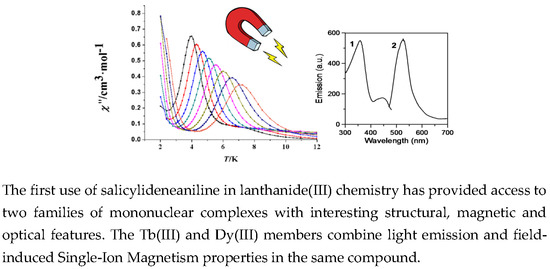
Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Comments
2.2. Conventional Characterization of the Complexes
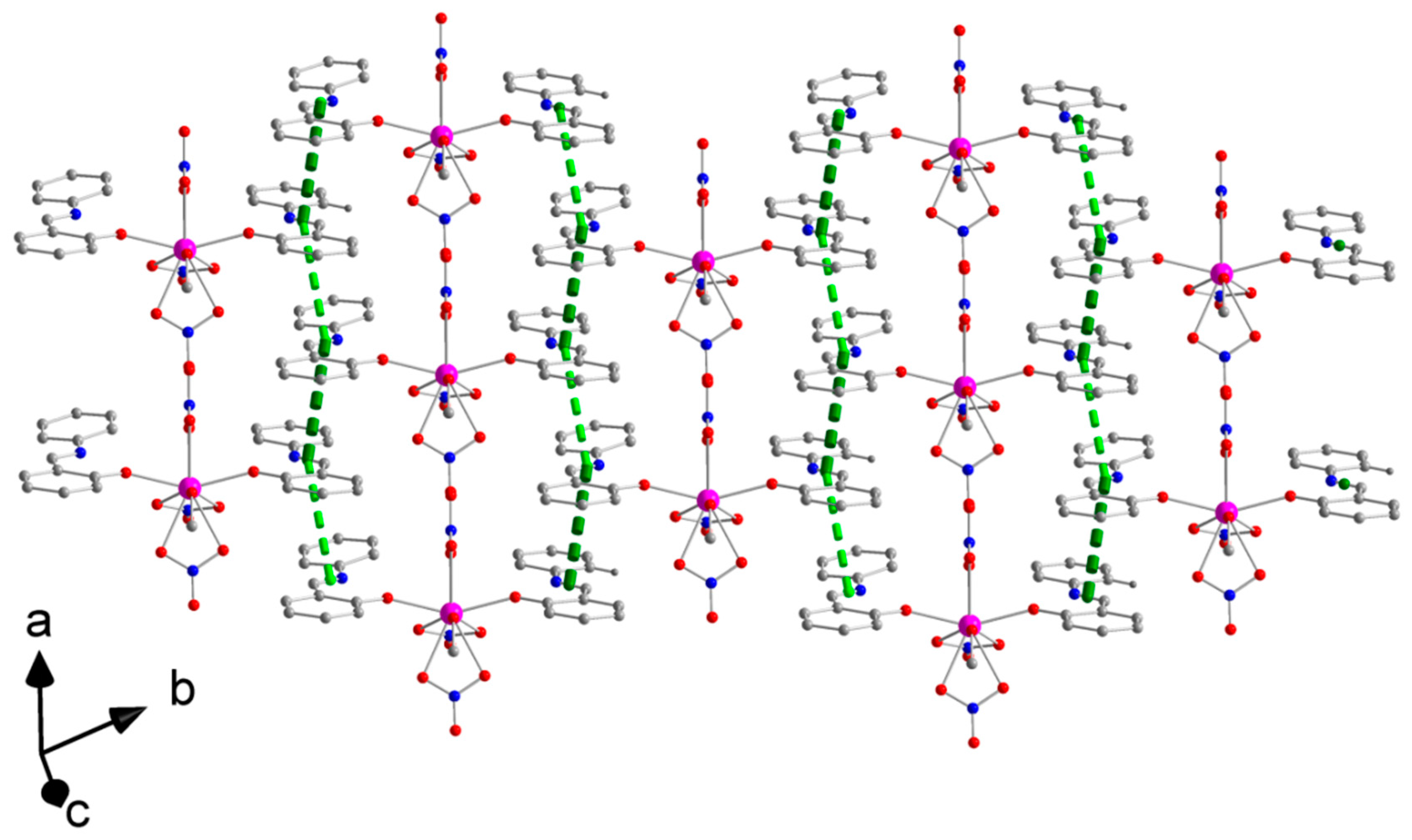
2.3. Description of Structures
2.4. Emission Studies
2.5. Magnetic Studies of the Tb(III) and Dy(III) Complexes
3. Experimental Section
3.1. Materials, Physical, and Spectroscopic Measurements
3.2. Synthesis of the Representative Complex [Dy(NO3)3(salanH)2(H2O)]·MeCN (7·MeCN_Dy)
3.3. Syntheses of [Pr(NO3)3(salanH)2(H2O)]·MeCN (1·MeCN_Pr), [Nd(NO3)3(salanH)2(H2O)]·MeCN (2·MeCN_Nd), [Sm(NO3)3(salanH)2(H2O)]·MeCN (3·MeCN_Sm), [Eu(NO3)3(salanH)2(H2O)]·MeCN (4·MeCN_Eu), [Gd(NO3)3(salanH)2(H2O)]·MeCN (5·MeCN_Gd), [Tb(NO3)3(salanH)2(H2O)]·MeCN (6·MeCN_Tb), [Ho(NO3)3(salanH)2(H2O)]·MeCN (8·MeCN_Ho), [Er(NO3)3(salanH)2(H2O)]·MeCN (9·MeCN_Er), [Yb(NO3)3(salanH)2(H2O)]·MeCN (10·MeCN_Yb) and [Y(NO3)3(salanH)2(H2O)]·MeCN (11·MeCN_Y)
3.4. Synthesis of the Representative Complex [Dy(NO3)3(salanH)2(MeOH)]· (salanH) (18_Dy)
3.5. Syntheses of [Pr(NO3)3(salanH)2(MeOH)]·(salanH) (12_Pr), [Nd(NO3)3(salanH)2(MeOH)]· (salanH) (13_Nd), [Sm(NO3)3(salanH)2(MeOH)]· (salanH) (14_Sm), [Eu(NO3)3(salanH)2(MeOH)]· (salanH) (15_Eu), [Gd(NO3)3(salanH)2(MeOH)]· (salanH) (16_Gd), [Tb(NO3)3(salanH)2(MeOH)]· (salanH) (17_Tb), [Ho(NO3)3(salanH)2(MeOH)]· (salanH) (19_Ho), [Er(NO3)3(salanH)2(MeOH)]· (salanH) (20_Er), [Yb(NO3)3(salanH)2(MeOH)]· (salanH) (21_Yb) and [Y(NO3)3(salanH)2(MeOH)]· (salanH) (22_Y)
3.6. Single-Crystal X-ray Crystallography
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Kahn, O. Molecular Magnetism; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Barra, A.L.; Brunel, L.C.; Guillot, M. Alternating Current Susceptibility, High Field Magnetization, and Millimeter Band EPR Evidence for a ground S=10 State in [Mn12O12(CH3COO)16(H2O)4]·2CH3COOH·4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- For a review on Mn Single-Molecule Magnets, see: Bagai, R.; Christou, G. The drosophila of single-molecule magnetism: [Mn12O12(O2CR)16(H2O)4]. Chem. Soc. Rev. 2009, 38, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- For an excellent comprehensive review, see: Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. Struct. Bond. 2015, 164, 1–109. [Google Scholar]
- For an excellent comprehensive review, see: Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(III) single-molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; Van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, L.; Tang, J. Hydrazone-based Dysprosium Single Molecule Magnets. Current Inorg. Chem. 2013, 3, 101–111. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Tang, J. Lanthanide single molecule magnets: Progress and perspective. Dalton Trans. 2015, 44, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Woodruff, D.N.; Bendix, J.; Clérac, R. Experimental Aspects of Lanthanide Single-Molecule Magnet Physics. In Lanthanides and Actinides in Molecular Magnetism, 1st ed.; Layfield, R.A., Murugesu, M., Eds.; Wiley-VCH: Berlin, Germany, 2015; pp. 125–152. [Google Scholar]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Luzon, J.; Sessoli, R. Lanthanides in molecular magnetism: So fascinating, so challenging. Dalton Trans. 2012, 41, 13556–13567. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Murugavel, R. Enriching lanthanide single-ion magnetism through symmetry and axiality. Chem. Commun. 2018, 54, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Pointillant, F.; Cador, O.; Le Guennic, B.; Quahab, L. Uncommon lanthanide ions in purely 4f Single Molecule Magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sujita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.T.; Monteiro, B.; Pereira, L.C.J. Ln(III)-based SIMs. In Lanthanide-Based Multifunctional Materials, 1st ed.; Martin-Ramos, P., Ramos-Silva, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–231. [Google Scholar]
