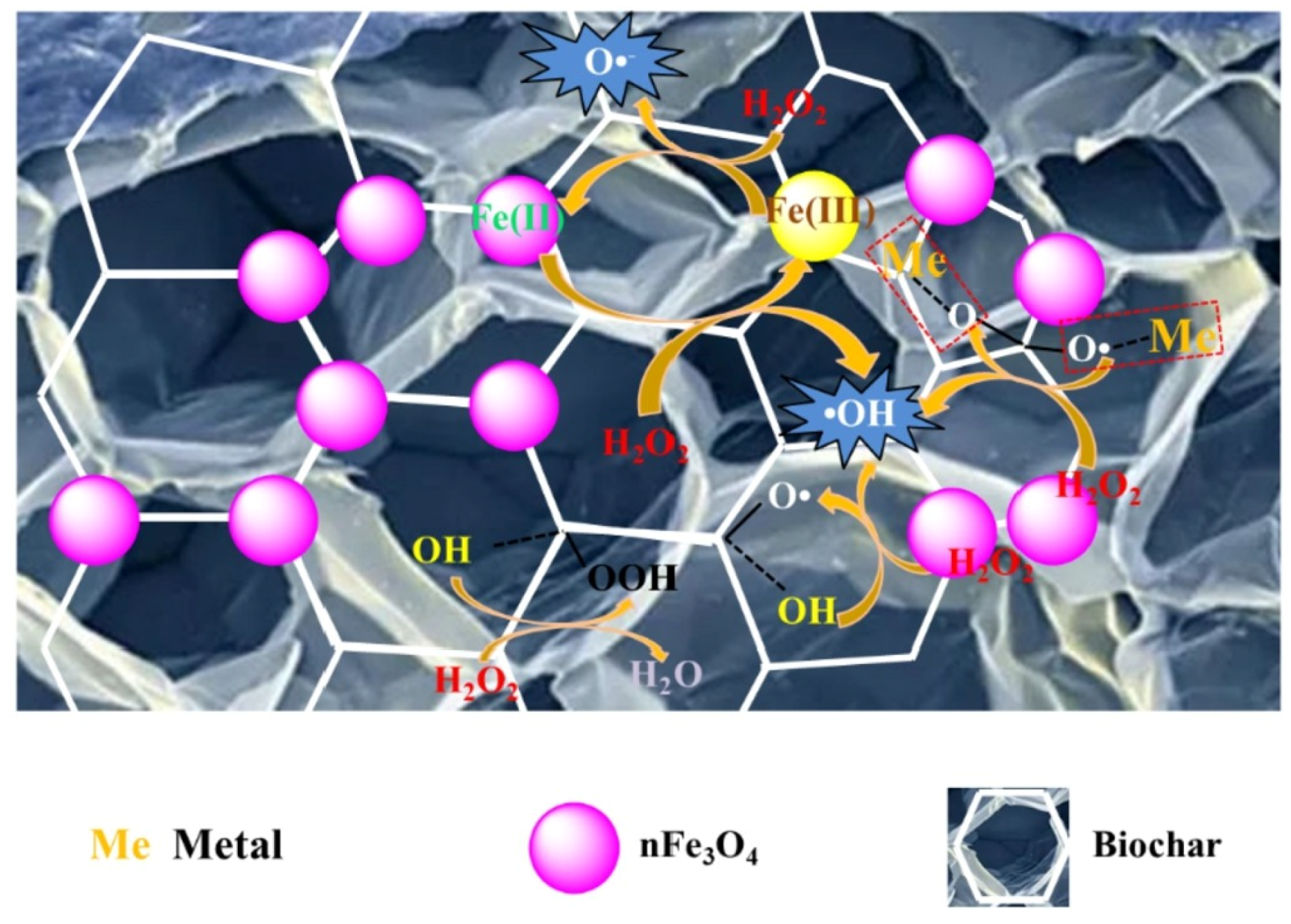
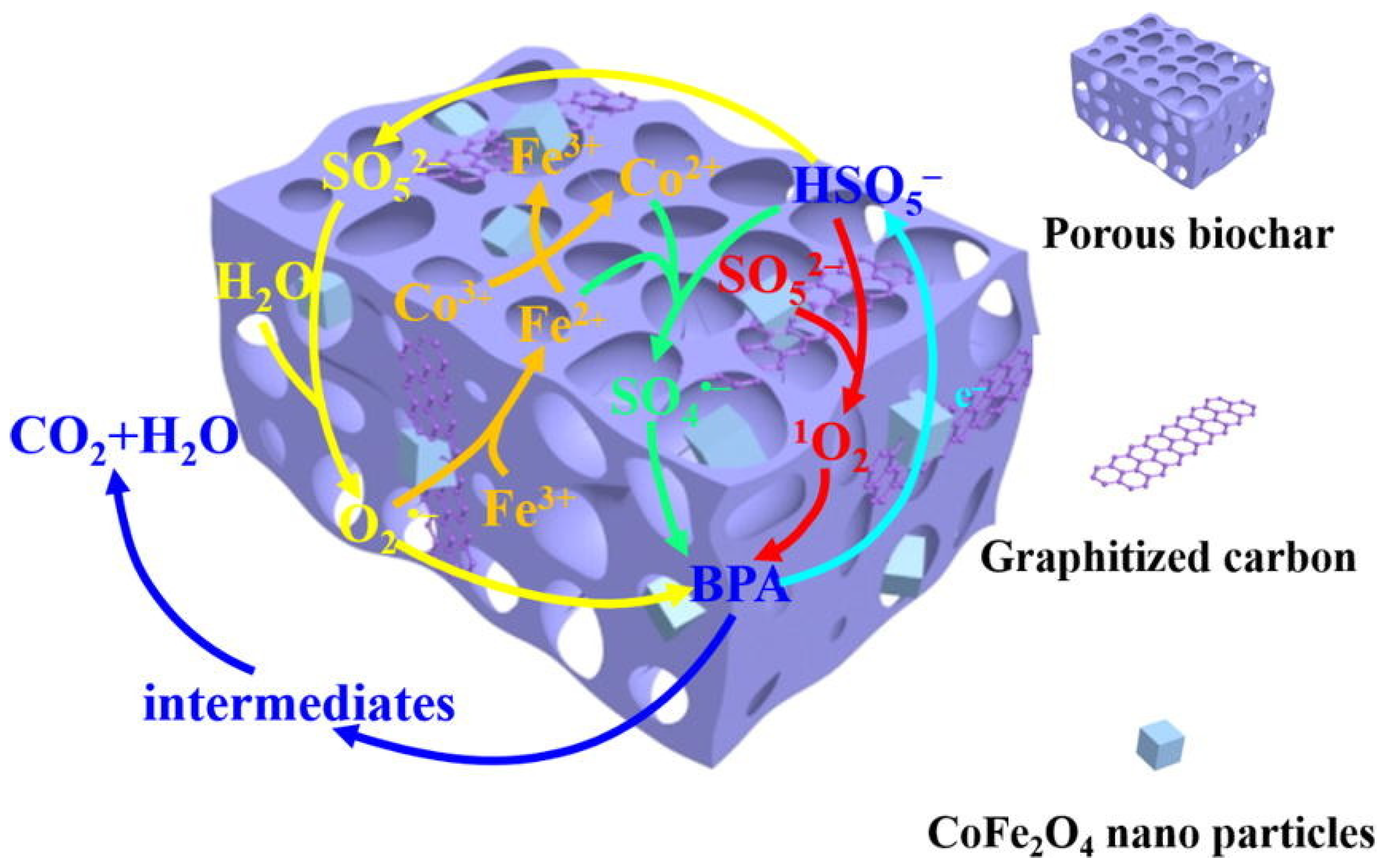
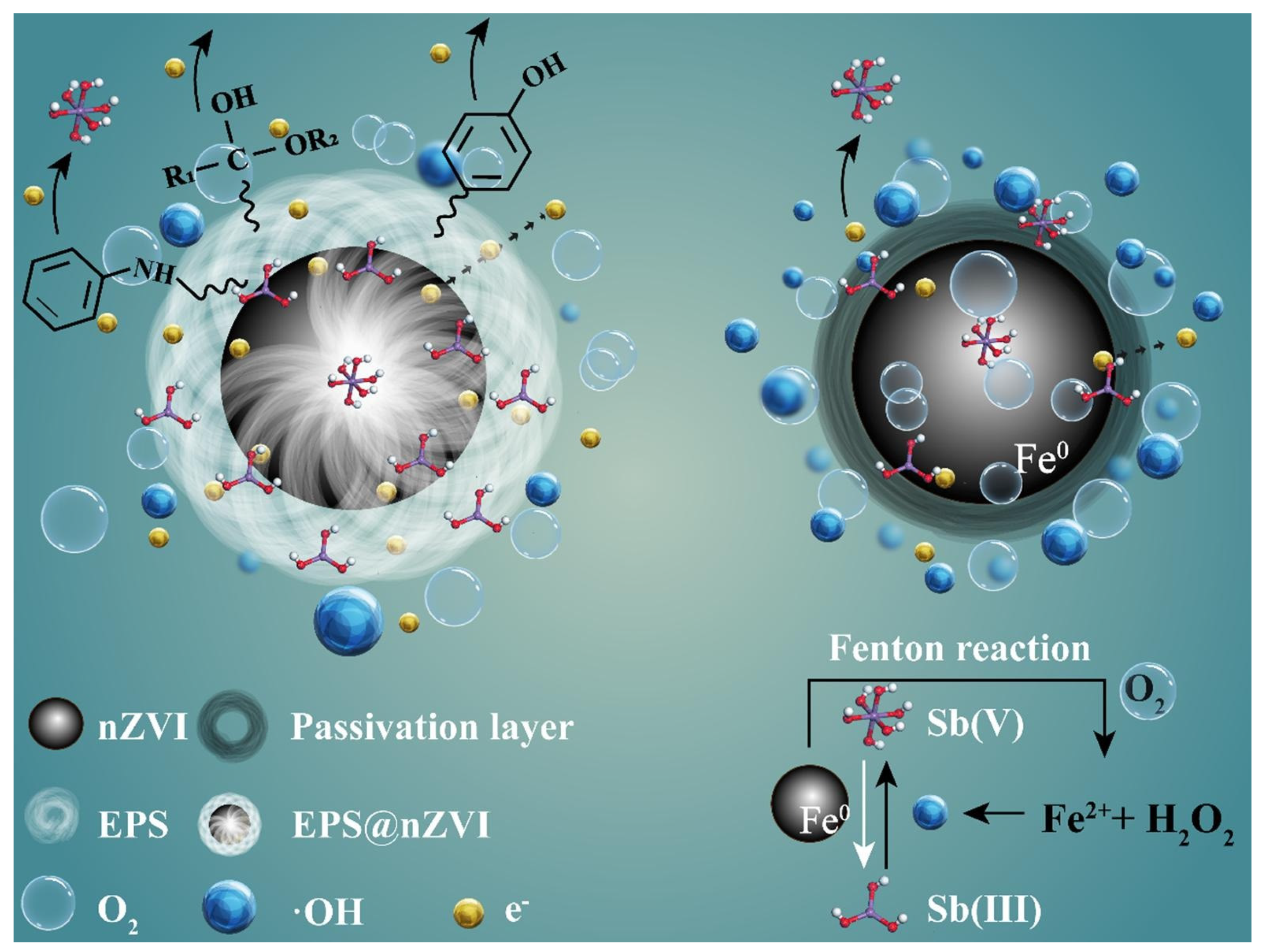
| Category of Contaminant | Subcategory | Selected Examples | Permitted Level (mgL−1) | Examples of Sources | Examples of Known Health Effects |
|---|---|---|---|---|---|
| Inorganic | Heavy metals | cadmium (Cd) lead (Pb) copper (Cu) | 0.005 0.015 0.05 | Mining and petroleum industries | Cancer, Cardiovascular disease, diabetes |
| Polyatomic | Arsenate (AsO43−) Chromate (CrO42−) Nitrate NO3− | 0.05 0.05 50 | Agricultural and steel industries | Renal failure, hypertension, Skeletal malformation | |
| Organic | Dyes | methylene blue crystal violet | 0.01 * 20.8 ** | Textile and Printing Industries | Mutagenicity, Genotoxicity, Cancer |
| Herbicides | 2,3-dichlorophenol | 0.01 *** | Agriculture | ||
| Pharmaceuticals | ibuprofen | NA | Pharmaceutical industry | ||
| Microorganisms | Bacteria | Escherichia coli | 1000 + | Fecal and soil pollution | Waterborne diseases, diarrhea |
| Viruses | Poliovirus-1 | 0 ++ |
| Material | Morphology | Surface Area m2 g−1 | Synthesis Method | Contaminant | Method of Removal | Ref |
|---|---|---|---|---|---|---|
| Fe3O4 | Ultrafine wires | 94.43 | Co-precipitation | Cd2+, Cu2+ Ni2+ | Adsorption | [36] |
| Fe3O4 | Nanorings | 109.3 | Hydrothermal synthesis | Methylene blue, rhodamine B and bromophenol blue | Fenton style catalysis | [37] |
| Mn/Fe oxycarbide | Rod-Like | 179.6 | Solvothermal synthesis + calcination | Butyl parabens | PMS activation | [38] |
| Fe3O4@C–COOH | Hollow spheres | N/A | Solvothermal synthesis | Pb2+ derived from contaminated soils | Adsorption | [39] |
| NixMgyZn(1-x-y)Fe2O4 | Spherical | 91.6 | Ethanol mediated combustion + calcination | Methylene blue dye | Adsorption | [40] |
