Stepwise Synthesis, Hydrogen-Bonded Supramolecular Structure, and Magnetic Property of a Co–Mn Heterodinuclear Complex
Abstract
1. Introduction
2. Results and Discussion
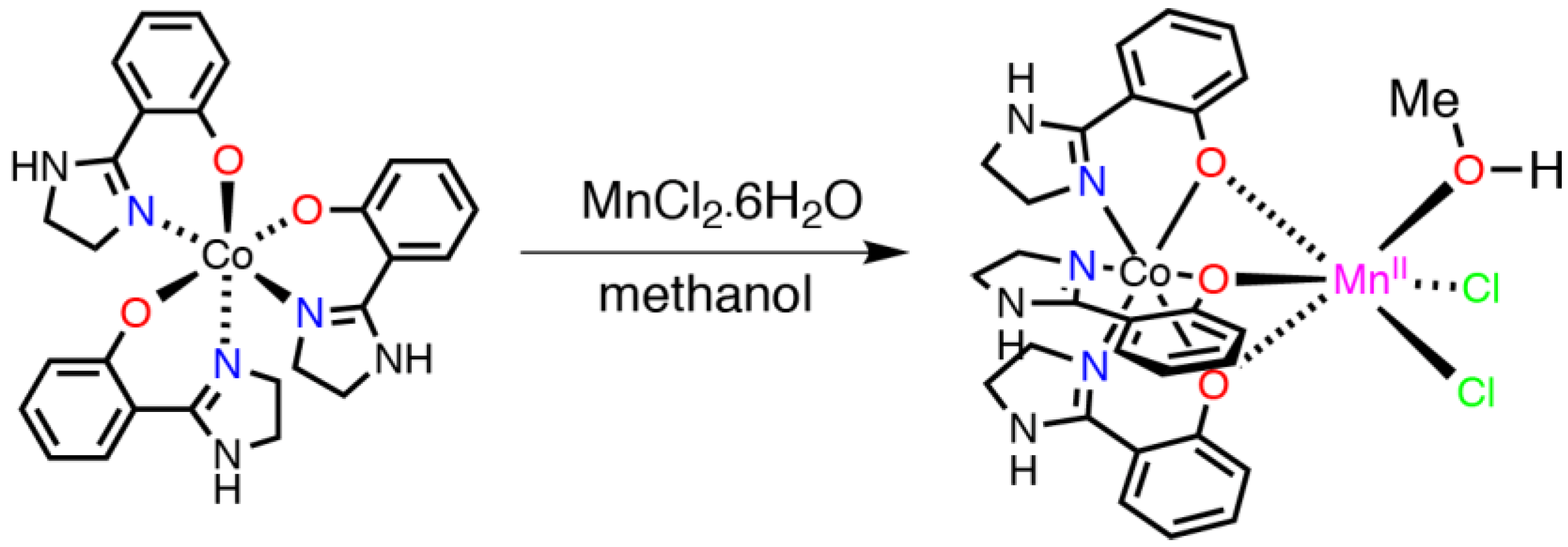
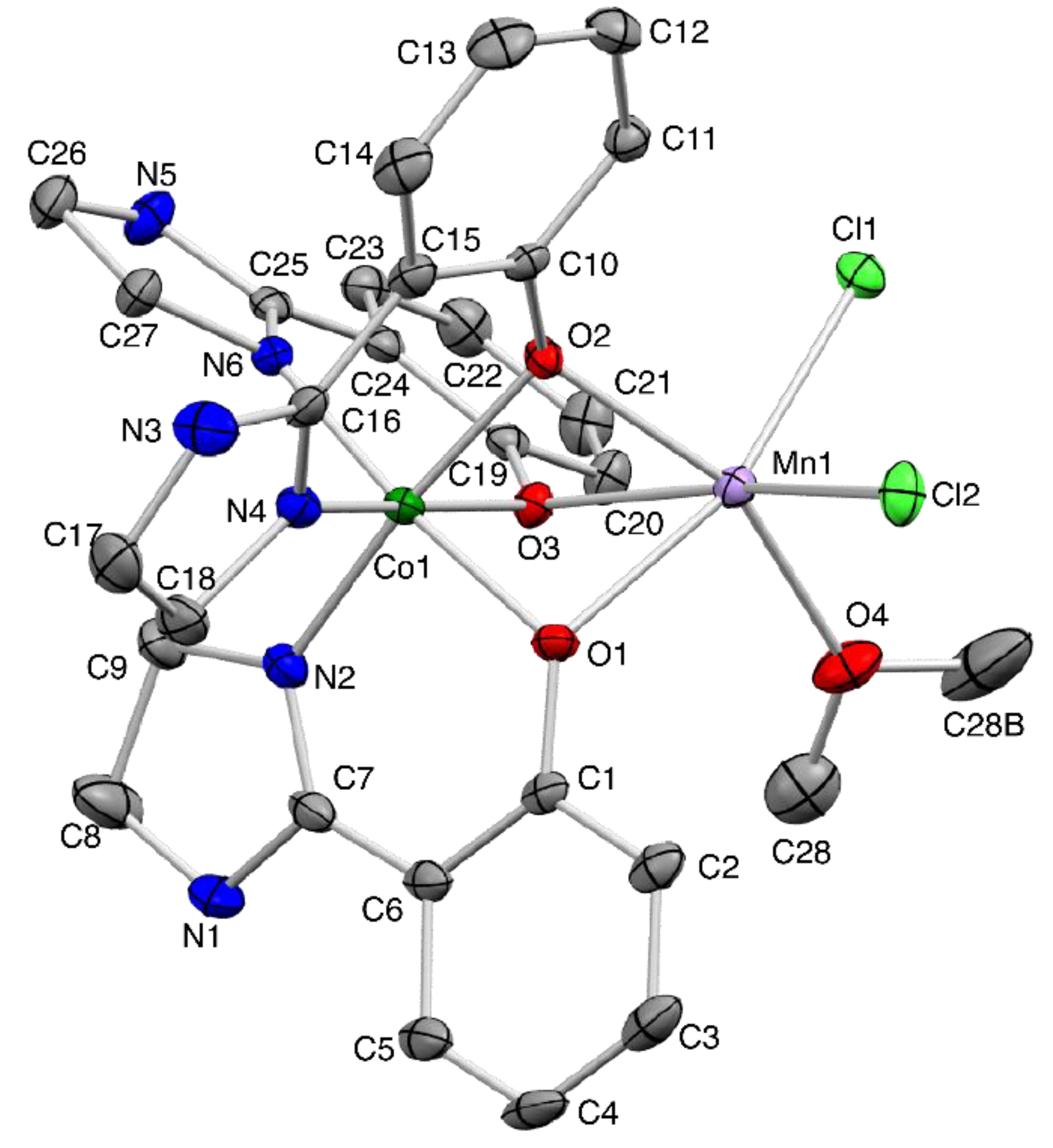
2.1. Synthesis and Characterization
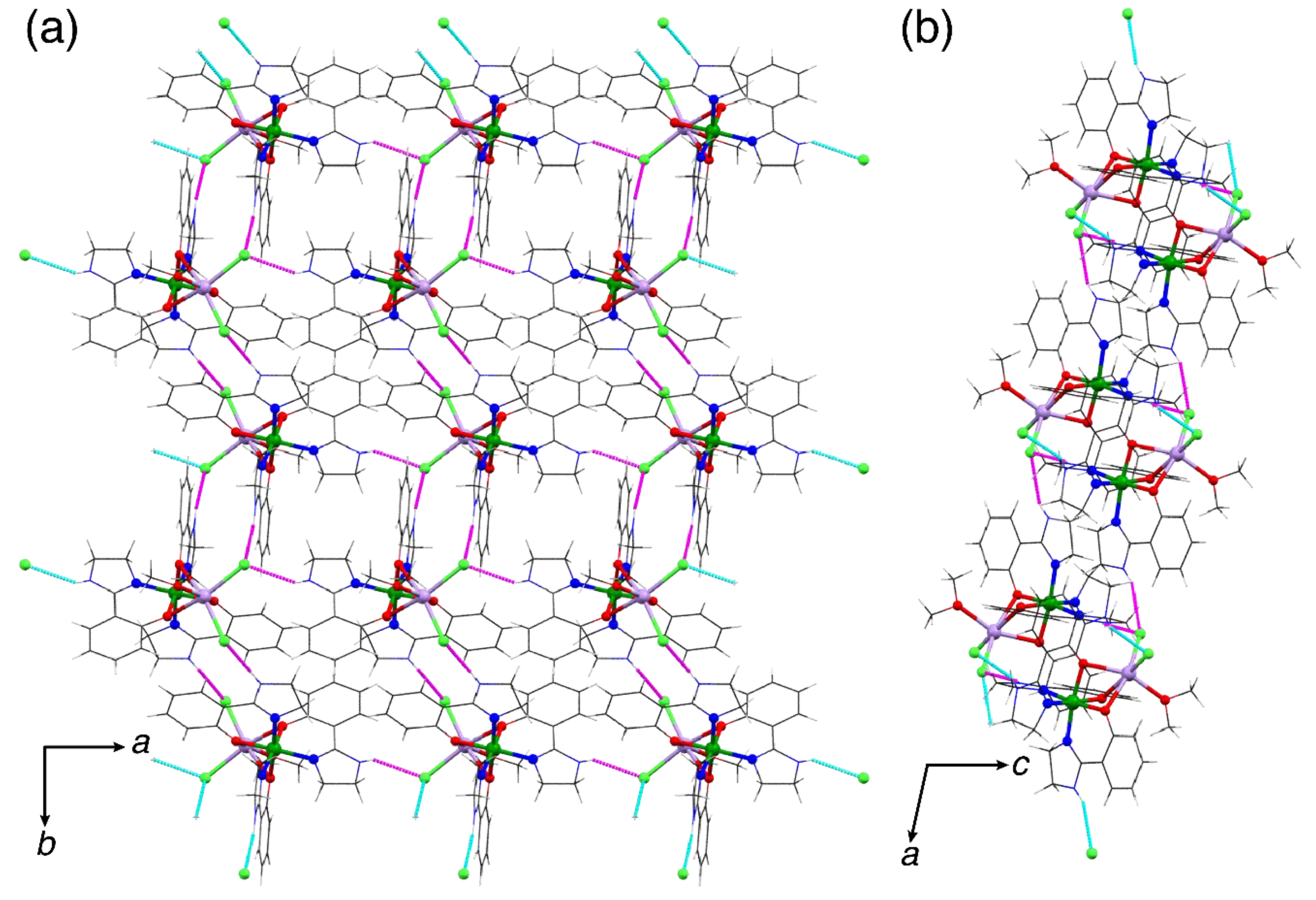
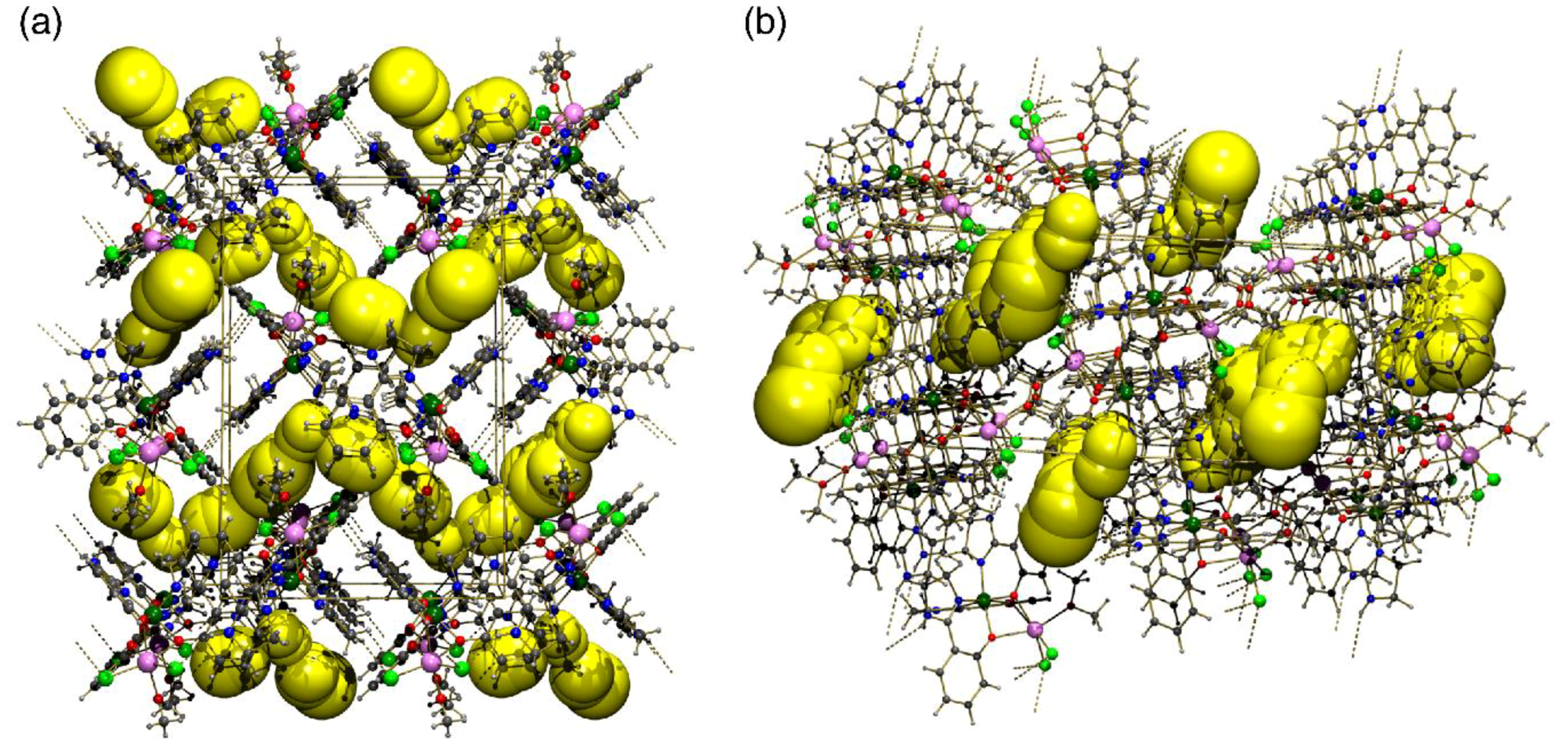
2.2. Supramolecular Structure
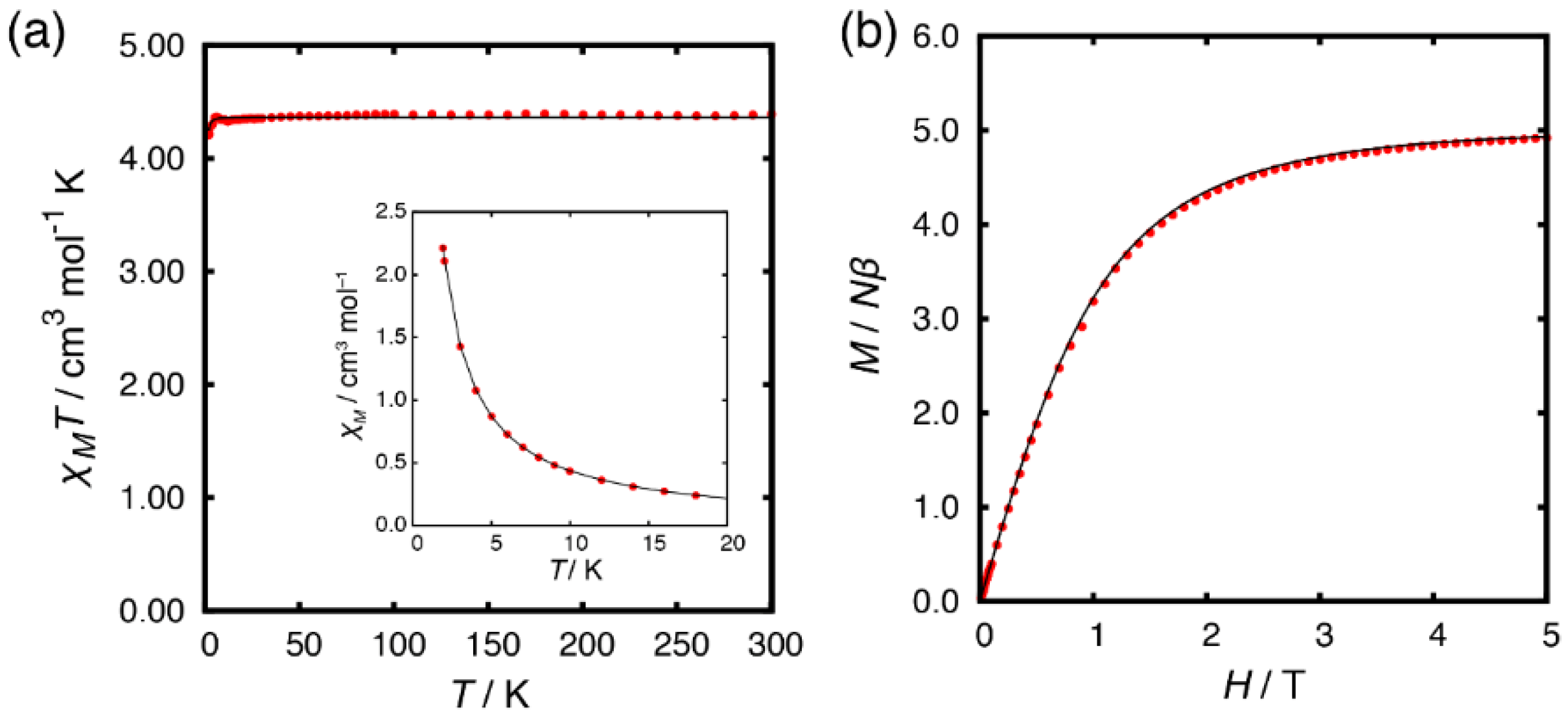
2.3. Magnetic Properties
3. Materials and Methods
3.1. General Consideration
3.2. Synthetic Method
3.3. Single-Crystal X-ray Crystallography
3.4. Magnetic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Long, J.R. Slow magnetic relaxation at zero field in the tetrahedral complex [Co(SPh)4]2−. J. Am. Chem. Soc. 2011, 133, 20732–20734. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.