Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Comments and IR Discussion
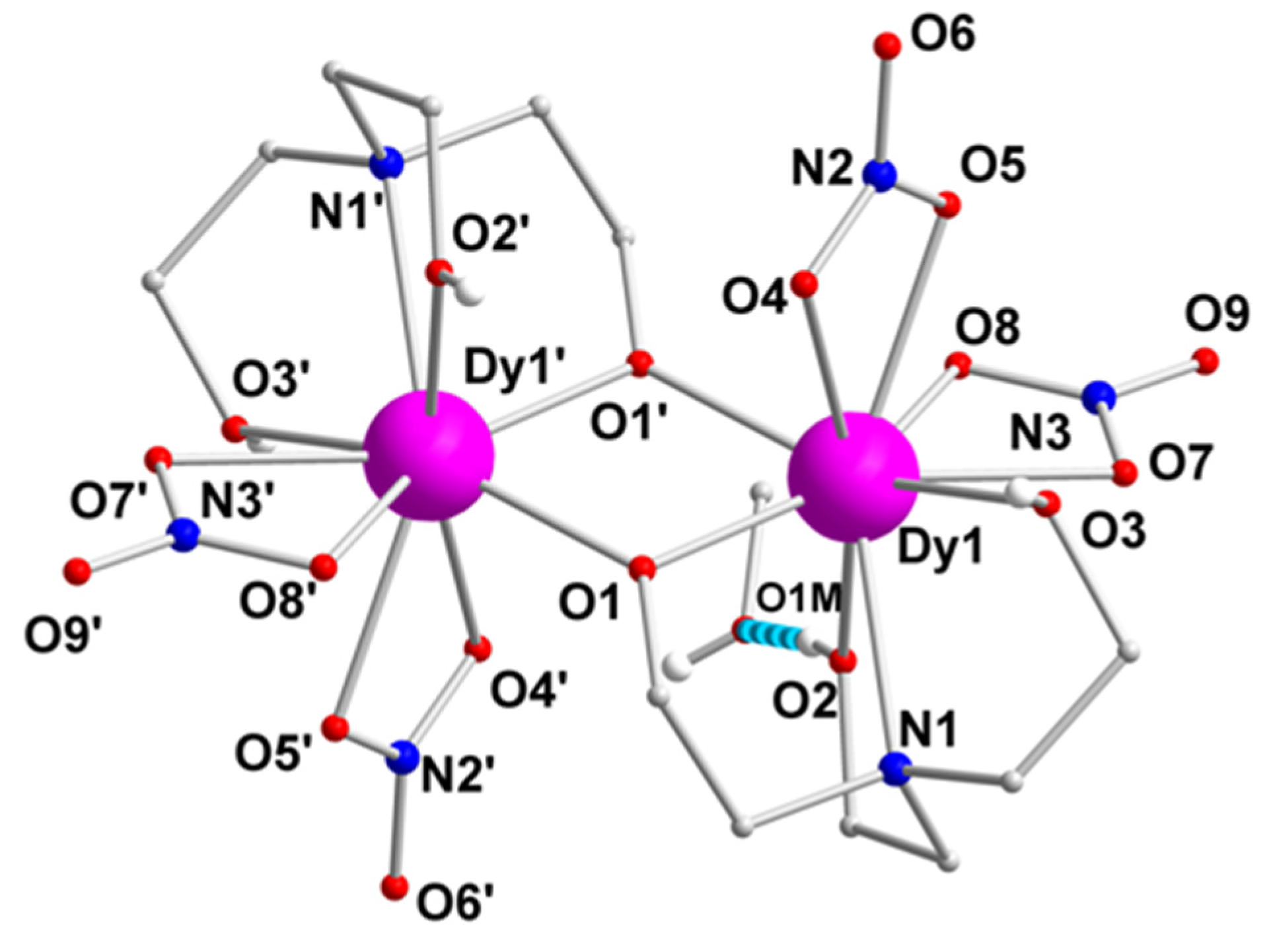
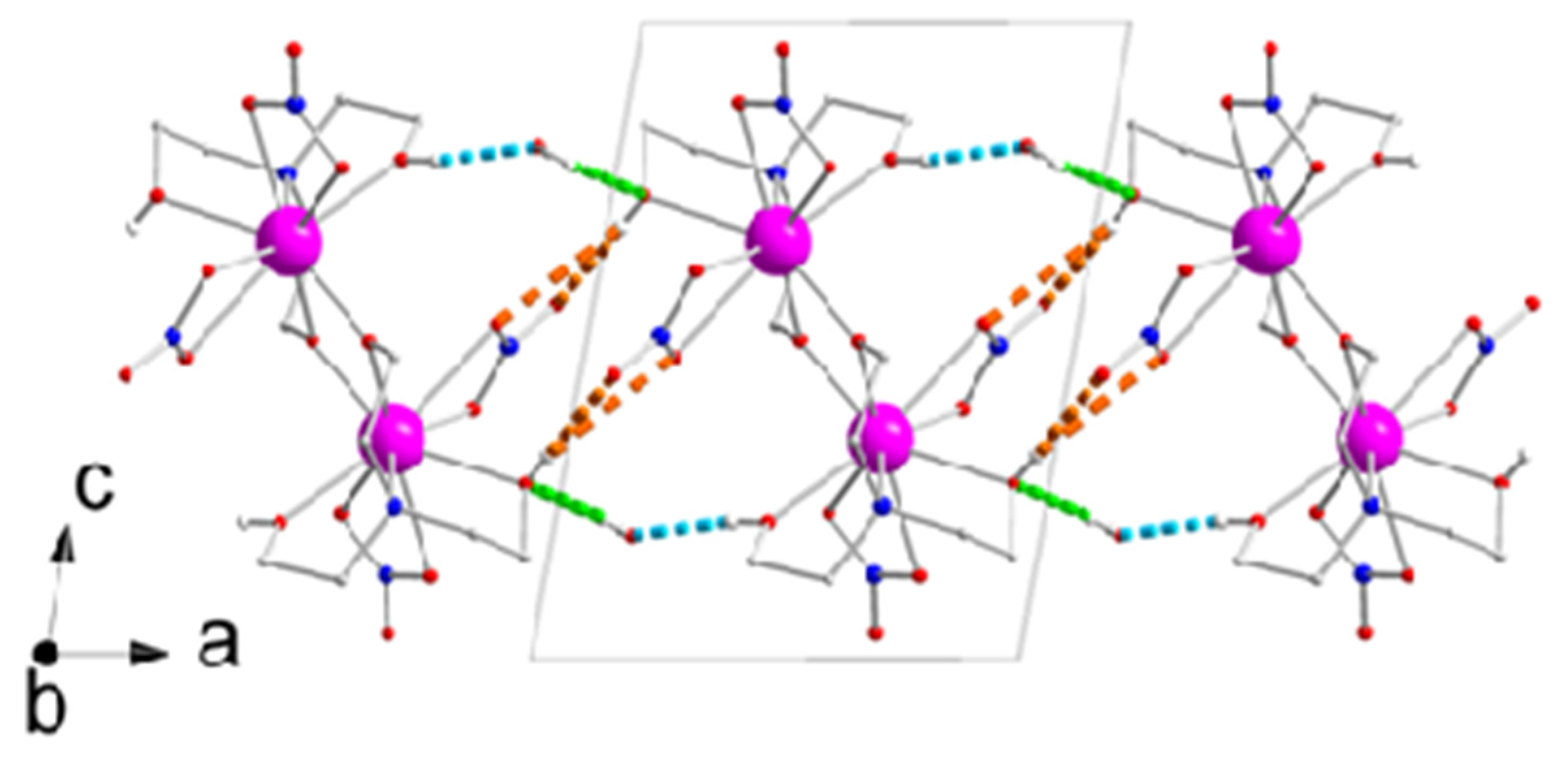
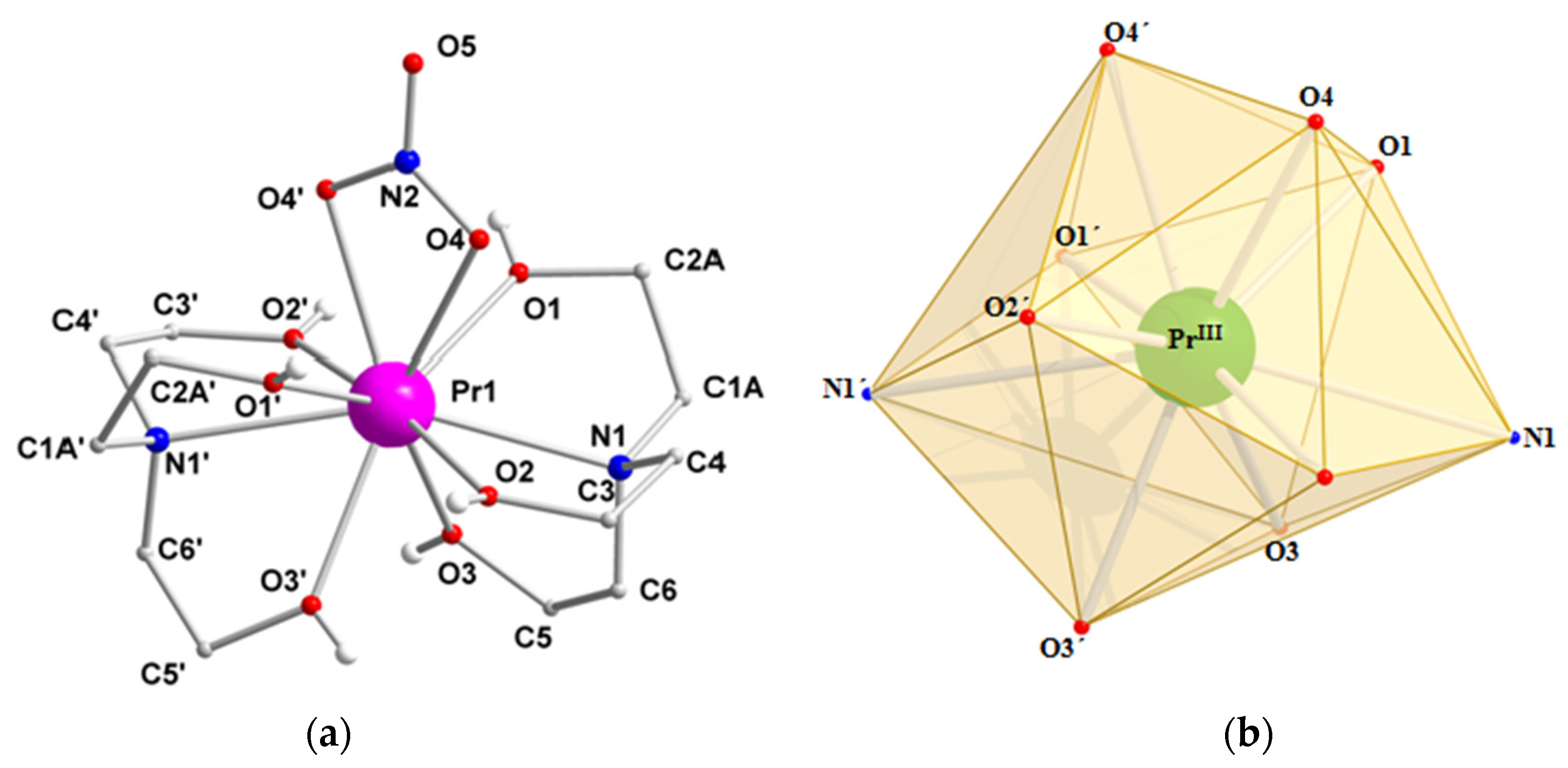
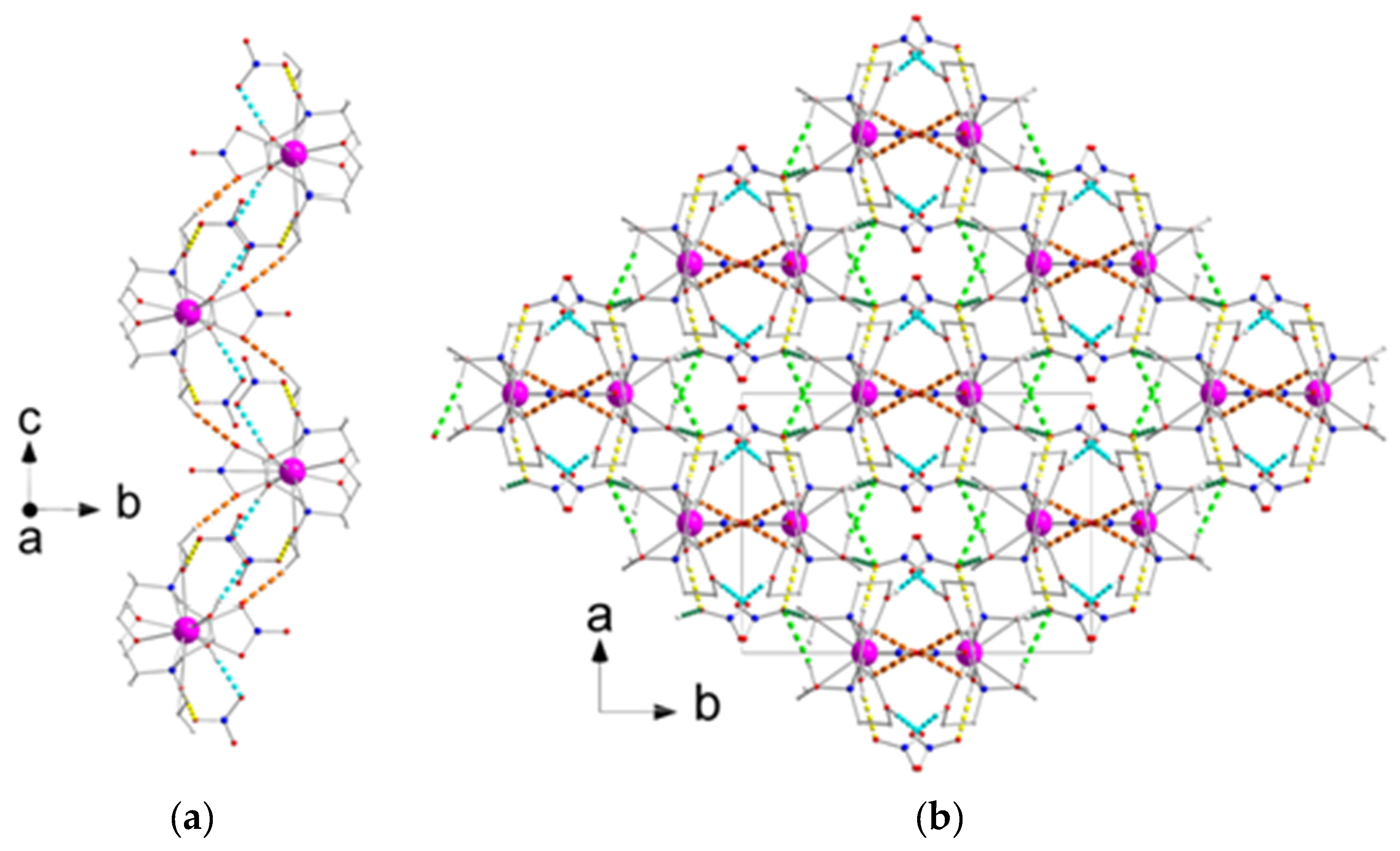
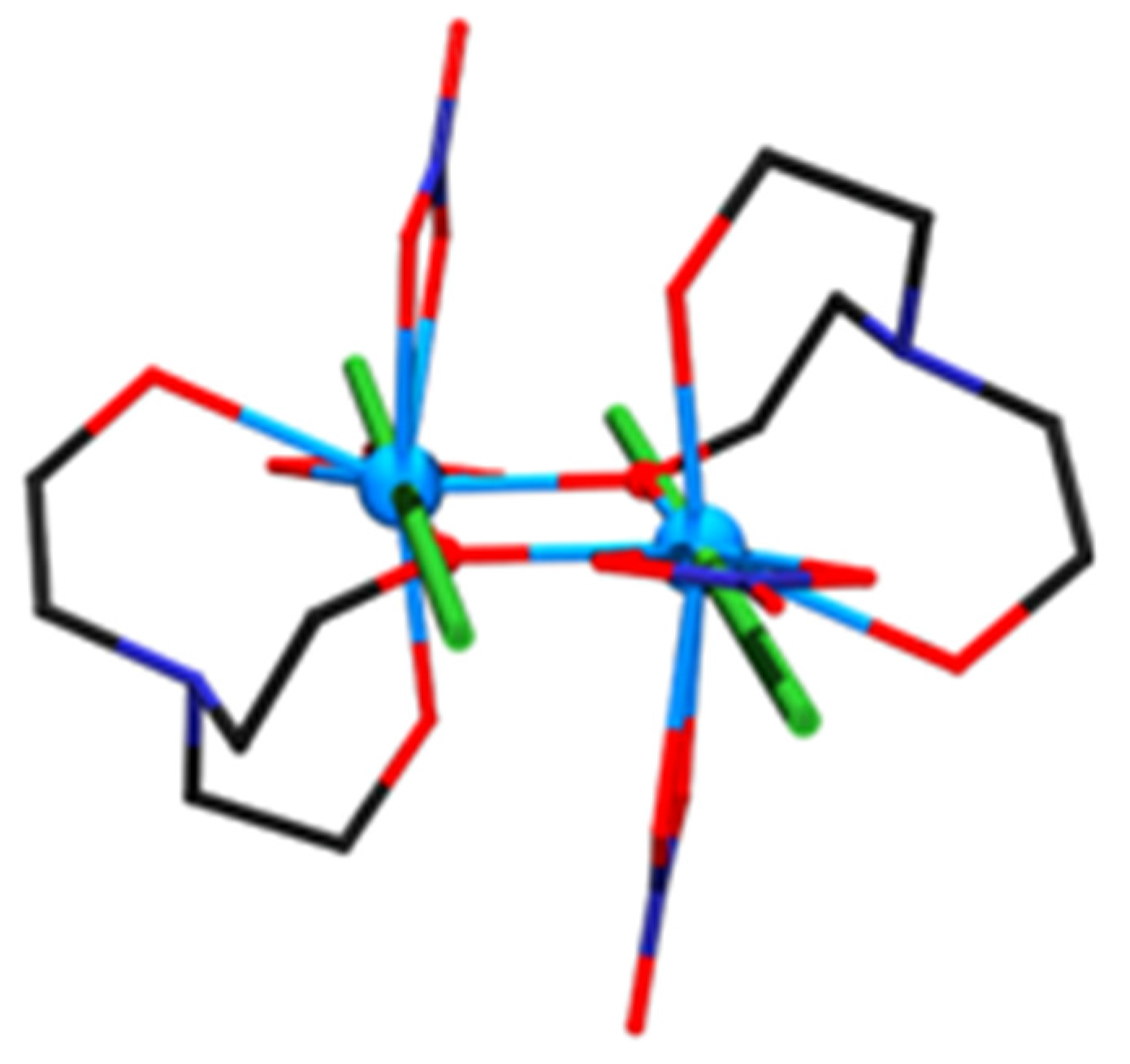
2.2. Description of Structures
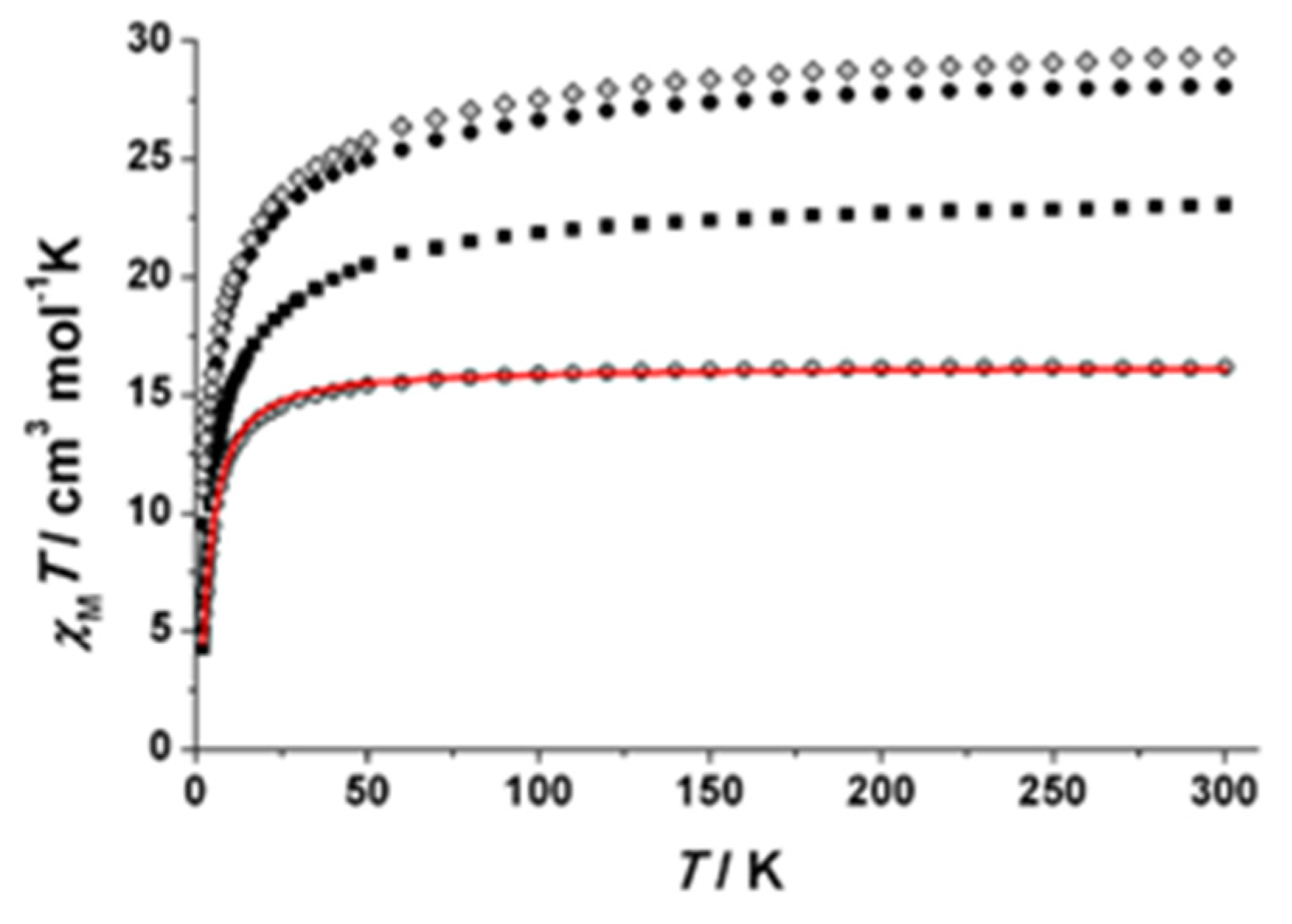
2.3. Magnetic Susceptibility Studies
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Synthesis of [Pr2(NO3)4(teaH2)2]∙2MeOH (1∙2MeOH)
3.3. Syntheses of [Gd2(NO3)4(teaH2)2]∙2MeOH (2∙2MeOH), [Tb2(NO3)4(teaH2)2]∙2MeOH (3∙2MeOH), [Dy2(NO3)4(teaH2)2]∙2MeOH (4∙2MeOH) and [Ho2(NO3)4(teaH2)2]∙2MeOH (5∙2MeOH)
3.4. Synthesis of [Pr(NO3)(teaH3)2](NO3)2 (6)
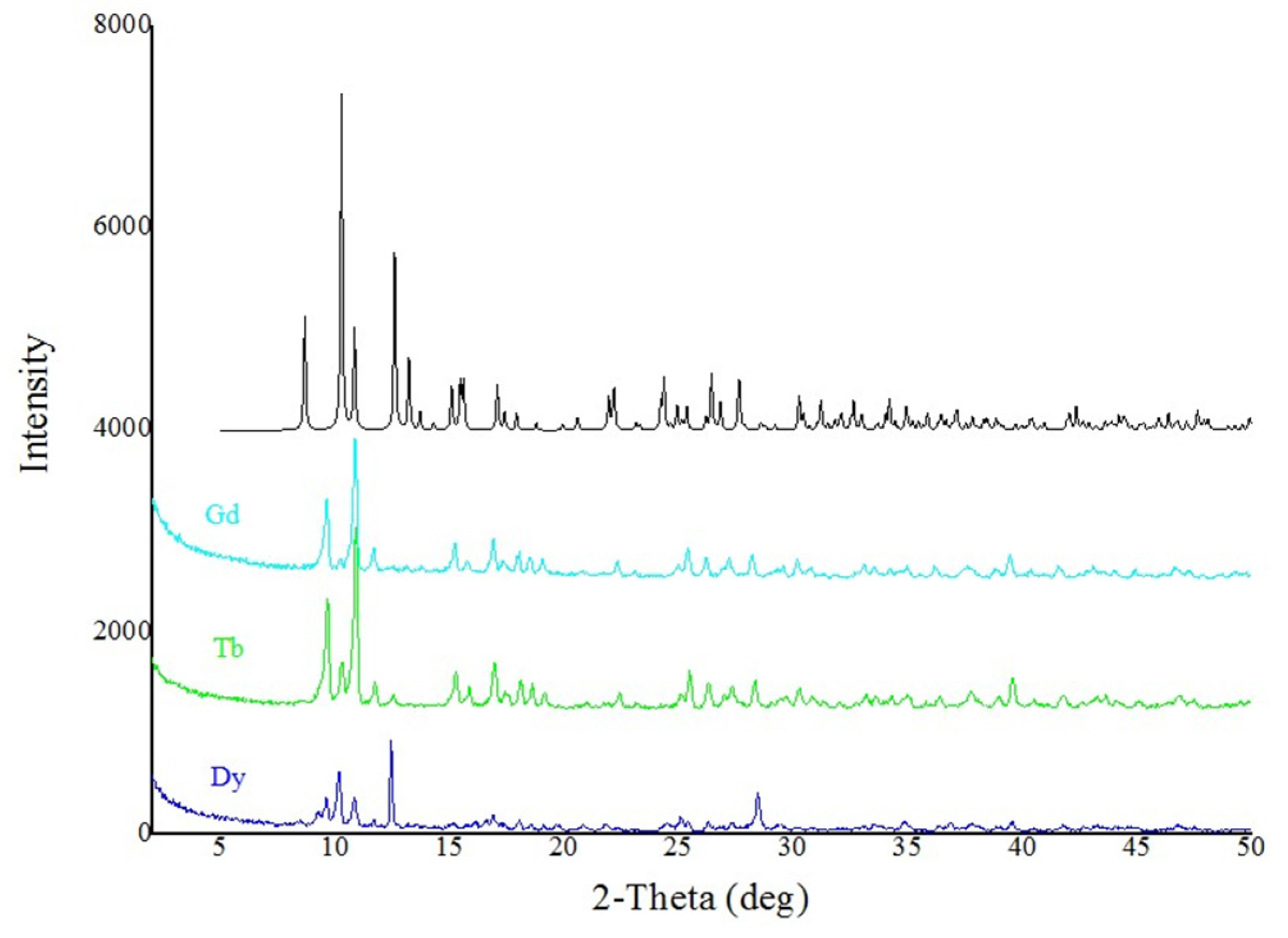
3.5. Single-Crystal and Powder X-ray Crystallography
4. Conclusions and Perspectives
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luzon, J.; Sessoli, R. Lanthanides in molecular magnetism: So fascinating, so challenging. Dalton Trans. 2012, 41, 13556–13567. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. Struct. Bond. 2015, 164, 1–109. [Google Scholar]
- For a review on Mn SMMs, see: Bagai, R.; Christou, G. The Drosophila of single-molecule magnetism: [Mn12O12(O2CR)16(H2O)4]. Chem. Soc. Rev. 2009, 38, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- For an excellent and comprehensive review, see: Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5138. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, X.; Meng, X.; Shi, W.; Cheng, P.; Powell, A.K. Constraining the coordination geometries of lanthanide centers and magnetic building blocks in frameworks: A new strategy for molecular nanomagnets. Chem. Soc. Rev. 2016, 45, 2423–2439. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, P. Polynuclear lanthanide Single Molecule Magnets. In Lanthanides and Actinides in Molecular Magnetism, 1st ed.; Layfields, R.A., Murugesu, M., Eds.; Wiley-VCH: Berlin, Germany, 2015; pp. 61–88. [Google Scholar]
