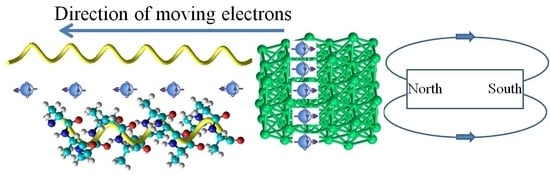
Chiral Magneto-Electrochemistry
Abstract
1. Introduction
2. Magnetic Effect on the Electrolyte Solution
3. Electrodeposition of Chiral Structures Induced by a Magnetic Field
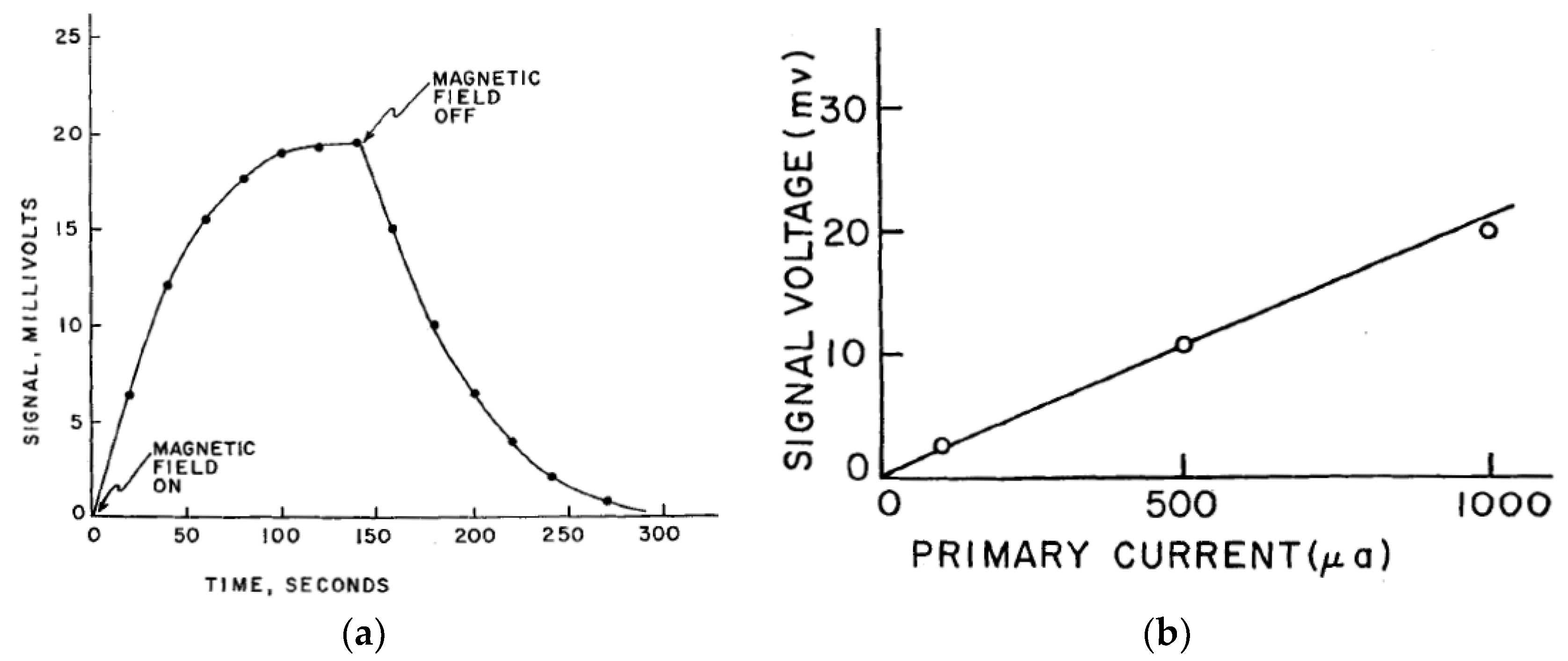
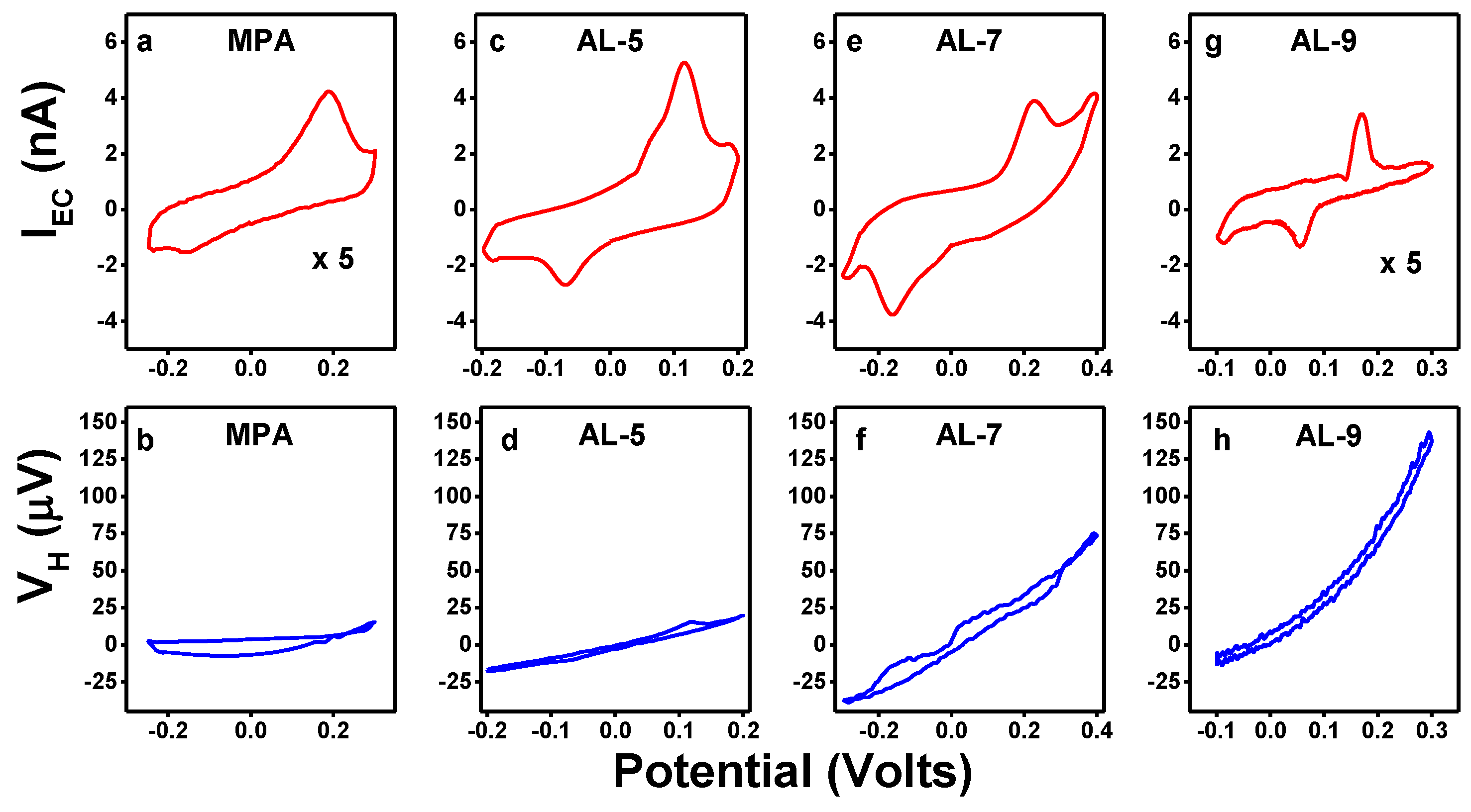
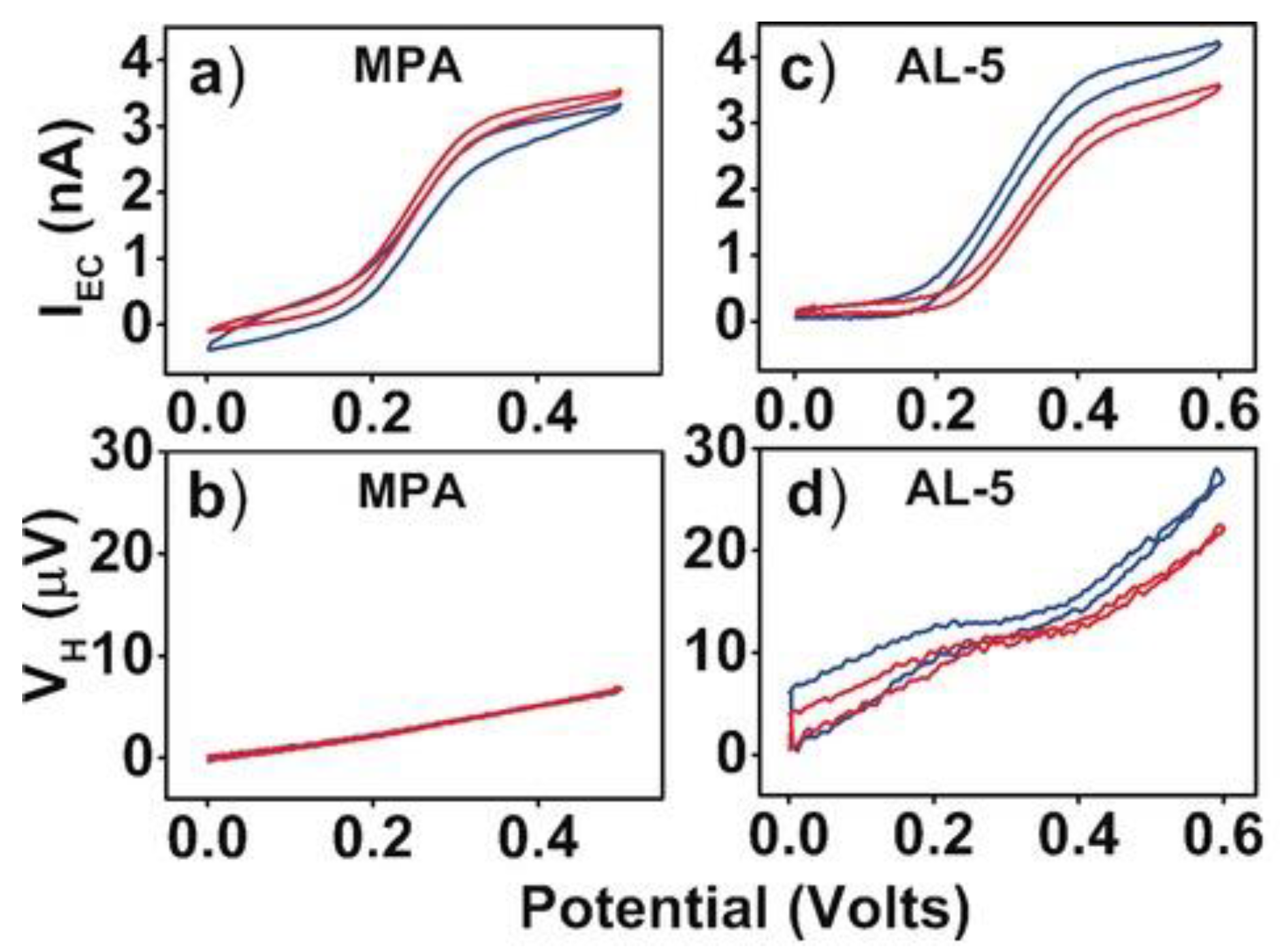
4. Hall Effect in Bulk Electrolyte
5. Induced Magnetic Field via Chiral Molecules
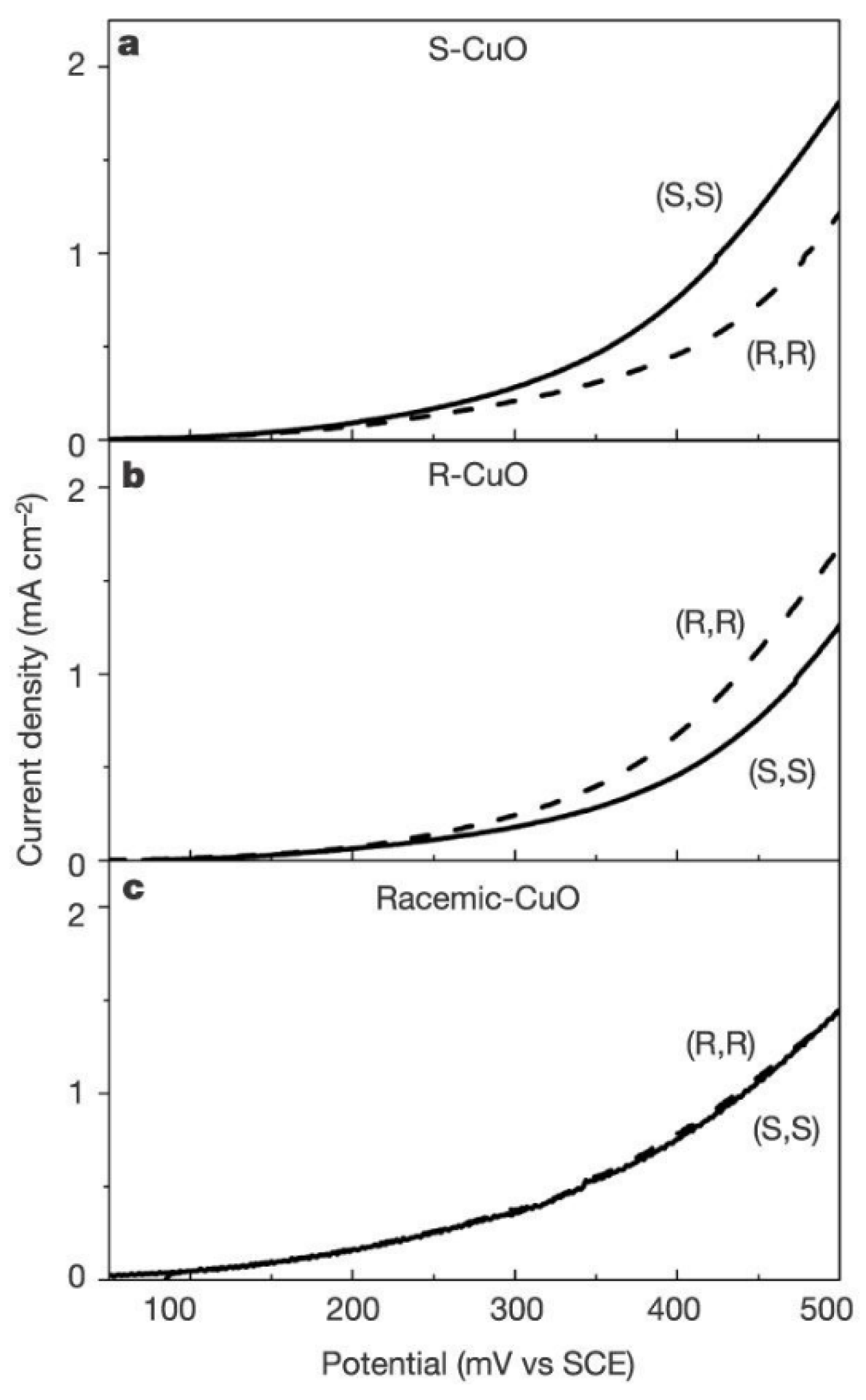
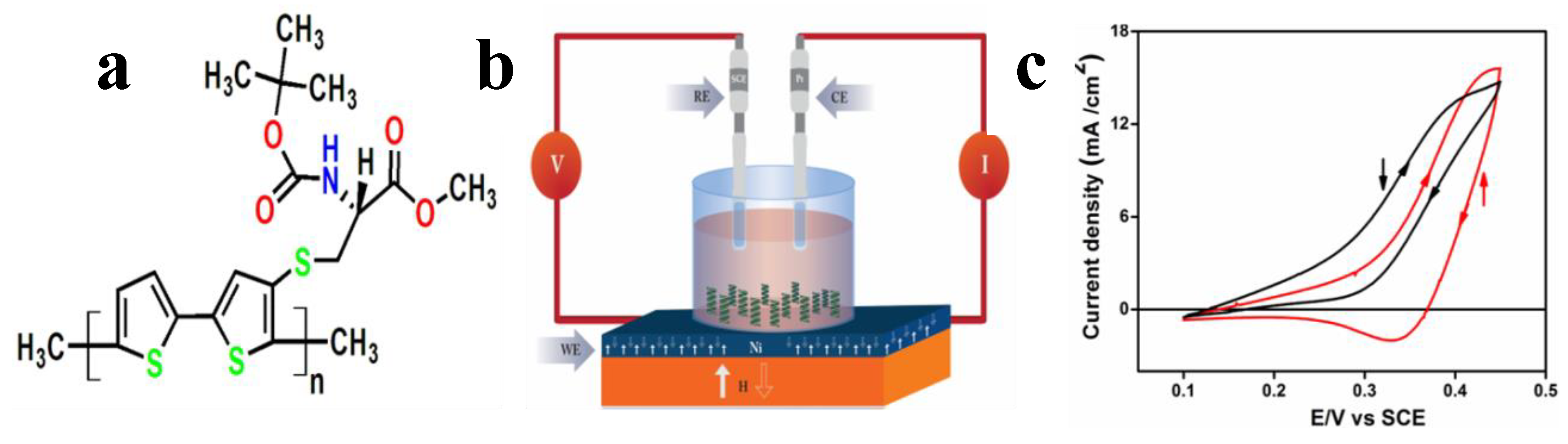
