Abstract
Due to the expanding occurrence of marine toxins, and their potential impact on human health, there is an increased need for tools for their rapid and efficient detection. We give an overview of the use of magnetic beads (MBs) for the detection of marine toxins in shellfish and fish samples, with an emphasis on their incorporation into electrochemical biosensors. The use of MBs as supports for the immobilization of toxins or antibodies, as signal amplifiers as well as for target pre-concentration, is reviewed. In addition, the exploitation of MBs in Systematic Evolution of Ligands by Exponential enrichment (SELEX) for the selection of aptamers is presented. These MB-based strategies have led to the development of sensitive, simple, reliable and robust analytical systems for the detection of toxins in natural samples, with applicability in seafood safety and human health protection.
1. Marine Toxins
Oceans and their resources have sustained nations for millennia, with seafood being a strong part of cultural identity and tradition. Marine toxins accumulate in shellfish, fish and other seafood, and, even if they do not all represent a threat for the hosting organism, they can be hazardous for human health, and have thus drawn attention from food safety agencies, the seafood industry and scientists worldwide [1]. The presence of marine toxins can have socio-economic impacts, including the closure of production and recreational areas, as well as enforcing changes in the diet of entire populations [2]. Diverse toxins cause different intoxications, which are grouped according to their effects: diarrheic shellfish poisoning (DSP), paralytic shellfish poisoning (PSP), amnesic shellfish poisoning (ASP), neurologic shellfish poisoning (NSP), ciguatera fish poisoning (CFP) and pufferfish poisoning [3]. The marine toxins responsible for these intoxications are produced by microalgae, except for pufferfish poisoning, in which the toxin producer is a bacterium [4].
In recent years, the use of traditional toxicity screening tests such as the mouse bioassay (MBA) is increasingly avoided due to their low sensitivity, low specificity and ethical problems. Chromatographic techniques coupled with several detection methods are powerful and accurate analysis tools, and are routinely used as reference methods for many marine toxins. However, the required instrumentation is expensive and requires trained personnel, and to address these shortcomings, the European Commission encourages the development and use of alternative or complementary methods [5], which are usually based on a functional or structural recognition of the toxin [6]. Cell-based assays (CBAs) are easy to perform, give an overall view of the toxicity of a sample and can detect the presence of unknown toxins. However, they show high variability, which hampers their harmonization, and may not be able to discriminate compounds that share the same mechanism of action. Some enzyme inhibition assays have been developed, and these assays are relatively easy to apply, but may suffer from enzyme instability as well as from matrix effects, which may interfere with the response. Receptor-based assays (RBAs) are based on the structural recognition of ligands, but the isolation of receptors from animals is not a trivial task, and, additionally, the affinity may not correlate with the toxicity. Immunoassays, based on the affinity between antibodies and target antigens, show high sensitivity. Whilst the structural recognition may not be necessarily related to the toxicity, antibodies are easier to obtain than receptors, and are also more robust, facilitating an easier implementation of immunoassays, as well as immunosensors, which have the added potential benefit of being miniaturisable and portable [7,8].
2. Magnetic Beads
Magnetic beads (MBs) are particles that consist of magnetite (Fe3O4) or maghemite (mostly in the face-centered cubic crystal modification γ-Fe2O3) and they have a superparamagnetic or a ferromagnetic behaviour, depending on their size and magnetic content [9]. Superparamagnetism is a particular kind of magnetism that occurs in sufficiently small ferromagnetic or ferrimagnetic particles, which exhibit magnetic properties only when placed in a magnetic field, with no residual magnetism once the magnetic field is removed or switched off. Because of the absence of a remnant magnetization, the previously magnetized superstructure decomposes into single particles. Ferromagnetic magnetism, instead, keeps a magnetic moment even when the magnetic field is removed, not allowing superstructures to decompose.
According to Laurent and co-workers [10], numerous chemical methods can be used to synthesize MBs, such as microemulsions, sonochemical reactions, sol-gel syntheses, hydrothermal reactions, hydrolysis and thermolysis of precursors, electrospray syntheses and flow injection syntheses. All these methods have been used to prepare particles with a regular composition and small size. Nevertheless, the most common method for the production of magnetite and maghemite MBs is still the chemical co-precipitation of iron salts.
MBs of different materials, sizes and functionalizations are now commercially available, enabling their conjugation to a broad range of biomolecules or compounds though different reaction chemistries or affinity interactions [11,12,13].