- Habib, E.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Guo, Y.-N.; Ungur, L.; Tang, J.; Chibotaru, L. Tuning the Magnetic Interactions and Relaxation Dynamics of Dy2 Single-Molecule Magnets. Chem. Eur. J. 2015, 21, 14099–14106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Brunet, G.; Holmberg, R.J.; Habib, F.; Karobkov, I.; Murugesu, M. Terminal solvent effects on the anisotropy barriers of Dy2 systems. Dalton Trans. 2016, 45, 16709–16715. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Ding, H.-Y.; Meng, Y.-S.; Gao, C.; Zhang, X.-J.; Meng, Z.-S.; Zhang, Y.-Q.; Shi, W.; Wang, B.-W.; Gao, S. Hydroxide-bridged five-coordinate DyIII single-molecule magnet exhibiting the record thermal relaxation barrier of magnetization among lanthanide-only dimmers. Chem. Sci. 2016. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F. Strong Axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, Y.-Y.; Liu, C.-M.; Li, Y.-Z.; Zuo, J.-L. A sandwich-type triple-decker lanthanide complex with mixed phthalocyanine and Schiff base ligands. Dalton Trans. 2013, 42, 11043–11046. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Bernot, K.; Cador, O.; Luzon, J.; Calvez, G.; Daiguebonne, C.; Guillou, O. Influence of ferromagnetic connection of Ising-type DyIII-based single ion magnets on their magnetic slow relaxation. Dalton Trans. 2013, 42, 6728–6731. [Google Scholar] [CrossRef] [PubMed]
- Nematirad, M.; Gee, W.J.; Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Murray, K.S.; Batten, S.R. Single molecule magnetism in a μ-phenolato dinuclear motif ligated by heptadentate Schiff base ligands. Dalton Trans. 2012, 41, 13711–13715. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-F.; Wang, Q.-L.; Gamez, P.; Ma, Y.; Clérac, R.; Tang, J.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. A promising new route towards single-molecule magnets based on the oxalate ligand. Chem. Commun. 