5.1. 3D Electrochemistry Using Chiral Molecules
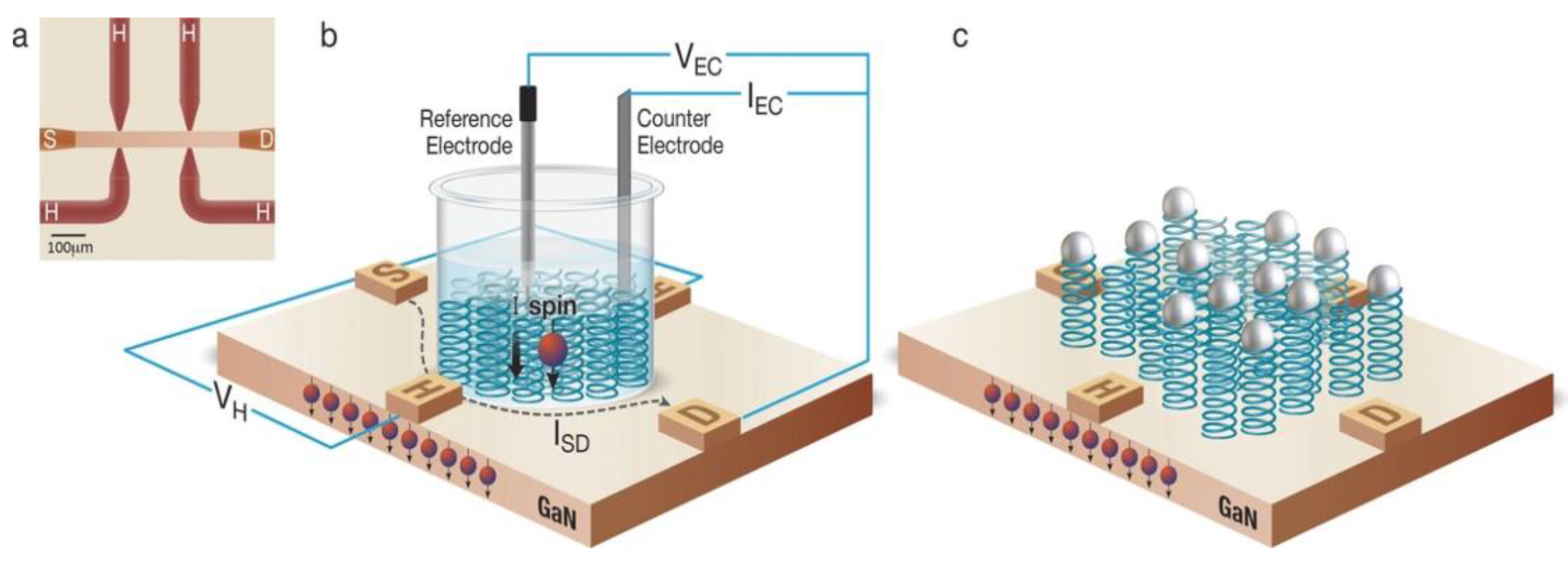
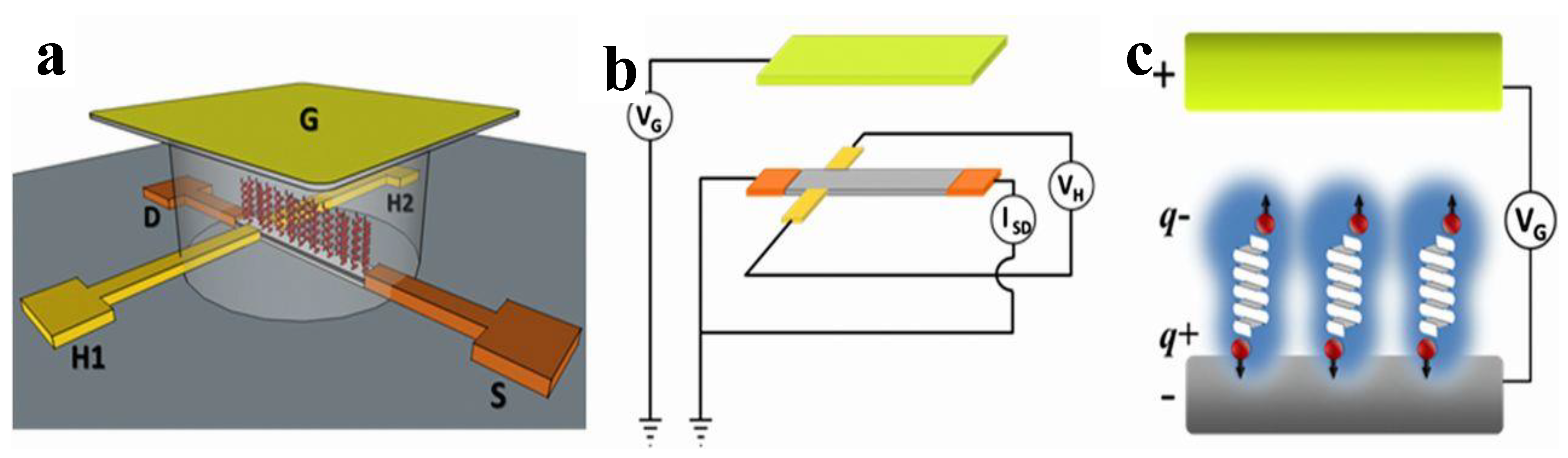
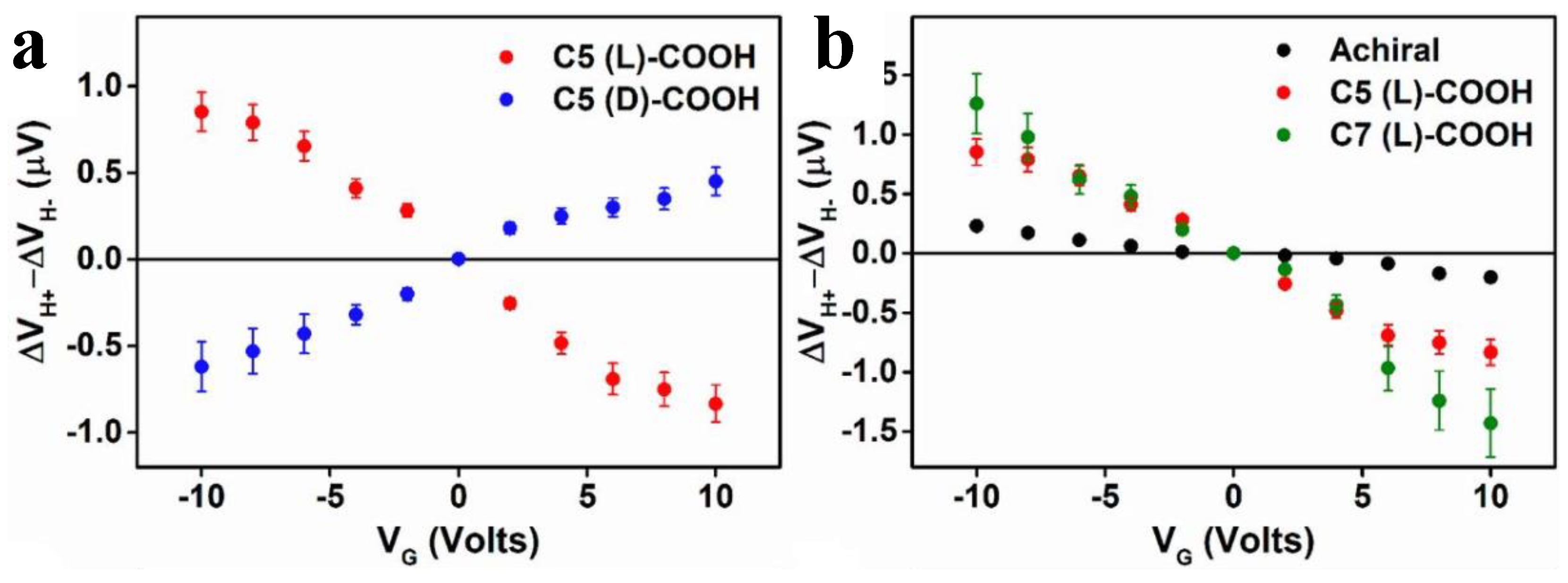
5.2. Magnetic Field Using Electrolytic Gated Chiral Molecules
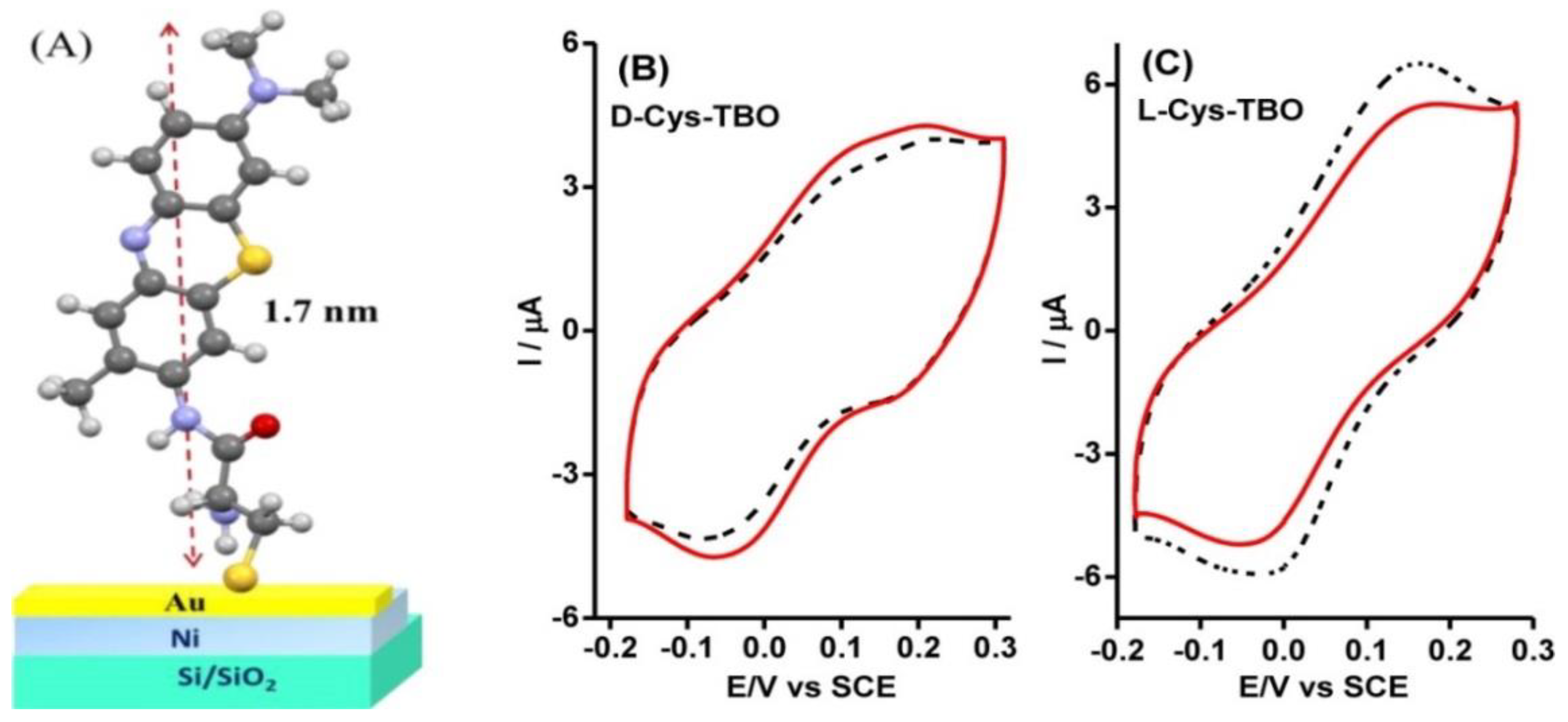
6. Spin-Controlled Charge Transport in Chiral Molecules
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Coey, J.M.D.; Hinds, G. Magnetoelectrolysis-the effect of magnetic fields in electrochemistry. In Proceedings of the 5th