3. Magnetic Beads in Marine Toxin Detection
The use of MBs, mainly superparamagnetic, in the development of immunoassays and immunosensors for food analysis and clinical diagnosis is garnering increasing interest [14,15,16], due to the various advantages that the use of MBs can entail, including an increased surface-to-volume ratio, improved assay kinetics, a higher washing efficiency and lower matrix effects. Herein, we describe the exploitation of MBs in different approaches related with the detection of marine toxins, classifying them according to their use as supports, signal amplifiers, capture agents and, finally, for the production of biorecognition molecules. Table 1 gives an overview of the MB uses and functionalizations taken in consideration for this manuscript.

Table 1.
Overview of the magnetic bead (MB) uses and functionalizations for their applicability in the detection of marine toxins.
3.1. Magnetic Beads as Supports
The first report of the use MBs as a support for marine toxin detection was in the development of an immunosensor for okadaic acid (OA) [17] (Figure 1A). OA is a lipophilic marine toxin produced by microalgae of the genera Dinophysis and Prorocentrum. This toxin is accumulated in shellfish and, since its mode of action is related to the inhibition of protein phosphatases (PPs), it can cause DSP in humans. OA was conjugated to biotin and then captured on streptavidin-coated MBs. Once OA was immobilized on the MBs, a colorimetric indirect competitive enzyme-linked immunosorbent assay (ELISA) was performed, where OA in solution competed for interaction with an anti-OA monoclonal antibody (mAb). The authors tested two different sizes of MBs, achieving limits of detection (LODs) of 0.8 µg/L with 2.8 µm-diameter MBs and 1.99 µg/L with 1 µm-diameter MBs. The functionalized MBs were then exploited in an electrochemical immunosensor, where they were magnetically immobilized on screen-printed electrodes (SPEs), and, again, a competitive assay performed. Differential pulse voltammetry (DPV) was used to measure the oxidation of 1-naphthol resulting from the dephosphorylation of 1-naphthyl phosphate by the alkaline phosphatase (ALP) enzyme label, and slightly lower LODs were obtained, with the larger MBs again performing better (0.38 µg/L vs. 0.99 µg/L). It should be noted that whilst larger MBs imply a higher surface area, the amount of MBs used was 10-fold lower and the whole available surface area was lower when using the larger MBs. This immunosensor was then easily integrated into an automated flow-through system [18], one of the advantages of using MBs, achieving an improved LOD of 0.15 µg/L.
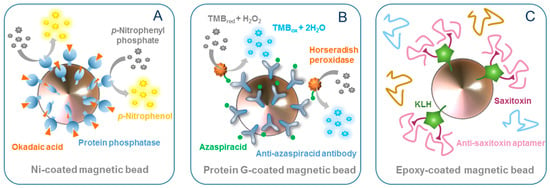
Figure 1.
Examples of uses and functionalizations of MBs: (A) MBs as supports for enzymes, (B) MBs as supports for antibodies, and (C) MBs for the production of aptamers.
Moving towards the development of portable devices for field analysis, Pan and collaborators [19,20] described fluorescence immunosensors for the detection of OA. In the first work [19], carboxylic acid-modified MBs were used as a support for the immobilization of OA–bovine serum albumin (OA–BSA), which competed with OA in the sample to bind with an anti-OA mAb. The fluorescence of CdTe quantum dots (QDs) linked to the reporter antibody was detected using a portable flow cytometer (Moxi-Flow), facilitating on-site OA detection and quantification of OA, and achieving an LOD of 0.05 µg/L [19]. In the second work [20], the authors modified the system, using streptavidin-coated MBs with biotinylated OA and a secondary antibody labelled with R-phycoerythrin (R-PE) dye, again achieving an LOD of 0.05 µg/L.
Hayat and co-workers [21] also exploited MBs in a direct immunoassay/immunosensor format for the detection of OA. Instead of conjugating the toxin to the MBs, the anti-OA mAb was immobilized on protein G-coated MBs. OA labelled with horseradish peroxidase (HRP) was used as a tracer in the colorimetric assay, whilst, for the electrochemical immunosensor, no label was used. DPV measurements in a 1 mM [Fe(CN)6]3−/4− solution showed that the interaction between the toxin and the antibody decreases the current peak of the ferri/ferrocyanide redox probe. Using this detection strategy, they obtained an LOD of 0.5 µg/L, lower than that obtained with the colorimetric immunoassay (1 µg/L).