2010, 46, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.E.; Hughbanks, T. Magnetic Coupling in Dinuclear Gd Complexes. J. Am. Chem. Soc. 2006, 128, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Luis, F.; Repollés, A.; Martínez-Pérez, M.J.; Aguilà, D.; Roubeau, O.; Zueco, D.; Alonso, P.J.; Evangelisti, M.; Camón, A.; Sesé, J.; et al. Molecular Prototypes for Spin-Based CNOT and SWAP Quantum Gates. Phys. Rev. Lett. 2011, 107, 117203-1–117203-5. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Ariciu, A.-M.; McAdams, S.; Weihe, H.; Bendix, J.; Tuna, F.; Piligkos, S. Towards Molecular 4f Single-Ion Magnet Qubits. J. Am. Chem. Soc. 2016, 138, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- For example, see: Anastasiadis, N.C.; Mylonas-Margaritis, I.; Psycharis, V.; Raptopoulou, C.P.; Kalofolias, D.A.; Milios, C.J.; Klouras, N.; Perlepes, S.P. Dinuclear, tetrakis(acetato)-bridged lanthanide(III) complexes from the use of 2-acetylpyridine hydrazone. Inorg. Chem. Commun. 2015, 51, 99–102. [Google Scholar] [CrossRef]
- Lin, P.-H.; Burchell, T.J.; Clérac, R.; Murugesu, M. Dinuclear Dysprosium(III) Single-Molecule Magnets with a large Anisotropic Barrier. Angew. Chem. Int. Ed. 2008, 47, 8848–8851. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, N.C.; Kalofolias, D.A.; Philippidis, A.; Tzani, S.; Raptopoulou, C.P.; Psycharis, V.; Milios, C.J.; Escuer, A.; Perlepes, S.P. A family of dinuclear lanthanide(III) complexes from the use of a tridentate Schiff base. Dalton Trans. 2015, 44, 10200–10209. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, N.C.; Granadeiro, C.M.; Klouras, N.; Cunha-Silva, L.; Raptopoulou, C.P.; Psycharis, V.; Bekiari, V.; Balula, S.S.; Escuer, A.; Perlepes, S.P. Dinuclear Lanthanide(III) Complexes by Metal-Ion-Assisted Hydration of Di-2-pyridyl Ketone Azine. Inorg. Chem. 2013, 52, 4145–4147. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, H.; Terzis, A.; Raptopoulou, C.P.; Psycharis, V.; Bekiari, V.; Perlepes, S.P. Unique Dinuclear, Tetrakis (nitrato-O,O′)-Bridged Lanthanide(III) Complexes from the Use of Pyridine-2-Amidoxime: Synthesis, Structural Studies and Spectroscopic Characterization. J. Surf. Interfaces Mater. 2014, 2, 311–318. [Google Scholar] [CrossRef]
