Abstract
This article describes the successful application of the DOSY method for the separation and analysis of the α- and β-anomers of carbohydrates with different diffusion coefficients. In addition, the DOSY method was found to effectively separate two kinds of glucopyranosides with similar aglycon structures from a mixture.
1. Introduction
High-resolution nuclear magnetic resonance (NMR) spectroscopy has become an excellent established tool for determining the molecular structures and conformations of compounds while preserving the sample integrity. In addition, pulsed gradient spin echo (PGSE) NMR is recognized as a powerful technique for the determination of diffusion coefficients and the separation of different species in a mixture on the basis of their diffusion coefficients [1]. Diffusion-ordered NMR spectroscopy (DOSY), which displays the PGSE NMR data in a two-dimensional spectrum, is a practical experiment for separating the 1H NMR spectra of different species [2]. In addition to the DOSY separation of a mixture, the DOSY method has been widely used for the characterization of high-molecular-weight polymeric compounds and the identification of supramolecular structures [3,4,5,6,7,8]. However, the DOSY method has generally failed to identify the isomeric species of similar size and structure because of their similar diffusion coefficients. Therefore, recent studies on the DOSY technique have focused on developing strategies for the separation of isomeric species [9,10,11,12,13,14]. The DOSY method has been also applied to carbohydrate chemistry as a tool for the separation and analysis of mono-, di-, oligo-, and polysaccharides, as well as for the structural analysis of metal-complexed carbohydrates [15,16,17,18,19,20]. However, reports on the application of the DOSY method for the separation of carbohydrate anomeric isomers are still scarce. With an aim of increasing the utility and applicability of the DOSY method in carbohydrate chemistry, we tackled its evaluation in the separation and analysis of the α- and β-anomers of carbohydrates.
2. Results and Discussion
The DOSY analysis for the isomer separation in a mixture of α- and β-anomers was investigated using several kinds of carbohydrate derivatives of glycopyranosides and glycopyranoses, as shown in Figure 1.
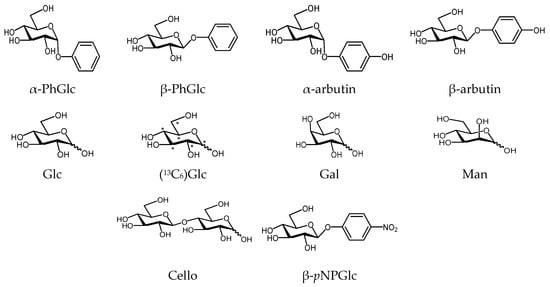
Figure 1.
Carbohydrate derivatives used for DOSY analysis on anomer isomers.
2.1. DOSY Separation of the α- and β-Anomeric Isomers of Glycopyranosides
The DOSY separation of the anomeric isomers in a mixture of 10 mM phenyl β-glucopyranoside (β-PhGlc) and 10 mM phenyl α-glucopyranoside (α-PhGlc) was firstly investigated. Figure 2 shows the DOSY spectrum of the mixture of α-PhGlc and β-PhGlc in D2O at 30 °C, together with the individual 1H NMR spectra of β-PhGlc and α-PhGlc in D2O. In the DOSY spectrum, two different species with diffusion coefficients (D) of 5.9 × 10−10 m2·s−1 and 5.6 × 10−10 m2·s−1 could be identified, whose resonances corresponded to the 1H NMR spectrum of β-PhGlc and α-PhGlc, respectively. It was thereby found that the apparent difference between the diffusion coefficients of β-PhGlc and α-PhGlc allow the DOSY separation of these glucopyranoside anomers.
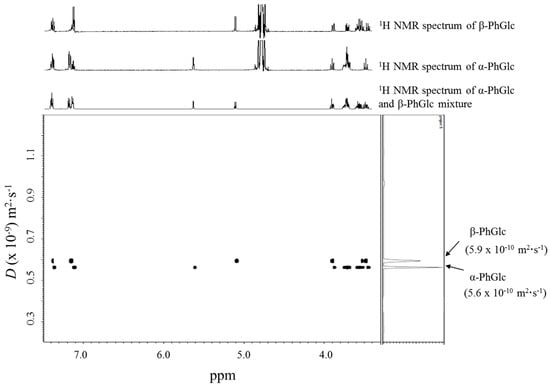
Figure 2.
DOSY spectrum of a 10 mM β-PhGlc and 10 mM α-PhGlc mixture.