An interesting and different approach for the detection of OA is presented in the work of Garibo et al. [22]. In this work, the PP inhibition was measured to detect and quantify the toxin. The authors used genetically engineered PPs with extra-His tails to conjugate the enzymes to Ni-modified MBs. The colorimetric assay attained an LOD of 30.1 µg/L. Although this LOD was more than an order of magnitude higher than that achieved with free enzymes, the immobilization of the PP on the MBs provided higher enzyme activity stability, a crucial parameter, especially when working with these enzymes.
Azaspiracids (AZAs) are lipophilic marine toxins produced by microalgae of the genera Azadinium and Amphiodioma. Those toxins accumulate in shellfish, and the ingestion of contaminated seafood can lead to azaspiracid shellfish poisoning (AZP), first reported in 1995 [42]. Leonardo and co-workers [23] developed an MBs-based direct immunoassay for AZA detection (Figure 1B). Protein G-coated MBs were functionalized with anti-AZA polyclonal antibody (pAb), and free AZA competed with HRP-labelled AZA (HRP–AZA) for binding to the immobilized antibody in suspension, achieving LODs of 1.1 and 1.0 µg/L, using 3,3′, 5,5′-tetramethylbezidine (TMB) as an enzyme mediator and optical and electrochemical detection, respectively. Additionally, the assay was completed in just 15 min, due to the faster kinetics provided by the use of MBs in suspension. When the biorecognition was performed, immobilizing the Ab-MBs magnetically on the electrode surface, the LOD increased to 3.7 µg/L, which could be attributable to mass transfer limitations. Furthermore, naturally-contaminated mussels were analyzed, and results were similar to the ones obtained with liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS), demonstrating the applicability of the system for monitoring purposes.
Tetrodotoxin (TTX) is a potent natural neurotoxin produced by bacteria that live in endosymbiosis with some other organisms such as pufferfish. Consumption of this contaminated animal may cause intoxication and even death, and the rapid and reliable detection of TTX in pufferfish is thus of enormous importance. Recently, an electrochemical MB-based immunosensor has been developed for the detection of TTX [4]. Oriented and stable TTX immobilization was achieved through the formation of a cysteine monolayer on maleimide-activated MBs, for the subsequent covalent biding of TTX. A competitive assay was again pursued, with TTX in solution competing with the immobilized TTX for binding to an anti-TTX mAb and using an HRP-labelled secondary antibody as a reporter antibody. The immunocomplexes were magnetically captured on an 8-electrode array, and using amperometric detection, an LOD of 1.2 µg/L was achieved. The authors applied the biosensor to the detection of TTX in muscle, skin and the internal organs of two juvenile pufferfishes (Lagocephalus sceleratus) from Greece, achieving a good degree of correlation with LC–MS/MS. It had previously been observed that the liver tissue matrix had a marked effect on assay performance, and this effect was almost completely eliminated due to the use of MBs as a support. This work thus demonstrates the advantages that MBs provide in terms of reduction of matrix effects. An alternative electrochemical immunoassay for TTX was described by Zhang and co-workers [24], who synthesized MBs and coated them with polyethylene glycol for subsequent reaction with BSA–TTX. After competition between immobilized TTX and free TTX for a primary anti-TTX antibody, and incubation with an enzyme-labelled secondary antibody, the enzyme product was electrochemically measured. The modification of the working electrode with ionic liquids and carbon nanotubes significantly avoided electrode surface fouling by the enzyme product and improved the sensitivity as compared to bare electrodes, achieving an LOD of 5 µg/L.
Aptamers have also been used for the detection of TTX, as described by Jin and co-workers [25], who conjugated an NH2-terminated anti-TTX aptamer to thiodiglycolic acid-stabilized Fe3O4 MBs. Carxboxylated carbon dots (CDs) were then added, forming Fe3O4/aptamer/CDs nanocomposites. When excited at 780 nM, those nanocomposites were observed to have a decreased up-conversion fluorescence emission at 475 nm, attributed to the photo-induced electron transfer (PET) from the CDs to the aptamer. The addition of TTX caused the unwinding of CDs from the aptamer and subsequent recovery of the up-conversion fluorescence. The system attained an LOD of 0.06 µg/L, and showed high selectivity when tested against other toxins (aflatoxin B1 and B2, botulin neurotoxin A and B and Staphylococcus aureus enterotoxin A and B), biomolecules (histidine, cysteine, uric acid, ascorbic acid, glucose, glutathione and thiohydracrylic acid) and anions (Cl−, PO43− and CO32−) that could interfere in the analysis of human body fluids. The good recoveries obtained in the analysis of spiked gastric juice, serum and urine samples demonstrated the applicability of this aptamer-based optical assay.
