Designing Magnetic NanoMOFs for Biomedicine: Current Trends and Applications
Abstract
1. Introduction
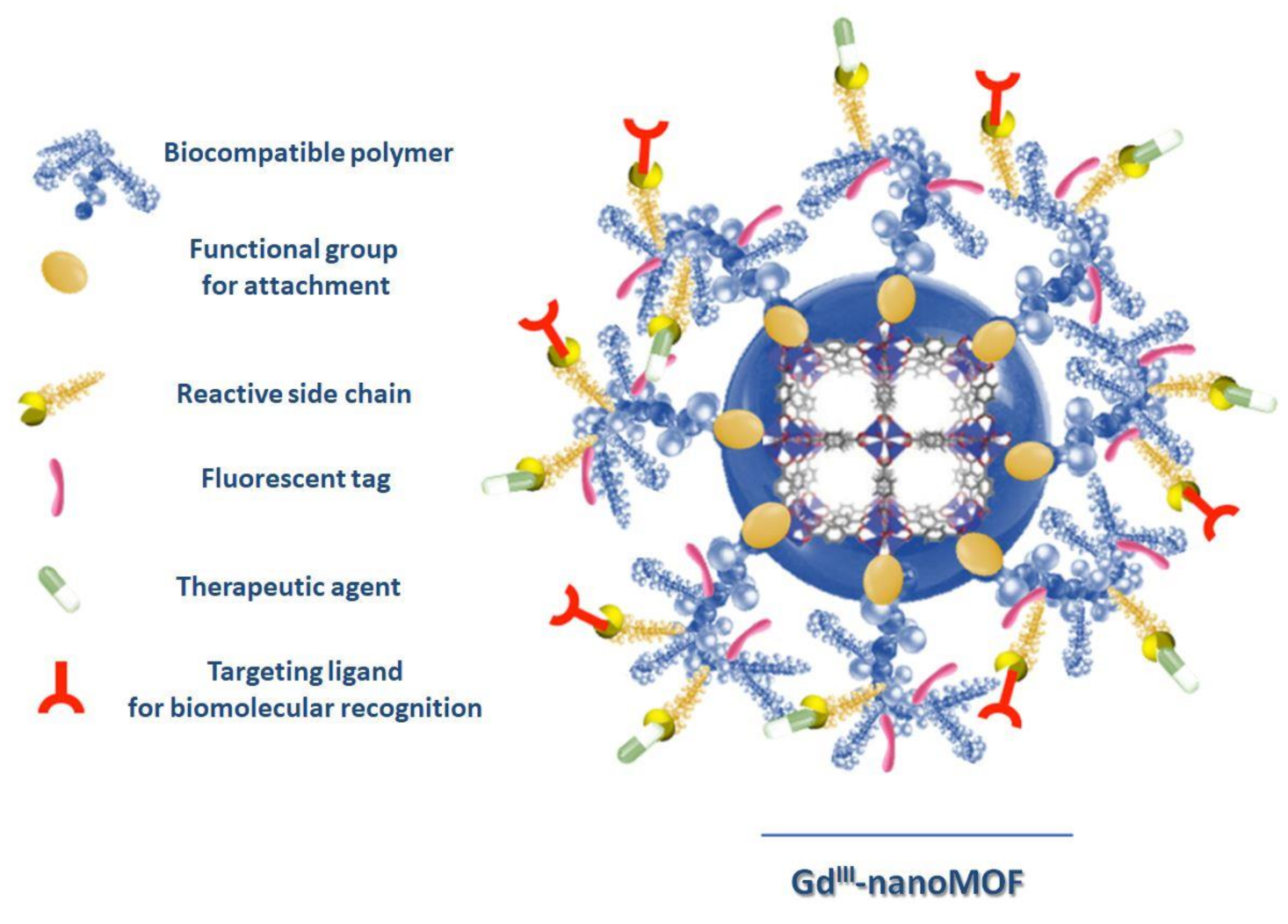
2. Biocompatible MOF Design
3. Miniaturization
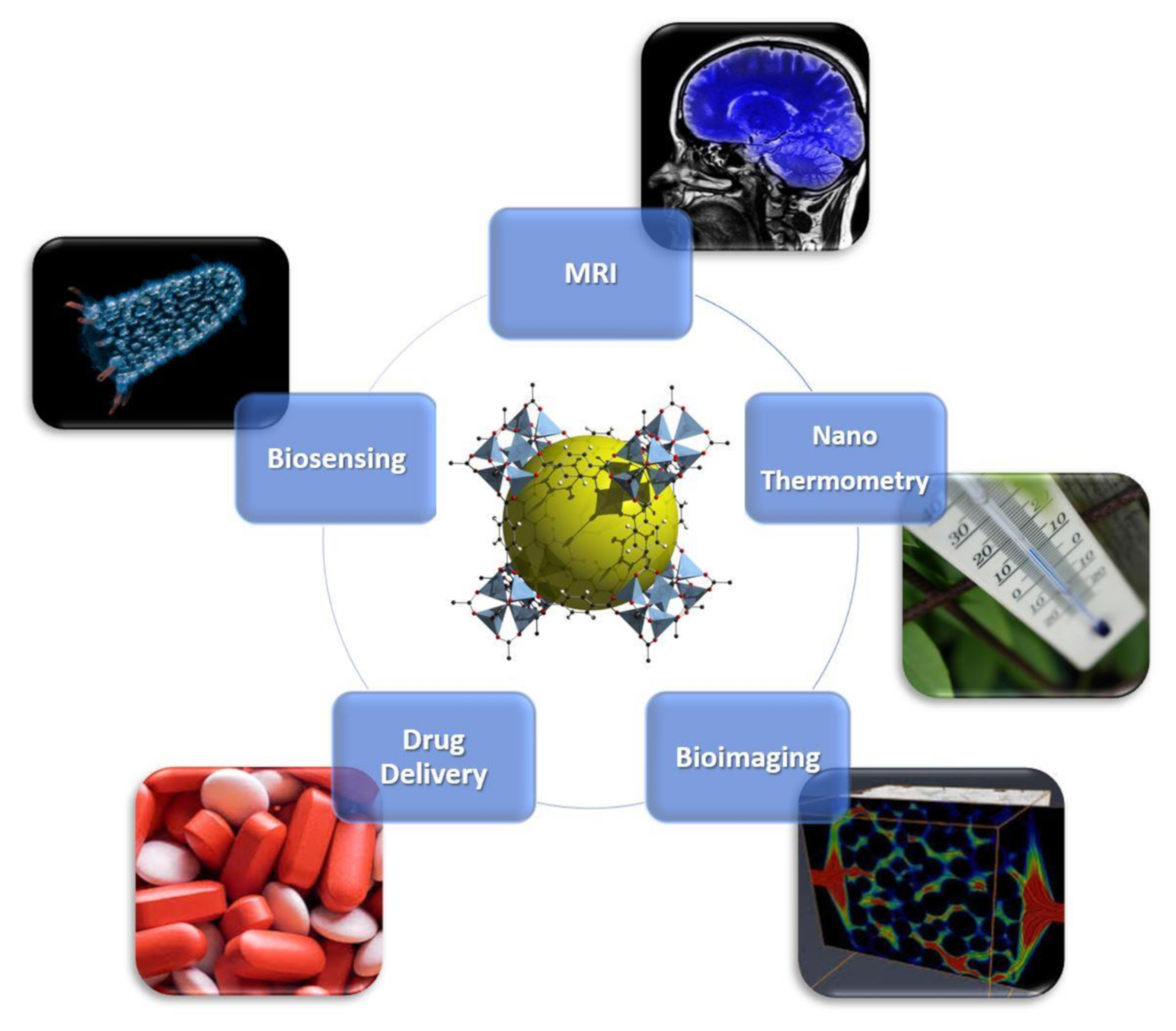
4. Magnetic MOF Applications
4.1. GdIII-Based MOFs
4.2. FeIII-Based MOFs
4.3. MnII-Based MOFs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mendes, R.F.; Almeida Paz, F.A. Transforming metal-organic frameworks into functional materials. Inorg. Chem. Front. 2015, 2, 495–509. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal-organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Gascón, V.; Carucci, C.; Jiménez, M.B.; Blanco, R.M.; Sánchez-Sánchez, M.; Magner, E. Rapid In Situ Immobilization of Enzymes in Metal–Organic Framework Supports under Mild Conditions. ChemCatChem 2017, 9, 1182–1186. [Google Scholar] [CrossRef]
- Ashoka Sahadevan, S.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-Based Neutral Coordination Polymer Nanosheets for Solvent Sensing. ACS Appl. Nano Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef]
- Carucci, C.; Bruen, L.; Gascón, V.; Paradisi, F.; Magner, E. Significant Enhancement of Structural Stability of the Hyperhalophilic ADH from Haloferax volcanii via Entrapment on Metal Organic Framework Support. Langmuir 2018, 34, 8274–8280. [Google Scholar] [CrossRef] [PubMed]
- Ashoka Sahadevan, S.; Monni, N.; Abhervé, A.; Marongiu, D.; Sarritzu, V.; Sestu, N.; Saba, M.; Mura, A.; Bongiovanni, G.; Cannas, C.; et al. Nanosheets of Two-Dimensional Neutral Coordination Polymers Based on Near-Infrared-Emitting Lanthanides and a Chlorocyananilate Ligand. Chem. Mater. 2018, 30, 6575–6586. [Google Scholar] [CrossRef]
- Ye, H.Q.; Li, Z.; Peng, Y.; Wang, C.C.; Li, T.Y.; Zheng, Y.X.; Sapelkin, A.; Adamopoulos, G.; Hernández, I.; Wyatt, P.B.; et al. Organo-erbium systems for optical amplification at telecommunications wavelengths. Nat. Mater. 2014, 13, 382–386. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Zhou, H.L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef]
