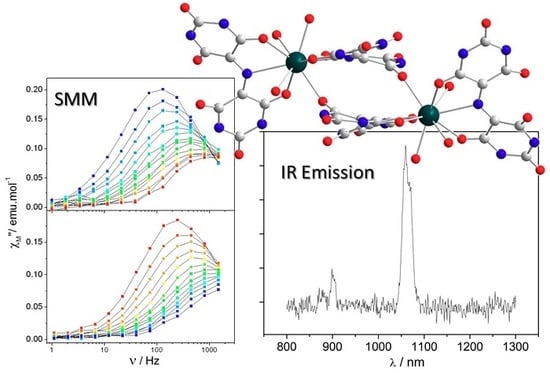
Closing the Circle of the Lanthanide-Murexide Series: Single-Molecule Magnet Behavior and Near-Infrared Emission of the NdIII Derivative
Abstract
:1. Introduction
2. Results
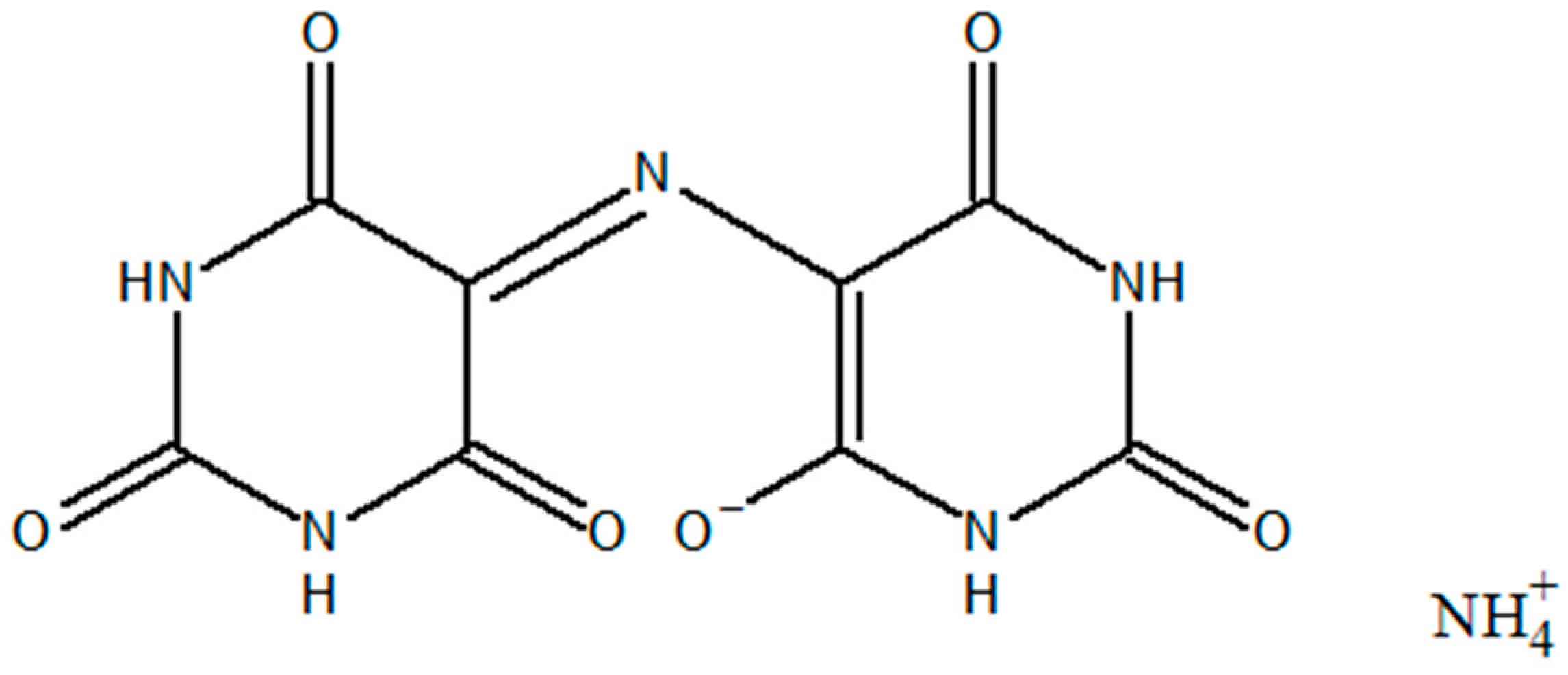
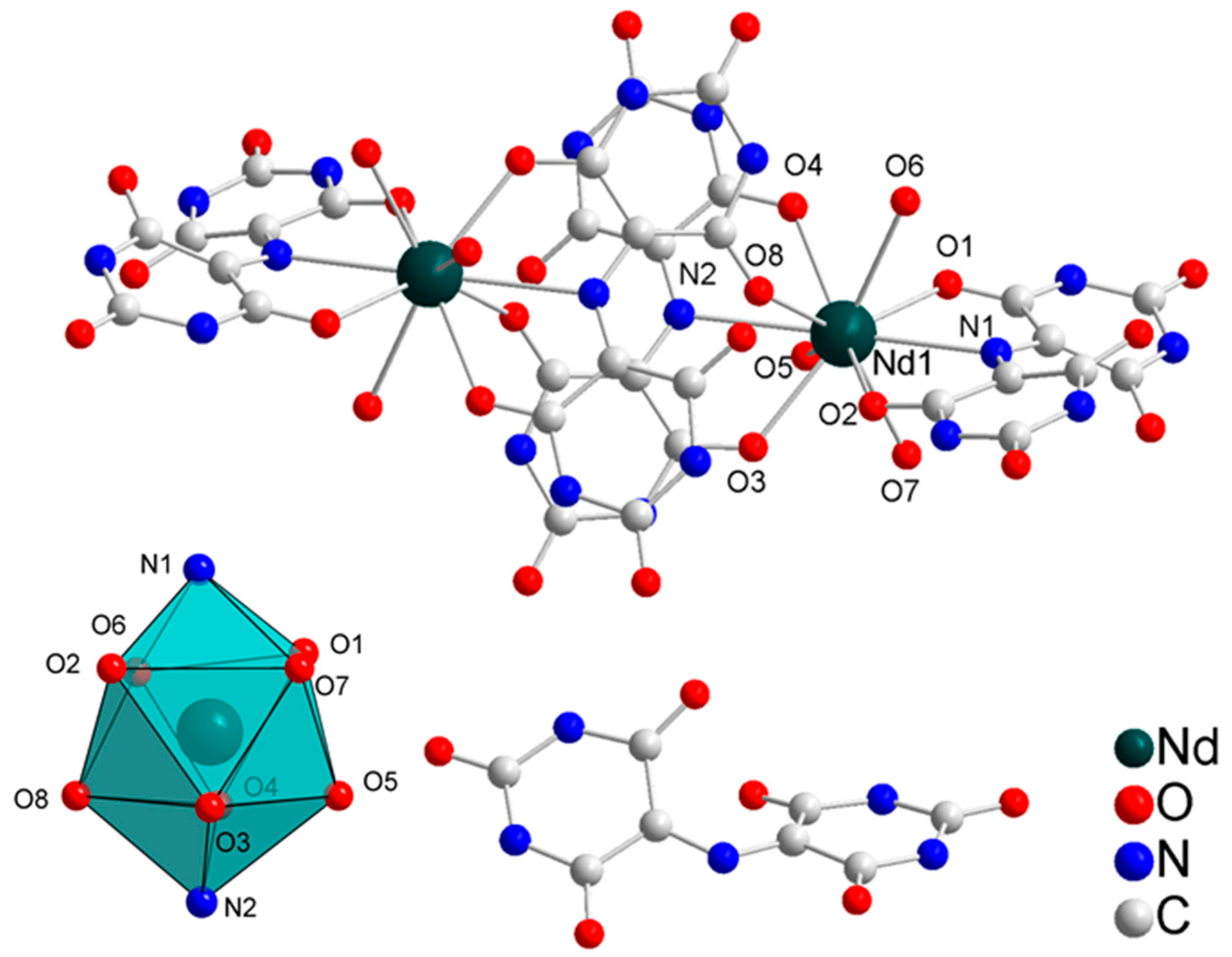
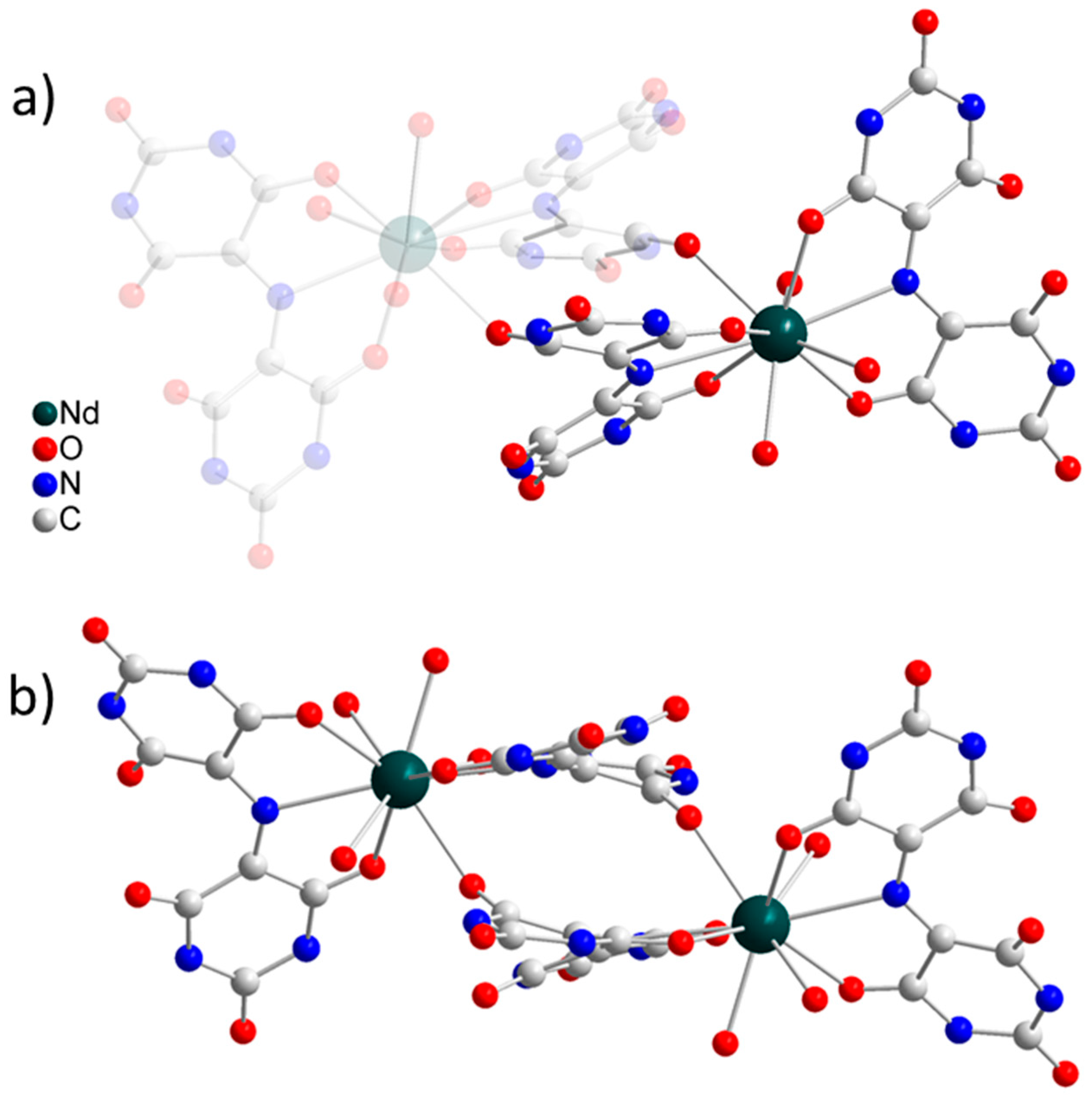
2.1. Crystal Structure
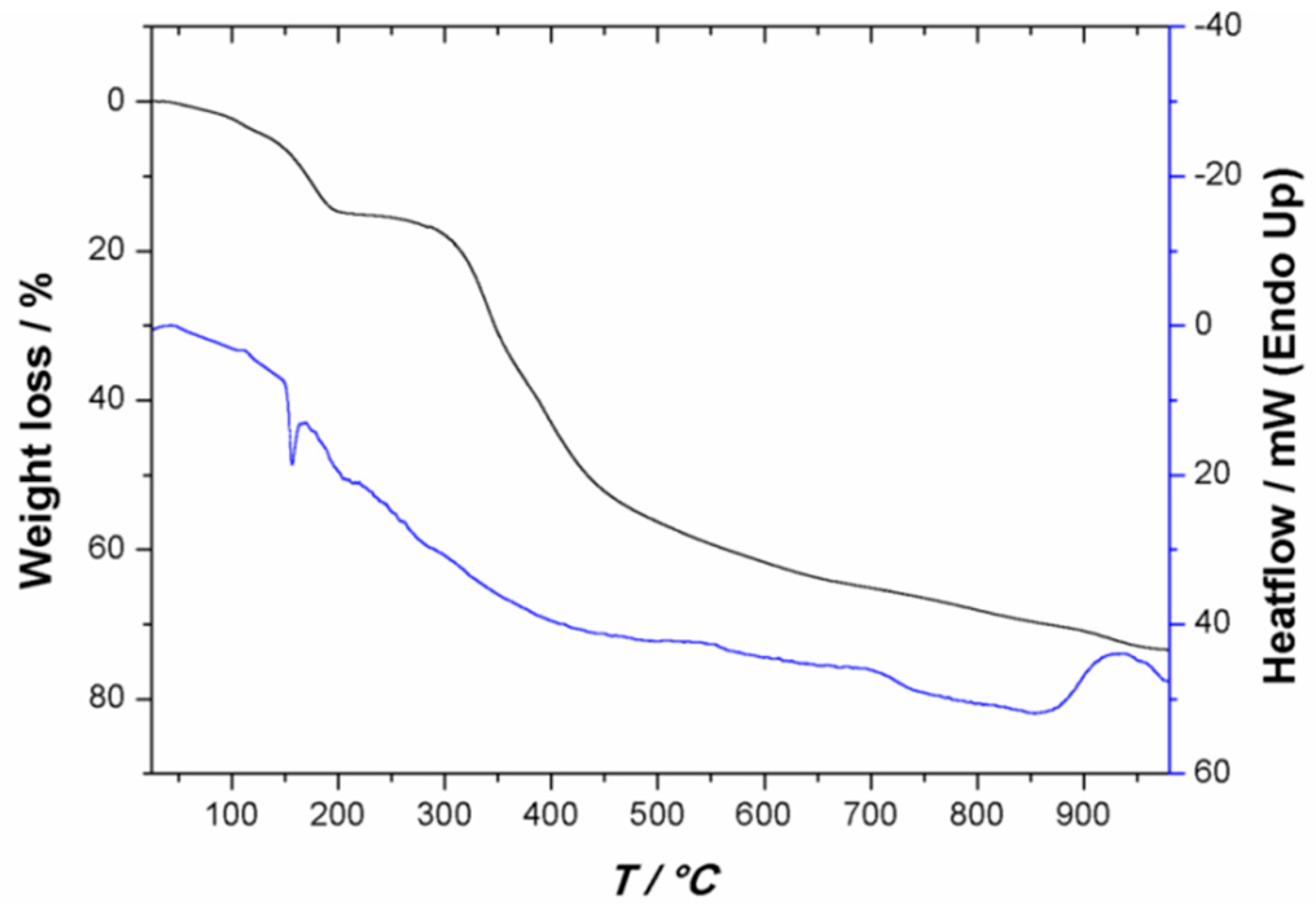
2.2. Thermal Stability
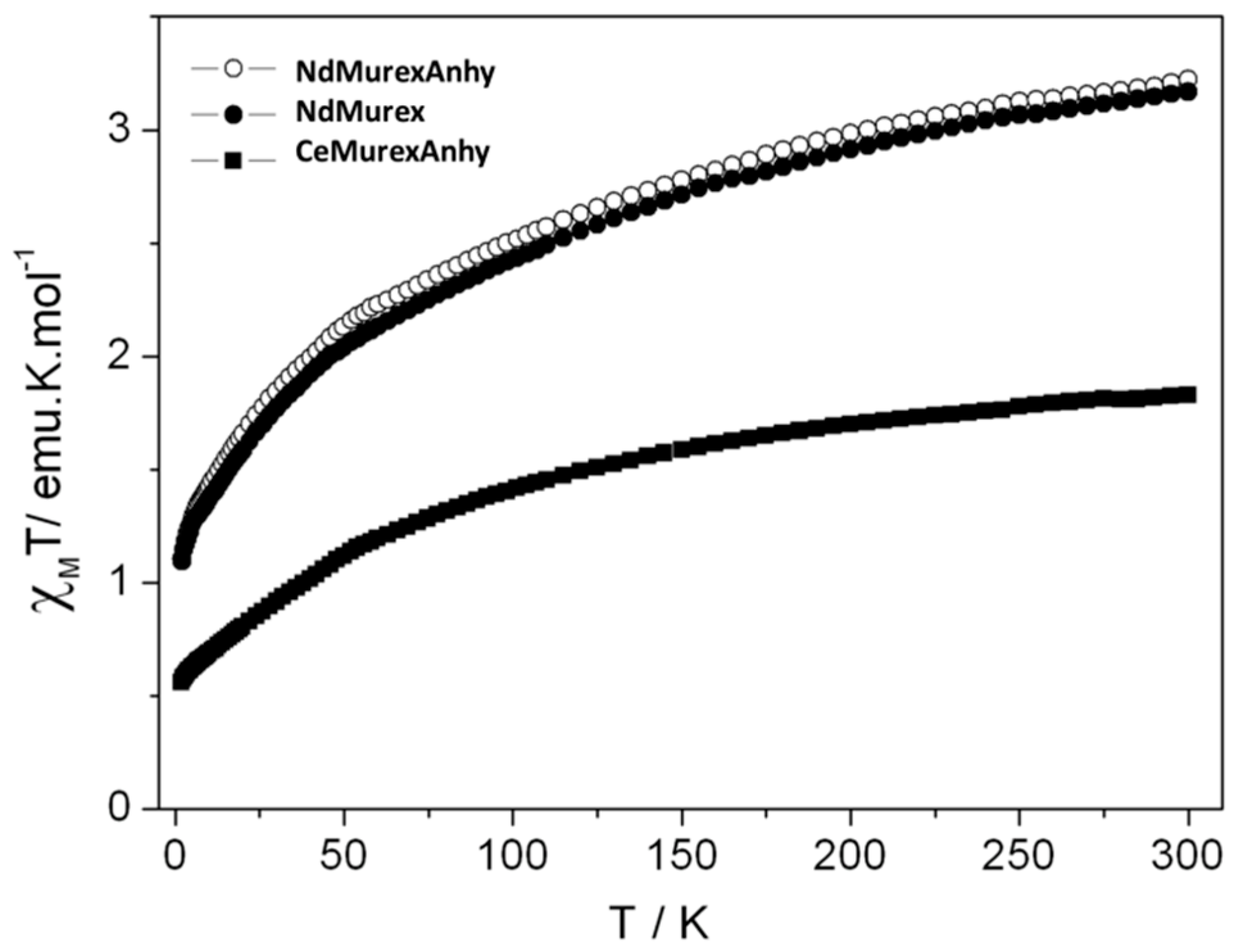
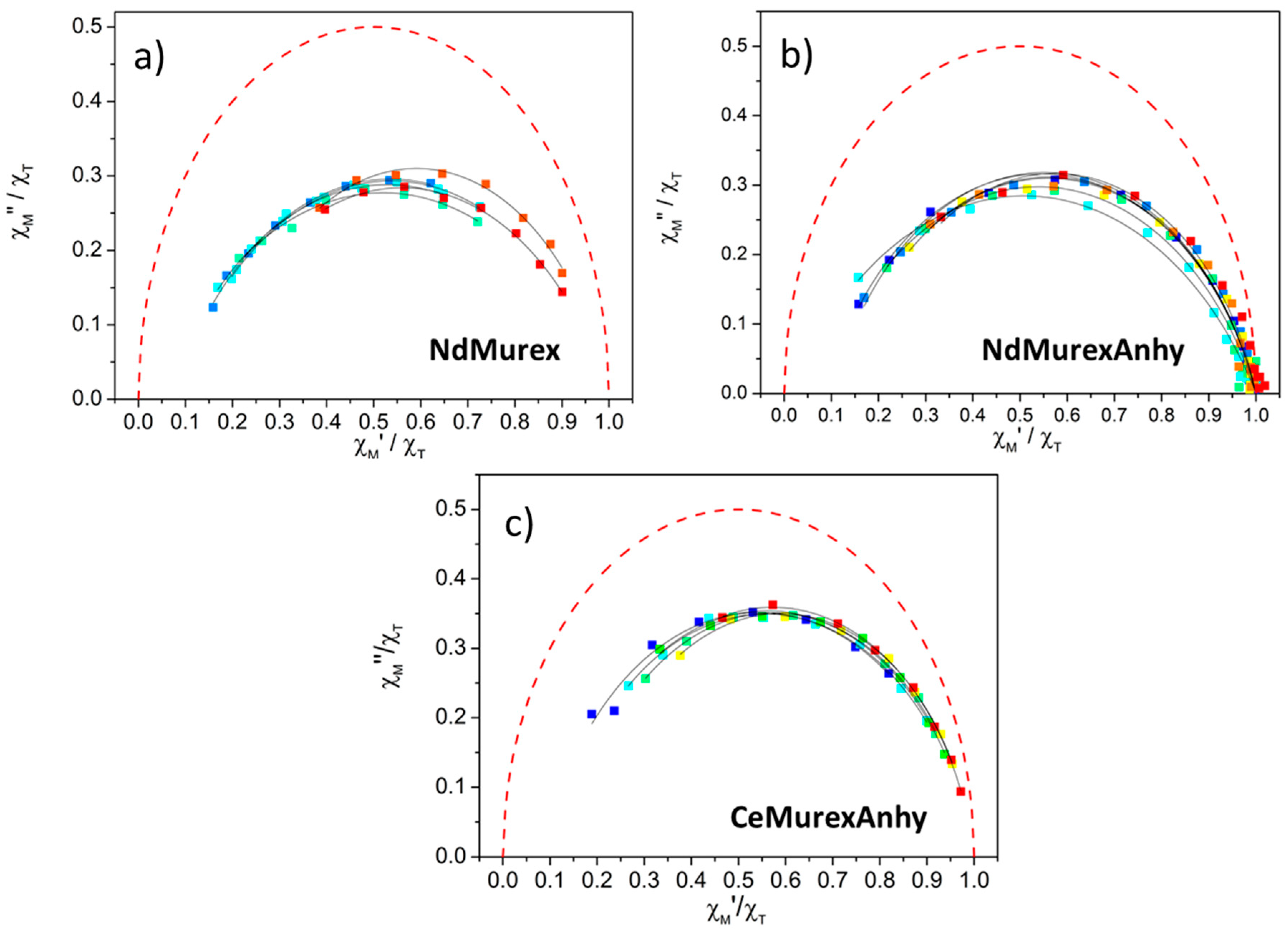
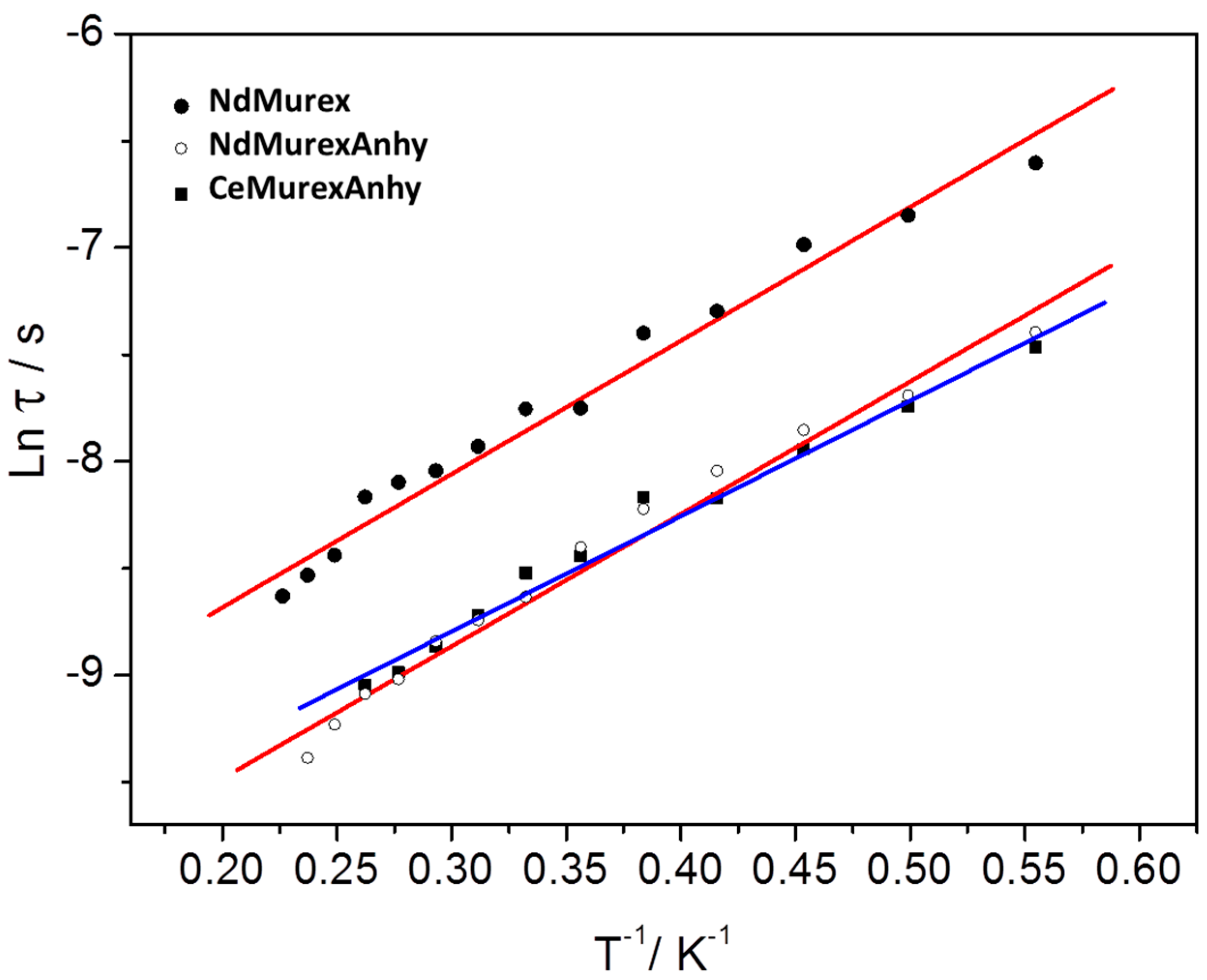
2.3. Magnetic Characterization
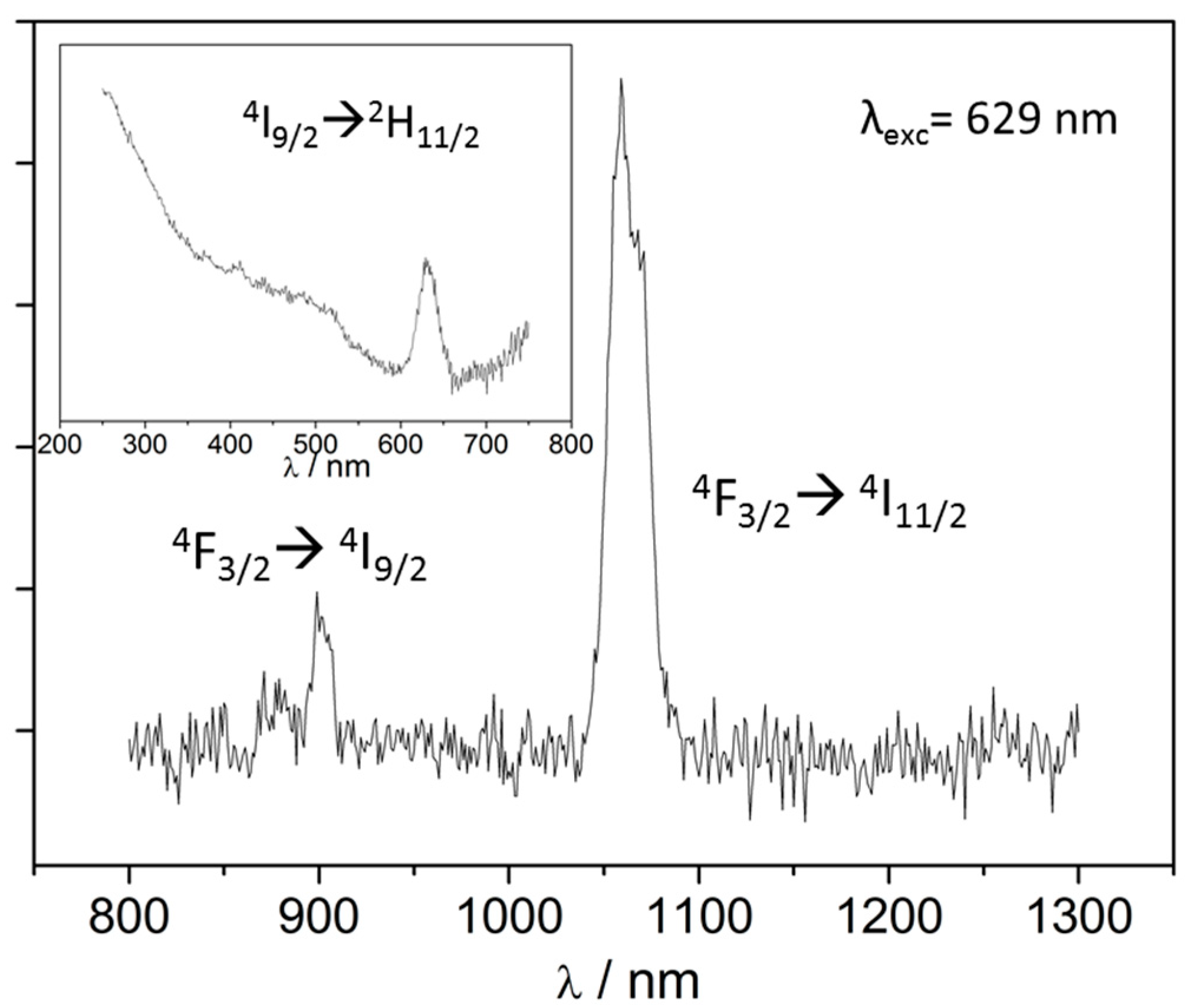
2.4. Luminescence Measurements
3. Materials and Methods
3.1. General Procedures and Methods
3.2. Synthetic Procedure
3.3. Crystal Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atwood, D. The Rare Earth Elements: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Clérac, R.; Winpenny, R.E.P. 50 Years of Structure and Bonding—The Anniversary Volume; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; p. 35. [Google Scholar]
- Prša, K.; Nehrkorn, J.; Corbey, J.; Evans, W.; Demir, S.; Long, J.; Guidi, T.; Waldmann, O. Perspectives on Neutron Scattering in Lanthanide-Based Single-Molecule Magnets and a Case Study of the Tb2(μ-N2) System. Magnetochemistry 2016, 2, 45. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bunzli, J.C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G. Lanthanide Luminescent Bioprobes (LLBs). Chem. Lett. 2009, 38, 104–109. [Google Scholar] [CrossRef]
- Kido, J.; Okamoto, Y. Organo Lanthanide Metal Complexes for Electroluminescent Materials. Chem. Rev. 2002, 102, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, G.; Flores Gonzalez, J.; Ou-Yang, J.-K.; Saleh, N.; Pointillart, F.; Cador, O.; Guizouarn, T.; Totti, F.; Ouahab, L.; Crassous, J.; et al. Slow Magnetic Relaxation in Chiral Helicene-Based Coordination Complex of Dysprosium. Magnetochemistry 2017, 3, 2. [Google Scholar] [CrossRef]
- Wada, H.; Ooka, S.; Iwasawa, D.; Hasegawa, M.; Kajiwara, T. Slow Magnetic Relaxation of Lanthanide(III) Complexes with a Helical Ligand. Magnetochemistry 2016, 2, 43. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283. [Google Scholar] [CrossRef] [PubMed]
- Mamontova, E.; Long, J.; Ferreira, R.; Botas, A.; Luneau, D.; Guari, Y.; Carlos, L.; Larionova, J. Magneto-Luminescence Correlation in the Textbook Dysprosium(III) Nitrate Single-Ion Magnet. Magnetochemistry 2016, 2, 41. [Google Scholar] [CrossRef]