Next, the DOSY separation of the anomeric isomers in a mixture of 10 mM α-arbutin (p-hydroxyphenyl α-glucopyranoside) and 10 mM β-arbutin in D2O at 30 °C was similarly investigated. The two glucopyranoside anomers—which exhibited diffusion coefficients (D) of 5.9 × 10−10 m2·s−1 (α-arbutin) and 5.8 × 10−10 m2·s−1 (β-arbutin), respectively—were also successfully separated by the DOSY technique, as shown in Figure S1.
2.2. DOSY Separation of the α- and β-Anomeric Isomers of Glycopyranoses
Glycopyranoses are known to undergo mutarotation; they interconvert their α- and β-anomers in water and an equilibrium mixture of the two forms is achieved. Figure 3 displays the mutarotation of d-glucopyranose (Glc). The 1H NMR spectrum of Glc at a concentration of 20 mM in D2O indicated that the anomer ratio of Glc was ca. 1:1. We investigated whether the DOSY method could separate the individual 1H NMR spectra of the anomeric isomers in an equilibrium mixture of α-Glc and β-Glc. The DOSY spectrum of a 20 mM solution of Glc in D2O at 30 °C revealed that two species with different diffusion coefficients (D) of 7.6 × 10−10 m2·s−1 and 5.8 × 10−10 m2·s−1 were present, the former corresponding to the 1H NMR spectrum of α-Glc, and the latter to the 1H NMR spectrum of β-Glc, as can be seen in Figure 4. We found that the difference between the diffusion coefficients of α-Glc and β-Glc was sufficient to separate the two anomeric isomers of Glc by using the DOSY technique.
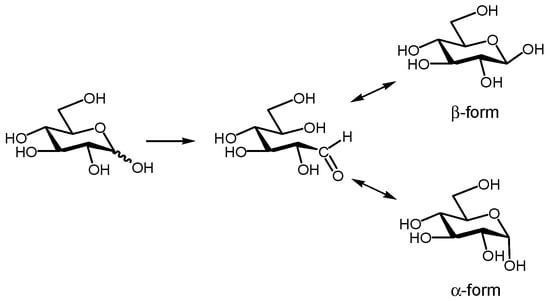
Figure 3.
Mutarotation of Glc to interconvert between α-anomer and β-anomer in water.
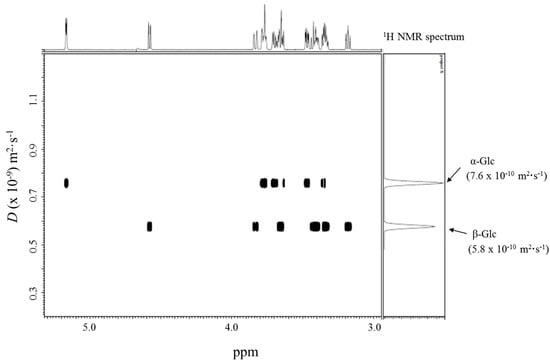
Figure 4.
DOSY spectrum of 20 mM Glc.
In order to confirm the applicability of the DOSY method for the determination of the diffusion coefficients of both anomers of glycopyranose showing mutarotation, the DOSY spectra of several kinds of glycopyranoses were measured. The DOSY measurements were performed using 20 mM solutions of (13C6)-d-glucopyranose ((13C6)Glc), d-galactopyranose (Gal), d-mannopyranose (Man), and cellobiose (Glcβ(1→4)Glc, Cello) in D2O at 30 °C. We had previously confirmed the presence of the α- and β-anomers of these glycopyranoses in D2O by 1H NMR measurements. The individual 1H NMR spectra of the α- and β-anomers were successfully separated in all the DOSY spectra, and their corresponding diffusion coefficients (D) were thereby obtained. The DOSY spectra are shown in Figures S2–S5, and the α- and β-anomers of these glycopyranoses are summarized in Table 1, together with the average diffusion coefficients of some of the α- and β-glycopyranose mixtures previously reported. Since the DOSY technique was able to separate the α- and β-anomers of glycopyranoses, it seems to be a reliable method for the estimation of their individual diffusion coefficients.

Table 1.
Diffusion coefficients of carbohydrate anomers measured in this study and their reported values.
2.3. DOSY Separation of a Mixture of Two Kinds of Glycopyranosides Having Similar Aglycon Structures
We also investigated the DOSY separation of a mixture of two kinds of glycopyranosides having a similar aglycon structures. The DOSY spectrum of a mixture of 10 mM α-PhGlc and 10 mM α-arbutin in D2O at 30 °C clearly separated the two kinds of glycopyranoside species, which exhibited different diffusion coefficients (D) of 5.77 × 10−10 m2·s−1 (α-PhGlc) and 5.49 × 10−10 m2·s−1 (α-arbutin), as shown in Figure S6. The DOSY spectrum in Figure S7 also evinces the successful separation of a mixture of 10 mM β-arbutin and 10 mM β-pNPGlc, whose diffusion coefficients (D) were 7.2 × 10−10 m2·s−1 and 5.6 × 10−10 m2·s−1, respectively.