- Taylor, K.M.L.; Jin, A.; Lin, W. Surfactant-assisted synthesis of nanoscale gadolinium metal-organic frameworks for potential multimodal imaging. Angew. Chem. Int. Ed. 2008, 47, 7722–7725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, R.B.; Wang, J.; Wang, B.; Liang, B.; Yildirim, T.; Zhang, J.; Zhou, W.; Chen, B. Optimization of the Pore Structures of MOFs for Record High Hydrogen Volumetric Working Capacity. Adv. Mater. 2020, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Frey, B.L.; Chen, Y.S.; Zhang, J. Topology-Guided Stepwise Insertion of Three Secondary Linkers in Zirconium Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Ng, E.P.; Bakhtiari, K.; Vinciguerra, M.; Ahmad, H.A.; Awala, H.; Mintova, S.; Daghighi, M.; Bakhshandeh Rostami, F.; De Vries, M.; et al. Zeolite Nanoparticles for Selective Sorption of Plasma Proteins. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mameli, V.; Musinu, A.; Ardu, A.; Ennas, G.; Peddis, D.; Niznansky, D.; Sangregorio, C.; Innocenti, C.; Thanh, N.T.K.; Cannas, C. Studying the effect of Zn-substitution on the magnetic and hyperthermic properties of cobalt ferrite nanoparticles. Nanoscale 2016, 8, 10124–10137. [Google Scholar] [CrossRef]
- Sanna Angotzi, M.; Musinu, A.; Mameli, V.; Ardu, A.; Cara, C.; Niznansky, D.; Xin, H.L.; Cannas, C. Spinel Ferrite Core-Shell Nanostructures by a Versatile Solvothermal Seed-Mediated Growth Approach and Study of Their Nanointerfaces. ACS Nano 2017, 11, 7889–7900. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Sanna Angotzi, M.; Mameli, V.; Cara, C.; Musinu, A.; Sangregorio, C.; Niznansky, D.; Xin, H.L.; Vejpravova, J.; Cannas, C. Coupled hard–soft spinel ferrite-based core–shell nanoarchitectures: Magnetic properties and heating abilities. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Zhao, H.; Serre, C.; Dumas, E.; Steunou, N. Functional MOFs as theranostics. Met. Fram. Biomed. Appl. 2020. [Google Scholar] [CrossRef]