- Farger, P.; Leuvrey, C.; Gallart, M.; Gilliot, P.; Rogez, G.; Rabu, P.; Delahaye, E. Elaboration of Luminescent and Magnetic Hybrid Networks Based on Lanthanide Ions and Imidazolium Dicarboxylate Salts: Influence of the Synthesis Conditions. Magnetochemistry 2017, 3, 1. [Google Scholar] [CrossRef]
- Davidson, D. Murexide and leucomurexide. J. Am. Chem. Soc. 1936, 58, 1821–1822. [Google Scholar] [CrossRef]
- Davidson, D.; Epstein, E. The murexide question. J. Org. Chem. 1936, 1, 305–314. [Google Scholar] [CrossRef]
- Kashanian, S.; Gholivand, M.B.; Madaeni, S.; Nikrahi, A.; Shamsipur, M. Spectrophotometric study of the complexation reactions between alkaline-earth cations and murexide in some non-aqueous solutions. Polyhedron 1988, 7, 1227–1230. [Google Scholar] [CrossRef]
- Shamsipur, M.; Esmaeili, A.; Amini, M.K. Formation of cobalt, nickel and copper-complexes with murexide in ethanol water mixtures. Talanta 1989, 36, 1300–1302. [Google Scholar] [CrossRef]
- Shamsipur, M.; Madaeni, S.; Kashanian, S. Spectrophotometric study of the alkali-metal murexide complexes in some non-aqueous solutions. Talanta 1989, 36, 773–776. [Google Scholar] [CrossRef]
- Parham, H.S.; Shamsipur, M. Spectrophotometric study of some alkali and alkaline-earth cryptates in dimethylformamide solution using murexide as a metallochromic indicator. Polyhedron 1992, 11, 987–991. [Google Scholar] [CrossRef]
- Favas, M.C.; Kepert, D.L.; White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 3. Crystal-structures of triaquapurpuratoiron(II) purpurate hexahydrate and tetra-aquapurpuratomanganese(II) purpurate hexahydrate—Stereochemistry of the [M(tridentate ligand)(unidentate ligand)3] complexes. J. Chem. Soc. Dalton Trans. 1977, 1350–1362. [Google Scholar] [CrossRef]
- Kepert, D.L.; White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 2. Crystal-structures of diaquabis (purpurato)-calcium and diaquabis(purpurato)-cadmium dihydrate—Stereochemistry of the [M(tridentate ligand)2(unidentate ligand)2] complexes. J. Chem. Soc. Dalton Trans. 1977, 1342–1349. [Google Scholar] [CrossRef]
- Martin, R.L.; White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 1. Crystal-structures of potassium purpurate trihydrate and ammonium purpurate monohydrate (murexide). J. Chem. Soc. Dalton Trans. 1977, 1336–1342. [Google Scholar] [CrossRef]
- Raston, C.L.; White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 5. Crystal-structure of di-mu-aqua-tetrakis(purpurato)distrontium tridecahydrate. J. Chem. Soc. Dalton Trans. 1977, 1368–1372. [Google Scholar] [CrossRef]