- Gupta, S.K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R. Is a strong axial crystal-field the only essential condition for a large magnetic anisotropy barrier? The case of non-Kramers Ho(III) versus Tb(III). Dalton Trans. 2018, 47, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; Meng, Y.-S.; Xiong, J.; Wang, B.-W.; Jiang, S.-D.; Gao, S. Magnetic anisotropy investigation on light lanthanide complexes. Dalton Trans. 2018, 47, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Michiwaki, S.; Noda, T.; Katoh, K.; Yamashita, M.; Matsubara, K.; Kawata, S. Field-Induced Slow Magnetic Relaxation of Mono- and Dinuclear Dysprosium(III) Complexes Coordinated by a Chloranilate with Different Resonance Forms. Inorganics 2018, 6, 7. [Google Scholar] [CrossRef]
- Harriman, K.L.M.; Brosmer, J.L.; Ungur, L.; Diaconescu, P.L.; Murugesu, M. Pursuit of Record Breaking Energy Barriers: A Study of Magnetic Axiality in Diamide Ligated DyIII Single-Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.-C.; Jia, J.-H.; Liu, J.-L.; Vieru, V.; Ungur, L.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal-Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Liu, J.-L.; Wernsdorfer, W.; Liu, D.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Hyperfine-Interaction-Driven Suppression of Quantum Tunneling at Zero Field in a Holmium(III) Single-Ion Magnet. Angew. Chem. Int. Ed. 2017, 56, 4996–5000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nakanishi, R.; Katoh, K.; Breedlove, B.K.; Kitagawa, Y.; Yamashita, M. Low coordinated mononuclear erbium(III) single-molecule magnets with C3v symmetry: A method for altering single-molecule magnet properties by incorporating hard and soft donors. Dalton Trans. 2018, 47, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Chibotaru, L.F. Strategies toward High-Temperature Lanthanide-Based Single-Molecule Magnets. Inorg. Chem. 2016, 55, 10043–10056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Liu, J.-L.; Ungur, L.; Liu, J.; Li, Q.-W.; Wang, L.-F.; Ni, Z.-P.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Symmetry Supported Magnetic Blocking at 20 K in the Pentagonal Bipyramidal Dy(III) Single-Ion Magnets. J. Am. Chem. Soc. 2016, 138, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. On Approaching the Limit of Molecular Magnetic Anisotropy: A Near Perfect Pentagonal Bipyramidal Dysprosium (III) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tang, J. Six-Coordinate Ln(III) Complexes with Various Coordination Geometries Showing Distinct Magnetic Properties. Inorganics 2018, 6, 16. [Google Scholar] [CrossRef]