- Murugesu, M.; Mishra, A.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Mixed 3d/4d and 3d/4f metal clusters: Tetranuclear FeIII2MIII2 (MIII = Ln, Y and MnIV2MIII2 (M = Yb,Y) complexes and the first Fe/4f single-molecule magnets. Polyhedron 2006, 25, 613–625. [Google Scholar] [CrossRef]
- Schray, D.; Abbas, G.; Lan, Y.; Mereacre, V.; Sundt, A.; Dreiser, J.; Waldmann, O.; Kostakis, G.E.; Anson, C.E.; Powell, A.K. Combined Magnetic Susceptibility Measurements and 57Fe Mössbauer Spectroscopy on a Ferromagnetic {FeIII4Dy4} Ring. Angew. Chem. Int. Ed. 2010, 49, 5185–5188. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Langley, S.K.; Moubaraki, B.; Murray, K.S. Synthesis, structural and magnetic studies of an isostructural family of mixed 3d/4f tetranuclear ‘star’ clusters. Chem. Commun. 2010, 46, 7787. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Wielechowski, D.P.; Moubaraki, B.; Abrahams, B.F.; Murray, K.S. Magnetic Exchange Effects in {CrIII2DyIII2} Single Molecule Magnets Containing Alcoholamine Ligands. Aust. J. Chem. 2014, 67, 1581–1587. [Google Scholar] [CrossRef]
- Hahn, F.E.; Mohr, J. Synthese und Strukturen von Bis(triethanolamin)lanthanoid-Komplexen. Chem. Ber. 1990, 123, 481–484. [Google Scholar] [CrossRef]
- Starynowicz, P. Synthesis and structure of bis(triethanolamine)europium(II) diperchlorate. J. Alloy. Compd. 2001, 323–324, 159–163. [Google Scholar] [CrossRef]
- Starynowicz, P.; Gatner, K. An Ytterbium(II) Complex with Triethanolamine. Z. Anorg. Allg. Chem. 2003, 629, 722–726. [Google Scholar] [CrossRef]