Brevetoxin B (BTX-2) is a neurotoxin produced by microalgae such as Ptychodiscus brevis and Gymnodinium breve. This toxin accumulates in shellfish and, when ingested, can result in death. Additionally, aerosol exposure to BTX-2 during microalgae blooms can cause respiratory irritation [43]. This particular toxin together with dinophysistoxin-1 (DTX-1), an OA analog also responsible for DSP and produced by some Prorocentrum and Dinophysis species, were selected as targets for the development of a flow-through electrochemical immunoassay [26]. Anti-BTX-2 and anti-DTX-1 mAbs were co-immobilized on MBs. Tracers were synthesized by conjugation of the toxins with cadmium and copper nanoclusters. The incubation of the functionalized MBs with both toxins and their tracers, and the subsequent dissolution of the metal labels and injection into the detection cell, allowed the selective detection of the two toxins using square wave anodic stripping voltammetry, with no cross-reactivity observed. The system showed high cross reactivity with BTX-1, BTX-3, DTX-2 and DTX-3, as expected, and no false positive results from OA, pectenotoxin-6 (PTX-6) or yessotoxin (YTX). LODs of 1.8 ng/L and 2.2 ng/L were achieved for BTX-2 and DTX-1, respectively.
The PSP toxin group comprises saxitoxin (STX) and related compounds produced by marine dinoflagellates of Alexandrium, Gymnodinium, and Pyrodinum species. PSP toxins can accumulate in bivalves, crabs, lobsters and even carnivorous snails [44]. The ingestion of contaminated vectors causes neurotoxic illness that can result in paralysis and, at its acute expression, death. With this target in mind, Jin and co-workers [27] developed a magnetic electrochemical immunosensor for the detection of STX in seawater and seafood. The immunosensor used anti-STX antibody-functionalized MBs and palladium-doped graphitic carbon nitride nanoparticles (peroxidase mimetic) to generate the electrochemical signal. Unlike the other approaches described so far, the assay was non-competitive, because they took advantage of the electrostatic interaction between the electro-positive STX and the electro-negative palladium nanoparticles. The immunosensor successfully detected trace STX amounts in seawater and shellfish samples with an LOD of 1.2 ng/L. Moving towards compact analytical devices, Kim and Choi [28] proposed a lab-on-a-chip (LOC) system for the immunodetection of STX. The LOC system was composed of a sample chamber and a detection chamber connected via a channel. MBs functionalized with anti-STX antibodies were added to the sample chamber together with STX-HRP and the sample containing STX. After incubation, a magnet was used to transport the MBs from the sample chamber to the detection chamber, which had been previously filled with enzyme substrate. The LOD was around 3 µg/L, far below the regulatory level of PSP toxins (800 µg STX per kg shellfish). In 2017, Yu and Choi [29] improved the system by adding an extra washing chamber between the two existing ones, resulting in a decrease in the LOD to around 6 ng/L.
CFP is a human intoxication caused by the ingestion of contaminated fish and is a worldwide health problem. This disease is characterized by severe neurological, gastrointestinal and cardiovascular disorders. Causative toxins of CFP are produced by marine dinoflagellates of the genera Gambierdiscus and Fukuyoa and are known as ciguatoxins (CTXs). An electrochemical immunoassay for the detection of CTX3C was developed by Zhang et al. [30], where sample injection, incubation, capillary electrophoresis separation and electrochemical detection were all performed in a capillary system. An anti-CTX3C antibody was immobilized on MBs and injected into the capillary system, followed by the addition of CTX3C standard/contaminated samples. A rotating magnetic field was applied to increase mixing efficiency and molecular binding rates. An anti-CTX3C antibody linked to HRP-functionalized gold nanoparticles was then added and sandwich immunocomplexes were formed. Finally, the enzyme product was electrochemically detected, and the system achieved a very low LOD (0.09 ng/L), almost 17,000 times lower than that obtained with high performance liquid chromatography coupled to mass spectrometry (HPLC–MS). The authors claim that the enhanced sensitivity can be attributed to the use of gold nanoparticles as multi-enzyme carriers, resulting in a high HRP/Ab molar ratio.
3.2. Magnetic Beads as Signal Amplifiers
One of the functionalities of MBs is their ability to amplify signals, as exemplified in the work of Garibo et al. [31], who described the development of a competitive surface plasmon resonance (SPR) optical immunosensor for OA. Protein G-coated MBs were used to immobilize anti-OA antibodies, whilst OA was immobilized on the sensor chip surface. The antibodies were added to the sensor together with a free OA standard/sample, and any binding of molecules to the immobilized OA generated a response proportional to the bound mass. SPR analysis demonstrated that, with conjugates, it is possible to attain similar responses to free antibodies, but using an 8-fold lower antibody concentration. The Ab–MBs resulted in a 3-fold lower LOD, even in the presence of mussel matrix (from 4.7 µg/L to 1.2 µg/L), demonstrating the ability of MBs to be used as signal amplifiers.