- Dickie, C.M.; Laughlin, A.L.; Wofford, J.D.; Bhuvanech, N.S.; Nippe, M. Transition metal redox switches for reversible “on/off” and “slow/fast” single-molecule magnet behavior in dysprosium and erbium bis-diamidoferrocene complexes. Chem. Sci. 2017, 8, 8039–8049. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, G.; Perfetti, M.; Luzon, J.; Etienne, M.; Car, P.-E.; Caneschi, A.; Calvez, G.; Bernot, K.; Sessoli, R. Magnetic Anisotropy in a Dysprosium/DOTA Single-Molecule Magnet: Beyond Simple Magneto-Structural Correlations. Angew. Chem. Int. Ed. 2012, 51, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Datta, S.; Friend, L.; Cardonna-Serra, S.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Multi-frequency EPR studies of a mononuclear holmium single-molecule magnet based on the polyoxometalate [HoIII(W5O18)2]9−. Dalton Trans. 2012, 41, 13697–13704. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Manget Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 Kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Reta, D.; Ortu, F.; Chilton, N.F.; Mills, D.P. Synthesis and Electronic Structures of Heavy Lanthanide Metallocenium Cations. J. Am. Chem. Soc. 2017, 139, 18714–18724. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Swavey, S.; Swavey, R. Dinuclear and polynuclear lanthanide coordination complexes containing polyazine ligands: Synthesis and luminescent properties. Coord. Chem. Rev. 2009, 253, 2627–2638. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- He, H. Near-infrared emitting lanthanide complexes of porphyrin and BODIPY dyes. Coord. Chem. Rev. 2014, 273–274, 87–99. [Google Scholar] [CrossRef]
- Ahmed, Z.; Iftikhar, K. Sensitization of Visible and NIR Emitting Lanthanide(III) Ions in Noncentrosymmetric Complexes of Hexafluoroacetylacetone and Unsubstituted Monodentate Pyrazole. J. Phys. Chem. A 2013, 117, 11183–11201. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Papagni, A.; Meinardi, F.; Tubino, R.; Ottonelli, M.; Musso, G.F.; Dellepione, G. Novel lanthanide complexes for visible and IR emission. Synth. Met. 2004, 147, 143–147. [Google Scholar] [CrossRef]
- Stacey, O.J.; Ward, B.D.; Amoroso, A.J.; Pope, S.J.A. Near-IR luminescent lanthanide complexes with 1,8-diaminoanthraquinone-based chromophoric ligands. Dalton Trans. 2016, 45, 6674–6681. [Google Scholar] [CrossRef] [PubMed]
- For an excellent paper covering the whole literature, see: Mamontova, E.; Long, J.; Ferreira, R.A.S.; Botas, A.M.P.; Luneau, D.; Guari, Y.; Carlos, L.D.; Larionova, J. Magneto-Luminescence Correlation in the Textbook Dysprosium(III) Nitrate Single-Ion Magnet. Magnetochemistry 2016, 2, 41. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; Herrera-Lanzós, A.; Garcia-Deibe, A.M.; Sanmartin-Matalobos, J.; Herrera, J.M.; Colacio, E.; Nuñez, C. Improving the SMM and luminescence properties of lanthanide complexes with LnO9 cores in the presence of ZnII: An emissive Zn2Dy single ion magnet. Dalton Trans. 2017, 46, 17000–17009, and refs. cited therein. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Vallat, R.; Ferreira, R.A.S.; Carlos, L.D.; Almeida Paz, F.A.; Guari, Y.; Larionova, J. A bifunctional luminescent single-ion magnet: Towards correlation between luminescence studies and magnetic slow relaxation processes. Chem. Commun. 2012, 48, 9974–9976. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Miyazaki, R.; Kataoka, Y.; Nakanishi, T.; Hasegawa, Y.; Nakano, M.; Yamasura, T.; Kajiwara, T. A luminescent single-molecule magnet: Observation of magnetic anisotropy using emission as a probe. Dalton Trans. 2013, 42, 1987–1990. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Le Guennic, B.; Colhen, S.; Cador, O.; Maury, O.; Quahab, L. A redox-active luminescent ytterbium-based single molecule magnet. Chem. Commun. 2013, 49, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Gavey, E.L.; Al Hareri, M.; Regier, J.; Carlos, L.D.; Ferreira, R.A.S.; Razavi, F.S.; Rawson, J.M.; Pilkington, M. Placing a crown on Dy(III)-A dual property LnIII crown ether complex displaying optical properties and SMM behaviour. J. Mater. Chem. C 2015, 3, 7738–7747. [Google Scholar] [CrossRef]