| MnxZn(1-x)Fe2O4 | Particles | N/A | Co-precipitation | Reactive yellow 15 dye | Photocatalytic Fenton reaction | [41] |
| Cu–nFe3O4 | Octagonal | 83.7 | Co-precipitation | Chlorotetracycline | Catalytic degradation | [42] |
| ZnFe0.8Co0.4O2.4 | Hexagonal | 10.6 | Sol–gel combustion | Bisphenol A and diphenhydramine | PMS activation | [43] |
| FeMnO3 | Porous plate | 11.2 | Sol–gel combustion | Bacillus subtilis bacteria | Mn mediated cell disruption | [44] |
| Mn0.5Zn0.5-xMgxFe2O4 | Spherical | 8.3 | Sol–gel + sintering | Rhodamine B various bacterial species | Photocatalytic oxidation | [45] |
| Ni0.6Zn0.4Fe2O4 and Ni0.6Zn0.2Ce0.2Fe2O4 | Cubic | 20.3 23.6 | Sol gel+ calcination | various bacterial species | Ion release mediated cell disruption | [46] |
| Zero valent iron (nZVI) | Spherical | 33 | Borohydride reduction | Congo red dye | Catalytic reduction | [47] |
| Sulfur-modified nZVIs (S–nZVIs) | Particles | N/A | Ball-milling | Acetaminophen and ren sulfadiazine | PMS activation | [48] |
| Carbon-modified nZVIs | Flakes | 6.6 | Ball-milling | Trichloroethylene | PMS activation | [49] |
| Fe3O4 | Nano needles | 23.4 | Chemical reduction | Pb2+ | Adsorption | [50] |
| Core Magnetic Nanoparticle (MNP) | Mineral Coating | Surface Area (m2 g−1) | Contaminant | Qmax (mg·g−1) | Ref |
|---|---|---|---|---|---|
| CoFe2O4 | Cu/Al LDO/LDO | 89 (17.6 calcined) | Methyl orange | 1355.1 | [74] |
| Fe3O4 | Ni/Fe LDH | 93.9 | Histidine | 3287.67 | [75] |
| Fe2O3 | Sericite Clay | 161.5 | Methylene blue crystal violet | 35.36 35.45 | [76] |
| Various iron oxides | Spent bleaching earth | 122.1 | Tetracycline | 106 | [77] |
| ZnFe2O4 | Hydroxyapatite | 60.2 | Cd2+ | 120.33 | [78] |
| Core NMP | Form of Carbon or Additional Material | Surface Area (m2 g−1) | Contaminant | Method of Removal | Ref |