- Chedid, G.; Yassin, A. Recent trends in covalent and metal organic frameworks for biomedical applications. Nanomaterials 2018, 8, 916. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Zhang, W.; Li, P.; Gao, X.; Zhang, W.; Wang, H.; Tang, B. Photodynamic therapy for hypoxic solid tumors via Mn-MOF as a photosensitizer. Chem. Commun. 2019, 55, 10792–10795. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Ray Chowdhuri, A.; Bhattacharya, D.; Sahu, S.K. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalt. Trans. 2016, 45, 2963–2973. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Monni, N.; Abhervé, A.; Cosquer, G.; Oggianu, M.; Ennas, G.; Yamashita, M.; Avarvari, N.; Mercuri, M.L. Dysprosium Chlorocyanoanilate-Based 2D-Layered Coordination Polymers. Inorg. Chem. 2019, 58, 13988–13998. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Abhervé, A.; Monni, N.; Sáenz De Pipaón, C.; Galán-Mascarós, J.R.; Waerenborgh, J.C.; Vieira, B.J.C.; Auban-Senzier, P.; Pillet, S.; Bendeif, E.E.; et al. Conducting Anilate-Based Mixed-Valence Fe(II)Fe(III) Coordination Polymer: Small-Polaron Hopping Model for Oxalate-Type Fe(II)Fe(III) 2D Networks. J. Am. Chem. Soc. 2018, 140, 12611–12621. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Hernandez-Paredes, A.; Mondal, A.; Lopez Martinez, G.; Canet-Ferrer, J.; Konar, S.; Gomez-Carcia, C. Slow relaxation of the magnetization, reversible solvent exchange and luminescence in 2D anilato-based frameworks. Chem. Commun. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mínguez Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor for Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Liang, C.; Feng, L.; Fu, T.; Dong, Z.; Chao, Y.; Li, Y.; Lu, G.; Chen, M.; et al. Nanoscale Metal-Organic Particles with Rapid Clearance for Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Nano 2016, 10, 2774–2781. [Google Scholar] [CrossRef]
- Du, T.; Zhao, C.; ur Rehman, F.; Lai, L.; Li, X.; Sun, Y.; Luo, S.; Jiang, H.; Gu, N.; Selke, M.; et al. In Situ Multimodality Imaging of Cancerous Cells Based on a Selective Performance of Fe2+-Adsorbed Zeolitic Imidazolate Framework-8. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host-guest interactions in Fe(III)-trimesate MOF nanoparticles loaded with doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine- and amino-decorated pores. J. Am. Chem. Soc. 2010, 132, 38–39. [Google Scholar] [CrossRef]
