Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines
Abstract
1. Introduction
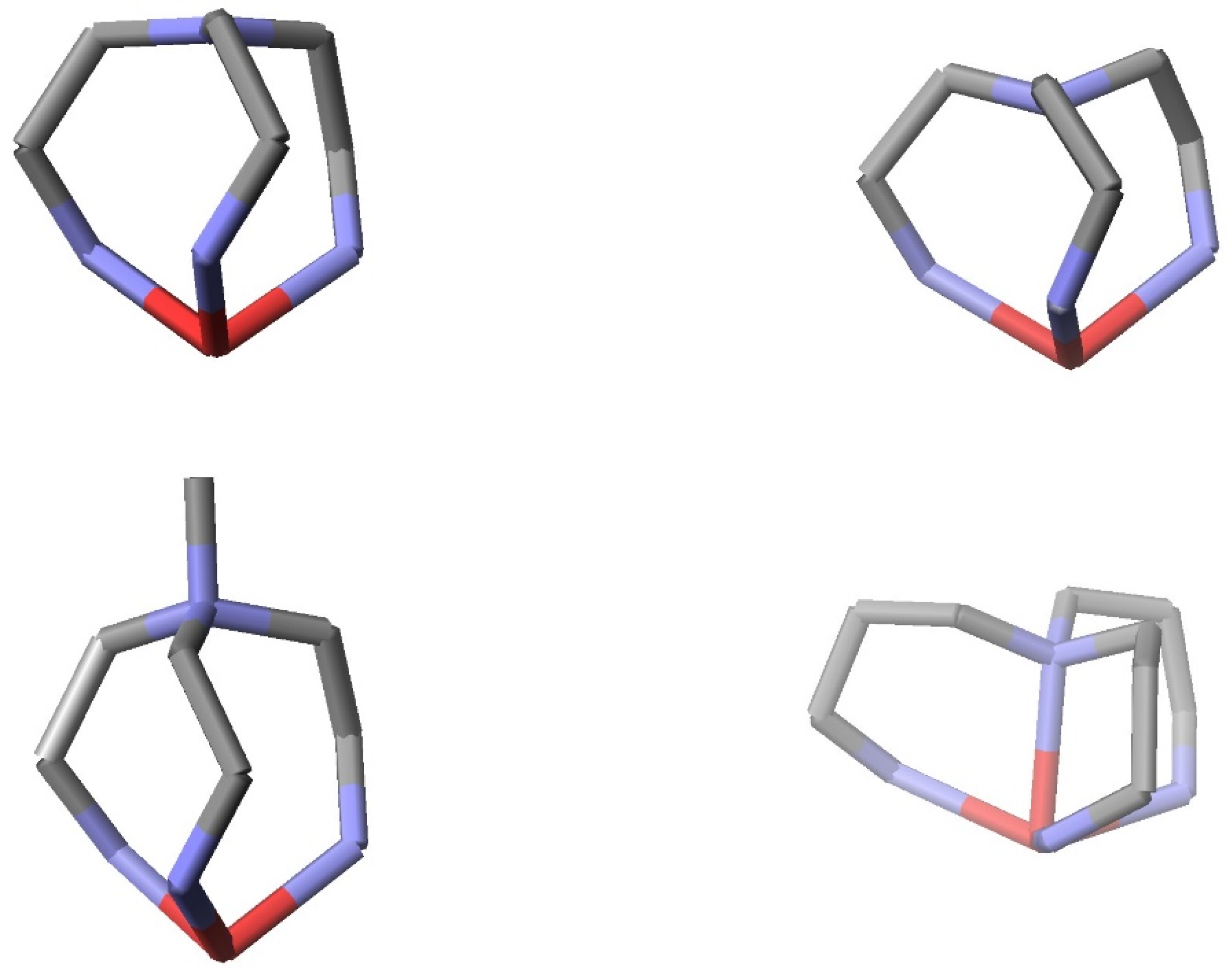
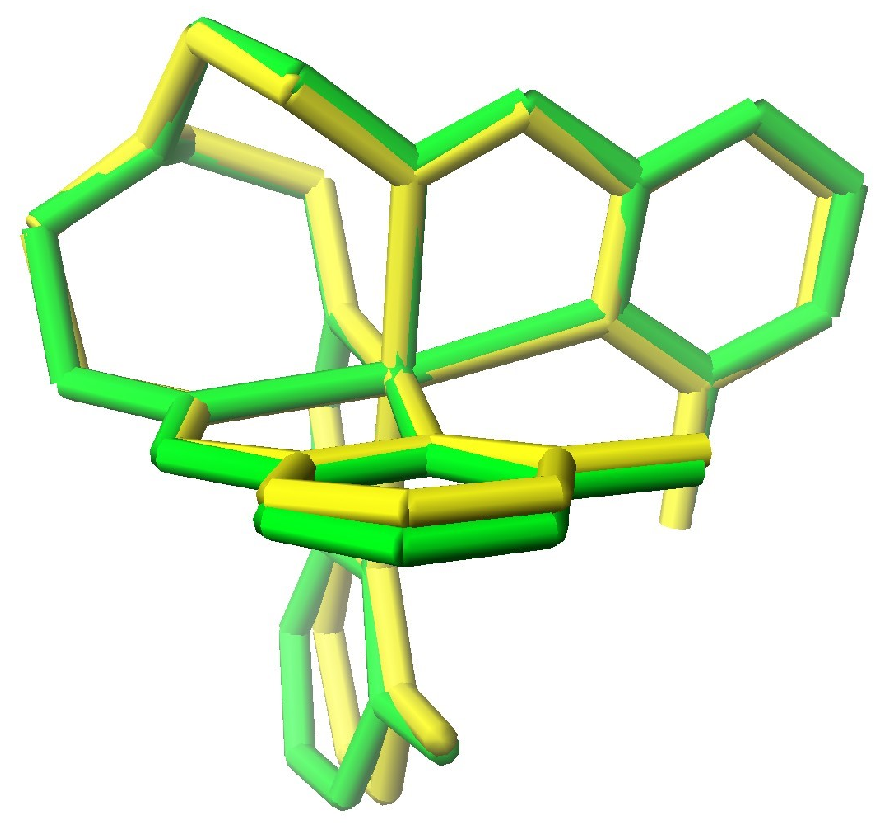
2. Structural Considerations
3. Mononuclear Metal Systems
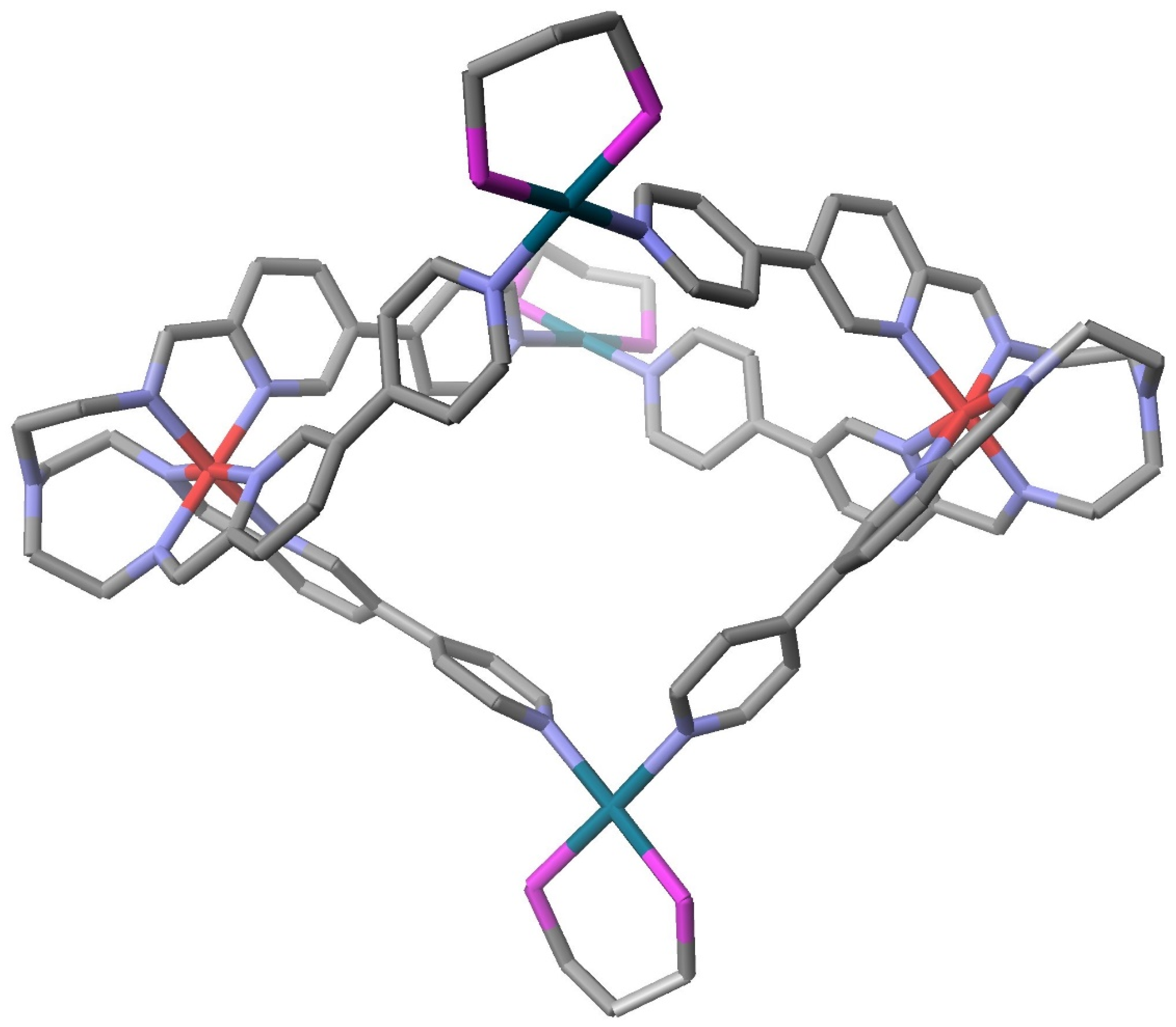
4. Supramolecular Systems
5. Summary and Perspective
Supplementary Materials
Funding
Conflicts of Interest
References
- Gutlich, P.; Goodwin, H.A. Springer Spin Crossover in Transition Metal Compounds. Top. Curr. Chem. 2004, 233, 1–47. [Google Scholar]
- Real, J.A.; Gaspar, A.B.; Munoz, M.C. Thermal pressure and light switchable spin crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Drago, R.S. Physical Methods in Chemistry; W.B. Saunders: Philadelphia, PA, USA, 1997. [Google Scholar]
- Gutlich, P.; Goodwin, H.A. Structural aspects of spin crossover, examples of the [FeIILn(NCS)2] Complexes. Springer Spin Crossover in Transition Metal Compounds. Top. Curr. Chem. 2004, 234, 97–128. [Google Scholar]
- Gutlich, P.; Goodwin, H.A. Structural investigation of tetrazole complexes of Fe(II). Springer Spin Crossover in Transition Metal Compounds. Top. Curr. Chem. 2004, 234, 129–154. [Google Scholar]
- Alvarez, S. Distortion pathways of transition metal coordination polyhedral induced by chelating topology. Chem. Rev. 2015, 115, 13477–13483. [Google Scholar] [CrossRef]
- Figg, D.C.; Herber, R.H.; Felner, I. Evidence for Intermediate spin (S = 1) state stabilization in FeII (4,4′-dpb)2 (NCS)2 and FeII (4,4′-dpb)2 (NCSe)2 (dpb=diphenyl-2,2′-bipyridyl) from susceptibility, infrared and Fe Mossbauer Data. Inorg. Chem. 1991, 30, 2535–2540. [Google Scholar] [CrossRef]
- Shankar, S.; Peters, M.; Steinborn, K.; Krahwinkel, B.; Sonnichsen, F.D.; Grote, D.; Sander., W.; Lohmiller, T.; Rudiger., O.; Herges, R. Light controlled switching of the spin sate of iron(III). Nat. Comm. 2018, 9, 4750. [Google Scholar] [CrossRef]
- Gutlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Hauser, A.; Jeftic, J.; Romstedt, H.; Hinek, R.; Spiering, H. Cooperative phenomena and light-induced bistability in iron(II) spin-crossover compounds. Coord. Chem. Rev. 1999, 190, 471. [Google Scholar] [CrossRef]
- Kahn, T.O.; Jay-Martinez, C. Spin-Transition Polymers: From Molecular Materials Toward Memory Devices. Science 1998, 279, 44. [Google Scholar] [CrossRef]
- Halder, G.J.; Kepert, C.J.; Moubara, B. Guest dependent spin crossover in a nonoporous molecular framework material. Science 2002, 298, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Yoneda, K.; Agusti, G.; Munoz, M.C.; Gaspar, A.B.; Real, J.A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional chemo-switching of spin state in a microporous Framework. Angew. Chem. Int. Ed. 2009, 48, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.G.; Sinn, E. First manganese(III) spin crossover and first d4 crossover. Comment on cytochrome oxidase. J. Am. Chem. Soc. 1981, 103, 241–243. [Google Scholar] [CrossRef]
- Brewer, G.; Luckett, C. Preparation and separation of asymmetric tripodal iron complexes ot tren and selected aldehydes. Inorg. Chim. Acta 2005, 358, 239–245. [Google Scholar] [CrossRef]
- Morgernstern-Badarau, I.; Lambert, F.; Renault, J.P.; Cesario, M.; Marechal, J.D.; Maseras, F. Amine conformational change and spin conversion induced by metal-assisted ligand oxidation: From the seven coordinate iron(II) TPAA complex to the two oxidized iron(II)–(py)3tren isomers. Characterization, crystal structures, and density functional study. Inorg. Chim. Acta 2000, 297, 338–350. [Google Scholar] [CrossRef]