- Raston, C.L.; White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 8. Crystal-structure of triaquapurpuratocalcium nitrate dihydrate. J. Chem. Soc. Dalton Trans. 1977, 1381–1384. [Google Scholar] [CrossRef]
- White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 4. Crystal-structures of aquabis(purpurato)lead(II) and aquabis(purpurato)lead(II) trihydrate. J. Chem. Soc. Dalton Trans. 1977, 1362–1368. [Google Scholar] [CrossRef]
- White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 6. Crystal-structures of bis(purpurato)-copper(II) and bis(purpurato)-zinc(II) hydrates. J. Chem. Soc. Dalton Trans. 1977, 1372–1377. [Google Scholar] [CrossRef]
- White, A.H.; Willis, A.C. Structural studies in metal-purpurate complexes. 7. Crystal-structures of diaquanitratopurpurato-cobalt(II) and diaquanitratopurpurato-zinc(II) dihydrate. J. Chem. Soc. Dalton Trans. 1977, 1377–1381. [Google Scholar] [CrossRef]
- Jung, J.; Yi, X.; Huang, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Cador, O.; Caneschi, A.; Roisnel, T.; Le Guennic, B.; et al. Analysis of the electrostatics in DyIII single-molecule magnet: The case study of Dy(Murex)3. Dalton Trans. 2015, 44, 18270–18275. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Bernot, K.; Le Corre, V.; Calvez, G.; Pointillart, F.; Cador, O.; Le Guennic, B.; Jung, J.; Maury, O.; Placide, V.; et al. Unraveling the Crystal Structure of Lanthanide–Murexide Complexes: Use of an Ancient Complexometry Indicator as a Near-Infrared-Emitting Single-Ion Magnet. Chem. Eur. J. 2014, 20, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Cador, O.; Le Guennic, B.; Ouahab, L. Uncommon lanthanide ions in purely 4f Single Molecule Magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Jürgen Buschow, K.H. Materials Science and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 2209–2233. [Google Scholar] [CrossRef] [PubMed]
- Mylonas-Margaritis, I.; Mayans, J.; Sakellakou, S.-M.; Raptopoulou, C.P.; Psycharis, V.; Escuer, A.; Perlepes, S.P. Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies. Magnetochemistry 2017, 3, 5. [Google Scholar] [CrossRef]
