1D Chains of Lanthanoid Ions and a Dithienylethene Ligand Showing Slow Relaxation of the Magnetization
Abstract
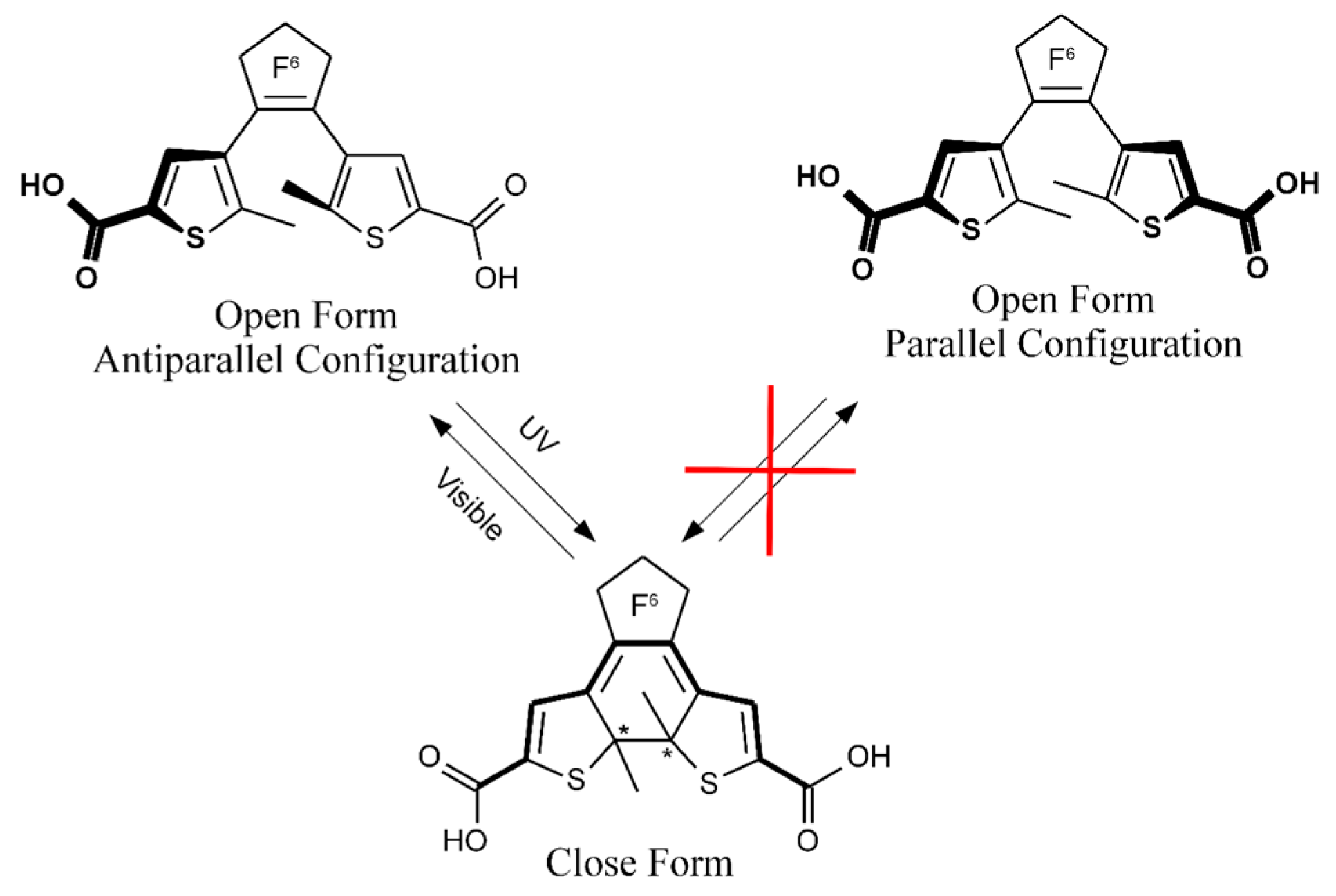
:1. Introduction
2. Materials and Methods
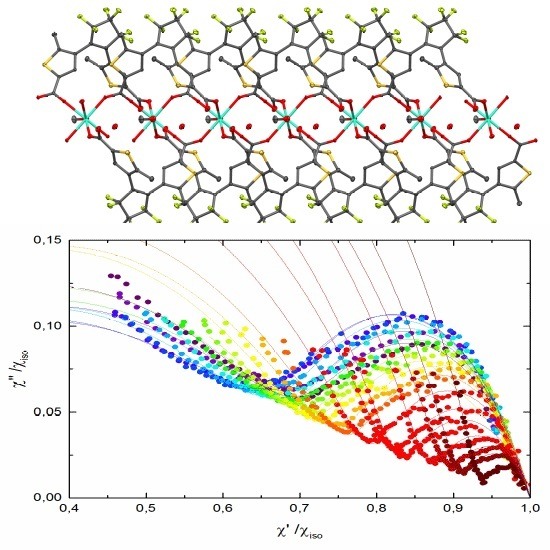
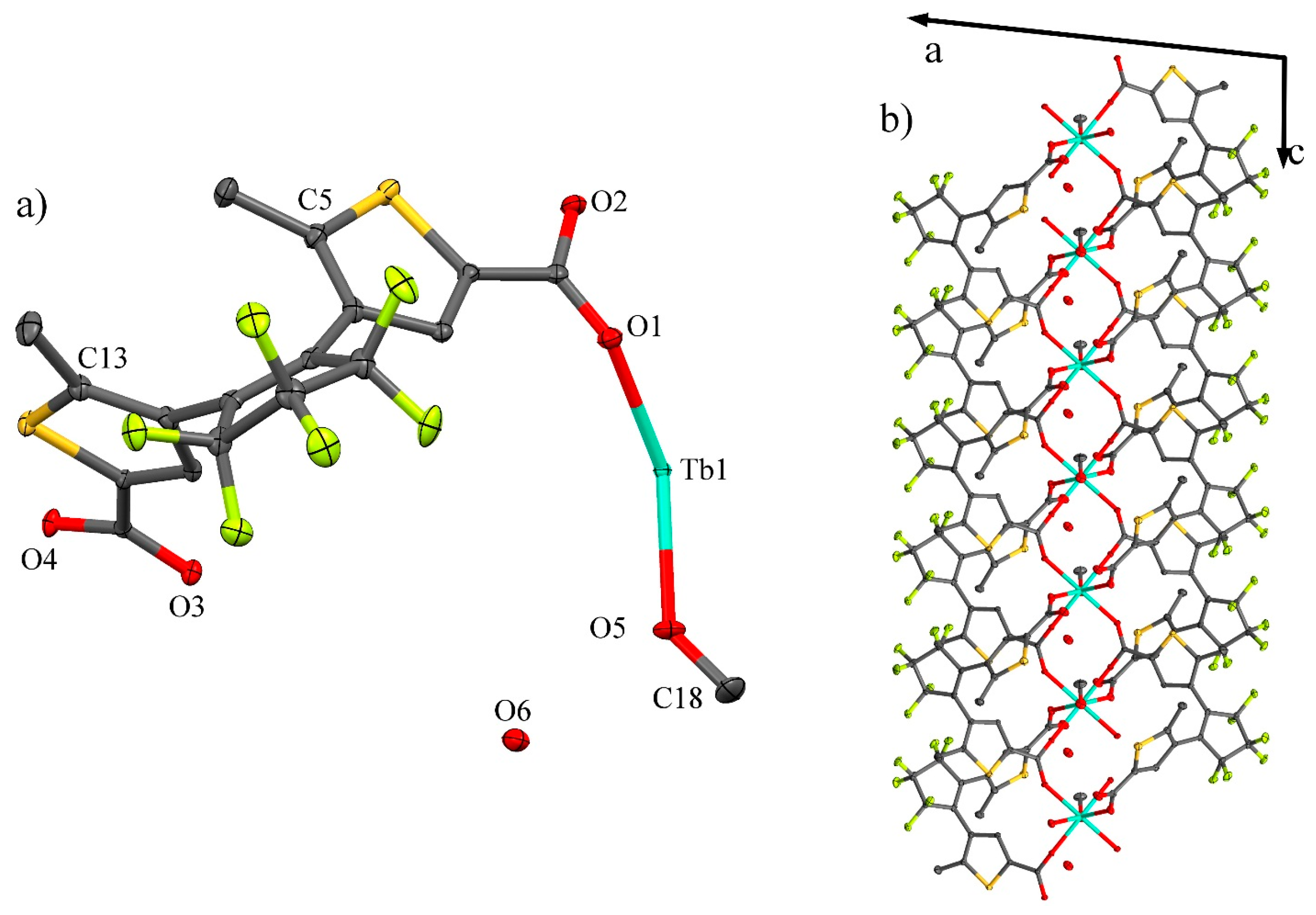
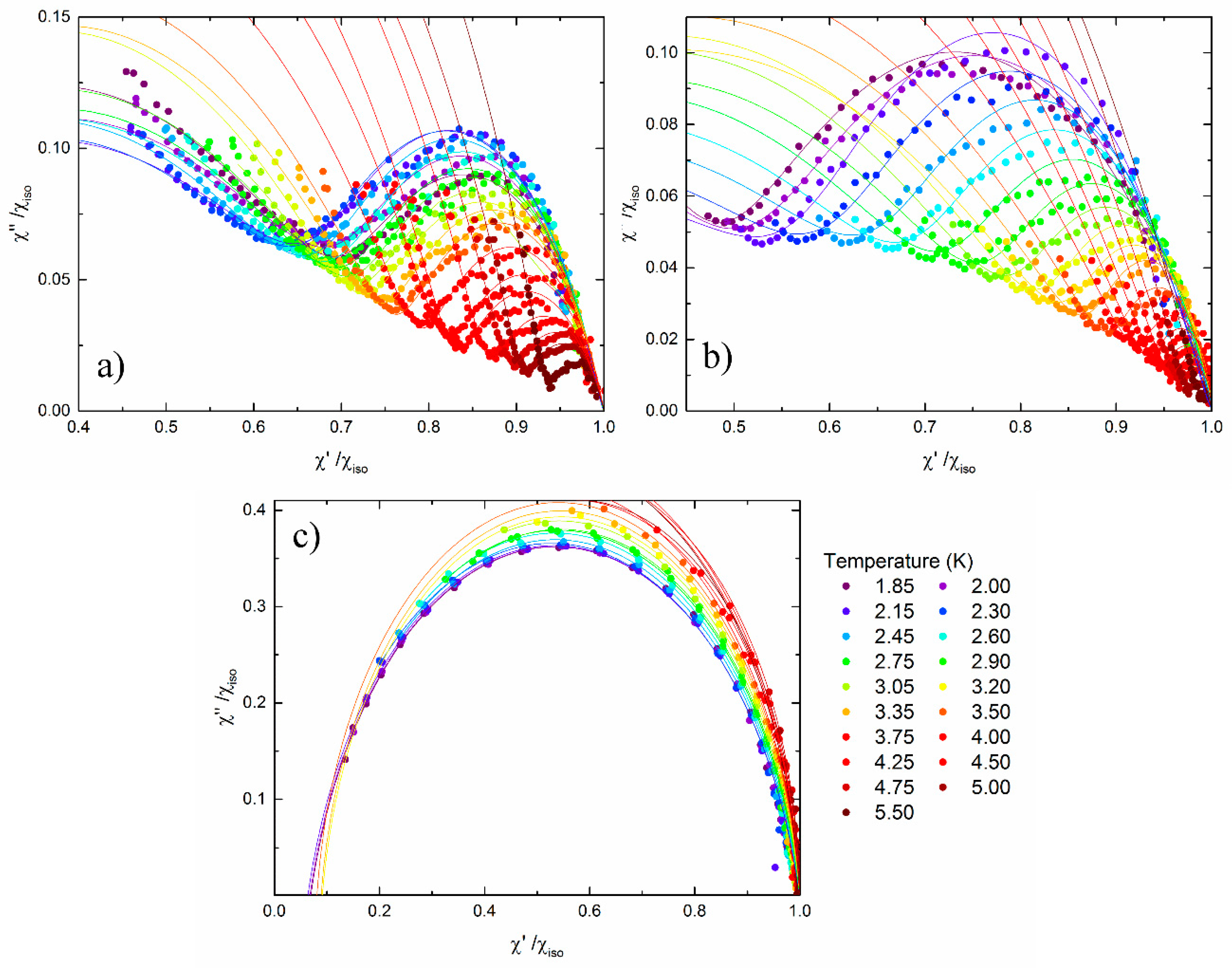
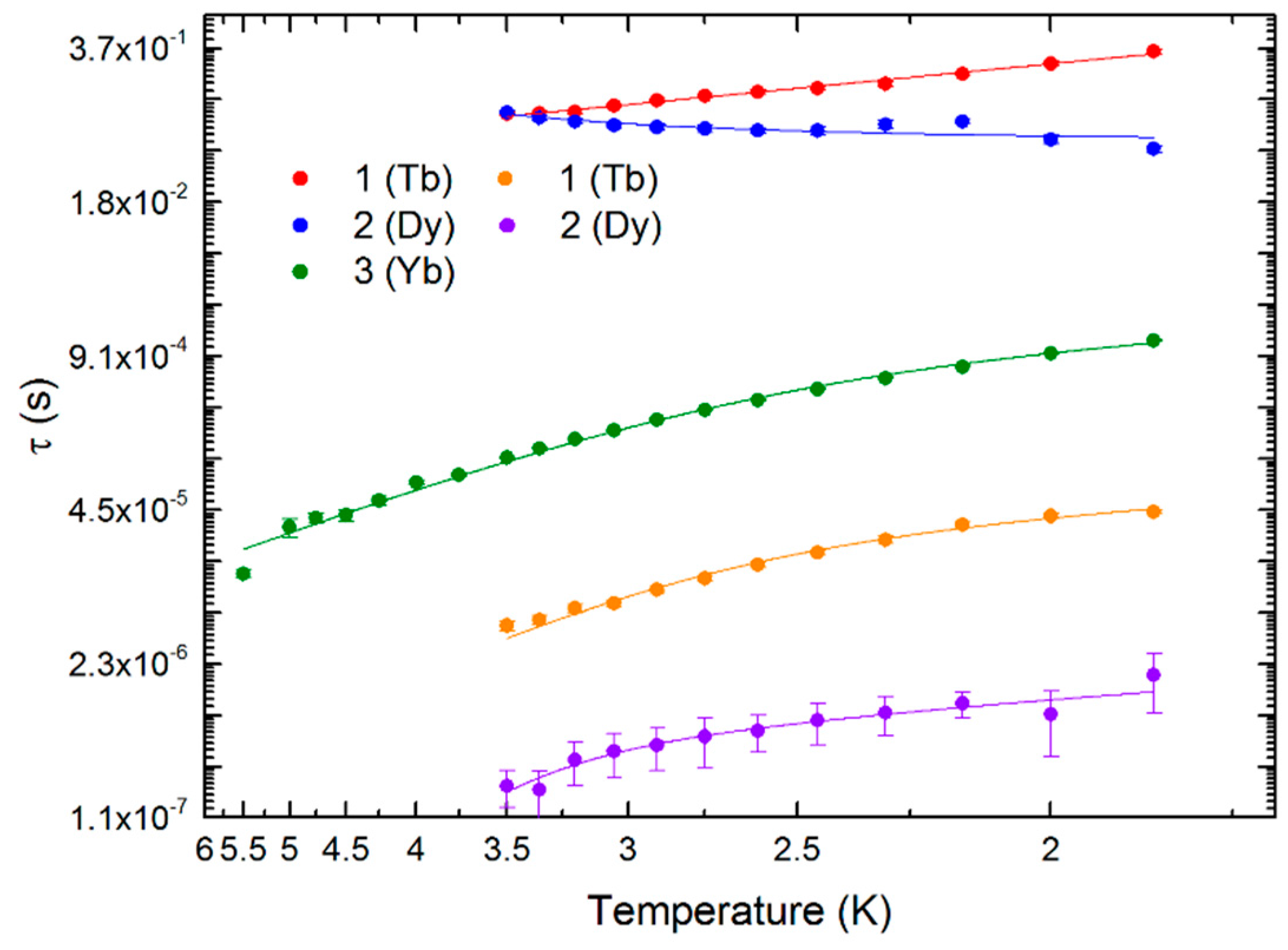
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Katoh, K.; Isshiki, H.; Komeda, T.; Yamashita, M. Molecular spintronics based on single-molecule magnets composed of multiple-decker phthalocyaninato terbium(III) complex. Chem. Asian J. 2012, 7, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.P.; Moro, F.; McMaster, J.; van Slageren, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. A delocalized arene-bridged diuranium single-molecule magnet. Nat. Chem. 2011, 3, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R. Quantum tunneling of magnetization and related phenomena in molecular materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; van Slageren, J. Improving f-element single molecule magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Sorace, L.; Wernsdorfer, W.; Thirion, C.; Barra, A.