- Brewer, C.; Brewer, G.; Viragh, C.; Patil, G.; Sun, Y.; Butcher, R.J. Conformational Control of Spin State in Iron(II) Tripodal Imidazole Complexes. Inorg. Chim. Acta 2005, 358, 3441–3448. [Google Scholar] [CrossRef]
- Brewer, G.; White, G.; Brewrer, C.T.; Butcher, R.; Schmiedekamp, A.; Viragh, C.; Carpenter, E. Synthesis and Characterization of Iron(II) and Iron(III) Complexes of a Tripodal Ligand Derived from Tris(2-aminoethhyl)methane. Inorg. Chim. Acta 2009, 362, 4158–4166. [Google Scholar] [CrossRef]
- Brewer, C.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Cuenca, L.; Schmiedekamp, A.M.; Viragh, C. Synthesis and Characterization of Seven-Coordinate Tripodal Imidazole Complexes of Iron(II) and Manganese(II). J. Chem. Soc. Dalton Trans. 2005, 22, 3617–3619. [Google Scholar] [CrossRef]
- Brewer, C.; Brewer, G.; Butcher, R.J.; Robichaux, G.T.; Viragh, C. Correlation of nitrogen chirality, R or S, with metal chelate chirality, delta or lambda, in a series of reduced tripodal Schiff base complexes. A route to total spontaneous resolution. Inorg. Chim. Acta. 2014, 410, 171–177. [Google Scholar]
- Brewer, G.; Olida, M.J.; Schmiedekamp, A.M.; Viragh, C.; Zavalij, P.Y. A DFT Computational Study of Spin Crossover in Iron(III) and Iron(II) Tripodal Imidazole Complexes. A Comparison of Experiment with Calculations. Dalton Trans. 2006, 5617–5629. [Google Scholar] [CrossRef]
- Brewer, G.; Olida, M.J.; Schmiedekamp, A.M.; Viragh, C.; Zavalij, P.Y. A DFT Computational Study of Spin Crossover in Iron(III) and Iron(II) Tripodal Imidazole Complexes. A Comparison of Experiment with Calculations, 2nd printing. Dalton Trans. 2007, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Halepoto, D.M.; Holt, D.G.L.; Larkworthy, L.F.; Leigh, G.L.; Povey, D.C.; Smith, G.W. Spin crossover in chromium(II) complexes and the crystal and molecular structure of the high spin form of bis[1,2-bis(diethylphosphino)ethane]di-iodochromium(II). J. Chem. Soc. Chem. Comm. 1989, 1322–1323. [Google Scholar] [CrossRef]
- Halepoto, D.M.; Holt, D.G.L.; Larkworthy, L.F.; Leigh, G.L.; Povey, D.C.; Smith, G.W. Spin crossover in chromium(II) complexes. Polyhedron 1989, 8, 1821–1822. [Google Scholar] [CrossRef]
- Hughes, A.K.; Murphy, V.J.; O’Hare, D.J. Synthesis, X-ray Structure and Spin-Crossover in the Triple-Decker Complex, [(η5-C5Me5)Cr(µ2:η5-P5)Cr(η5-C5Me5)]+[A]–(A = PF6, SbF6). Chem. Soc. Chem. Comm. 1994, 2, 163. [Google Scholar] [CrossRef]
- Mc Daniel, A.M.; Rappe, A.K.; Shores, M.P. Structural and Electronic comparison of 1st row transition metal complex of a tripodal iminopyridine ligand. Inorg. Chem. 2012, 51, 12493–12502. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.M.; Tseng, H.W.; Hill, E.A.; Damrauer, N.H.; Rappe, A.K.; Shores, M.P. Syntheses and Photophysical Investigations of Cr(III) Hexadentate Iminopyridine Complexes and Their Tris(Bidentate) Analogues. Inorg. Chem. 2013, 52, 1368–1378. [Google Scholar] [CrossRef]
- Sickerman, N.; Park, Y.J.; Ng, G.K.Y.; Bates, J.E.; Hilkert, M.; Ziller, J.W.; Furche, F.; Borovik, A. Synthesis, structure, and physical properties for a series of trigonal bipyramidal MII–Cl complexes with intramolecular hydrogen bonds. Dalton Trans. 2012, 41, 4358–4364. [Google Scholar] [CrossRef]
- Sim, P.G.; Sinn, E. Synthesis and crystal structure of tris[1-(2-azolyl)-2-azabuten-4-yl]amineiron(III), [Fe((pyrol)3tren)]. Inorg. Chem. 1978, 17, 1288–1290. [Google Scholar] [CrossRef]
- Guionneau, P.; Marchivie, M.; Garcia, Y.; Howard, J.A.K.; Chasseau, D. Spin crossover in [MnIII(pyrol)3tren] probed by high-pressure and low-temperature x-ray diffraction. Phys. Rev. B Condens. Mat. 2005, 72, 214408. [Google Scholar] [CrossRef]