- Xu, M.-X.; Meng, Y.-S.; Xiong, J.; Wang, B.-W.; Jiang, S.-D.; Gao, S. Magnetic anisotropy investigation on light lanthanide complexes. Dalton Trans. 2018, 47, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Jassal, A.K.; Sran, B.S.; Suffren, Y.; Bernot, K.; Pointillart, F.; Cador, O.; Hundal, G. Structural diversity and photo-physical and magnetic properties of dimeric to 1D polymeric coordination polymers of lighter lanthanide(III) dinitrobenzoates. Dalton Trans. 2018, 47, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Ooka, S.; Yamamura, T.; Kajiwara, T. Light Lanthanide Complexes with Crown Ether and Its Aza Derivative Which Show Slow Magnetic Relaxation Behaviors. Inorg. Chem. 2017, 56, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, A.B.; Belkhiria, M.S.; Huang, G.; Freslon, S.; Guillou, O.; Bernot, K. Single-molecule magnet behaviour in polynuclear assembly of trivalent cerium ions with polyoxomolybdates. Dalton Trans. 2015, 44, 16458–16464. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, J.J.; Gorelsky, S.I.; Korobkov, I.; Murugesu, M. Slow Magnetic Relaxation in Uranium(III) and Neodymium(III) Cyclooctatetraenyl Complexes. Organometallics 2015, 34, 1415–1418. [Google Scholar] [CrossRef]
- Li, Q.-W.; Wan, R.-C.; Chen, Y.-C.; Liu, J.-L.; Wang, L.-F.; Jia, J.-H.; Chilton, N.F.; Tong, M.-L. Unprecedented hexagonal bipyramidal single-ion magnets based on metallacrowns. Chem. Commun. 2016, 52, 13365–13368. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Slow magnetic relaxation in homoleptic trispyrazolylborate complexes of neodymium(III) and uranium(III). Dalton Trans. 2012, 41, 13572–13574. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Meihaus, K.R.; Long, J.R. Slow magnetic relaxation in a neodymium metallocene tetraphenylborate complex. J. Organomet. Chem. 2018, 857, 164–169. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Clemente-Juan, J.M.; Coronado, E.; Duan, Y.; Gaita-Ariño, A.; Giménez-Saiz, C. Construction of a General Library for the Rational Design of Nanomagnets and Spin Qubits Based on Mononuclear f-Block Complexes. The Polyoxometalate Case. Inorg. Chem. 2014, 53, 9976–9980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Wu, X.-Y.; Liao, J.-Z.; Kuang, X.-F.; Yang, W.; Lu, C.-Z. A novel trigonal propeller-shaped hybrid tri-neodymium-polyoxometalate exhibiting single-molecule magnet behavior. Dalton Trans. 2018, 47, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Berta, C.; Mercè, F.-B.; Saskia, S.; Salah, E.F.M.; Ramon, V. Field-Induced SMM and Visible/NIR-Luminescence Behaviour of Dinuclear LnIII Complexes with 2-Fluorobenzoate. Eur. J. Inorg. Chem. 2018, 2018, 1928–1937. [Google Scholar]