- Fowkes, A.; Harrison, W.T.A. (Nitrato-κ2O, O′)bis(triethanolamine-κ4N, O,O′,O″)lanthanum(III) dinitrate. Acta Crystallogr. Sect. C 2006, 62, m232–m233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Obrai, S.; Jassal, A.K.; Hundai, M.S. Supramolecular architectures of N, N, N′, N′-tetrakis(2-hydroxyethyl)ethylenediamine and tris(2-hydroxyethyl)amine with La(III) picrate. RSC Adv. 2014, 4, 59248–59264. [Google Scholar] [CrossRef]
- Langley, S.K.; Moubaraki, B.; Forsyth, G.M.; Gass, I.A.; Murray, K.S. Structure and magnetism of new lanthanide 6-wheel compounds utilizing triethanolamine as a stabilizing ligand. Dalton Trans. 2010, 39, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Moubaraki, B.; Murray, K.S. Magnetic Properties of Hexanuclear Lanthanide(III) Clusters Incorporating a Central μ6-Carbonate Ligand Derived from Atmospheric CO2 Fixation. Inorg. Chem. 2012, 51, 3947–3949. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Moubaraki, B.; Murray, K.S. Trinuclear, octanuclear and decanuclear dysprosium(III) complexes: Synthesis, structural and magnetic studies. Polyhedron 2013, 64, 255–261. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Zhao, L.; Lin, S.-Y.; Xue, S.; Tang, J.; Liu, Z. Two Locally Chiral Dysprosium Compounds with Salen-Type Ligands That Show Slow Magnetic Relaxation Behaviour. Eur. J. Inorg. Chem. 2013, 1351–1357. [Google Scholar] [CrossRef]
- Naini, A.A.; Young, V.; Verkade, J.G. New complexes of Triethanolamine (TEA): Novel Structural Features of [Y(TEA)2](ClO4)3·3C5H5N and [Cd(TEA)2](NO3)2. Polyhedron 1995, 14, 393–400. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Zagoraiou, E.; Savvidou, A.; Raptopoulou, C.P.; Psycharis, V.; Szyrwiel, L.; Holyńska, M.; Perlepes, S.P. Binding of oxime group to uranyl ion. Dalton Trans. 2016, 45, 9307–9319. [Google Scholar] [CrossRef] [PubMed]
- Kitos, A.A.; Efthymiou, C.G.; Manos, M.J.; Tasiopoulos, A.J.; Nastopoulos, V.; Escuer, A.; Perlepes, S.P. Interesting Copper(II)-assisted transformations of 2-acetylpyridine and 2-benzoylpyridine. Dalton Trans. 2016, 45, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-M.; Yu, B.-Y.; Hecke, V.K.; Cui, G.-H. A series of d10 metal coordination polymers based on a flexible bis(2-methylbenzimidazole) ligand and different carboxylates: Synthesis, structures, photoluminescence and catalytic properties. CrystEngComm 2015, 17, 2279–2293. [Google Scholar] [CrossRef]