International Pamir Conference, Ramatuelle, France, 16–20 September 2002; pp. 1–7. [Google Scholar]
- Fahidy, T.Z. Magnetoelectrolysis. J. Appl. Electrochem. 1983, 13, 553–563. [Google Scholar] [CrossRef]
- Bund, A.; Koehler, S.; Kuehnlein, H.H.; Plieth, W. Magnetic field effects in electrochemical reactions. Electrochim Acta 2003, 49, 147–152. [Google Scholar] [CrossRef]
- Fahidy, T.Z. Characteristics of surfaces produced via magnetoelectrolytic deposition. Prog. Surf. Sci. 2001, 68, 155–188. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Chiral-Induced Spin Selectivity Effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Capua, E.; Kesharwani, M.K.; Martin, J.M.L.; Sitbon, E.; Waldeck, D.H.; Naaman, R. Chirality-induced spin polarization places symmetry constraints on biomolecular interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of Tyrosine Based on a Novel Magnetic Electrochemical Chiral Sensor. Electrochim Acta 2017, 241, 386–394. [Google Scholar] [CrossRef]
- Mishra, D.; Markus, T.Z.; Naaman, R.; Kettner, M.; Gohler, B.; Zacharias, H.; Friedman, N.; Sheves, M.; Fontanesi, C. Spin-dependent electron transmission through bacteriorhodopsin embedded in purple membrane. Proc. Natl. Acad. Sci. USA 2013, 110, 14872–14876. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties, 4th ed.; Graduate Texts in Physics; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-000709-5. [Google Scholar]
- Alfven, H. Existence of Electromagnetic-Hydrodynamic Waves. Nature 1942, 150, 405–406. [Google Scholar] [CrossRef]
- Rhen, F.M.F.; Coey, J.M.D. Magnetic Field Effect on Autocatalysis: Ag and Cu in Concentrated Nitric Acid. J. Phys. Chem. B 2006, 110, 6274–6278. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Watanabe, K. Surface chirality induced by rotational electrodeposition in magnetic fields. Sci. Rep. 2013, 3, 2574. [Google Scholar] [CrossRef] [PubMed]
- Mhíocháin, T.R.N.; Coey, J.M.D. Chirality of electrodeposits grown in a magnetic field. Phys. Rev. E 2004, 69. [Google Scholar] [CrossRef] [PubMed]
- Ispas, A.; Matsushima, H.; Plieth, W.; Bund, A. Influence of a magnetic field on the electrodeposition of nickel–iron alloys. Electrochim Acta 2007, 52, 2785–2795. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Electrocatalytic Chirality on Magneto-Electropolymerized Polyaniline Electrodes. Available online: http://www.ingentaconnect.com/content/ssam/14328488/2007/00000011/00000006/art00010;jsessionid=bchasa5ilpue2.x-ic-live-01 (accessed on 1 August 2018).