- For example, see: Anastasiadis, N.C.; Kalofolias, D.A.; Philippidis, A.; Tzani, S.; Raptopoulou, C.P.; Psycharis, V.; Milios, C.J.; Escuer, A.; Perlepes, S.P. A family of dinuclear lanthanide(III) complexes from the use of a tridentate Schiff base. Dalton Trans. 2015, 44, 10200–10209. [Google Scholar] [CrossRef] [PubMed]
- For example, see: Mylonas-Margaritis, I.; Mayans, J.; Sakellakou, S.-M.; Raptopoulou, C.P.; Psycharis, V.; Escuer, A.; Perlepes, S.P. Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies. Magnetochemistry 2017, 3, 5. [Google Scholar] [CrossRef]
- For example, see: Anastasiadis, N.C.; Granadeiro, C.M.; Klouras, N.; Cunha-Silva, L.; Raptopoulou, C.P.; Psycharis, V.; Bekiari, V.; Balula, S.S.; Escuer, A.; Perlepes, S.P. Dinuclear Lanthanide(III) Complexes by Metal-Ion-Assisted Hydration of Di-2-pyridyl Ketone Azine. Inorg. Chem. 2013, 52, 4145–4147. [Google Scholar] [CrossRef] [PubMed]
- For example, see: Bekiari, V.; Thiakou, K.A.; Raptopoulou, C.P.; Perlepes, S.P.; Lianos, P. Structure and photophysical behavior of 2,2′-bipyrimidine/lanthanide ion complexes in various environments. J. Lumin. 2008, 128, 481–488. [Google Scholar] [CrossRef]
- For example, see: Mylonas-Margaritis, I.; Kitos, A.A.; Panteli, C.C.; Skordi, K.; Tasiopoulos, A.J.; Bekiari, V.; Escuer, A.; Perlepes, S.P. 2-hydroxybenzophenone-controlled self-assembly of enneanuclear lanthanide(III) hydroxo coordination clusters with an “hourglass”-like topology. Inorg. Chem. Commun. 2017, 83, 118–122. [Google Scholar] [CrossRef]
- For example, see: Nikolaou, H.; Terzis, A.; Raptopoulou, C.P.; Psycharis, V.; Bekiari, V.; Perlepes, S.P. Unique Dinuclear, Tetrakis(nitrato-O,O’)-Bridged Lanthanide(III) Complexes from the Use of Pyridine-2-Amidoxime: Synthesis, Structural Studies and Spectroscopic Characterization. J. Surf. Interface Mater. 2014, 2, 311–318. [Google Scholar] [CrossRef]
- For example, see: Anastasiadis, N.C.; Mylonas-Margaritis, I.; Psycharis, V.; Raptopoulou, C.P.; Kalofolias, D.A.; Milios, C.J.; Klouras, N.; Perlepes, S.P. Dinuclar, tetrakis(acetato)-bridged lanthanide(III) complexes form the use of 2-acetylpyridine hydrazone. Inorg. Chem. Commun. 2015, 51, 99–102. [Google Scholar] [CrossRef]
- Maniaki, D.; Mylonas-Margaritis, I.; Mayans, J.; Savvidou, A.; Raptopoulou, C.P.; Bekiari, V.; Psycharis, V.; Escuer, A.; Perlepes, S.P. Slow magnetic relaxation and luminescence properties in lanthanide(III)/anil complexes. Dalton Trans. 2018, 47, 11859–11872. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord. Chem. Rev. 1999, 190–192, 537–555. [Google Scholar] [CrossRef]
- Nematirad, M.; Gee, W.J.; Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Murray, K.S.; Batten, S.R. Single molecule magnetism in a μ-phenolato dinuclear motif ligated by heptadentate Schiff base ligands. Dalton Trans. 2012, 41, 13711–13715. [Google Scholar] [CrossRef] [PubMed]
- Lacelle, T.; Brunet, G.; Pialat, A.; Holmberg, R.J.; Lan, Y.; Gabidullin, B.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. Single-molecule magnet behaviour in a tetranuclear DyIII complex formed from a novel tetrazine-centered hydrazone Schiff base ligand. Dalton Trans. 2017, 46, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, V.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- For a comprehensive review, see: Hadjoudis, E.; Mavridis, I.M. Photochromism and thermochromism of Schiff bases in the solid state: Structural aspects. Chem. Soc. Rev. 2004, 33, 579–588. [Google Scholar] [PubMed]