- Cui, G.-H.; He, C.-H.; Jiao, C.-H.; Geng, J.-C.; Blatov, V.A. Two metal-organic frameworks with unique high-connected binodal network topologies: Synthesis, structures, and catalytic properties. CrystEngComm 2012, 14, 4210–4216. [Google Scholar] [CrossRef]
- For example, see: Polyzou, C.D.; Nikolaou, H.; Papatriantafyllopoulou, C.; Psycharis, V.; Terzis, A.; Raptopoulou, C.P.; Escuer, A.; Perlepes, S.P. Employment of methyl 2-pyridyl ketone oxime in 3d/4f-metal chemistry: Dinuclear nickel(II)/lanthanide(III) species and complexes containing the metals in separate ions. Dalton Trans. 2012, 41, 13755–13764. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Girera, J.; Alemany, P.; Alvarez, S. SHAPE, Continuous Shape Measures Calculation, version 2.0; Universitat de Barcelona: Barcelona, Spain, 2010. [Google Scholar]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kepert, D.L. Inorganic Stereochemistry; Springer: Berlin, Germany, 1982; pp. 179–187. [Google Scholar]
- Long, J.; Habib, F.; Lin, P.-H.; Korobkov, I.; Enright, G.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Single-Molecule Magnet Behaviour for an Antiferromagnetically Superexchange-Coupled Dinuclear Dysprosium(III) Complex. J. Am. Chem. Soc. 2011, 133, 5319. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Chilton, N.F.; Collison, D.; McInnes, E.J.I.; Winpenny, R.E.P.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Kettle, S.F.A. Physical Inorganic Chemistry-A Coordination Chemistry Approach; Oxford University Press: Oxford, UK, 1998; pp. 462–465. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Polynuclear d- and f-block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- CrystalClear, version 1.40; Rigaku/MSC: The Woodlands, TX, USA, 2005.