- Sarkar, S.; Moon, D.; Lah, M.S.; Lee, H.I. Structure and Heme-Independent Peroxidase activity of a fully coordinated mononuclear Mn(II) complex with a Schiff base tripodal ligand containing three imidazole groups. Bull. Korean Chem. Soc. 2010, 31, 3173–3179. [Google Scholar] [CrossRef]
- Halcrow, M.A. The spin states and spin transitions of mononuclear iron(II) complexes of nitrogen-donor ligands. Polyhedron 2007, 26, 3523–3576. [Google Scholar] [CrossRef]
- Brewer, G.; Luckett, C.E.; May, L.; Beatty, A.M.; Scheidt, W.R. Synthesis and Characterization of Tripodal Iron(II) Complexes Prepared from 2-Pyridinecarboxaldehyde and 1-Methyl-2-imidazolecarboxaldehyde: Stabilization of Iron(II) Cations with N6 Donor Sets. Inorg. Chim. Acta 2004, 357, 2390–2396. [Google Scholar] [CrossRef]
- Hardie, M.J.; Kilner, C.A.; Halcrow, M.A. Tris[4-(1H-pyrazol-3-yl)-3-azabut-3-enyl]amine iron(II) diperchlorate monohydrate. Acta Crystallogr. Sect. C. 2004, C60, m177–m179. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.C.; Barrett, T.F.S.A.; Kliner, C.A.; Letard, J.F.; Halcrow, M.A. Thermal and light-induced spin-crossover in salts of the heptadentate complex [tris(4-{pyrazol-3-yl}-3-aza-3-butenyl)amine]iron(ii). Dalton Trans 2007, 4276. [Google Scholar] [CrossRef]
- Yamada, M.; Fukumoto, E.; Ooidemizu, M.; Brefuel, N.; Matsumoto, N.; Iijima, S.; Kojima, M.; Re, N.; Dahan, F.; Tuchauges, J.P. A 2D layered spin crossover complex constructed by NH…Cl− hydrogen bonds: [FeIIH3 LMe]Cl I3 (H3 LMe = tris[2-(((2-methylimidazoyl-4-yl)methylidene)aminoethyl]amine. Inorg. Chem. 2005, 44, 6967–6974. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, M.; Dahan, F.; Tuchauges, J.P. A variety of Spin Crossover behaviors depending on the counter anion: Two dimensional complexes constructed by NH…Cl- hydrogen bonds, [FeIIH3LMe]Cl⋅X (X = PF6−, AsF6−, SbF6−, CF3SO3−; H3LMe = Tris[2-{[(2-methylimidazol-4-yl)methylidene]amino}ethyl]amine). Chem. Eur. J. 2006, 12, 4544–4549. [Google Scholar] [CrossRef]
- Struch, N.; Wagner, N.; Schnakenburg, G.; Weisbarth, R.; Klos, S.; Beck, J.; Lutzen, A. Thiazolylimines as novel lifand systems for spin crossover centered near room temperature. Dalton Trans. 2016, 45, 14023–14029. [Google Scholar] [CrossRef]
- Kulmaczewski, R.; Cespedes, O.; Halcrow, M.A. Gradual thermal spin crossover mediated by a reentrant Z’=1 → Z’ =6 → Z’ =1 Phase transition. Inorg. Chem. 2017, 56, 3144–3148. [Google Scholar] [CrossRef]
- Seredyuk, M.; Carmen-Munoz, M.; Castro, M.; Romero-Morcillo, T.; Gaspar, A.B.; Real, J.A. Unprecedented Multi stable spin crossover molecular material with two thermal memory channels. Chem. Eur. J. 2013, 19, 6591–6596. [Google Scholar] [CrossRef]
- Decurtins, S.; Gutlich, P.; Cohler, C.P.; Spiering, H.; Hauser, A. Light-induced excited spin state trapping in a transition-metal complex: The hexa-1-propyltetrazole-iron (II) tetrafluoroborate spin-crossover system. Chem. Phys. Lett. 1984, 105, 1–4. [Google Scholar] [CrossRef]
- Delgado, T.; Tissot, A.; Guenee, L.; Hauser, A.; Valverdo-Munoz, F.J.; Seredyuk, M.; Real, J.A.; Pillet, S.; Bendeif, E.E.; Besnard, C. Very long lived photogenerated high spin phase of a multistable spin crossover molecular material. J. Am. Chem. Soc. 2018, 140, 12870–12876. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M.; Carmen-Munoz, M.; Ksenofontov, V.; Gutlich, P.; Galyametdinov, Y.; Real, J.A. Spin crossover star shaped metallomesogens of iron(II). Inorg. Chem. 2014, 53, 8442–8454. [Google Scholar] [CrossRef] [PubMed]