- For a review, see: Hadjoudis, E. Photochromic and Thermochromic Anils. Mol. Eng. 1995, 5, 301–337. [Google Scholar] [CrossRef]
- Shen, M.Y.; Zhao, L.Z.; Goto, T.; Mordzinski, A. Polarization dependence of photochromism in a N-salicylideneaniline. J. Chem. Phys. 2000, 112, 2490–2497. [Google Scholar] [CrossRef]
- Harada, J.; Uekusa, H.; Ohashi, Y. X-ray Analysis of Structural Changes in Photochromic Salicylidene Crystals. Solid-State Reaction Induced by Two-Photon Excitation. J. Am. Chem. Soc. 1999, 121, 5809–5810. [Google Scholar] [CrossRef]
- Wei, L.; Stogsdill, R.M.; Lingafelter, E.C. The crystal structure of bis-(N-phenylsalicylaldiminato)copper(II). Acta Crystallogr. 1964, 17, 1058–1062. [Google Scholar] [CrossRef]
- Sadikov, G.G.; Tkachev, V.V.; Antsyshkina, A.S.; Sergienko, V.S.; Burlov, A.S.; Vasil’chenko, I.S.; Garnovskii, A.D. Coordination compounds of metals with azomethine ligands: The crystal and molecular structure of a zinc complex with 2-hydroxy-1-naphthalidene-aniline. Russ. J. Inorg. Chem. 2005, 50, 353–359. [Google Scholar]
- Van Wyk, J.L.; Mapolie, S.F.; Lennartson, A.; Håkansson, M.; Jagner, S. The catalytic oxidation of phenol in aqueous media using cobalt(II) complexes derived from N-(aryl) salicylaldimines. Inorg. Chim. Acta 2008, 361, 2094–2100. [Google Scholar] [CrossRef]
- McAuliffe, C.A.; Pritchard, R.G.; Luaces, L.; Garcia-Vazquez, J.A.; Romero, J.; Bermejo, M.R.; Sousa, A. Chlorobis(N-phenylsalicylideneaminato-O,N)manganese(III): A Manganese Schiff-Base Complex Derived by an Electrochemical Route. Acta Crystallogr. 1993, C49, 587–589. [Google Scholar] [CrossRef]
- Jones, M.D.; Davidson, M.G.; Keir, C.G.; Hughes, L.M.; Mahon, M.F.; Apperley, D.C. Zinc(II) Homogeneous and Heterogeneous Species and Their Application for the Ring-Opening Polymerization of rac-Lactide. Eur. J. Inorg. Chem. 2009, 635–642. [Google Scholar] [CrossRef]
- Ren, K.; Shang, X.; Fu, J.; Zhao, P.; Zhang, J. Copper complex based on 2-(phenylimino-methyl)-phenol as a high selective fluorescent probe for hydrogen sulfide. Polyhedron 2016, 104, 99–105. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, A.; Bohra, R.; Lorenz, I.-P.; Mayer, P. Synthesis and structural elucidation of some novel aluminium(III) complexes with Schiff bases: Crystal and molecular structure of [Al{O(C6H4)CH=NC6H5}2{HO(C6H4)CH=NC6H5}2]Br. Polyhedron 2006, 25, 654–662. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Zang, S.-L.; Gu, X.-J.; Yu, Y.-J.; Jin, H. Schiff Base Complex of Gallium: Synthesis and Crystal Structure. Chin. J. Inorg. Chem. 2010, 26, 763–768. [Google Scholar]
- Zhou, M.-D.; Zhao, J.; Li, J.; Yue, S.; Bao, C.-N.; Mink, J.; Zang, S.-L.; Kühn, F.E. MTO Schiff-Base Complexes: Synthesis, Structures and Catalytic Applications in Olefin Epoxidation. Chem. Eur. J. 2007, 13, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Destro, R.; Gavezzotti, A.; Simonetta, M. Salicylideneaniline. Acta Crystallogr. 1978, B34, 2867–2869. [Google Scholar] [CrossRef]
- Arod, F.; Gardon, M.; Pattison, P.; Chapuis, G. The α2-polymorph of salicylideneaniline. Acta Crystallogr. 2005, C61, o317–o320. [Google Scholar] [CrossRef] [PubMed]