- Horvath, J.D.; Koritnik, A.; Kamakoti, P.; Sholl, D.S.; Gellman, A.J. Enantioselective Separation on a Naturally Chiral Surface. J. Am. Chem. Soc. 2004, 126, 14988–14994. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.O.; Baddeley, C.J.; Muryn, C.; Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 2000, 404, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.D.; Gellman, A.J. Enantiospecific Desorption of Chiral Compounds from Chiral Cu(643) and Achiral Cu(111) Surfaces. J. Am. Chem. Soc. 2002, 124, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Switzer, J.A.; Kothari, H.M.; Poizot, P.; Nakanishi, S.; Bohannan, E.W. Enantiospecific electrodeposition of a chiral catalyst. Nature 2003, 425, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huber, C.O. Electrocatalysis and amperometric detection using an electrode made of copper oxide and carbon paste. Anal. Chem. 1991, 63, 1714–1719. [Google Scholar] [CrossRef]
- Hinds, G.; Spada, F.E.; Coey, J.M.D.; Ní Mhíocháin, T.R.; Lyons, M.E.G. Magnetic Field Effects on Copper Electrolysis. J. Phys. Chem. B 2001, 105, 9487–9502. [Google Scholar] [CrossRef]
- Wattanakit, C.; Côme, Y.B.S.; Lapeyre, V.; Bopp, P.A.; Heim, M.; Yadnum, S.; Nokbin, S.; Warakulwit, C.; Limtrakul, J.; Kuhn, A. Enantioselective recognition at mesoporous chiral metal surfaces. Nat. Commun. 2014, 5, 3325. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.L.; Hoffman, J.G. Hall voltage in electrolyte solutions. Phys. Chem. Liq. 1972, 3, 191–204. [Google Scholar] [CrossRef]
- Hubbard, J.B.; Wolynes, P.G. An electrohydrodynamic contribution to the Hall effect in electrolyte solutions. J. Chem. Phys. 1981, 75, 3051–3054. [Google Scholar] [CrossRef]
- Newman, D.S.; Frank, C.; Matlack, R.W.; Twining, S.; Krishnan, V. The ionic hall effect in the solid electrolyte C5H6NAg5I6. Electrochim Acta 1977, 22, 811–814. [Google Scholar] [CrossRef]
- Hall, E.H. On a New Action of the Magnet on Electric Currents. Am. J. Math. 1879, 2, 287–292. [Google Scholar] [CrossRef]
- Eckshtain-Levi, M.; Capua, E.; Refaely-Abramson, S.; Sarkar, S.; Gavrilov, Y.; Mathew, S.P.; Paltiel, Y.; Levy, Y.; Kronik, L.; Naaman, R. Cold denaturation induces inversion of dipole and spin transfer in chiral peptide monolayers. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Capua, E.; Vankayala, K.; Fontanesi, C.; Naaman, R. Magnetless Device for Conducting Three-Dimensional Spin-Specific Electrochemistry. Angew. Chem. 2017, 129, 14779–14782. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Coey, J.M.D. Magnetic fields in electrochemistry: The Lorentz force. A mini-review. Electrochem. Commun. 2014, 42, 38–41. [Google Scholar] [CrossRef]
- Walsh, D.A.; Lovelock, K.R.J.; Licence, P. Ultramicroelectrode voltammetry and scanning electrochemical microscopy in room-temperature ionic liquid electrolytes. Chem. Soc. Rev. 2010, 39, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.C.; Fontanesi, C.; Waldeck, D.H.; Naaman, R. Spin-Dependent Transport through Chiral Molecules Studied by Spin-Dependent Electrochemistry. Acc. Chem. Res. 2016, 49, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, C.; Tassinari, F.; Parenti, F.; Cohen, H.; Mondal, P.C.; Kiran, V.; Giglia, A.; Pasquali, L.; Naaman, R. New One-Step Thiol Functionalization Procedure for Ni by Self-Assembled Monolayers. Langmuir 2015, 31, 3546–3552. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.C.; Kantor-Uriel, N.; Mathew, S.P.; Tassinari, F.; Fontanesi, C.; Naaman, R. Chiral Conductive Polymers as Spin Filters. Adv. Mater. 2015, 27, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.C.; Fontanesi, C.; Waldeck, D.H.; Naaman, R. Field and Chirality Effects on Electrochemical Charge Transfer Rates: Spin Dependent Electrochemistry. ACS Nano 2015, 9, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, C. Spin-dependent electrochemistry: A novel paradigm. Curr. Opin. Electrochem. 2018, 7, 36–41. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Mondal, P.C.; Fontanesi, C. Chiral Magneto-Electrochemistry. Magnetochemistry 2018, 4, 36. https://doi.org/10.3390/magnetochemistry4030036
Kumar A, Mondal PC, Fontanesi C. Chiral Magneto-Electrochemistry. Magnetochemistry. 2018; 4(3):36. https://doi.org/10.3390/magnetochemistry4030036
Chicago/Turabian StyleKumar, Anup, Prakash Chandra Mondal, and Claudio Fontanesi. 2018. "Chiral Magneto-Electrochemistry" Magnetochemistry 4, no. 3: 36. https://doi.org/10.3390/magnetochemistry4030036
APA StyleKumar, A., Mondal, P. C., & Fontanesi, C. (2018). Chiral Magneto-Electrochemistry. Magnetochemistry, 4(3), 36. https://doi.org/10.3390/magnetochemistry4030036