- Haglwara, H.; Minoura, R.; Okada, S.; Sunatsuki, Y. Synthesis structure and magnetic property of a new mononuclear iron(II) spin crossover complex with a tripodal ligand containing three 1,2,3 triazole groups. Chem. Lett. 2014, 43, 950–952. [Google Scholar] [CrossRef]
- Gasper, A.B.; Seredyuk, M.; Gutlich, P. Spin crossover in metallomesogens. Coord. Chem. Rev. 2009, 253, 2399–2413. [Google Scholar] [CrossRef]
- Chakraborty, P.; Tissot, A.; Peterhans, L.; Guenee, L.; Besnard, C.; Pattison, P.; Hauser, A. Determination of the molecular structure of the short-lived light-induced high-spin state in the spin-crossover compound [Fe(6-mepy)3tren](PF6)2. Phys. Rev. B Cond. Matt. 2013, 87, 214306. [Google Scholar] [CrossRef]
- Tissot, A.; Riviere, E.; Guillot, R.; Toupet, L.; Collett, L.; Boillot, M.l. Loght induced Excited spin state trapping effect on [Fe(6MePy)2 tren](PF6)2. Dalton Trans. 2014, 43, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Klug, C.M.; McDaniel, A.M.; Fiedler, S.R.; Schulte, K.A.; Newell, B.S.; Shores, M.P. Anion dependence in the spin-crossover properties of a Fe(II) podand complex. Dalton Trans. 2012, 41, 12577–12585. [Google Scholar] [CrossRef]
- Howson, S.E.; Allen, L.E.N.; Chmel, N.P.; Clarkson, G.J.; Deeth, R.J.; Faulkner, A.D.; Simpson, D.H.; Scott, P. Origins of stereoselevtivity in optically pure phenylethaniminopyridine tris chelates M(NN’)3n+ (M=Mn, Fe, Co, Ni and Zn). Dalton Trans. 2011, 40, 10416–10433. [Google Scholar] [CrossRef]
- McDaniel, A.M.; Klug, C.M.; Shores, M.P. Synthesis of Functionalized Hexadentate Iminopyridine FeII Complexes–Toward Anion-Dependent Spin Switching in Polar Media. Eur. J. Inorg. Chem. 2013, 2013, 943–950. [Google Scholar] [CrossRef]
- Ozumerzifon, T.J.; Higgins, R.F.; Joyce, J.P.; Kolanowski, J.L.; Rappe, A.K.; Shores, M.P. Evidence for reagent induced spin state switching in tripodal Fe(II) iminopyridine complexes. Inorg. Chem. 2019, 58, 7785–7793. [Google Scholar] [CrossRef]
- Hardy, M.; Struch, N.; Topic, F.; Schnakenburg, G.; Rissanen, K.; Lutzen, A. Stepwise construction of heterobimetallic cages by an extended molecular library approach. Inorg. Chem. 2018, 57, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M.; Gasper, A.B.; Ksenofontov, V.; Galyametdinov, Y.; Kusz, J.; Gutlich, P. Does the solid-liquid crystal phase transition provoke the spin state change in spin crossover metallomesogens? J. Am. Chem. Soc. 2008, 130, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Struch, N.; Topic, F.; Schnakenburg, G.; Rissanen, K.; Lutzen, A. Electron deficient pyridylimines: Versatile building blocks for functional metallosupramolecular chemistry. Inorg. Chem. 2018, 57, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Plicar, C.; Durot, S.; Cesario, M.; Yuwei, L.; Korri-Youssoufi, H.; Kelta, B.; Nadjo, L. Imidazole and Imidazolate iron complexes: On the way for tuning 3D Structural Characteristics and reactivity. Redox interconversions controlled by protonation state. Inorg. Chem. 2004, 43, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Sunatsuki, Y.; Kojima, M.; Iijima, S.; Akashi, H.; Matsumoto, N. A tripodal ligand containing three imidazole groups inducing spin crossover in both Fe(II) and Fe(III) complexes; Structures and spin crossover behavior of the complexes. Chem. Lett. 2004, 33, 350–351. [Google Scholar] [CrossRef]
- Sunatsuki, S.M.; Matsuzaki, S.; Kojima, M.H.; Matsumoto, N. Thermal and pressure induced spin crossover of a novel iron(III) complex with a tripodal ligand involving three imidazole groups. Chem. Lett. 2001, 30, 1254–1255. [Google Scholar] [CrossRef]