- Arod, F.; Pattison, P.; Schenk, K.J.; Chapuis, G. Polymorphism in N-Salicylideneaniline Reconsidered. Cryst. Growth Des. 2007, 7, 1679–1685. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, S.; Sreekumar, A.; Choudhury, A.R. The evaluation of the role of C-H···F hydrogen bonds in crystal altering the packing modes in the presence of strong hydrogen bond. J. Mol. Struct. 2016, 1106, 154–169. [Google Scholar] [CrossRef]
- Camp, C.; Guidal, V.; Biswas, B.; Pécaut, J.; Dubois, L.; Mazzanti, M. Multielectron redox chemistry of lanthanide Schiff-base complexes. Chem. Sci. 2012, 3, 2433–2448. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Ariciu, A.-M.; McAdams, S.; Weihe, H.; Bendix, J.; Tuna, F.; Piligkos, S. Towards Molecular 4f Single-Ion Magnet Qubits. J. Am. Chem. Soc. 2016, 138, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Allodi, G.; Chiesa, A.; Garlatti, E.; Mitcov, D.; Konstantatos, A.; Pedersen, K.S.; De Renzi, R.; Piligkos, S.; Carretta, S. Coherent manipulation of a molecular Ln-based nuclear qubit coupled to an electron qubit. J. Am. Chem. Soc. 2018, 140, 9814–9818. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Girera, J.; Alemany, P.; Alvarez, S. SHAPE, Continuous Shape Measures Calculation; Version 2.0; Universitat de Barcelona: Barcelona, Spain, 2010. [Google Scholar]
- Alexandropoulos, D.I.; Fournet, A.; Cunha-Silva, L.; Mowson, A.M.; Bekiari, V.; Christou, G.; Stamatatos, T.C. Fluorescent Naphthalene Diols as Bridging Ligands in LnIII Cluster Chemistry: Synthetic, Structural, Magnetic, and Photophysical Characterization of LnIII8 “Christmas Stars”. Inorg. Chem. 2014, 53, 5420–5422. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, Y.; Liu, T.; Feng, J.; Deng, W.; Shi, L. Visible-near-infrared luminescent lanthanide ternary complexes based on beta-diketonate using visible-light excitation. Luminescence 2015, 30, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Reineke, S.; Baldo, M.A. Room temperature triplet state spectroscopy of organic semiconducors. Sci. Rep. 2014, 4, 3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- We thank one of the referees for her/his advice concerning the calculation of the triplet state of salanH.
- Ning, Y.; Liu, Y.-W.; Meng, Y.-S.; Zhang, J.-L. Design of Near-Infrared Luminescent Lanthanide Complexes Sensitive to Environmental Stimulus through Rationally Tuning the Secondary Coordination Sphere. Inorg. Chem. 2018, 57, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R. Spin-lattice relaxation in rare-earth salts. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1961, 264, 458–484. [Google Scholar] [CrossRef]
- Bartolomé, J.; Filoti, G.; Kuncser, V.; Schienteie, G.; Mereacre, V.; Anson, C.E.; Powell, A.K.; Prodius, D.; Turta, C. Magnetostructural correlations in the tetranuclear series of {Fe3LnO2} butterfly core clusters: Magnetic and Mössbauer spectroscopic study. Phys. Rev. B Condens. Matter Phys. 2009, 80, 014430. [Google Scholar] [CrossRef]
- Chilton, N.F.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.nfchilton.com/magellan.html (accessed on 7 September 2018).
- Kettle, S.F.A. Physical Inorganic Chemistry—A Coordination Chemistry Approach; Oxford University Press: Oxford, UK, 1998; pp. 462–465. [Google Scholar]
- CrystalClear, ver. 1.40; Rigaku/MSC: The Woodlands, TX, USA, 2005.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Diamond, Crystal and Molecular Structure Visualization, ver. 3.1; Crystal Impact: Bonn, Germany, 2018.