- Casanovas, B.; Speed, S.; Maury, O.; El Fallah, M.S.; Font-Bardía, M.; Vicente, R. Dinuclear LnIII Complexes with 9-Anthracenecarboxylate Showing Field-Induced SMM and Visible/NIR Luminescence. Eur. J. Inorg. Chem. 2018, 34, 3859–3867. [Google Scholar] [CrossRef]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolome, E.; Cases, R.; Luzon, J.; Bartolome, J.; Turta, C. Structural and magnetic properties of some lanthanide (Ln = Eu(III), Gd(III) and Nd(III)) cyanoacetate polymers: Field-induced slow magnetic relaxation in the Gd and Nd substitutions. Dalton Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef] [PubMed]
- Jassal, A.K.; Aliaga-Alcalde, N.; Corbella, M.; Aravena, D.; Ruiz, E.; Hundal, G. Neodymium 1D systems: Targeting new sources for field-induced slow magnetization relaxation. Dalton Trans. 2015, 44, 15774–15778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Huang, X.-S.; Liu, J.-L.; Tong, M.-L. Magnetic Dynamics of a Neodymium(III) Single-Ion Magnet. Inorg. Chem. 2018, 57, 11782–11787. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R. An unprecedented zero field neodymium(III) single-ion magnet based on a phosphonic diamide. Chem. Commun. 2016, 52, 7168–7171. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.K.; Kalita, P.; Singh, M.K.; Rajaraman, G.; Chandrasekhar, V. Low-coordinate mononuclear lanthanide complexes as molecular nanomagnets. Coord. Chem. Rev. 2018, 367, 163–216. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Yi, X.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A Luminescent and Sublimable Dy-III-Based Single-Molecule Magnet. Chem.-Eur. J. 2012, 18, 11379–11387. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–70. [Google Scholar] [CrossRef]
- Boulon, M.E.; Cucinotta, G.; Luzon, J.; Degl’Innocenti, C.; Perfetti, M.; Bernot, K.; Calvez, G.; Caneschi, A.; Sessoli, R. Magnetic Anisotropy and Spin-Parity Effect Along the Series of Lanthanide Complexes with DOTA. Angew. Chem. Int. Edit. 2013, 52, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, G.; Perfetti, M.; Luzon, J.; Etienne, M.; Car, P.-E.; Caneschi, A.; Calvez, G.; Bernot, K.; Sessoli, R. Magnetic Anisotropy in a Dysprosium/DOTA Single-Molecule Magnet: Beyond Simple Magneto-Structural Correlations. Angew. Chem. Int. Ed. 2012, 51, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guegan, F.; Riobe, F.; Maury, O.; Jung, J.; Le Guennic, B.; Morell, C.; Luneau, D. Teaching an old molecule new tricks: Evidence and rationalisation of the slow magnetisation dynamics in [DyTp2Acac]. Inorg. Chem. Front. 2018, 5, 1346–1353. [Google Scholar] [CrossRef]