- Brewer, C.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Cuenca, L.; Noll, B.C.; Scheidt, W.R.; Viragh, C.; Zavalij, P.Y.; Zielaski, D. Synthesis and characterization of manganese(ii) and iron(iii) d5 tripodal imidazole complexes. Effect of oxidation state, protonation state and ligand conformation on coordination number and spin state. Dalton. Trans. 2006, 1009. [Google Scholar] [CrossRef]
- Timken, M.D.; Hendrickson, D.N.; Sinn, E. Dynamics of spin state interconversion and cooperativity for ferric spic crossover complexes in the solid state. Inorg. Chem. 1985, 24, 3947–3955. [Google Scholar] [CrossRef]
- Brewer, C.T.; Brewer, G.; Luckett, C.; Marbury, G.S.; Viragh, C.; Beatty, A.M.; Scheidt, W.R. Proton control of oxidation and spin state in a series of iron tripodal imidazole complexes. Inorg. Chem. 2004, 43, 2402. [Google Scholar] [CrossRef]
- Goodwin, H.A. Spin crossover in cobalt(II) systems. Top. Curr. Chem. 2004, 234, 23–47. [Google Scholar]
- Krivopapic, I.; Zerara, M.; Daku, M.L.; Vargas, A.; Enachescu, C.; Ambrus, C.; Tregenna-Piggott, P.; Amstutz, N.; Krausz, E.; Hauser, A. Spin Crossover in cobalt(II) imine complexes. Coord. Chem. Rev. 2006, 251, 364–378. [Google Scholar] [CrossRef]
- Beckmann, U.; Brooker, S. Cobalt(II) complexes of pyridazine or triazole containing ligands: Spin state control. Coord. Chem. Rev. 2003, 245, 17–29. [Google Scholar] [CrossRef]
- Sayami, S.; Shigeyoshi, Y.; Akita, M.; Inoue, K.; Kato, K.; Osaka, K.; Takata, M.; Kawajiri, R.; Mitani, T.; Maedo, Y. Reverse spin transition triggered by a structural phase transition. Angew. Chem. Int. Ed. 2005, 44, 4899–4901. [Google Scholar]
- Sieber, R.; Decurtins, S.; Stoeckli-Evans, H.; Wilson, C.; Yufit, D.; Howard, J.A.K.; Capelli, S.C.; Hauser, A. A thermal spin transition in [Co(bpy)3][LiCr(ox)3] (ox= C2O42−; bpy=2,2′-bipyridine). Chem. Eur. J. 2000, 6, 361–368. [Google Scholar] [CrossRef]
- He, H.S. {Tris[2-(1H-imidazol-2-ylmethyleneamino-[kappa]2N,N’)ethyl]amine}cobalt(II) bis(perchlorate) hemihydrate. Acta Crystallogr. Sect. E 2007, E63, m1933–m1994. [Google Scholar] [CrossRef]
- Alvarado, L.; Brewer, G.; Carpenter, E.E.; Viragh, C.; Zavalij, P. Use of acid–base and redox chemistry to synthesize cobalt(III) and iron(III) complexes of a partially deprotonated triprotic imidazole-containing Schiff base ligand: Hydrogen bound 1D linear homochiral and zig-zag heterochiral supramolecular complexes. Inorg. Chim. Acta 2010, 363, 817. [Google Scholar] [CrossRef]
- Brewer, G.; Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley and Sons: Weinheim, Germany, 2000; unpublished work. [Google Scholar]
- Saalfrank, R.W.; Demleitner, B.; Sauvage, J.P. Transition Metals in Supramolecular Chemistry. Sauvage, J.P., Ed.; John Wiley and Sons: Weinheim, Germany, 1999. [Google Scholar]
- Dodziuk, H. Introduction to Supramolecular Chemistry; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley and Sons: West Sussex, UK, 2000. [Google Scholar]
- Clark, R.J.H. The Chemistry and spectroscopy of Mixed-Valence complexes. Chem Soc. Rev. 1984, 13, 219. [Google Scholar] [CrossRef]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Viragh, C. Synthesis and characterization of a spin crossover iron(ii)–iron(iii) mixed valence supramolecular pseudo-dimer exhibiting chiral recognition, hydrogen bonding, and π–π interactions. Dalton Trans. 2007, 295. [Google Scholar] [CrossRef]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Schmiedekamp, C.; Straka, A.; Viragh, C.; Yuzafpolskiy, Y.; Zavalij, P. Synthesis and Characterization of Homo- and Heterodinuclear M(II)-M(III)’ (M(II) = Mn or Fe, M(III)’ = Fe or Co) Mixed Valence Supramolecular Pseudo-dimers. The Effect of Hydrogen Bonding on Spin State Selection of M(II). Dalton Trans. 2011, 40, 181. [Google Scholar] [CrossRef]