3.3. Magnetic Beads as Capture Agents
Immunomagnetic capture (IMC) represents an innovative technique for toxin extraction and purification from complex environmental or biological matrices and is much simpler and more rapid than the use of chromatographic columns. The first example of IMC with marine toxins was reported by Devlin and co-workers [32], who covalently immobilized an antibody to MBs using glutaraldehyde crosslinking for the immunoaffinity extraction of PSP toxins from cultures of the dinoflagellate Alexandrium tamarense. After steel ball bearing beating for cell lysis, HPLC measurements showed that toxin recovery increased with increasing amounts of MBs (up to 96.2%), and that the process could be completed within an hour. Recently, Bragg and collaborators [33] coupled IMC with LC–MS/MS for the extraction and detection of STX from human urine. The method showed advantages over conventional protocols, such as an improved selectivity (reducing matrix interference), a 5-fold increase in sensitivity, and requirement of only one third of the sample volume.
IMC combined with LC–MS/MS has also been used by Chen and collaborators [34], in this case for the extraction of OA from shellfish samples. MBs were able to capture the toxin in just 10 min, due to their use of suspension. Additionally, shellfish matrix effects were minimized, and recovery values between 82.2% and 95.5% were obtained for the analysis of oysters, mussels and scallops.
MBs have also been used as capture agents in the work of Neely et al. [35]. In their study, the researchers reported the exposure of C8-coated MBs to blood serum samples from California sea lions to identify patterns of domoic acid (DA) toxicosis. DA can cause ASP and can affect not only humans but also common predators that live in and around marine habitats. Detection of DA was achieved using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. Artificial neuronal networks (ANN) were trained using MALDI-TOF data from serum analysis, and the obtained models were good predictors of acute DAT. The strategy resulted in a highly sensitive (100% negative predictive value) and a highly specific (100% positive predictive value) diagnostic tool.
3.4. Magnetic Beads to Produce Biorecognition Molecules
Marine toxins are not always easy to find and isolate from field samples. The limited availability of marine toxins has hindered the development of biorecognition molecules and, consequently, of systems for their detection. To address this problem, specifically for CTXs, the use of synthetic toxin fragments has been exploited in the production of antibodies [36]. In this work, streptavidin-coated MBs were used for the panning of phages. In the experiment, a biotinylated synthetic ABC-ring fragment of CTX3C (ABC-PEG-biotin) was incubated with a phage library, and then captured on the streptavidin-coated MBs together with the positive phages expressing hapten-binding antibodies. To select antibodies to the left side of CTX3C, elution was performed with a synthetic CTX3C fragment, instead of the scarce CTX3C. Following three rounds of selection and amplification, the authors observed an increased recovery of eluted phages, as well as the enrichment of phages bearing Fab fragments. The gene fragments from the sorted phage were sub-cloned for the production of three soluble recombinant Fabs, which had dissociation constants (Kd) of about 10−5 M.