- Sheldrick, G.M. SHELXS 97, Program for Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C. 2005, 71, 3–8. [Google Scholar]
- DIAMOND, Crystal and Molecular Structure Visualization, version 3.1; Crystal Impact: Bonn, Germany.

| Parameter | 1·2MeOH | 2·2MeOH | 4·2MeOH | 6 |
|---|---|---|---|---|
| Formula | C14H36N6Pr2O20 | C14H36N6Gd2O20 | C14H36N6Dy2O20 | C12H30N5PrO15 |
| Formula weight | 890.31 | 922.99 | 933.49 | 652.32 |
| Crystal system | triclinic | triclinic | triclinic | monoclinic |
| Space group | ||||
| Radiation | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| T/K | 160 | 230 | 160 | 160 |
| a/Å | 8.3271(2) | 8.3299(4) | 8.2897(4) | 11.5500(2) |
| b/Å | 8.6978(2) | 8.6424(4) | 8.6055(4) | 14.8428(3) |
| c/Å | 10.3787(3) | 10.3733(5) | 10.2855(5) | 13.8550(3) |
| α/° | 86.893(1) | 86.964(2) | 86.892(1) | 90 |
| β/° | 80.373(1) | 79.310(1) | 79.141(1) | 107.393(1) |
| γ/° | 84.632(1) | 84.494(2) | 84.532(1) | 90 |
| V/Å3 | 737.30(3) | 729.96(6) | 716.83(6) | 2266.62(8) |
| Z | 1 | 1 | 1 | 4 |
| Dcalc/g·cm−3 | 2.005 | 2.100 | 2.162 | 1.832 |
| μ/mm−1 | 3.36 | 4.60 | 5.27 | 2.23 |
| Reflns measured | 15028 | 13695 | 9845 | 21716 |
| Reflns unique (Rint) | 3210(0.018) | 3184(0.024) | 3119(0.026) | 2463(0.021) |
| Reflns with I > 2σ(I) | 3143 | 3099 | 3042 | 2424 |
| GOF on F2 | 1.12 | 1.07 | 1.07 | 1.08 |
| R1a [I > 2σ(I)] | 0.0129 | 0.0129 | 0.0142 | 0.0134 |
| wR2 b (all data) | 0.0305 | 0.0292 | 0.0332 | 0.0347 |
| Interatomic Distances (Å) a | |||
|---|---|---|---|
| Dy1···Dy1' | 3.669(1) | Dy1–O8 | 2.527(2) |
| Dy1–O1 | 2.265(1) | Dy1–N1 | 2.653(2) |
| Dy1···O1' | 2.236(1) | N2–O4 | 1.261(2) |
| Dy1–O2 | 2.363(1) | N2–O5 | 1.261(2) |
| Dy1–O3 | 2.466(2) | N2–O6 | 1.233(2) |
| Dy1–O4 | 2.564(2) | N3–O7 | 1.280(2) |
| Dy1–O5 | 2.492(2) | N3–O8 | 1.263(2) |
| Dy1–O7 | 2.463(2) | N3–O9 | 1.216(2) |
| Bond Angles (Å) a | |||
| O1–Dy1–O1' | 70.8(1) | O5–Dy1–O1 | 122.3(1) |
| O1–Dy1–O2 | 95.5(1) | O5–Dy1–O7 | 73.7(1) |
| O1–Dy1–O7 | 157.3(1) | O7–Dy1–O8 | 51.2(1) |
| O1'–Dy1–O3 | 149.6(1) | O8–Dy1–O5 | 67.7(1) |
| O2–Dy1–O5 | 136.2(1) | N1–Dy1–O2 | 65.8(1) |
| O2–Dy1–O7 | 78.4(1) | N1–Dy1–O5 | 144.5(1) |
| O3–Dy1–O5 | 77.9(1) | Dy1–O1–Dy1' | 109.2(1) |
| O3-Dy1-O8 | 114.8(1) | O4–N2–O5 | 117.4(2) |
| O4–Dy1–O5 | 50.4(1) | O4–N2–O6 | 120.9(2) |
| O4–Dy1–O7 | 113.2(1) | O5–N2–O6 | 121.7(2) |
| Bond Lengths (Å) | |||
|---|---|---|---|
| Pr1–O1 | 2.514(1) | N2–O5 | 1.224(2) |
| Pr1–O2 | 2.502(1) | N1–C1A | 1.487(4) |
| Pr1–O3 | 2.521(1) | C1A–C2A | 1.518(4) |
| Pr1–O4 | 2.584(1) | C2A–O1 | 1.429(2) |
| Pr1–N1 | 2.743(1) | C3–O2 | 1.434(2) |
| N2–O4 | 1.271(1) | C5–O3 | 1.430(2) |
| Bond Angles (°) a | |||