- Brewer, G.; Butcher, R.J.; Zavalij, P. Use of pyrazole hydrogen bonding in tripodal complexes to form self assembled homochiral dimers. Materials 2020, 13, 1595. [Google Scholar] [CrossRef]
- Ohta, H.; Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ijima, S.; Akashi, H.; Kambee, T.; Kojima, M. Spin crossover in a supramolecular Fe(II)-Fe(III) system. Mater. Sci. 2003, 21, 191–198. [Google Scholar]
- Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ohta, H.; Kojima, M.; Iijima, S.; Hayami, S.; Maeda, Y.; Kaizaki, S.; Dahan, F.; et al. An unprecedented homochiral mixed-valence spin-crossover compound. Angew. Chem. Int. Ed. 2003, 42, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Sunatsuki, Y.; Ohta, H.; Kojima, M.; Ikuta, Y.; Goto, Y.; Matsumoto, N.; Iijima, S.; Akashi, H.; Kaizaki, S.; Dahan, F.; et al. Supramolecular Spin-Crossover Iron Complexes Based on Imidazole−Imidazolate Hydrogen Bonds. Inorg. Chem. 2004, 43, 4154–4171. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A New Family of Spin Crossover Complexes with a Tripod Ligand Containing Three Imidazoles: Synthesis, Characterization, and Magnetic Properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar] [PubMed]
- Yamada, M.; Ooidemizu, M.; Ikuta, Y.; Osa, S.; Matsumoto, N.; Iijima, S.; Kojima, M.; Dahan, F.; Tuchagues, J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X- = ClO4−, BF4-, PF6−, AsF6−, and SbF6−; H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 8406–8416. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Matsumoto, N.; Iijima, S.; Kojima, M. Layered iron(II) spin crossover complex constructed by NH…Br− hydrogen bonds with 2 K wide thermal hysteris, [FeIIH3LMe]BrCF3SO3 (H3LMe = tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine. Inorg. Chem. Acta 2011, 366, 283–289. [Google Scholar] [CrossRef]
- Brewer, G.; Alvorado, L.J.; Brewer, C.T.; Butcher, R.J.; Cipresi, J.; Viragh, C.; Zavalij, P.Y. Synthesis, structure and supramolecular features of homonuclear iron(III) and heteronuclear iron(III)–cobalt(III) complexes, [FeH3L][ML](ClO4)3 (M = Fe(III) or Co(III). 2D sheet and tetrahedral arrays. Inorg. Chim. Acta 2014, 421, 100–109. [Google Scholar] [CrossRef]
- Lenartson, A. Absolute Asymmetric Synthesis. Ph.D. Thesis, University of Gothenberg, Gothenberg, Sweden, 2009. [Google Scholar]
- Brewer, G.; Alvorado, L.; Lear, S.; Viragh, C.; Zavalij, P. Synthesis, Structure and Supramolecular Features of Heteronuclear Iron(III)-Copper(II) Complexes of a tripodal imidazole containing ligand. Inorg. Chim. Acta 2016, 439, 111. [Google Scholar]
- Lambert, F.; Renault, J.P.; Policar, C.; Morgenstern-Badarau, I.; Cesario, M. A polymeric layered bimetallic Mn(II) Fe(III) imidazolate network; crystal structure and magnetic properties. Chem. Comm. 2000, 35–36. [Google Scholar] [CrossRef]
- Brewer, G.; Butcher, R.J.; Viragh, C.; White, G. Supramolecular assemblies prepared from an iron(II) tripodal imidazole complex. A molecular scaffolding for the self assembly of icosahedral complexes of K+, Rb+, Cs+ and NH4+ cations †‡. Dalton Trans. 2007, 4132. [Google Scholar] [CrossRef]
- Alvarado, L.; Brewer, C.; Brewer, G.; Butcher, R.J.; Straka, A.; Viragh, C. Supramolecular assemblies prepared from an iron(II) tripodal complex, tetrafluoroborate, and alkali metal cations. The effect of cation size on coordination number, anion disorder and hydrogen bonding. CrystEngComm 2009, 11, 2297. [Google Scholar] [CrossRef]





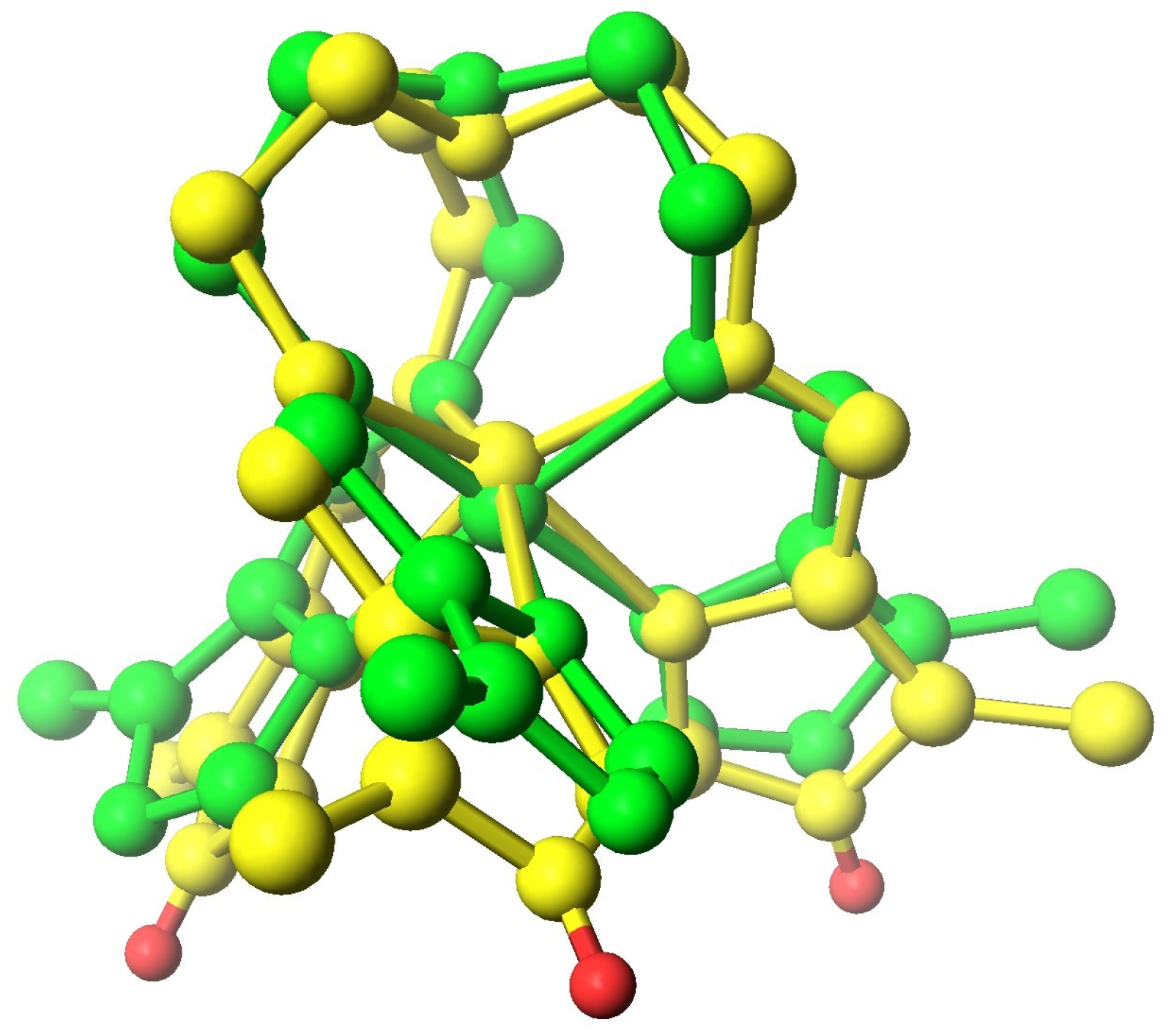
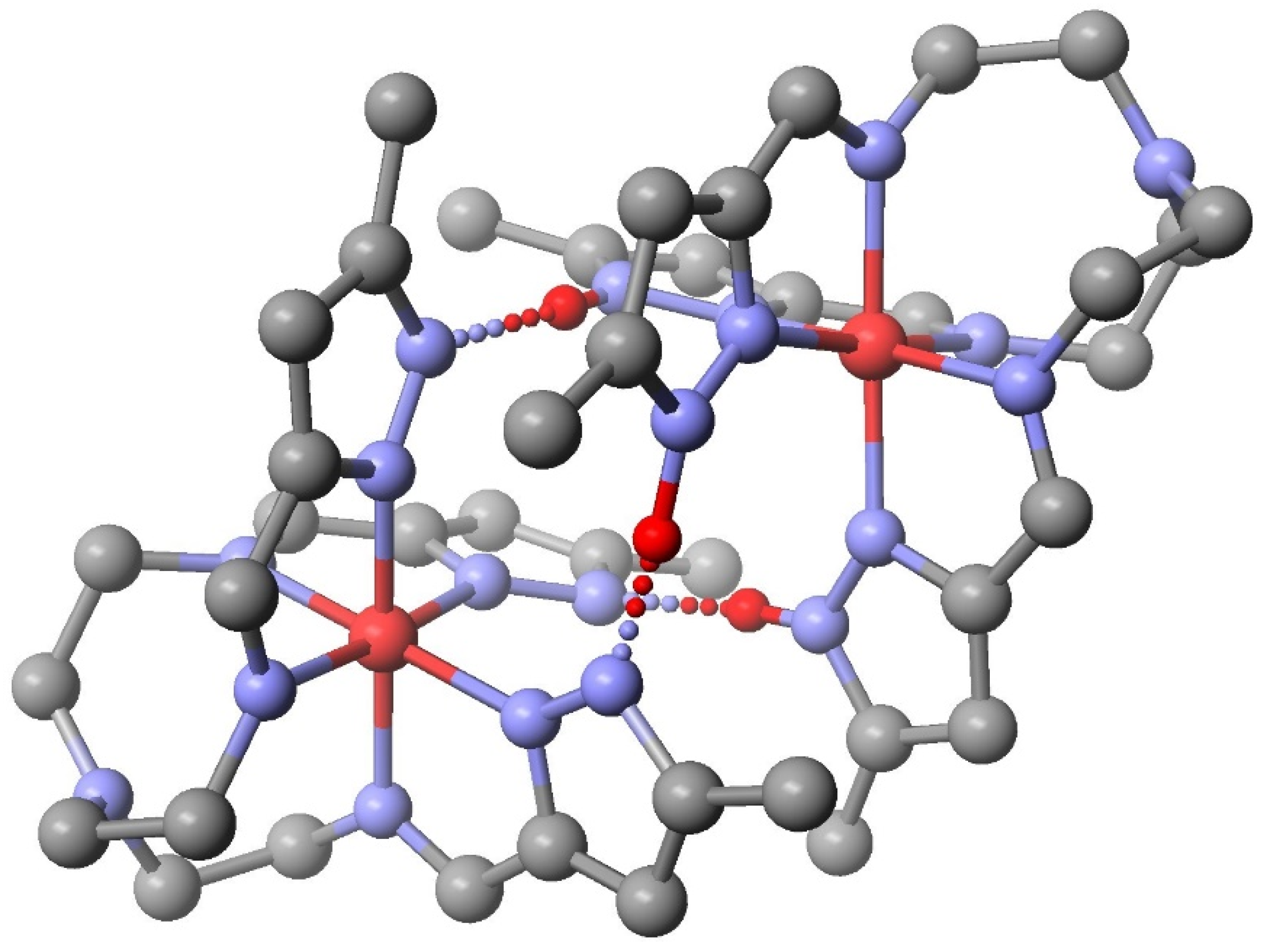