MBs have also been used for the production of aptamers, oligonucleotides able to bind to specific target molecules with high affinity and specificity and used as biorecognition molecules in bioanalysis. The in vitro process to obtain aptamers is termed systematic evolution of ligands by exponential enrichment (SELEX), and MBs are frequently used as a support and for the effective partitioning of bound and unbound DNA because they improve the binding kinetics and the washing steps. The first example was described by Handy and co-workers [37], who conjugated STX to keyhole limpet hemocyanin (KLH) using 2,2′-(ethylenedioxy)bis(ethylamine) (Jeffamine) as a spacer compound, for its subsequent covalent binding to epoxy-coated MBs (Figure 1C). The modified MBs were incubated with a random ssDNA library. Bound and unbound DNA were magnetically separated, and the bound ssDNA was eluted from the MBs, PCR-amplified and finally used to enrich the ssDNA library for the following round of selection. After 10 rounds, the PCR product was cloned and sequenced. Preliminary results using SPR showed the affinity of the selected aptamer for STX. A sensor chip modified with DA was used to evaluate the specificity of the aptamer towards this marine toxin, which often co-occurs with STX. Binding was not observed, further supporting that the selected aptamer was specific to STX. Gao and co-workers [38] used a SELEX with MBs to produce aptamers for gonyautoxins 1/4 (GTX1/4). They immobilized the GTX1/4-carboxylated derivative on amine-modified MBs via the EDC/NHS chemistry. In round 2, negative MBs were introduced to remove the ssDNA that bound non-specifically to improve the screening efficiency. In round 3, free competitive counter-molecules were added in the positive incubation system to improve the specificity of screening. After eight rounds of selection, appropriate sequences were obtained. However, these sequences were not further investigated. The same research group developed an aptamer for the detection of palytoxin (PlTX), a toxin initially isolated from soft corals and later found in shellfish, sea urchins and crabs, usually associated with Ostreopsis blooms [39]. Counter SELEX was performed against potential interferents, including OA, microcystin-LR (MC-LR), STX, and brevetoxin-A/B, resulting in a highly selective aptamer. The selected aptamer was used to develop an optical biosensor based on biolayer interferometry, where PlTX was immobilized on the biosensor surface, and competed with free PlTX for binding to HRP-labelled aptamer. The addition of 3,3’-diaminobenzidine substrate solution resulted in the formation of a precipitated polymeric product directly on the biosensor surface. Changes in the optical thickness and mass density of biosensor layer were measured, resulting in an LOD of 0.04 ng/L.
Gu and collaborators [40] developed a magnetic separation-based multiple SELEX to simultaneously select aptamers against three different marine biotoxins: DA, STX and TTX. The first 12 rounds entailed mixed screening against the three toxins, and the subsequent four rounds of single screening were against each individual toxin. Additionally to the multiplexing strategy, the authors provided the novelty of combining the advantages of MBs and graphene oxide (GO) for efficient partitioning. A fluorescence assay was developed to determine the affinity of the aptamers, showing Kd values of of 62, 44 and 61 nM for DA, TTX and STX, respectively. Additionally, two multi-target aptamers, which can bind with either DA or TTX, were also obtained.
Finally, an aptamer specific to the antigen binding site of a mAb against OA has been produced using MB–SELEX [41]. The aptamer produced following this strategy mimics the OA structure. In this approach, F(ab’)2 fragments (obtained by pepsin digestion of the anti-OA mAb) were conjugated to MBs and subsequently incubated with the ssDNA library. Negative selection with bare MBs and six additional mAbs (against STX, BTX-2, TTX, DA, nodularin (NOD) and MC-LR) was applied to remove non-specifically bound ssDNA. The produced aptamer was used in two different immunoassays. In the first one, biotinylated aptamer competed with free OA for binding to immobilized anti-OA mAb, followed by the addition of streptavidin-HRP, with the aptamer thus acting as a tracer. In the second assay, immobilized OA competed with the aptamer for binding to anti-OA mAb, which was subsequently detected using a secondary antibody.
4. Conclusions and Perspectives
Marine toxins play a crucial role in shellfish poisoning, and reliable, rapid and cost effective detection of very low concentrations of these toxins is critical. Currently, MBs have been used in the field of marine toxin detection as supports in assays and biosensors, capture agents for toxin pre-concentration and as tools to produce biorecognition molecules such as phages and aptamers. Because of their advantages in terms of increased surface-to-volume ratio, improved assay kinetics, increased washing efficiency and reduced matrix effects, efficient and highly sensitive analytical systems for the detection of marine toxins have been developed.
The use of MB-based strategies in marine environments can facilitate the confirmation of toxin presence in shellfish at the occurrence of harmful algal blooms (HABs), and speed up monitoring programs. However, to provide biotechnological tools for seafood safety and human health protection, it will be necessary to validate these MB-based approaches. Validation studies will include analyses of multiple samples, of different natures and from different geographic locations, some of them with multi-toxin profiles, and maybe with emerging toxins as challenging targets.
Author Contributions
M.C. conception; G.G. and M.C. literature search and collection; G.G., C.K.O. and M.C. design, writing and critical reviewing.