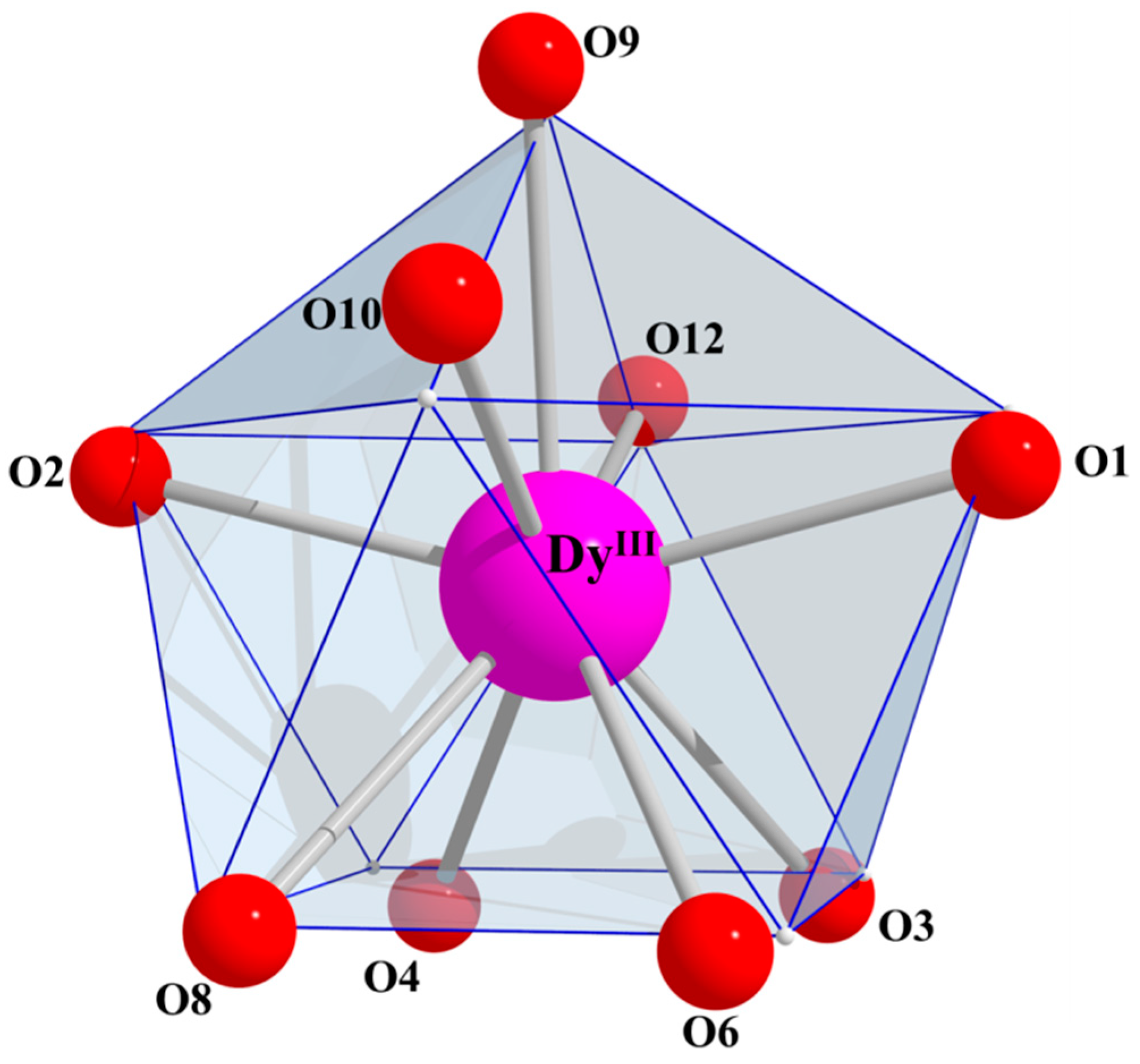








| Bond Distances (Å) | 4·MeCN_Eu b | 7·MeCN_Dy c | 10·MeCN_Yb d |
|---|---|---|---|
| Ln-O1 | 2.310(1) | 2.264(3) | 2.224(1) |
| Ln-O2 | 2.300(1) | 2.262(3) | 2.218(1) |
| Ln-O1W | 2.403(2) | 2.366(3) | 2.312(2) |
| Ln-O3 | 2.511(1) | 2.479(3) | 2.452(1) |
| Ln-O4 | 2.480(1) | 2.439(3) | 2.399(1) |
| Ln-O6 | 2.572(1) | 2.548(3) | 2.526(1) |
| Ln-O8 | 2.536(1) | 2.491(2) | 2.445(1) |
| C1-C6 | 1.431(2) | 1.441(5) | 1.435(3) |
| C6-C7 | 1.417(2) | 1.415(6) | 1.419(3) |
| C7-N1 | 1.298(2) | 1.299(6) | 1.294(3) |
| C1-O1 | 1.302(2) | 1.302(5) | 1.302(2) |
| C14-C19 | 1.428(3) | 1.433(5) | 1.433(3) |
| C19-C20 | 1.419(3) | 1.414(6) | 1.420(3) |
| C20-N2 | 1.307(2) | 1.304(6) | 1.304(3) |
| C14-O2 | 1.309(2) | 1.311(5) | 1.305(2) |
| Bond angles (°) | |||
| O1-Ln-O2 | 77.6(1) | 77.8(1) | 78.0(1) |
| O1W-Ln-O1 | 79.8(1) | 79.7(1) | 79.6(1) |
| O1W-Ln-O2 | 87.8(1) | 87.6(1) | 87.3(1) |
| O3-Ln-O4 | 51.3(1) | 51.9(1) | 52.7(1) |
| O3-Ln-O6 | 114.7(1) | 115.0(1) | 115.5(1) |
| O8-Ln-O2 | 153.5(1) | 152.7(1) | 152.2(1) |
| O11-Ln-O1 | 147.8(1) | 147.1(1) | 147.1(1) |
| Angles (°) | 4·MeCN_Eu | 7·MeCN_Dy | 10·MeCN_Yb |
|---|---|---|---|
| φ(A,B) | 28.7 b, 11.1 c | 28.9 b, 11.5 c | 28.8 b, 11.8 c |
| ψ(A,C) | 4.8 b, 3.5 c | 5.3 b, 3.4 c | 5.2 b, 3.4 c |
| ω(B,C) | 24.4 b, 7.7 c | 24.2 b, 8.1 c | 24.1 b, 8.5 c |
| Bond Distances (Å) | 17_Tb d | 18_Dy e |
|---|---|---|
| Ln-O1 | 2.304(3) | 2.292(2) |
| Ln-O2 | 2.307(3) | 2.296(2) |
| Ln-O12 b | 2.365(4) | 2.361(3) |
| Ln-O3 | 2.491(4) | 2.482(3) |
| Ln-O4 | 2.475(4) | 2.461(3) |
| Ln-O6 | 2.434(4) | 2.420(3) |
| Ln-O8 | 2.475(5) | 2.469(3) |
| Ln-O9 | 2.528(4) | 2.520(3) |
| Ln-O10 | 2.460(4) | 2.444(2) |
| C1-C6 | 1.436(7) | 1.437(5) |
| C6-C7 | 1.415(8) | 1.414(6) |
| C7-N1 | 1.296(7) | 1.304(5) |
| C1-O1 | 1.306(6) | 1.307(4) |
| C14-C19 | 1.435(7) | 1.431(5) |
| C19-C20 | 1.420(8) | 1.421(5) |
| C20-N2 | 1.298(7) | 1.295(5) |