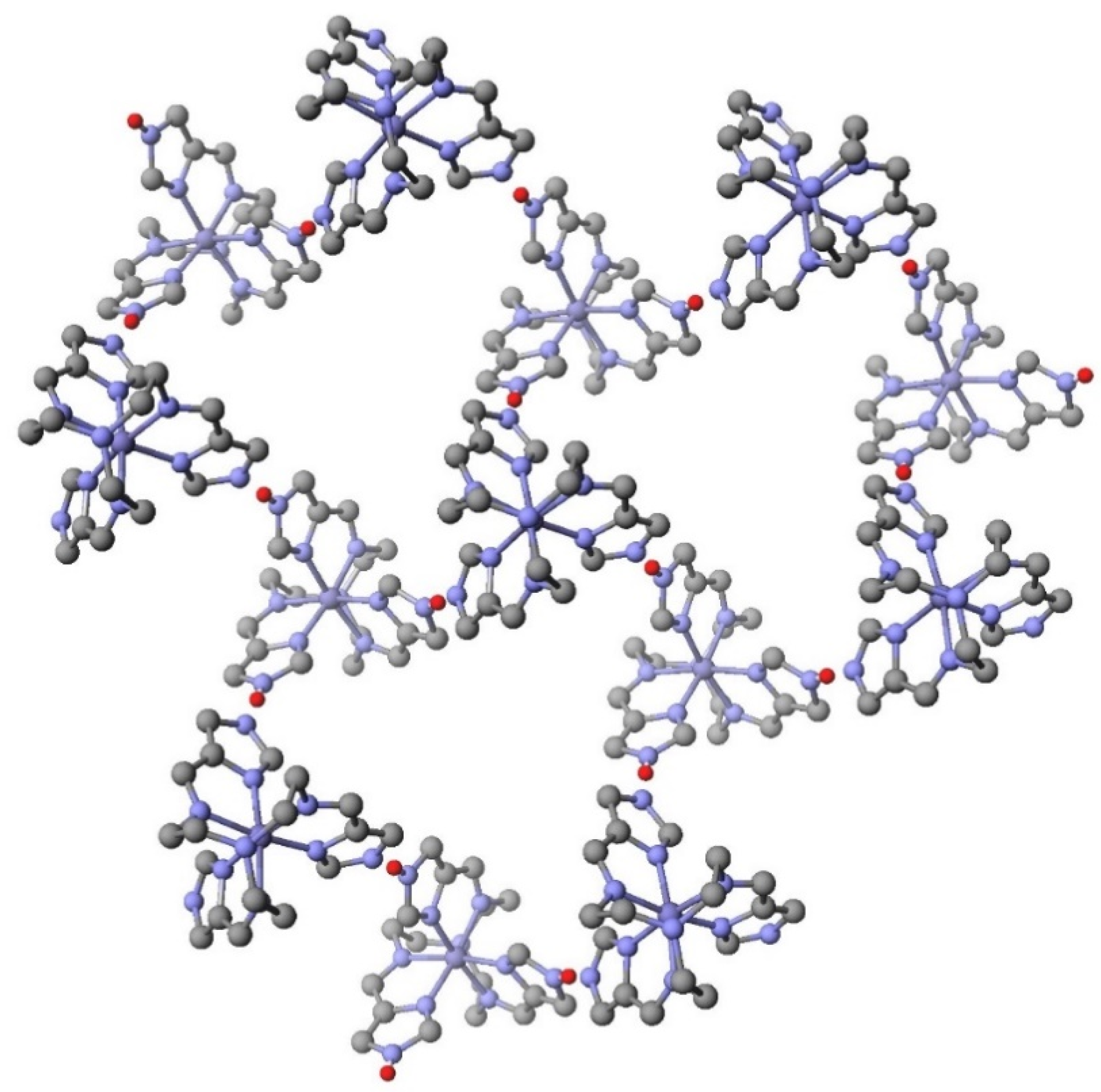
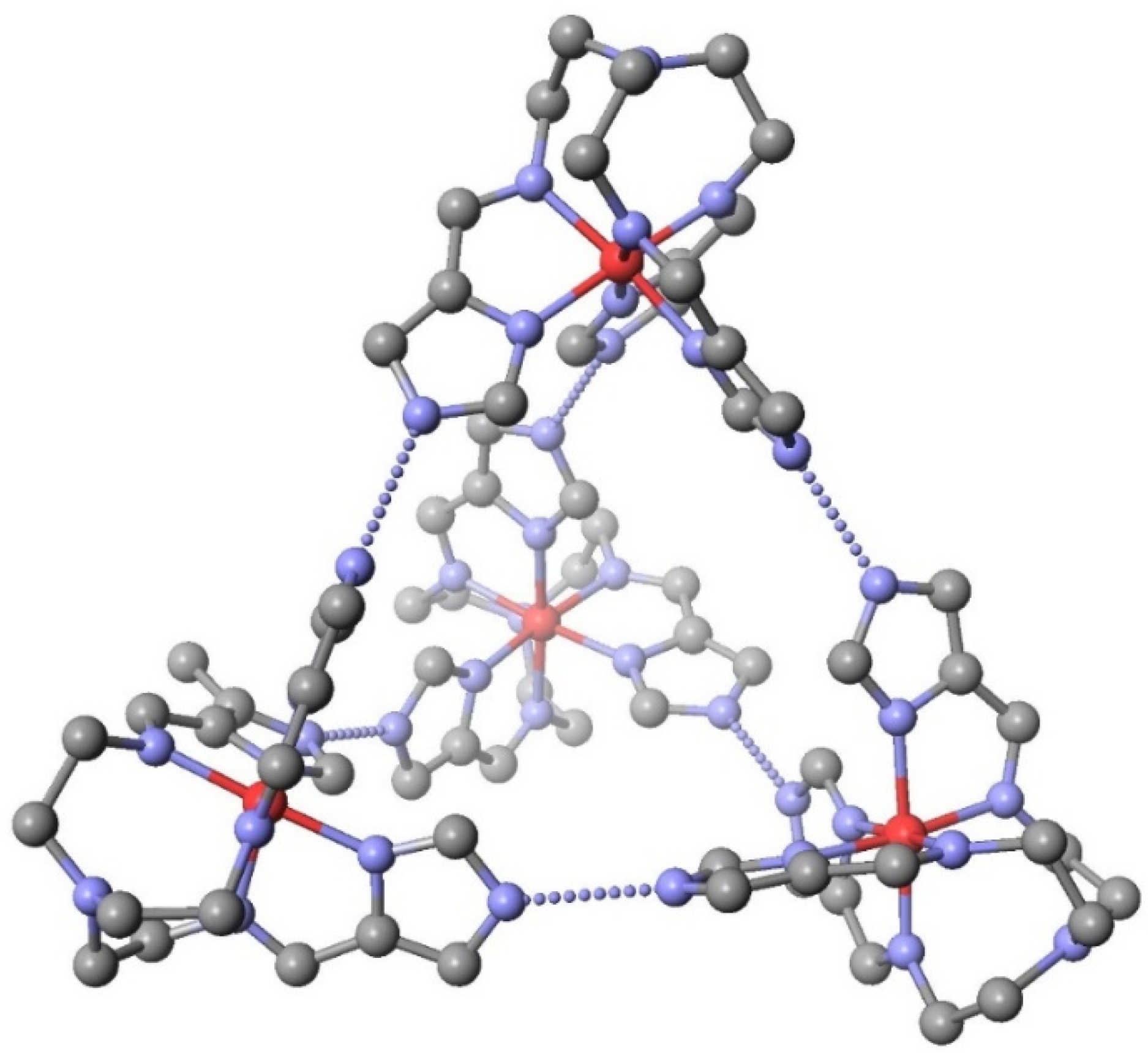
| System | Number of Compounds | System | Number of Compounds |
|---|---|---|---|
| Nap(CH2CH2N=C)3 | 890 | C-Nap+(CH2CH2N=C)3 | 3 |
| Nap(CH2CH2N=C)3 + any metal | 764 | HC(CH2CH2N=C)3 | 2 |
| Nap(CH2CH2N=C)3 + any transition metal | 563 | Nap(CH2CH2CH2N=C)3 | 19 |
| Nap(CH2CH2N=C)3 + any transition metal bound to 3 Schiff base N atoms | 517 | Nap(CH2CH2N=C)3 + any transition metal bound to 3 Schiff base N atoms + 3 mixed N, O, or 3 S atoms | none |
| Nap(CH2CH2N=C)3 + any transition metal bound to 3 Schiff base N atoms + 3 other non-metal atoms | 431 | Nap(CH2CH2N=C)3 + any transition metal bound to 3 Schiff base N atoms + 3 other O | 92 |
| Nap(CH2CH2N=C)3 + any transition metal bound to 3 Schiff base N atoms + 3 other N atoms | 339 | Nap(CH2CH2NH-CH)3 + any transition metal bound to 3 reduced Schiff base N atoms + 3 other non metals | 19 |
| Structural Parameter | Low Spin | High Spin |
|---|---|---|
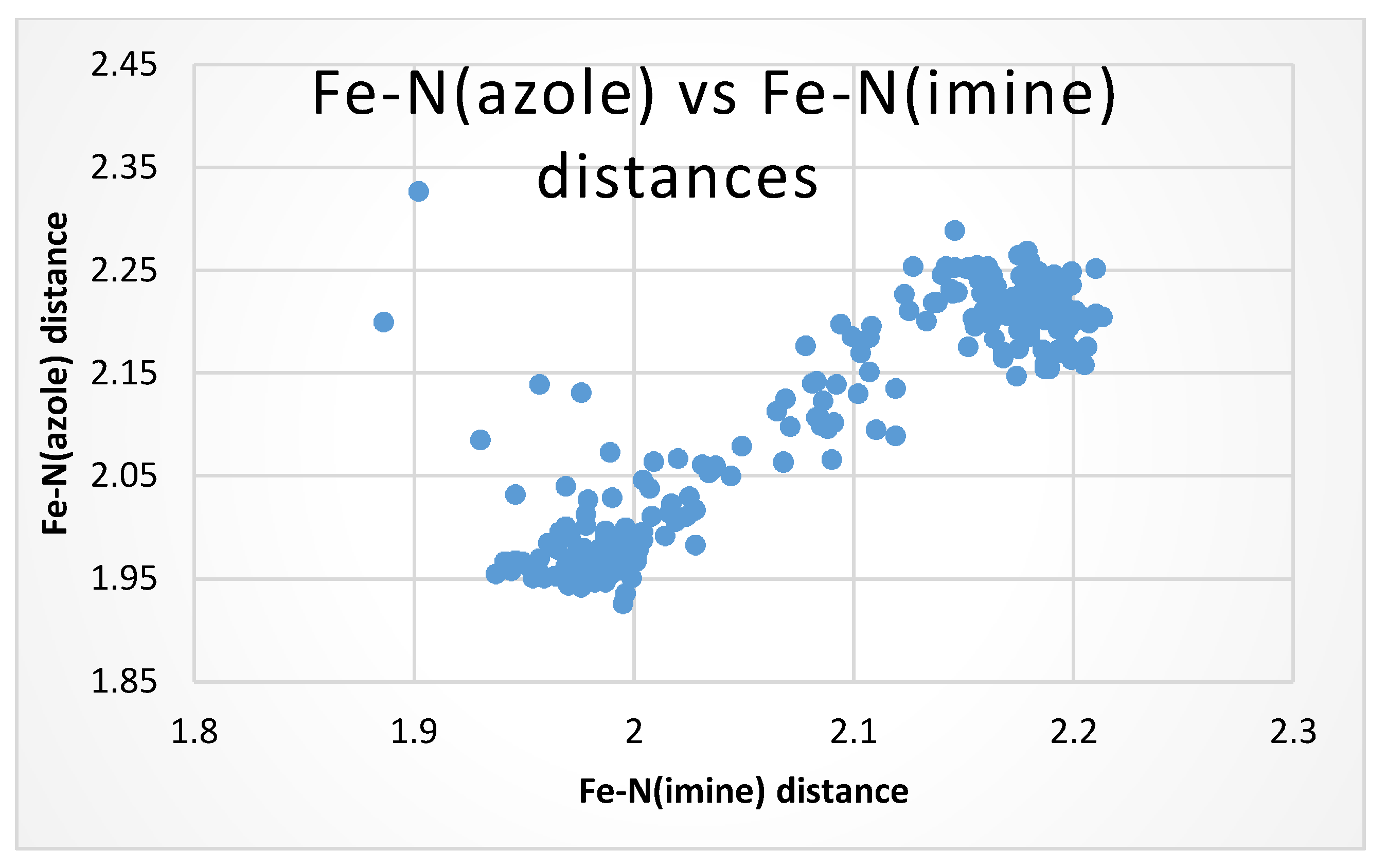
| M-Nimine (Å) | <2.00 Å | >2.10 Å |
| M-N(Imidazole, pyrazole, pyrrole) (Å) | <2.00 Å | >2.10 Å |
| M-Nap (Å) | 3.50 Å | 3.00 Å |
| Nimine–M–N bite angleo | 81o | 76o |
| C-Nap-C o | “P” 120o | “Nap in” 114o |
| NAME/CSD File | T | Fe-Nimine | Fe-Nazole | Fe-Nap | Nimine-Fe-Nazole | C-Nap-C |
|---|---|---|---|---|---|---|
| Fe[tren(4ImH)3](BF4)2 | ||||||
| EBUQAU | 108 | 1.989 | 1.981 | 3.522 | 81.084 | 119.955 |
| EBUQAU0 | 293 | 2.196 | 2.189 | 2.888 | 75.776 | 113.91 |
| Fe[tren(2ImH)3](BF4)2 | ||||||
| ENUBAR | 100 | 1.985 | 1.961 | 3.473 | 81.163 | 119.494 |
| ENUBAR02 | 295 | 2.175 | 2.174 | 2.976 | 76.218 | 114.781 |
| Fe [tren(1-nBu-2Im)3](PF6)2 | ||||||
| GIGGUA07 | 25 | 1.986 | 1.978 | 3.423 | 81.002 | 119.265 |
| GIGGUA09 | 230 | 2.174 | 2.225 | 3.001 | 74.753 | 113.68 |
| Fe[tren(PyrzH)3](NO3)2 | ||||||
| JIQDET05 | 30 | 2.017 | 2.023 | 3.413 | 79.288 | 118.934 |
| JIQDET | 300 | 2.184 | 2.249 | 2.96 | 75.025 | 114.401 |
| Fe[tren(1-nBu-2Im)3](ClO4)2 | ||||||
| JOMMOP | 120 | 1.981 | 1.959 | 3.348 | 80.464 | 119.833 |
| JOMMOP01 | 250 | 2.152 | 2.176 | 2.865 | 75.562 | 114.132 |
| Fe[tren(1-nHx-2BzIm)3](ClO4)2 | ||||||
| JOMNAC | 120 | 1.963 | 1.985 | 3.441 | 80.169 | 120.092 |
| JOMNAC01 | 298 | 2.086 | 2.123 | 3.121 | 76.594 | 116.606 |
| Fe [tren(2Me-4ImH)3]Cl(I)3 | ||||||
| NAVYEP01 | 90 | 2.02 | 2.067 | 3.417 | 81.299 | 124.275 |
| NAVYEP | 180 | 2.187 | 2.202 | 3.085 | 77.041 | 116.333 |
| Fe[tren(2Thia)3](ClO4)2 | ||||||
| OSABOB01 | 100 | 1.959 | 1.951 | 3.394 | 80.937 | 119.492 |
| OSABOB | 296 | 2.142 | 2.254 | 2.837 | 74.562 | 113.107 |
| Fe[tren(5Me-4ImH)3](ClO4)2 | ||||||
| PASDOD | 100 | 1.978 | 1.972 | 3.527 | 80.997 | 119.829 |
| PASDOD01 | 293 | 2.185 | 2.206 | 3.05 | 75.665 | 115.454 |
| Fe[tren(1-nBu-2Im)3]PF6)(AsF6) | ||||||
| QITZIF02 | 157 | 1.969 | 1.963 | 3.311 | 80.615 | 119.496 |
| QITZIF | 230 | 2.18 | 2.222 | 2.989 | 74.996 | 113.913 |
| Fe[tren(2Me-4ImH)3]Br(CF3 SO3) | ||||||
| ULODIJ01 | 93 | 2.037 | 2.06 | 3.228 | 79.727 | 118.606 |
| ULODIJ | 296 | 2.161 | 2.231 | 2.95 | 76.157 | 114.551 |
| Fe[tren(4Thia)3](BF4)2 | ||||||
| YAQVIY09 | 100 | 1.973 | 1.972 | 3.511 | 81.049 | 120.147 |
| YAQVIY07 | 300 | 2.155 | 2.249 | 2.809 | 74.355 | 113.524 |
| Fe[tren(2Me-4ImH)3](PF6)Cl | ||||||
| YELWOC01 | 93 | 1.978 | 2.002 | 3.376 | 82.156 | 119.71 |
| YELWOC | 296 | 2.18 | 2.232 | 3.004 | 76.4 | 115.002 |
| Fe[tren(2Me-4ImH)3](AsF6)Cl | ||||||
| YELWUI02 | 90 | 1.979 | 1.972 | 3.372 | 81.142 | 117.44 |
| YELWUI | 180 | 2.177 | 2.212 | 2.983 | 76.634 | 114.903 |
| Fe[tren(1Phtriazole)3](C2F6 NO4 S2)2 | ||||||