- Cattaneo, D.; Warrender, S.J.; Duncan, M.J.; Kelsall, C.J.; Doherty, M.K.; Whitfield, P.D.; Megson, I.L.; Morris, R.E. Tuning the nitric oxide release from CPO-27 MOFs. RSC Adv. 2016, 6, 14059–14067. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Q.; Chen, Q.; Tang, L.L.; Zhuang, C.; Zhu, W.R.; Lin, N. A porous metal-organic framework with a unique hendecahedron-shaped cage: Structure and controlled drug release. Dalt. Trans. 2016, 45, 3694–3697. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mittal, D.; Verma, A.K.; Roy, I. Copper-Gallic Acid Nanoscale Metal-Organic Framework for Combined Drug Delivery and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 2092–2101. [Google Scholar] [CrossRef]
- Hatakeyama, W.; Sanchez, T.J.; Rowe, M.D.; Serkova, N.J.; Liberatore, M.W.; Boyes, S.G. Synthesis of gadolinium nanoscale metal-organic framework with hydrotropes: Manipulation of particle size and magnetic resonance imaging capability. ACS Appl. Mater. Interfaces 2011, 3, 1502–1510. [Google Scholar] [CrossRef]
- Kundu, T.; Mitra, S.; Díaz Díaz, D.; Banerjee, R. Gadolinium(III)-Based Porous Luminescent Metal–Organic Frameworks for Bimodal Imaging. Chempluschem 2016, 81, 728–732. [Google Scholar] [CrossRef]
- Kawano, T. Use of swimming cells of green paramecia for detection of toxic rare earth ions at lethal and sub-lethal concentration. Adv. Mater. Res. 2014, 875–877, 2229–2237. [Google Scholar] [CrossRef]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martínez, M.; An, J.; Solé-Font, I.; Rosi, N.L.; Maspoch, D. Metal-biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302. [Google Scholar] [CrossRef]
- Oggianu, M.; Mameli, V.; Monni, N.; Ashoka Sahadevan, S.; Sanna Angotzi, M.; Cannas, C.; Mercuri, M.L. Nanoscaled Metal-Organic Frameworks (Nano-MOFs): Challenges towards Biomedical Applications. J. Nanosci. Nanotechnol. 2019, in press. [Google Scholar]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Yan, L.; Wang, G.; Liu, A. Recent advances in the synthesis of spherical and nanoMOF-derived multifunctional porous carbon for nanomedicine applications. Coord. Chem. Rev. 2019, 391, 69–89. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Mameli, V.; Angotzi, M.S.; Cara, C.; Cannas, C. Liquid Phase Synthesis of Nanostructured Spinel Ferrites—A Review. J. Nanosci. Nanotechnol. 2019, 19, 4857–4887. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Flügel, E.A.; Ranft, A.; Haase, F.; Lotsch, B.V. Synthetic routes toward MOF nanomorphologies. J. Mater. Chem. 2012, 22, 10119–10133. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Lamer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Wang, X.G.; Cheng, Q.; Yu, Y.; Zhang, X.Z. Controlled Nucleation and Controlled Growth for Size Predicable Synthesis of Nanoscale Metal–Organic Frameworks (MOFs): A General and Scalable Approach. Angew. Chem. Int. Ed. 2018, 57, 7836–7840. [Google Scholar] [CrossRef] [PubMed]
- Simon-Yarza, T.; Giménez-Marqués, M.; Mrimi, R.; Mielcarek, A.; Gref, R.; Horcajada, P.; Serre, C.; Couvreur, P. A Smart Metal–Organic Framework Nanomaterial for Lung Targeting. Angew. Chem. Int. Ed. 2017, 56, 15565–15569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Morris, W.; Liu, Y.; McGuirk, C.M.; Zhou, Y.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Surface-specific functionalization of nanoscale metal-organic frameworks. Angew. Chem. Int. Ed. 2015, 54, 14738–14742. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykaç, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Cutrone, G.; Qiu, J.; Menendez-Miranda, M.; Casas-Solvas, J.M.; Aykaç, A.; Li, X.; Foulkes, D.; Moreira-Alvarez, B.; Encinar, J.R.; Ladavière, C.; et al. Comb-like dextran copolymers: A versatile strategy to coat highly porous MOF nanoparticles with a PEG shell. Carbohydr. Polym. 2019, 223, 115085. [Google Scholar] [CrossRef]
- Bellido, E.; Hidalgo, T.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; Santander-Ortega, M.J.; González-Fernández, Á.; Serre, C.; Alonso, M.J.; Horcajada, P. Heparin-Engineered Mesoporous Iron Metal-Organic Framework Nanoparticles: Toward Stealth Drug Nanocarriers. Adv. Healthc. Mater. 2015, 4, 1246–1257. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Huskens, J. The effect of PEG length on the size and guest uptake of PEG-capped MIL-88A particles. J. Mater. Chem. B 2016, 4, 1108–1115. [Google Scholar] [CrossRef]