-L.; Pacchioni, M.; Mailly, D.; Barbara, B. Photon-assisted tunneling in a Fe8 single-molecule magnet. Phys. Rev. 2003, 68. [Google Scholar] [CrossRef]
- Inose, T.; Tanaka, D.; Tanaka, H.; Ivasenko, O.; Nagata, T.; Ohta, Y.; de Feyter, S.; Ishikawa, N.; Ogawa, T. Switching of single-molecule magnetic properties of TbIII–porphyrin double-decker complexes and observation of their supramolecular structures on a carbon surface. Chem. Eur. J. 2014, 20, 11362–11369. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Ren, M.; Zheng, L.-M.; Sato, S.; Hasegawa, M.; Morimoto, M.; Irie, M.; Breedlove, B.K.; Cosquer, G.; Katoh, K.; et al. Control of the single-molecule magnet behavior of lanthanide-diarylethene photochromic assemblies by irradiation with light. Chem. Eur. J. 2014, 20, 12502–12513. [Google Scholar] [CrossRef] [PubMed]
- Irie, M. Diarylethenes for memories and switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Irie, M. Photochromic reactions of diarylethenes in single crystals with intermolecular O–H···N hydrogen-bonding networks. Chem. Eur. J. 2006, 12, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- CrystalClear-SM, 1.4.0 SP1, Rigaku Corporation: Tokyo, Japan, 17 April 2008.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M. Modeling hydroxyl and water H atoms. J. Appl. Crystallogr. 1999, 32, 563–571. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal′s constants. J. Chem. Educ. 2008, 85. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers: Weinheim, Germany, 1993. [Google Scholar]
- Katoh, K.; Horii, Y.; Yasuda, N.; Wernsdorfer, W.; Toriumi, K.; Breedlove, B.K.; Yamashita, M. Multiple-decker phthalocyaninato dinuclear lanthanoid(III) single-molecule magnets with dual-magnetic relaxation processes. Dalton Trans. 2012, 41, 13582–13600. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Ungur, L.; Sigrist, M.; Sundt, A.; Schau-Magnussen, M.; Vieru, V.; Mutka, H.; Rols, S.; Weihe, H.; Waldmann, O.; et al. Modifying the properties of 4f single-ion magnets by peripheral ligand functionalisation. Chem. Sci. 2014, 5, 1650–1660. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]

| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C35H22F12O10S4Tb | C35H22F12O10S4Dy | C35H22F12O10S4Yb |
| Mr/g mol−1 | 1117.70 | 1121.27 | 1131.80 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| a/Å | 35.9505(106) | 35.8577(39) | 36.0071(168) |
| b/Å | 10.3997(30) | 10.3531(8) | 10.3359(46) |
| c/Å | 10.4496(30) | 10.4158(11) | 10.3599(48) |
| β/° | 95.9205(35) | 96.0006(50) | 95.5797(64) |
| V/Å3 | 3886.0(2) | 3845.6(5) | 3837.3(3) |
| Z | 4 | 4 | 4 |
| T/K | 93.15 | 93.15 | 96.15 |
| Diffraction reflection | 4.118 ≤ 2θ ≤ 27.576 | 4.099 ≤ 2θ ≤ 27.523 | 4.005 ≤ 2θ ≤ 27.431 |
| ρcalcd/g cm−3 | 1.910 | 1.937 | 1.959 |
| μ/mm−1 | 2.150 | 2.277 | 2.771 |
| Number of reflections | 7573 | 15193 | 7322 |
| Independent reflections | 4395 | 4439 | 4243 |
| F02 ˃ 2σ(F0)2 | 3847 | 3941 | 2506 |
| Number of variables | 322 | 322 | 285 |
| Rint, R1, wR2 | 0.0226, 0.0271, 0.0599 | 0.0321, 0.0252, 0.0544 | 0.0850, 0.0827, 0.2130 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatoo, M.A.; Cosquer, G.; Morimoto, M.; Irie, M.; Breedlove, B.K.; Yamashita, M. 