-E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A Trigonal Prismatic Mononuclear Cobalt(II) Complex Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Pedersen, K.S.; Ueda, T.; Suzuki, T.; Bendix, J.; Mikuriya, M. Field-induced single-molecule magnet behavior in ideal trigonal antiprismatic cobalt(II) complexes: precise geometrical control by a hydrogen-bonded rigid metalloligand. Chem. Commun. 2018, 54, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Hosoya, S.; Suzuki, T.; Sunatsuki, Y.; Sakiyama, H.; Mikuriya, M. Hydrogen-bonding interactions and magnetic relaxation dynamics in tetracoordinated cobalt(II) single-ion magnets. Dalton Trans. 2019, 48, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Rosado Piquer, L.; Sañudo, E.C. Heterometallic 3d–4f single-molecule magnets. Dalton Trans. 2015, 44, 8771–8780. [Google Scholar] [CrossRef] [PubMed]
- Colacio, E.; Ruiz, J.; Ruiz, E.; Cremades, E.; Krzystek, J.; Carretta, S.; Cano, J.; Guidi, T.; Wernsdorfer, W.; Brechin, E.K. Slow Magnetic Relaxation in a CoII-YIII Single-Ion Magnet with Positive Axial Zero-Field Splitting. Angew. Chemie Int. Ed. 2013, 52, 9130–9134. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Weihe, H.; Vinum, M.G.; Mortensen, J.S.; Doerrer, L.H.; Bendix, J. Imposing high-symmetry and tuneable geometry on lanthanide centres with chelating Pt and Pd metalloligands. Chem. Sci. 2017, 8, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical tunnel-splitting-engineering in a dysprosium-based molecular nanomagnet. Nat. Commun. 2018, 9, 1292. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Suzuki, T.; Hosoya, S.; Mikuriya, M. Hydrogen-Bonded Supramolecular Structures of Cobalt(III) Complexes with Unsymmetrical Bidentate Ligands: mer/fac Interconversion Induced by Hydrogen-Bonding Interactions. Cryst. Growth Des. 2017, 17, 207–213. [Google Scholar] [CrossRef]
- Liu, W.; Thorp, H.H. Bond Valence Sum Analysis of Metal-Ligand Bond Lengths in Metalloenzymes and Model Complexes. 2. Refined Distances and Other Enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Suzuki, T.; Sunatsuki, Y. Four-Electron Oxidative Dehydrogenation Induced by Proton-Coupled Electron Transfer in Ruthenium(III) Complex with 2-(1,4,5,6-Tetrahydropyrimidin-2-yl)phenolate. Inorg. Chem. 2013, 52, 10183–10190. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SADABS, Program for Absorption Correction; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
| [Co(Himn)3MnCl2(CH3OH)]·0.78CH3OH·1.26H2O | |
|---|---|
| Chemical formula | C28.78H36.64Cl2CoMnN6O6.04 |
| Formula weight | 748.05 |
| Color and shape | brown, prism |
| Size of specimen/mm3 | 0.18 × 0.12 × 0.08 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 10.4888(13) |
| b/Å | 14.4208(18) |
| c/Å | 22.026(3) |
| β/° | 102.460(2) |
| V/Å3 | 3253.1(7) |
| Z | 4 |
| T/K | 90(2) |
| Dcalc/g cm−3 | 1.527 |