| O1–Pr1–O2 | 115.6(1) | O4–Pr1–O4' | 49.6(1) |
| O1–Pr1–O3 | 77.1(1) | O4–Pr1–N1 | 82.5(1) |
| O1–Pr1–O4 | 69.6(1) | O4'–Pr1–N1 | 122.5(1) |
| O1–Pr1–N1 | 63.4(1) | O4–N2–O4' | 116.9(2) |
| O2–Pr1–O2' | 172.0(5) | O4–N2–O5 | 121.6(1) |
| O3–Pr1–O3' | 70.2(1) | C1A–C2A–O1 | 107.4(2) |
| N1–Pr1–Nq' | 154.1(1) | C6–C5–O3 | 108.1(1) |
| Complex a | Coordination Mode b | Coordination Polyhedra | Ref. |
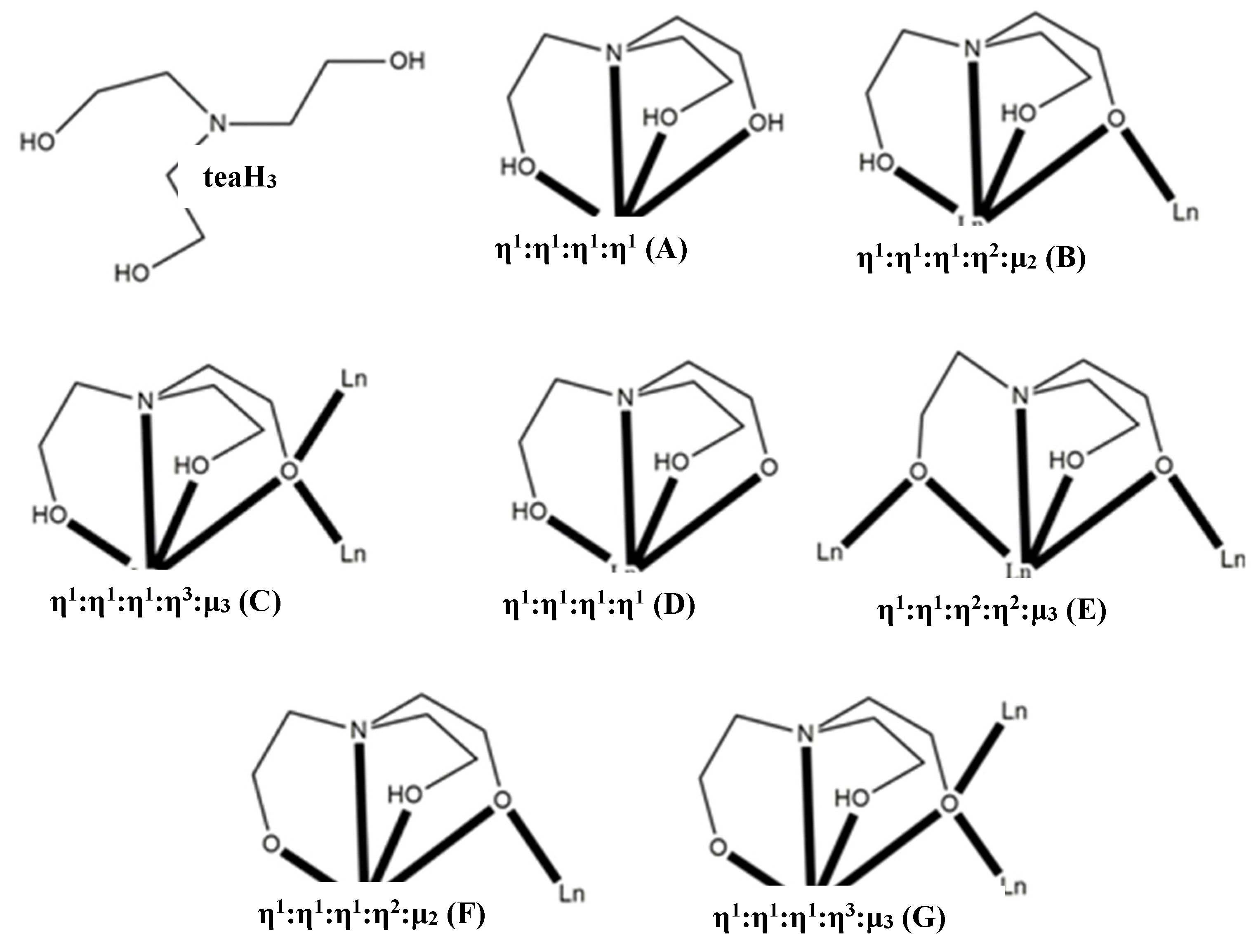
|---|---|---|---|
| [LnIII(teaH3)2](CF3SO3)3 (Ln = Pr, Yb, Lu) | η1:η1:η1:η1 (Α) | CSAPR j | [32] |
| [LnII(teaH3)2](ClO4)2 (Ln = Eu, Yb) | η1:η1:η1:η1 (Α) | n.r. k, BCATAPR l | [33,34] |
| [LnIII(NO3)(teaH3)2](NO3)2 (Ln = La, Pr) | η1:η1:η1:η1 (Α) | SPC m | [35], this work |
| [LnIII(teaH3)2(H2O)2](pic)2 (Ln = La) c | η1:η1:η1:η1 (Α) | BCSASPR n | [36] |
| [Ln6III(NO3)6(teaH3)6] (Ln = Gd, Dy) | η1:η1:η2:η2:μ3 (Ε) | SAPR o | [37] |
| [Ln6III(CO3)(NO3)2(chp)7(teaH2)2(teaH)2(H2O)](NO3) (Ln = Gd, Tb, Dy) d | η1:η1:η1:η3:μ3 (C) e,η1:η1:η2:η2:μ3 (E) f | SAPR o, TCTPR p | [38] |
| [Ln3III(OH)(teaH2)3(paa)3]Cl2 (Ln = Dy) g | η1:η1:η1:η2:μ2 (B) | SAPR o | [39] |
| [Ln8III(OH)6(teaH)6(teaH2)2(teaH3)2](CF3SO3)4 (Ln = Dy) | η1:η1:η1:η3:μ3 (G)f,η1:η1:η1:η2:μ2 (F) f, η1:η1:η1:η1 (A,D) h | SAPR o, CSAPR j | [39] |
| [Ln2III(L)(teaH2)6(o-van)(H2L)(H2O)](ClO4)2i | η1:η1:η1:η2:μ2 (B) | TCTPR p | [40] |
| [Ln2III(NO3)4(teaH2)2] (Ln = Pr, Gd, Dy) | η1:η1:η1:η2:μ2 (B) | CCU q, TCTPR p (Ln = Gd), CSAPR j (Ln = Dy) | this work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylonas-Margaritis, I.; Mayans, J.; Sakellakou, S.-M.; P. Raptopoulou, C.; Psycharis, V.; Escuer, A.; P. Perlepes, S. Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies. Magnetochemistry 2017, 3, 5. https://doi.org/10.3390/magnetochemistry3010005
Mylonas-Margaritis I, Mayans J, Sakellakou S-M, P. Raptopoulou C, Psycharis V, Escuer A, P. Perlepes S. Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies. Magnetochemistry. 2017; 3(1):5. https://doi.org/10.3390/magnetochemistry3010005
Chicago/Turabian StyleMylonas-Margaritis, Ioannis, Julia Mayans, Stavroula-Melina Sakellakou, Catherine P. Raptopoulou, Vassilis Psycharis, Albert Escuer, and Spyros P. Perlepes. 2017. "Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies" Magnetochemistry 3, no. 1: 5. https://doi.org/10.3390/magnetochemistry3010005
APA StyleMylonas-Margaritis, I., Mayans, J., Sakellakou, S.-M., P. Raptopoulou, C., Psycharis, V., Escuer, A., & P. Perlepes, S. (2017). Using the Singly Deprotonated Triethanolamine to Prepare Dinuclear Lanthanide(III) Complexes: Synthesis, Structural Characterization and Magnetic Studies. Magnetochemistry, 3(1), 5. https://doi.org/10.3390/magnetochemistry3010005