- Errulat, D.; Gabidullin, B.; Hemmer, E.; Murugesu, M. Probing Optical Anisotropy and Polymorph-Dependent Photoluminescence in [Ln2] Complexes via Hyperspectral Imaging on Single Crystals. Chem. Eur. J. 2018, 24, 10146–10155. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.; Moro, F.; Dorfel, M.; Ungur, L.; Waters, M.; Jiang, S.D.; Orlita, M.; Taylor, J.; Frey, W.; Chibotaru, L.F.; et al. Spectroscopic determination of crystal field splittings in lanthanide double deckers. Chem. Sci. 2014, 5, 3287–3293. [Google Scholar] [CrossRef]
- Gorczynski, A.; Marcinkowski, D.; Kubicki, M.; Loffler, M.; Korabik, M.; Karbowiak, M.; Wisniewski, P.; Rudowicz, C.; Patroniak, V. New field-induced single ion magnets based on prolate Er(III) and Yb(III) ions: Tuning the energy barrier Ueff by the choice of counterions within an N3-tridentate Schiff-base scaffold. Inorg. Chem. Front. 2018, 5, 605–618. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Nakamura, T.; Murakami, R.; Ishida, T. Spectroscopic and magnetic studies of erbium(III)-TEMPO complex as a potential single-molecule magnet: Interplay of the crystal-field and exchange coupling effects. Chem. Phys. Lett. 2016, 662, 163–168. [Google Scholar] [CrossRef]
- Ren, M.; Bao, S.-S.; Ferreira, R.A.S.; Zheng, L.-M.; Carlos, L.D. A layered erbium phosphonate in pseudo-D5h symmetry exhibiting field-tunable magnetic relaxation and optical correlation. Chem. Commun. 2014, 50, 7621–7624. [Google Scholar] [CrossRef] [PubMed]
- Palacios, M.A.; Titos-Padilla, S.; Ruiz, J.; Herrera, J.M.; Pope, S.J.A.; Brechin, E.K.; Colacio, E. Bifunctional ZnIILnIII Dinuclear Complexes Combining Field Induced SMM Behavior and Luminescence: Enhanced NIR Lanthanide Emission by 9-Anthracene Carboxylate Bridging Ligands. Inorg. Chem. 2014, 53, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Díaz-Ortega, I.F.; Ruiz, E.; Aravena, D.; Pope, S.J.A.; Colacio, E.; Herrera, J.M. Lanthanide Tetrazolate Complexes Combining Single-Molecule Magnet and Luminescence Properties: The Effect of the Replacement of Tetrazolate N3 by β-Diketonate Ligands on the Anisotropy Energy Barrier. Chem. Eur. J. 2016, 22, 14548–14559. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ramos, P.; Coutinho, J.T.; Ramos Silva, M.; Pereira, L.C.J.; Lahoz, F.; da Silva, P.S.P.; Lavin, V.; Martin-Gil, J. Slow magnetic relaxation and photoluminescent properties of a highly coordinated erbium(III) complex with dibenzoylmethane and 2,2’-bipyridine. New J. Chem. 2015, 39, 1703–1713. [Google Scholar] [CrossRef]