|---|---|---|---|---|---|
| MnFe2O4 | Graphene oxide + sand | N/A | Methylene blue | Fenton reaction | [93] |
| Fe3O4 | Reduced graphene oxide | 177 | Indigo carmine | Fenton reaction | [94] |
| γ-Fe2O3 | Graphene oxide | 177 | 17β-estradiol 17α-ethinylestradiol | adsorption | [95] |
| Fe3O4 | Graphene oxide + SiO2 | N/A | Cd2+ + Pb2+ | adsorption | [96] |
| Fe3O4 | Graphene oxide aerogel | 157 | Rhodamine B, methyl orange methylene blue | Photocatalytic oxidation | [97] |
| CoFe2O4 | Graphene oxide aerogel | 341.6 | Benzotriazole | PMS activation | [98] |
| Fe3O4 | Reduced graphene oxide polypyrrole | 33 | Pb2+ | Adsorption | [99] |
| ZnFe2O4 | Reduced graphene oxide tio2 nanosheets | N/A | p-nitrophenol | Photocatalytic oxidation | [100] |
| Fe3O4 | S and n-doped graphene oxide with l-cysteine | 96.3 | Methylene blue, Congo red and neutral red | Adsorption | [101] |
| Fe3O4 | Graphite poly polyethyleneimine | 12.8 | Humic acid | Adsorption | [102] |
| Co0.5 M0.5 Fe2O4 | Carbon nanotubes | 112 | Pentachlorophenol | Adsorption | [103] |
| Fe3O4 | Carbon nanotubes | N/A | Microcystis aeruginosa | Agglutination | [104] |
| Fe | Carbon nanotubes Cu, CuO, Al | 111.3 | Sulfamerazine | Oxygen reduction, Fenton reaction | [105] |
| Fe20Cu80 | Nanodiamond | N/A | Phenol | Fenton reaction | [106] |
| NinFe | Activated carbon | N/A | Perfluorooctanesulfonate | Reduction | [107] |
| nZVIs | Carbon black | 619.8 | Chloramphenicol | Adsorption + reduction | [108] |
| Core MNP | Polymer of Additional Coating | Surface Area (m2 g−1) | Contaminant | Method of Removal | Ref |
|---|---|---|---|---|---|
| Fe3O4 | β-cyclodextrin | 85 | Nd, Gd, ions | Adsorption | [112] |
| Fe3O4 | Poly-vinyl alcohol, sodium alginate | N/A | Nannochloropsis oculata | Flocculant | [113] |