| C14-O2 | 1.297(6) | 1.304(4) |
| C28 c-C33 c | 1.411(5) | 1.427(6) |
| C33 c-C34 c | 1.414(8) | 1.416(5) |
| C34 c-N6 c | 1.307(7) | 1.305(5) |
| C28 c-O13 c | 1.308(6) | 1.303(5) |
| Bond angles (°) | ||
| O1-Ln-O2 | 151.1(1) | 150.9(1) |
| O12 b-Ln-O1 | 87.0(1) | 87.2(1) |
| O12 b-Ln-O2 | 87.2(1) | 87.1(1) |
| O3-Ln-O4 | 51.4(1) | 51.6(1) |
| O3-Ln-O6 | 73.6(1) | 73.6(1) |
| O8-Ln-O2 | 72.0(1) | 71.9(1) |
| O10-Ln-O1 | 79.5(1) | 79.2(1) |
| Angles (°) | 17_Tb | 18_Dy |
|---|---|---|
| φ(A,B) | 1.1 b, 9.6 c, 2.4 d | 1.0 b, 9.8 c, 2.4 d |
| ψ(A,C) | 1.2 b, 2.6 c, 1.0 d | 0.9 b, 2.0 c, 1.1 d |
| ω(B,C) | 1.6 b, 11.4 c, 1.8 d | 0.9 b, 11.0 c, 1.7 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylonas-Margaritis, I.; Maniaki, D.; Mayans, J.; Ciammaruchi, L.; Bekiari, V.; P. Raptopoulou, C.; Psycharis, V.; Christodoulou, S.; Escuer, A.; P. Perlepes, S. Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies. Magnetochemistry 2018, 4, 45. https://doi.org/10.3390/magnetochemistry4040045
Mylonas-Margaritis I, Maniaki D, Mayans J, Ciammaruchi L, Bekiari V, P. Raptopoulou C, Psycharis V, Christodoulou S, Escuer A, P. Perlepes S. Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies. Magnetochemistry. 2018; 4(4):45. https://doi.org/10.3390/magnetochemistry4040045
Chicago/Turabian StyleMylonas-Margaritis, Ioannis, Diamantoula Maniaki, Julia Mayans, Laura Ciammaruchi, Vlasoula Bekiari, Catherine P. Raptopoulou, Vassilis Psycharis, Sotirios Christodoulou, Albert Escuer, and Spyros P. Perlepes. 2018. "Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies" Magnetochemistry 4, no. 4: 45. https://doi.org/10.3390/magnetochemistry4040045
APA StyleMylonas-Margaritis, I., Maniaki, D., Mayans, J., Ciammaruchi, L., Bekiari, V., P. Raptopoulou, C., Psycharis, V., Christodoulou, S., Escuer, A., & P. Perlepes, S. (2018). Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies. Magnetochemistry, 4(4), 45. https://doi.org/10.3390/magnetochemistry4040045