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
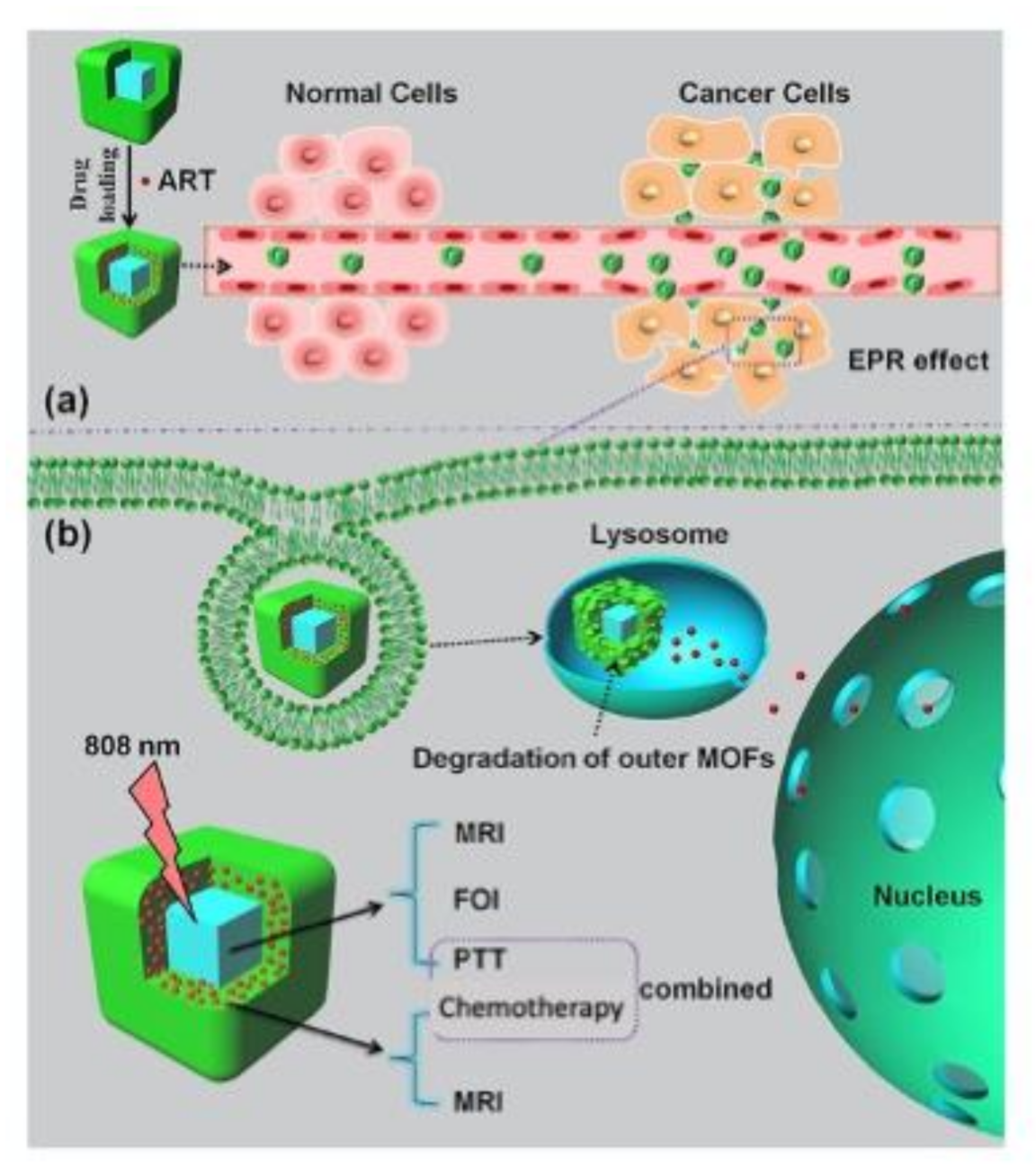
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Rowe, M.D.; Tham, D.H.; Kraft, S.L.; Boyes, S.G. Polymer-modified gadolinium metal-organic framework nanoparticles used as multifunctional nanomedicines for the targeted imaging and treatment of cancer. Biomacromolecules 2009, 10, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, Y.; Li, Y.H.; Sun, S.K.; Yin, X.B. Smart Metal-Organic Framework-Based Nanoplatforms for Imaging-Guided Precise Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Shang, Y.; Cao, P.; Cui, J.; Li, Z.; Yin, X.; Li, Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: Fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int. J. Nanomed. 2019, 14, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug deliveryand imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Wang, X.G.; Dong, Z.Y.; Cheng, H.; Wan, S.S.; Chen, W.H.; Zou, M.Z.; Huo, J.W.; Deng, H.X.; Zhang, X.Z. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef]
- Mao, D.; Hu, F.; Kenry; Ji, S.; Wu, W.; Ding, D.; Kong, D.; Liu, B. Metal–Organic-Framework-Assisted In Vivo Bacterial Metabolic Labeling and Precise Antibacterial Therapy. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Chen, H.; Zhang, J.; Zhang, H.; Wang, Z. Gram-scale synthesis of coordination polymer nanodots with renal clearance properties for cancer theranostic applications. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Xia, G.; Zhou, S.; Liu, Z.; Zhang, N.Q.; Wang, H.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Poon, C.; Lin, W. Theranostic nanoscale coordination polymers for magnetic resonance imaging and bisphosphonate delivery. J. Mater. Chem. B 2014, 2, 8249–8255. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.T.; Shang, Y.; Li, Y.H.; Yin, X.B. Theranostic Mn-Porphyrin Metal-Organic Frameworks for Magnetic Resonance Imaging-Guided Nitric Oxide and Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. [Google Scholar] [CrossRef]