| CuO and FeOOH | Ion exchange resin | 18.9 | As(V) | Adsorption + reduction | [114] |
| Fe3O4 | Thiourea formaldehyde resin | 132.6 | CrO42− and AsO43− | Adsorption | [115] |
| Fe3O4 | Molecular sieves carbon | 522.7 | Methyl orange, methylene blue rhodamine B dyes | Adsorption | [116] |
| Fe/Mn binary oxide | Polyvinylidene fluoride | N/A | As(V) | Adsorption | [117] |
| Fe3O4 | α,α’-dichloro-p-xylene carbonized | 180 | U(VI) | Adsorption | [118] |
| Core MNP | Metal oxide Component | Surface Area (m2.g−1) | Contaminant | Method of Removal | Ref |
|---|---|---|---|---|---|
| CoFe2O4 | Ag2O | N/A | Methyl orange | Photocatalysis | [119] |
| Co2O3 | Cu2O3:Al2O2 | N/A | Various bacteria | Bacterial inhibition | [120] |
| MnFe2O4 | ZrO2 | N/A | Methylene blue | Decolorization | [121] |
| Fe3O4 | SiO2 | 275 | Pb2+ | Adsorption | [122] |
| nZVIs | SiO2 | N/A | Organic arsenic compounds | Fenton reaction + adsorption of waste product | [123] |
| nZVIs | Pd | 10.9 | Carbon tetrachloride | Reduction | [124] |
| Fe3O4 | SiO2 | N/A | Aniline | Adsorption | [125] |
| FeNi3 | SiO2+TiO2 | N/A | Humic acid | Photocatalysis | [126] |
| FeNi3 | SiO2+ZnO | N/A | Tetracycline tamoxifen | Photocatalysis | [127,128] |
| FeNi3 | SiO2 | 481.6 | Tetracycline metronidazole | Fenton catalysis | [128,129] |
| Fe3O4 | TiO2 | 29.4 | Rhodamine B | Photocatalysis | [130] |
| Iron oxide (industrial waste) | TiO2 | N/A | Organic dyes and bacteria | Photocatalysis | [131] |
| ZnFe2O4 | V2O5 (e-waste) | N/A | BTEX | Fenton catalysis | [132] |
| γ-Fe2O3 | Mn–Fe Oxide | 109 | As(v) + as(iii) | Adsorption | [133] |
| CoFe2O4 | ZnO2 | N/A | Acid blue 113 | Radical generation | [134] |
| CuFe2O4 | CuO | N/A | Methylene blue | Photocatalysis | [135] |
| FeOOH | Al2O3 | 267 | Humic acid | PMS activation | [136] |