3. Materials and Methods
d-glucose, d-galactose, d-mannose, d-cellobiose, β-arbutin, phenyl α-glucopyranoside, phenyl β-glucopyranoside, and pNP β-glucopyranoside were purchased from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Tokyo, Japan) and (13C6)-d-glucose was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). α-Arbutin was purchased from Wako Pure Chemical Industries, Ltd. (Chuo-Ku, Osaka, Japan). D2O was purchased from Kanto Chemical Co., INC. (99.8% minimum in d, Chuo-ku, Tokyo, Japan).
The NMR spectra were obtained using a JEOL ECA-600 spectrometer (JEOL Ltd., Akishima, Tokyo, Japan), using 20 mM concentrations for the individual samples and 10 mM concentrations for the mixtures. The 1H NMR spectra were recorded at 600 MHz. The chemical shifts were referenced to the solvent values (δ 4.70 ppm for HOD). The spectra were analyzed after 16 scans and 4 dummy scans. The 2D DOSY experiments were performed at 30 °C using the bipolar pulse pair and longitudinal eddy current delay sequence. Gradient amplitudes were 20–247 mT/m. The spectral width was 6000 Hz. Bipolar rectangular gradients were used with total durations of 1 to 2 ms. Gradient recovery delays were 0.1 ms. Diffusion times were between 50 and 200 ms. The relaxation delay was 7.0 s. The spectra were analyzed after 256 scans and 16 dummy scans. The spectral analyses were processed by the Delta NMR processing software version 4.3.6. (JEOL USA, Inc., Peabody, MA, USA).
4. Conclusions
This article describes the evaluation of the DOSY method for the separation and analysis of the α- and β-anomers of carbohydrates. We found that the α- and β-anomers of carbohydrates having different diffusion coefficients can be separated by using the DOSY technique, and their individual diffusion coefficients can be determined. In addition, the DOSY method was also applicable to the separation of two kinds of glucopyranosides having similar aglycon structures from a mixture.
Supplementary Materials
The following are available online at www.mdpi.com/2312-7481/3/4/38/s1, Figure S1. DOSY spectrum of a 10 mM α-arbutin and 10 mM β-arbutin mixture in D2O at 30 °C, Figure S2. DOSY spectrum of 20 mM (13C6)Glc in D2O at 30 °C, Figure S3. DOSY spectrum of 20 mM Gal in D2O at 30 °C, Figure S4. DOSY spectrum of 20 mM Man in D2O at 30 °C, Figure S5. DOSY spectrum of 20 mM Cello in D2O at 30 °C, Figure S6. DOSY spectrum of a 10 mM α-PhGlc and 10 mM α-arbutin mixture in D2O at 30 °C, Figure S7. DOSY spectrum of a 10 mM β-arbutin and 10 mM β-pNPGlc mixture in D2O at 30 °C.
Author Contributions
T.Y. conceived idea of the article and wrote the paper. Y.O. performed the experiments. K.K. conceived and designed the experiments. All authors approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, C.S. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Antalek, B. Using Pulsed Gradient Spin Echo NMR for Chemical Mixture Analysis: How to Obtain Optimum Results. Concepts Magn. Reson. 2002, 14, 225–258. [Google Scholar] [CrossRef]
- Kavakka, J.S.; Kilpeläinen, I.; Heikkinen, S. General Chromatographic NMR Method in Liquid State for Synthetic Chemistry: Polyvinylpyrrolidone Assisted DOSY Experiments. Org. Lett. 2009, 6, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Kavakka, J.S.; Parviainen, V.; Wahala, K.; Kilpeläinen, I.; Heikkinen, S. Enhanced chromatographic NMR with polyethyleneglycol. A novel resolving agent for diffusion ordered spectroscopy. Magn. Reson. Chem. 2010, 48, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, R.; Bai, Z.; Yang, Y.; Li, S.; Dou, X. Evaluation of the separation performance of polyvinylpyrrolidone as a virtual stationary phase for chromatographic NMR. Magn. Reson. Chem. 2014, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, T.; Kenne, L.; Sandström, C. The use of diffusion-ordered spectroscopy and complexation agents to analyze mixtures of catechins. New J. Chem. 2009, 33, 1057–1063. [Google Scholar] [CrossRef]