Funding
The authors acknowledge support from the Ministerio de Ciencia, Innovación y Universidades through the CIGUASENSING (BIO2017-87946-C2-2-R) project and from CERCA Programme/Generalitat de Catalunya. G. Gaiani acknowledges IRTA-Universitat Rovira i Virgili for her PhD grant (2018PMF-PIPF-19).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, L.; Huang, Y.; Dong, Y.; Han, X.; Wang, S.; Liang, X. Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins 2018, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Rongo, T.; van Woesik, R. Socioeconomic consequences of ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2012, 20, 92–100. [Google Scholar] [CrossRef]
- Campàs, M.; Garibo, D.; Prieto-Simón, B. Novel nanobiotechnological concepts in electrochemical biosensors for the analysis of toxins. Analyst 2012, 137, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Kiparissis, S.; Rambla-Alegre, M.; Almarza, S.; Roque, A.; Andree, K.B.; Christidis, A.; Flores, C.; Caixach, J.; Campbell, K. Detection of tetrodotoxins in juvenile pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the North Aegean Sea (Greece) by an electrochemical magnetic bead-based immunosensing tool. Food Chem. 2019, 290, 255–262. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Regulation (EU) No 15/2011 of 10 January 2011 amending Regulation (EC) No 2074/2005 as regards recognised testing methods for detecting marine biotoxins in live bivalve molluscs. Off. J. Eur. Union 2011, 6, 3–6. [Google Scholar]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative methods for the detection of emerging marine toxins: Biosensors, biochemical assays and cell-based assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Prieto-Simón, B.; Campàs, M. New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal. Chim. Acta 2016, 908, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Toldrà, A.; Campàs, M. Trends and prospects on electrochemical biosensors for the detection of marine toxins. In Recent Advances in the Analysis of Marine Toxins, Comprehensive Analytical Chemistry, 1st ed.; Diogène, J., Campàs, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 303–341. [Google Scholar] [CrossRef]
- Ruffert, C. Magnetic bead—Magic bullet. Micromachines 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Duan, M.; Shapter, J.G.; Qi, W.; Yang, S.; Gao, G. Recent progress in magnetic nanoparticles: Synthesis, properties, and applications. Nanotechnology 2018, 29, 452001. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Xianyu, Y.; Wang, Q.; Chen, Y. Magnetic particles-enabled biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikušová, Z.; Skládal, P. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Microchim. Acta 2019, 186, 312. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Barthelmebs, L.; Marty, J.-L. Enzyme-linked immunosensor based on super paramagnetic nanobeads for easy and rapid detection of okadaic acid. Anal. Chim. Acta 2011, 690, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.B.; Hayat, A.; Sassolas, A.; Alonso, G.A.; Munoz, R.; Marty, J.-L. Automated flow-through amperometric immunosensor for highly sensitive and on-line detection of okadaic acid in mussel sample. Talanta 2012, 99, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, J.; Su, K.; Hu, N.; Wang, P. A novel quantum dot fluorescence immunosensor based on magnetic beads and portable flow cytometry for detection of okadaic acid. Procedia Technol. 2017, 27, 214–216. [Google Scholar] [CrossRef]
- Pan, Y.; Wei, X.; Liang, T.; Zhou, J.; Wan, H.; Hu, N.; Wang, P. A magnetic beads-based portable flow cytometry immunosensor for in-situ detection of marine biotoxin. Biomed. Microdevices 2018, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Barthelmebs, L.; Sassolas, A.; Marty, J.-L. Development of a novel label-free amperometric immunosensor for the detection of okadaic acid. Analytica Chim. Acta 2012, 724, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Devic, E.; Marty, J.-L.; Diogène, J.; Unzueta, I.; Blázquez, M.; Campàs, M. Conjugation of genetically engineered protein phosphatases to magnetic particles for okadaic acid detection. J. Biotechnol. 2012, 157, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Rambla-Alegre, M.; Samdal, I.A.; Miles, C.O.; Kilcoyne, J.; Diogène, J.; O’Sullivan, C.K.; Campàs, M. Immunorecognition magnetic supports for the development of an electrochemical immunoassay for azaspiracid detection in mussels. Biosens. Bioelectron. 