| F (000) | 1543 |
| µ(Mo − Kα)/mm−1 | 1.113 |
| Rint | 0.0361 |
| 2θmax/° | 55 |
| No. of independent reflection | 7454 |
| R1 (F2:Fo2 > 2s(Fo2)) | 0.0533 |
| wR2 (F2: all data) | 0.1282 |
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Co1–O1 | 1.909(3) | Mn1–O1 | 3.229(3) |
| Co1–O2 | 1.898(2) | Mn1–O2 | 3.317(3) |
| Co1–O3 | 1.902(3) | Mn1–O3 | 2.263(2) |
| Co1–N2 | 1.906(3) | Mn1–O4 | 2.160(2) |
| Co1–N4 | 1.899(3) | Mn1–Cl1 | 2.464(1) |
| Co1–N6 | 1.903(3) | Mn1–Cl2 | 2.459(1) |
| D–H···A | D–H | H···A | D···A | D–A···A |
|---|---|---|---|---|
| N1–H1···Cl1 i | 0.75(4) | 2.59(5) | 3.229(3) | 145(5) |
| N3–H3A···Cl2 ii | 0.75(4) | 2.63(4) | 3.317(3) | 153(4) |
| N5–H5A···Cl1 (iii) | 0.88 | 2.50 | 3.268(3) | 146 |
| O5–H5M···Cl2 (iv) | 0.84 | 2.44 | 3.272(7) | 169 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsuhashi, R.; Ueda, T.; Mikuriya, M. Stepwise Synthesis, Hydrogen-Bonded Supramolecular Structure, and Magnetic Property of a Co–Mn Heterodinuclear Complex. Magnetochemistry 2019, 5, 5. https://doi.org/10.3390/magnetochemistry5010005
Mitsuhashi R, Ueda T, Mikuriya M. Stepwise Synthesis, Hydrogen-Bonded Supramolecular Structure, and Magnetic Property of a Co–Mn Heterodinuclear Complex. Magnetochemistry. 2019; 5(1):5. https://doi.org/10.3390/magnetochemistry5010005
Chicago/Turabian StyleMitsuhashi, Ryoji, Takaaki Ueda, and Masahiro Mikuriya. 2019. "Stepwise Synthesis, Hydrogen-Bonded Supramolecular Structure, and Magnetic Property of a Co–Mn Heterodinuclear Complex" Magnetochemistry 5, no. 1: 5. https://doi.org/10.3390/magnetochemistry5010005
APA StyleMitsuhashi, R., Ueda, T., & Mikuriya, M. (2019). Stepwise Synthesis, Hydrogen-Bonded Supramolecular Structure, and Magnetic Property of a Co–Mn Heterodinuclear Complex. Magnetochemistry, 5(1), 5. https://doi.org/10.3390/magnetochemistry5010005