- Kishi, Y.; Cornet, L.; Pointillart, F.; Riobé, F.; Lefeuvre, B.; Cador, O.; Guennic, B.L.; Maury, O.; Fujiwara, H.; Ouahab, L. Luminescence and Single-Molecule-Magnet Behaviour in Lanthanide Coordination Complexes Involving Benzothiazole-Based Tetrathiafulvalene Ligands. Eur. J. Inorg. Chem. 2018, 2018, 458–468. [Google Scholar] [CrossRef]
- Soussi, K.; Jung, J.; Pointillart, F.; Le Guennic, B.; Lefeuvre, B.; Golhen, S.; Cador, O.; Guyot, Y.; Maury, O.; Ouahab, L. Magnetic and photo-physical investigations into DyIII and YbIII complexes involving tetrathiafulvalene ligand. Inorg. Chem. Front. 2015, 2, 1105–1117. [Google Scholar] [CrossRef]
- Jung, J.; da Cunha, T.T.; Le Guennic, B.; Pointillart, F.; Pereira, C.L.M.; Luzon, J.; Golhen, S.; Cador, O.; Maury, O.; Ouahab, L. Magnetic Studies of Redox-Active Tetrathiafulvalene-Based Complexes: Dysprosium vs. Ytterbium Analogues. Eur. J. Inorg. Chem. 2014, 2014, 3888–3894. [Google Scholar] [CrossRef] [Green Version]
- Cosquer, G.; Pointillart, F.; Jung, J.; Le Guennic, B.; Golhen, S.; Cador, O.; Guyot, Y.; Brenier, A.; Maury, O.; Ouahab, L. Alkylation Effects in Lanthanide Complexes Involving Tetrathiafulvalene Chromophores: Experimental and Theoretical Correlation between Magnetism and Near-Infrared Emission. Eur. J. Inorg. Chem. 2014, 2014, 69–82. [Google Scholar] [CrossRef]
- Pointillart, F.; Le Guennic, B.; Golhen, S.; Cador, O.; Maury, O.; Ouahab, L. A redox-active luminescent ytterbium based single molecule magnet. Chem. Commun. 2013, 49, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-W.; Liu, J.-L.; Jia, J.-H.; Chen, Y.-C.; Liu, J.; Wang, L.-F.; Tong, M.-L. “Half-sandwich” YbIII single-ion magnets with metallacrowns. Chem. Commun. 2015, 51, 10291–10294. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Lorusso, G.; Evangelisti, M.; Brechin, E.K.; Pope, S.J.A.; Colacio, E. Closely-Related ZnII2LnIII2 Complexes (LnIII = Gd, Yb) with Either Magnetic Refrigerant or Luminescent Single-Molecule Magnet Properties. Inorg. Chem. 2014, 53, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-W.; Liu, J.-L.; Jia, J.-H.; Leng, J.-D.; Lin, W.-Q.; Chen, Y.-C.; Tong, M.-L. Fluorescent single-ion magnets: Molecular hybrid (HNEt3)[DyxYb1-x(bpyda)2] (x = 0.135–1). Dalton Trans. 2013, 42, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]