- Qin, L.; Sun, Z.Y.; Cheng, K.; Liu, S.W.; Pang, J.X.; Xia, L.M.; Chen, W.H.; Cheng, Z.; Chen, J.X. Zwitterionic Manganese and Gadolinium Metal-Organic Frameworks as Efficient Contrast Agents for in Vivo Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 41378–41386. [Google Scholar] [CrossRef] [PubMed]


| Metal Ion | Magnetic Moment (μ) | Ionic Radius (pm) | LD50 | Biomedical Application | References |
|---|---|---|---|---|---|
| CrIII | 3 μB | 69 | - | Drug delivery | [34,35] |
| MnII | 5 μB | 80 | 1.5 g/kg | Contrast agent MRI | [35,36,37] |
| FeII | 4 μB | 83 | 30 g/kg | CT imaging, optical imaging | [35,36,38] |
| FeIII | 5 μB | 63 | 30 g/kg | Drug delivery, optical imaging | [16,35,36,39] |
| CoII | 3 μB | 72 | - | Biosensors, bactericidal agents | [35,40] |
| NiII | 2 μB | 69 | - | Drug delivery | [35,41] |
| CuII | 1 μB | 72 | 25 μg/kg | Drug delivery | [35,36,42,43] |
| GdIII | 8 μB | 94 | 58.2 μM (LC50) | Contrast agent MRI | [35,44,45,46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oggianu, M.; Monni, N.; Mameli, V.; Cannas, C.; Ashoka Sahadevan, S.; Mercuri, M.L. Designing Magnetic NanoMOFs for Biomedicine: Current Trends and Applications. Magnetochemistry 2020, 6, 39. https://doi.org/10.3390/magnetochemistry6030039
Oggianu M, Monni N, Mameli V, Cannas C, Ashoka Sahadevan S, Mercuri ML. Designing Magnetic NanoMOFs for Biomedicine: Current Trends and Applications. Magnetochemistry. 2020; 6(3):39. https://doi.org/10.3390/magnetochemistry6030039
Chicago/Turabian StyleOggianu, Mariangela, Noemi Monni, Valentina Mameli, Carla Cannas, Suchithra Ashoka Sahadevan, and Maria Laura Mercuri. 2020. "Designing Magnetic NanoMOFs for Biomedicine: Current Trends and Applications" Magnetochemistry 6, no. 3: 39. https://doi.org/10.3390/magnetochemistry6030039
APA StyleOggianu, M., Monni, N., Mameli, V., Cannas, C., Ashoka Sahadevan, S., & Mercuri, M. L. (2020). Designing Magnetic NanoMOFs for Biomedicine: Current Trends and Applications. Magnetochemistry, 6(3), 39. https://doi.org/10.3390/magnetochemistry6030039