| ZOKTAW | 135 | 1.999 | 1.951 | 3.295 | 80.489 | 119.164 |
| ZOKTAW01 | 296 | 2.083 | 2.142 | 2.94 | 76.6 | 115.288 |
| CCDC Code | Complex | Fe-Nimine | Fe-N | Fe-Nap | Nimine-Fe-N (O) | C-N-C |
|---|---|---|---|---|---|---|
| BAKSOV | Fetren(5Me-4Im)3 | 1.987 | 1.941 | 3.346 | 81.108 | 119.016 |
| DEYNEB | Fetren(2Im)3 a | 1.988 | 1.933 | 3.194 | 81.059 | 118.559 |
| EYIHUP | Fetren(4Im)3 | 1.988 | 1.938 | 3.257 | 80.959 | 119.004 |
| EYIJAX | Fetren(2Im)3 b | 1.99 | 1.933 | 3.186 | 80.966 | 119.269 |
| FEJBIG | Fetren(4Im)3.HPF6 | 1.987 | 1.953 | 3.165 | 81.116 | 117.93 |
| IMANAL | Fetren(2Me-2Im)3 | 2.03 | 2.001 | 3.346 | 80.063 | 119.595 |
| TAZBFE | Fetren(Pyr)3 | 1.99 | 1.936 | 3.304 | 81.041 | 119.467 |
| average | 1.992 | 1.944 | 3.254 | 80.875 | 118.980 | |
| CESALF10 | Fetren(5ClSal) | 2.185 | 1.954 | 3.261 | 85.6 | 117.837 |
| KENXOS | Fetren((4-5-MeS)PhSal)3 | 2.174 | 1.94 | 3.353 | 87.493 | 119.271 |
| ZOHMOY | Fetren(Sal)3 | 2.174 | 1.976 | 3.127 | 87.166 | 113.839 |
| average | 2.177 | 1.956 | 3.247 | 86.753 | 116.982 |
| Complex | Co-N imine | Co-N(O) | Co-Nap | N imine-Co-N(O) | C-Nap-C |
|---|---|---|---|---|---|
| [Cotren(5MeCO2Py)3] (CoCl4)2 | 2.079 | 2.228 | 2.626 | 74.495 | 113.104 |
| [Cotren(py)3] (ClO4)2 | 2.119 | 2.17 | 2.884 | 76.121 | 114.574 |
| [Cotren(5-tBuNHCO-2Py)3]Cl2 | 2.121 | 2.262 | 2.574 | 74.083 | 111.623 |
| [Cotren(5tBuNHCO-2Py)3](ClO4)2 | 2.11 | 2.264 | 2.706 | 74.333 | 113.752 |
| [Cotren(5tBuNHCO-2Py)3]I | 2.105 | 2.258 | 2.633 | 74.578 | 112.356 |
| [Cotren(5-tBuNHCO-2Py)3]Br2 | 2.107 | 2.253 | 2.592 | 74.596 | 111.809 |
| [Cotren(py)3](BF4)2 | 2.091 | 2.227 | 2.87 | 75.422 | 113.719 |
| [Cotren(pyrz)3](BF4)2 | 2.08 | 2.346 | 2.503 | 72.703 | 110.887 |
| [Cotren(N-oxyPy)3](PF6)2 | 2.149 | 2.162 | 2.443 | 78.768 | 111.715 |
| [Cotren(2Ph-2ImH)3](NO3)2 | 2.133 | 2.237 | 3.267 | 78.068 | 116.627 |
| [Cotren(1Me-2ImH)3](ClO4)2 | 2.131 | 2.164 | 2.946 | 76.18 | 114.819 |
| [Cotren(5Me-4ImH)3](ClO4)2 | 2.124 | 2.205 | 2.72 | 75.627 | 113.212 |
| [Cotren(2ImH)3](ClO4)2 | 1.914 | 1.862 | 3.331 | 82.188 | 119.51 |
| Component | T | Fe-Nimine | Fe-Nimid | Fe-Nap | N-imine-Fe-N-imid. | C-N-ap-C |
|---|---|---|---|---|---|---|
| Fetren(2Me-4Im)3- | 90 K | 2.03 | 2.059 | 3.366 | 79.998 | 119.316 |
| 130 K | 2.066 | 2.095 | 3.28 | 79.43 | 118.248 | |
| 293 K | 2.171 | 2.184 | 3.122 | 77.004 | 115.393 | |
| Fetren(2Me-4ImH)32+ | 90 K | 2.066 | 2.082 | 3.371 | 79.575 | 119.424 |
| 130K | 2.077 | 2.107 | 3.314 | 78.732 | 119.067 | |
| 293 K | 2.173 | 2.199 | 3.169 | 76.932 | 116.777 | |
| Fetren(4Im)3 | 100 K | 1.988 | 1.947 | 3.302 | 80.908 | 119.43 |
| 220 K | 2.021 | 2.005 | 3.143 | 79.667 | 117.836 | |
| 300 K | 2.013 | 1.952 | 3.202 | 80.82 | 118.388 | |
| Fetren)(4ImH)32+ | 100 K | 1.986 | 1.951 | 3.314 | 81.034 | 119.56 |
| 220 K | 2.023 | 2.008 | 3.157 | 79.755 | 118.277 | |
| 300 K | 2.026 | 1.979 | 3.16 | 79.642 | 118.086 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, G. Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines. Magnetochemistry 2020, 6, 28. https://doi.org/10.3390/magnetochemistry6020028
Brewer G. Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines. Magnetochemistry. 2020; 6(2):28. https://doi.org/10.3390/magnetochemistry6020028
Chicago/Turabian StyleBrewer, Greg. 2020. "Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines" Magnetochemistry 6, no. 2: 28. https://doi.org/10.3390/magnetochemistry6020028
APA StyleBrewer, G. (2020). Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines. Magnetochemistry, 6(2), 28. https://doi.org/10.3390/magnetochemistry6020028