| Compound | Hdc | Ueff (cm−1) | Reference |
|---|---|---|---|
| NdTp3 | 100 | 2.8 | [41] |
| Li(DME)3][Nd(COT″)2] | 1000 | 15 | [39] |
| [Nd(NO3)3(18-crown-6)] | 1000 | 21 | [37] |
| [Nd(NO3)3(1,10-diaza-18-crown-6)] | 1000 | 51 | [37] |
| [NdCd3(Hquinha)3(n-Bu3PO)2I3]·3EtOH·2H2O | 1000 | 15 | [40] |
| [Nd(W5O18)2]9− | 1000 | 51 | [43] |
| Nd2(2-FBz)4−(NO3)2(phen)2 | 1500 | 9.5 | [45] |
| Nd(fdh)3(bpy) | 500 | 20 | [35] |
| {[Nd(μ2-L1)3·(H2O)2]·H2O}n | 2000 | 19.5 | [36] |
| [Nd(μ2-L2)2·(CH3COO)·(H2O)2]n | 2000 | 14 | [36] |
| Nd2(μ2–9-AC)4(9-AC)2(bpy)2 | 2000 | 8 | [46] |
| {[Nd2(CNCH2COO)6(H2O)4]·2H2O}n | 1500 | 19 | [47] |
| [Nd(μ2-L1)3(H2O)2]·C2H3N}n | 2000 | 19 | [48] |
| [Nd(μ2-L2)(L2) (CH3COO)(H2O)2]n | 3500 | 15 | [48] |
| (NH2Me2)3{[Nd(Mo4O13)(DMF)4]3(BTC)2}·8DMF Cp*2Nd(BPh4) | 500 | 34 | [44] |
| 1000 | 29 | [42] | |
| [Nd(CyPh2PO)2(H2O)5]I3·2(CyPh2PO)·3EtOH | 0 | n.a. * | [49] |
| {[tBuPO(NHiPr)2Nd(H2O)5]-[I]3·tBuPO(NHiPr)2·(H2O)} | 0 | 11/17 | [50] |
| - | 1000 | 27 | [50] |
| - | 2000 | n.a. ** | [49] |
| NdMurex | 1200 | 4.3 | This study |
| NdMurexAnhy | 1200 | 4.3 | This study |
| CeMurexAnhy | 400 | 3.75 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Calvez, G.; Suffren, Y.; Daiguebonne, C.; Freslon, S.; Guillou, O.; Bernot, K. Closing the Circle of the Lanthanide-Murexide Series: Single-Molecule Magnet Behavior and Near-Infrared Emission of the NdIII Derivative. Magnetochemistry 2018, 4, 44. https://doi.org/10.3390/magnetochemistry4040044
Huang G, Calvez G, Suffren Y, Daiguebonne C, Freslon S, Guillou O, Bernot K. Closing the Circle of the Lanthanide-Murexide Series: Single-Molecule Magnet Behavior and Near-Infrared Emission of the NdIII Derivative. Magnetochemistry. 2018; 4(4):44. https://doi.org/10.3390/magnetochemistry4040044
Chicago/Turabian StyleHuang, Gang, Guillaume Calvez, Yan Suffren, Carole Daiguebonne, Stéphane Freslon, Olivier Guillou, and Kevin Bernot. 2018. "Closing the Circle of the Lanthanide-Murexide Series: Single-Molecule Magnet Behavior and Near-Infrared Emission of the NdIII Derivative" Magnetochemistry 4, no. 4: 44. https://doi.org/10.3390/magnetochemistry4040044
APA StyleHuang, G., Calvez, G., Suffren, Y., Daiguebonne, C., Freslon, S., Guillou, O., & Bernot, K. (2018). Closing the Circle of the Lanthanide-Murexide Series: Single-Molecule Magnet Behavior and Near-Infrared Emission of the NdIII Derivative. Magnetochemistry, 4(4), 44. https://doi.org/10.3390/magnetochemistry4040044