- Oda, Y.; Kobayashi, N.; Yamanoi, T.; Katsuraya, K.; Takahashi, K.; Hattori, K. β-Cyclodextrin Conjugates with Glucose Moieties Designed as Drug Carriers: Their Syntheses, Evaluations Using Concanavalin A and Doxorubicin, and Structural Analyses by NMR Spectroscopy. Med. Chem. 2008, 4, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Matsuda, S.; Yamanoi, T.; Murota, A.; Katsuraya, K. Identification of the inclusion complexation between phenyl β-d-(13C6)-glucopyranoside and α-cyclodextrin using 2D 1H or 13C DOSY spectrum. Supramol. Chem. 2009, 21, 638–642. [Google Scholar] [CrossRef]
- Haiber, S.; Nilsson, M.; Morris, G.A. Matrix-assisted diffusion-ordered spectroscopy: Application of surfactant solutions to the resolution of isomer spectra. Magn. Reson. Chem. 2012, 50, 458–465. [Google Scholar]
- Reile, I.; Aspers, R.L.E.G.; Feiters, M.C.; Rutjes, F.P.J.T.; Tessari, M. Resolving DOSY spectra of isomers by methanol-d4 solvent effects. Magn. Reson. Chem. 2017, 55, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Gramosa, N.V.; Ricardo, N.M.S.P.; Adams, R.W.; Morris, G.A.; Nilsson, M. Matrix-assisted diffusion-ordered spectroscopy: Choosing a matrix. Magn. Reson. Chem. 2016, 54, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.W.; Aguilar, J.A.; Cassani, J.; Morris, G.A.; Nilsson, M. Resolving natural product epimer spectra by matrix-assisted DOSY. Org. Biomol. Chem. 2011, 20, 7062–7064. [Google Scholar] [CrossRef] [PubMed]
- Codling, D.J.; Zheng, G.; Stait-Gardner, T.; Yang, S.; Nilsson, M.; Price, W.S. Diffusion Studies of Dihydroxybenzene Isomers in Water−Alcohol Systems. J. Phys. Chem. B 2013, 117, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.R.; Suryaprakash, N. Diffusion ordered spectroscopy for resolution of double bonded cis, trans-isomers. J. Mol. Struct. 2012, 1017, 106–108. [Google Scholar] [CrossRef]
- Viel, S.; Capitani, D.; Mannina, L.; Segre, A. Diffusion-Ordered NMR Spectroscopy: A Versatile Tool for the Molecular Weight Determination of Uncharged Polysaccharides. Biomacromolecules 2003, 4, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.; Rasmussen, M.O.; Molero, M.D.; Samain, E.; Cañada, F.J.; Driguez, H.; Jiménez-Barbero, J. Diffusion ordered spectroscopy as a complement to size exclusion chromatography in oligosaccharide analysis. Glycobiology 2004, 14, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Komura, F.; Nonaka, A.; Kato, T.; Fukumashi, J.; Matsui, T. Quantitative analysis of D-(+)-glucose in fruit juices using diffusion ordered-1H nuclear magnetic resonance spectroscopy. Anal. Sci. 2014, 30, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Groves, P.; Chávez, M.I.; Cañada, F.J.; Jiménez-Barberoa, J. Useful applications of DOSY experiments for the study of mushroom polysaccharides. Carbohydr. Res. 2006, 341, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kählig, H.; Dietrich, K.; Dorner, S. Analysis of Carbohydrate Mixtures by Diffusion Difference NMR Spectroscopy. Monatsh. Chem. 2002, 133, 589–598. [Google Scholar] [CrossRef]
- Díaz, M.D.; Berger, S. Studies of the complexation of sugars by diffusion-ordered NMR spectroscopy. Carbohydr. Res. 2000, 329, 1–5. [Google Scholar] [CrossRef]
- Nagy, L.; Gyetvai, G.; Nagy, G. Determination of the Diffusion Coefficient of Monosaccharides with Scanning Electrochemical Microscopy (SECM). Electroanalysis 2009, 21, 542–549. [Google Scholar] [CrossRef]
- Mori, N.; Sugai, E.; Fuse, Y.; Funazukuri, T. Infinite Dilution Binary Diffusion Coefficients for Six Sugars at 0.1 MPa and Temperatures from (273.2 to 353.2). J. Chem. Eng. Data 2007, 52, 40–43. [Google Scholar]
- Ihnat, M.; Goring, D.A.I. Shape of the cellodextrins in aqueous solution at 25 °C. Can. J. Chem. 1967, 45, 2353–2361. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).