1D Chains of Lanthanoid Ions and a Dithienylethene Ligand Showing Slow Relaxation of the Magnetization. Magnetochemistry 2016, 2, 21. https://doi.org/10.3390/magnetochemistry2020021
Yatoo MA, Cosquer G, Morimoto M, Irie M, Breedlove BK, Yamashita M. 1D Chains of Lanthanoid Ions and a Dithienylethene Ligand Showing Slow Relaxation of the Magnetization. Magnetochemistry. 2016; 2(2):21. https://doi.org/10.3390/magnetochemistry2020021
Chicago/Turabian StyleYatoo, Mudasir Ahmad, Goulven Cosquer, Masakazu Morimoto, Masahiro Irie, Brian K. Breedlove, and Masahiro Yamashita. 2016. "1D Chains of Lanthanoid Ions and a Dithienylethene Ligand Showing Slow Relaxation of the Magnetization" Magnetochemistry 2, no. 2: 21. https://doi.org/10.3390/magnetochemistry2020021
APA StyleYatoo, M. A., Cosquer, G., Morimoto, M., Irie, M., Breedlove, B. K., & Yamashita, M. (2016). 1D Chains of Lanthanoid Ions and a Dithienylethene Ligand Showing Slow Relaxation of the Magnetization. Magnetochemistry, 2(2), 21. https://doi.org/10.3390/magnetochemistry2020021