2017, 92, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Y.; Wu, J.; Wang, X.; Liu, Y. An Amperometric Immunosensor based on an ionic liquid and single-walled carbon nanotube composite electrode for detection of Tetrodotoxin in pufferfish. J. Agric. Food Chem. 2016, 64, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gui, R.; Sun, J.; Wang, Y. Facilely self-assembled magnetic nanoparticles/aptamer/carbon dots nanocomposites for highly sensitive up-conversion fluorescence turn-on detection of tetrodotoxin. Talanta 2018, 176, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hou, L.; Tang, D.; Liu, B.; Li, J.; Chen, G. Simultaneous multiplexed stripping voltammetric monitoring of marine toxins in seafood based on distinguishable metal nanocluster-labeled molecular tags. J. Agric. Food Chem. 2012, 60, 8974–8982. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, J.; Zeng, X.; Xu, L.; Wu, Y.; Fu, F. A signal-on magnetic electrochemical immunosensor for ultra-sensitive detection of saxitoxin using palladium-doped graphitic carbon nitride-based non-competitive strategy. Biosens. Bioelectron. 2019, 128, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Choi, S.-J. Immunoassay of paralytic shellfish toxins by moving magnetic particles in a stationary liquid-phase lab-on-a-chip. Biosens. Bioelectron. 2015, 66, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Choi, S.-J. Development of an improved stationary liquid-phase lab-on-a-chip for the field monitoring of paralytic shellfish toxins. BioChip J. 2017, 11, 30–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Luan, W.; Li, X.; Liu, Y.; Luo, X. Ultrasensitive and accelerated detection of ciguatoxin by capillary electrophoresis via on-line sandwich immunoassay with rotating magnetic field and nanoparticles signal enhancement. Anal. Chim. Acta 2015, 888, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Campbell, K.; Casanova, A.; De La Iglesia, P.; Fernández-Tejedor, M.; Diogène, J.; Elliott, C.; Campàs, M. SPR immunosensor for the detection of okadaic acid in mussels using magnetic particles as antibody carriers. Sensors and Actuators B Chem. 2014, 190, 822–828. [Google Scholar] [CrossRef]
- Devlin, R.; Campbell, K.; Kawatsu, K.; Elliott, C. Physical and immunoaffinity extraction of paralytic shellfish poisoning toxins from cultures of the dinoflagellate Alexandrium tamarense. Harmful Algae 2011, 10, 542–548. [Google Scholar] [CrossRef]
- Bragg, W.A.; Garrett, A.; Hamelin, E.I.; Coleman, R.M.; Campbell, K.; Elliott, C.T.; Johnson, R.C. Quantitation of saxitoxin in human urine using immunocapture extraction and LC–MS. Bioanalysis 2018, 10, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, Z.; Wu, H.; Peng, J.; Zhai, Y.; Guo, M. Selective enrichment and quantification of okadaic acid in shellfish using an immunomagnetic-bead-based liquid chromatography with tandem mass spectrometry assay. J. Sep. Sci. 2019, 42, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Neely, B.A.; Soper, J.L.; Greig, D.J.; Carlin, K.P.; Favre, E.G.; Gulland, F.M.; Almeida, J.S.; Janech, M.G. Serum profiling by MALDI-TOF mass spectrometry as a diagnostic tool for domoic acid toxicosis in California sea lions. Proteome Sci. 2012, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Oguri, H.; Tsumoto, K.; Shindo, Y.; Hirama, M.; Tsumuraya, T.; Fujii, I.; Tomioka, Y.; Mizugaki, M.; Kumagai, I. Phage-display selection of antibodies to the left end of CTX3C using synthetic fragments. J. Immunol. Methods 2004, 289, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Handy, S.M.; Yakes, B.J.; DeGrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; DeGrasse, S.L. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, B.; Zheng, X.; Cao, Y.; Liu, D.; Sun, M.; Jiao, B.; Wang, L. Gonyautoxin 1/4 aptamers with high-affinity and high-specificity: From efficient selection to aptasensor application. Biosens. Bioelectron. 2016, 79, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, X.; Hu, B.; Sun, M.; Wu, J.; Jiao, B.; Wang, L. Enzyme-linked, aptamer-based, competitive biolayer interferometry biosensor for palytoxin. Biosens. Bioelectron. 2017, 89, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Duan, N.; Xia, Y.; Hun, X.; Wang, H.; Wang, Z. Magnetic Separation-Based Multiple SELEX for Effectively Selecting Aptamers against Saxitoxin, Domoic Acid, and Tetrodotoxin. J. Agric. Food Chem. 2018, 66, 9801–9809. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, Z.-S.; Wang, D.-X.; Li, L.; Hu, P.; Gong, S.; Li, Y.-S.; Cui, C.; Wu, Z.-C.; Gao, Y. Generation of internal-image functional aptamers of okadaic acid via magnetic-bead SELEX. Mar. Drugs 2015, 13, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.; Silke, J. Winter toxicity of unknown aetiology in mussels. Harmful Algae News 1996, 14, 2. [Google Scholar]
- Mello, D.F.; De Oliveira, E.S.; Vieira, R.C.; Simoes, E.; Trevisan, R.; Dafre, A.L.; Barracco, M.A. Cellular and transcriptional responses of Crassostrea gigas hemocytes exposed in vitro to brevetoxin (PbTx-2). Mar. Drugs 2012, 10, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.; Landsberg, J.; Etheridge, S.; Pitcher, G.; Longan, S. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).