| Co0.5Ni0.5FeCrO4 | UiO-66 MOF SWCNT-GO | 210.5 | 4-nitrophenol 4-aminophenol | Reduction | [137] |
| nZVIs | ZIF-67 MOF PVP | 254.5 | Cr(VI) | Adsorption + reduction | [138] |
| Fe3O4 | Ag | N/A | Rhodamine B methylene blue nitrophenol | Reduction | [139] |
| Fe3O4 | SiO2 | 568 | Pb2+ + methyl orange | Adsorption | [140] |
| Core MNP | Biopolymer | Surface Area (m2 g−1) | Contaminant | Method of Removal | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | Humic acid | 68 | Malachite green | Adsorption + degradation | [144] |
| Fe3O4 | Humic acid | N/A | Carbamazepine, ibuprofen, bisphenol A, 5-tolylbenzotriazole | Fenton photocatalysis | [145] |
| Fe3O4 | Lignosulfonate | 53.8 | Congo red, titan yellow | Adsorption | [146] |
| Fe–Mn binary oxide | Starch | N/A | As(III) and Cd(II) | Adsorption | [147] |
| Fe3O4 | Bean extract | 78.6 | Indigo carmine | Adsorption | [148] |
| Iron oxides | Chitosan | N/A | Sr90 | Adsorption | [149] |
| Fe3O4 | Chitosan | N/A | Microcystin LR | Adsorption | [150] |
| iron | Chitosan | N/A | As(V) | Adsorption | [151] |
| Fe3O4 | Chitosan/polyaniline | N/A | CrO42− | Adsorption | [152] |
| Fe3O4 | Chitosan | N/A | Sunset yellow dye | Adsorption | [153] |
| Fe3O4 | chitosan + SnO2 | 3.6 | Reactive brilliant red | Adsorption | [154] |
| α-Fe2O3 | Cellulose acetate | N/A | Bacteria | Inhibition | [155] |
| nZVIs | Microcrystalline cellulose | 23.4 | Pb2+ | Adsorption | [156] |
| CoFe2O4 | Pectin (from orange juice production) | N/A | Oil emulsion | Adsorption | [157] |
| CuO/Fe2O3 | Loquat extract | 13.4 | Norfloxacin, ciprofloxacin | Adsorption | [158] |
| ZnFe2O4 | Black pepper extract | N/A | Methylene blue | Photocatalysis | [159] |
| Ni0.1CuO | Gum acacia | N/A | Various bacteria | Inhibition | [160] |
| Iron oxides | Myrrh gum | N/A | Crude oil | Adsorption | [161] |
| Fe3O4 | Onion peels, corn-silk husks | 243 261 | As species | Adsorption | [162] |
| Magnetic iron oxide | Bovine serum albumin | 70.6 | PO43− | Adsorption | [163] |
| nZVI | Extracellular polymeric substances (EPS) | N/A | SB(V) | Reduction | [164] |
| S–nZVI | EPS from sewage sludge | N/A | Nitrobenzene | Reduction | [165] |
| Source of Biochar | MNP (Other nps) | Surface Area (m2 g−1) | Contaminant | Method of Removal | Ref. |
|---|---|---|---|---|---|
| Palm fibers | nZVIs | 574 | Cd2+, As3+ | Adsorption | [170] |
| Oakwood | nZVIs | 10.97 | Cr(VI) | Adsorption | [171] |
| Rice straw | nZVIs | 392.8 | Cr(VI) Trichlorobenzene | Adsorption reduction | [172,173] |
| Pine needles | Fe3O4 | 175.3 | Ethylbenzene | Fenton catalysis | [174] |
| Sewage sludge | nZVI | 44 | Sb(III) | Adsorption | [175] |
| Sewage sludge | CoFe2O4 | N/A | norfloxacin | PMS activation | [176] |
| Wheat straw | nZVIs | 60.3 | Cr(VI) | Adsorption | [177] |
| Corn stalk | CoFe2O4 | 664.8 | Bisphenol A | PMS activation | [178] |
| Rice straw | CoFe2O4 | 150.7 | Metolachlor | PMS activation | [179] |
| Organism | Processing | MNP | Surface Area (m2 g−1) | Contaminant | Removal Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Aspergillus niger | Deactivated | Reduced iron | N/A | Cr(VI) | Adsorption | [183] |
| Brewer’s yeast | Dried | Fe3O4 | 67 | Oil | Adsorption | [184,185] |
| Bacillus cereus SO-14 | Dried and ground | γ-Fe2O3 | N/A | U and Th ions | Adsorption | [186] |
| Ulva prolifera | Crushed | S–nZVI | 56.3 | BrO3− | Adsorption | [187] |
| Shewanella putrefaciens | None | nZVI | N/A | Pentachlorophenol | Reduction | [188] |
| Aeromonas hydrophila | None | nZVI | N/A | Cr(VI) | Reduction | [189] |
| Pleurotus ostreatus | Dried and powdered | iron oxide | N/A | Ni2+ + Pb2+ | Adsorption | [190] |
| Var. spp. | None | Fe3O4 | N/A | Di-(2-ethylhexyl) phthalate | Reduction | [191] |
| Waste Source | Material Generated Using Waste | Resulting Composite | Contaminant | Ref. |
|---|---|---|---|---|
| E-waste | V2O5 | ZnFe2O4@V2O5 | BTEX | [132] |
| Water treatment waste sludge | Sludge-based activated carbon | Mg–Fe LDH/SBAC MgFe composite | NO3− and PO43− | [81] |
| Magnetic fly ash | C@magnetic fly ash | chitosan/polydopamine @C@magnetic fly ash | Ag+ | [169] |
| Orange fruit residue | Pectin based stabilizer | Pectin stabilized CoFe2O4 | Oil | [157] |
| Wheat straw | Biochar | Biochar-CMC-nZVI | Cr(VI) | [177] |
| Coal fly ash | Na-X Zeolite | Magnetic nanoparticle/ Zeolite composite | Cd2+ + Pb2+ | [87] |
| Iron oxide powders from mineral processing | Raw-waste iron oxide | Fe2O3@TiO2 composite | Water-soluble dyes | [131] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govan, J. Recent Advances in Magnetic Nanoparticles and Nanocomposites for the Remediation of Water Resources. Magnetochemistry 2020, 6, 49. https://doi.org/10.3390/magnetochemistry6040049
Govan J. Recent Advances in Magnetic Nanoparticles and Nanocomposites for the Remediation of Water Resources. Magnetochemistry. 2020; 6(4):49. https://doi.org/10.3390/magnetochemistry6040049
Chicago/Turabian StyleGovan, Joseph. 2020. "Recent Advances in Magnetic Nanoparticles and Nanocomposites for the Remediation of Water Resources" Magnetochemistry 6, no. 4: 49. https://doi.org/10.3390/magnetochemistry6040049
APA StyleGovan, J. (2020). Recent Advances in Magnetic Nanoparticles and Nanocomposites for the Remediation of Water Resources. Magnetochemistry, 6(4), 49. https://doi.org/10.3390/magnetochemistry6040049





