Spin Transition Kinetics in the Salt [H2N(CH3)2]6[Fe3(L)6(H2O)6] (L = 4-(1,2,4-triazol-4-yl)ethanedisulfonate)
Abstract
:1. Introduction
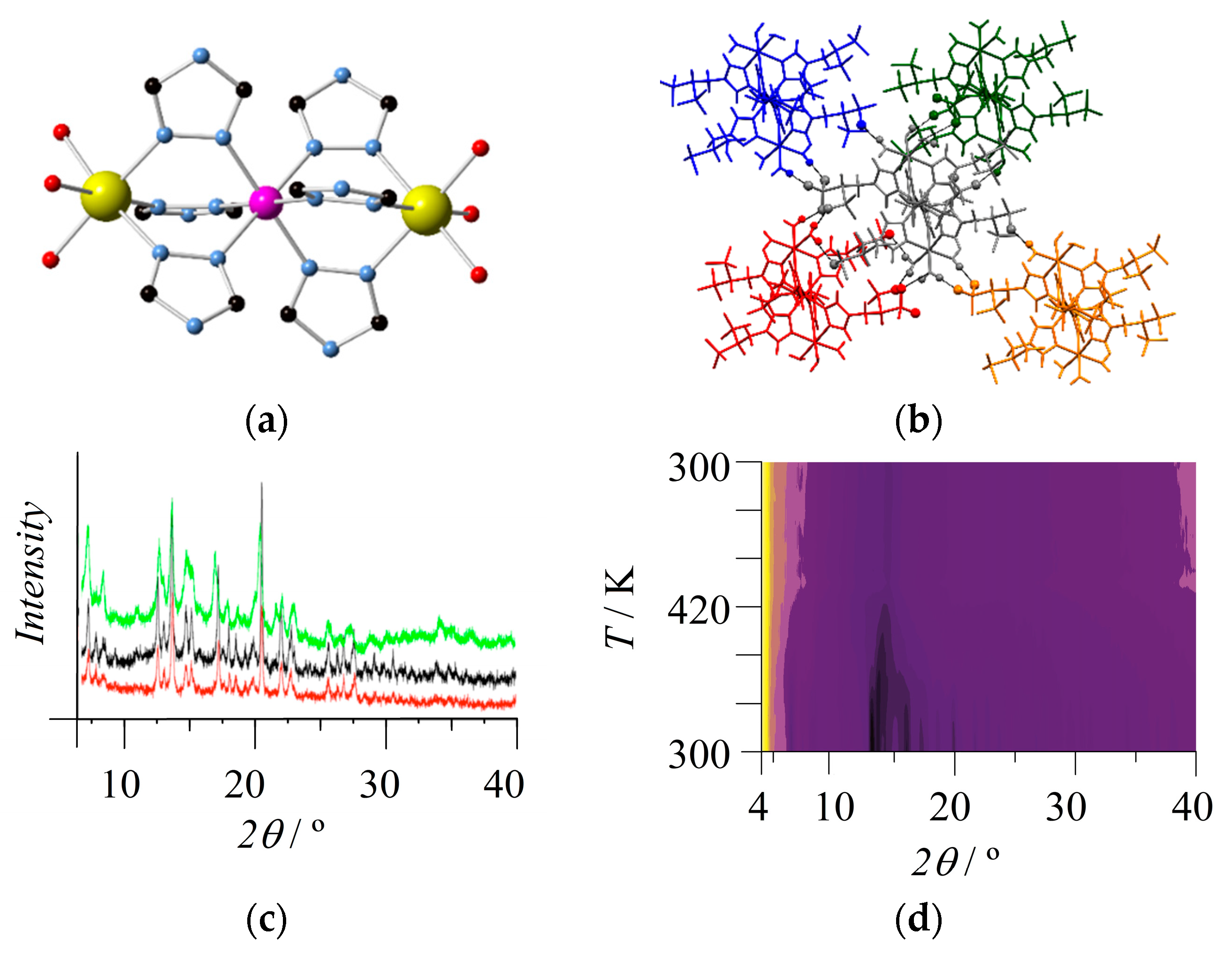
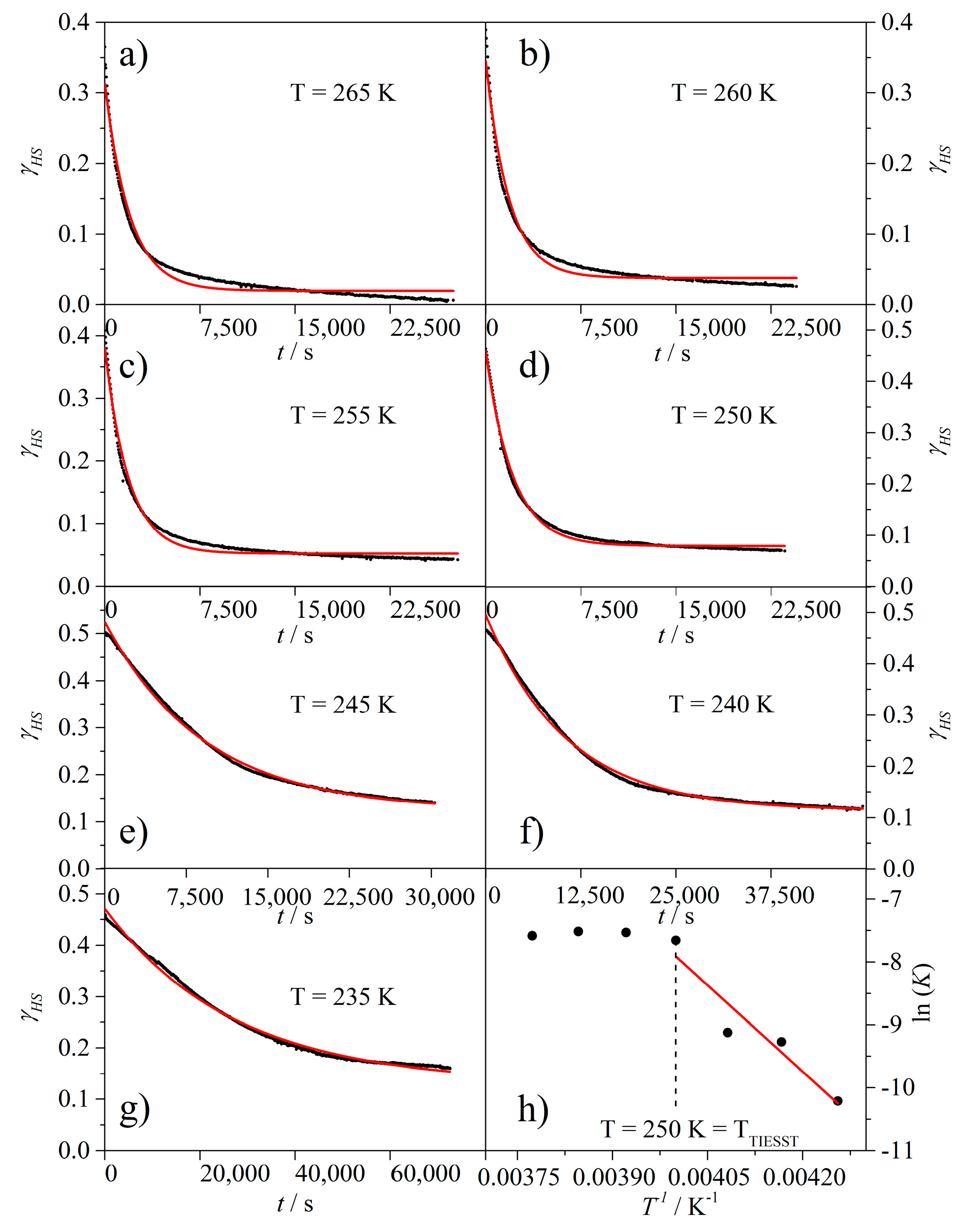
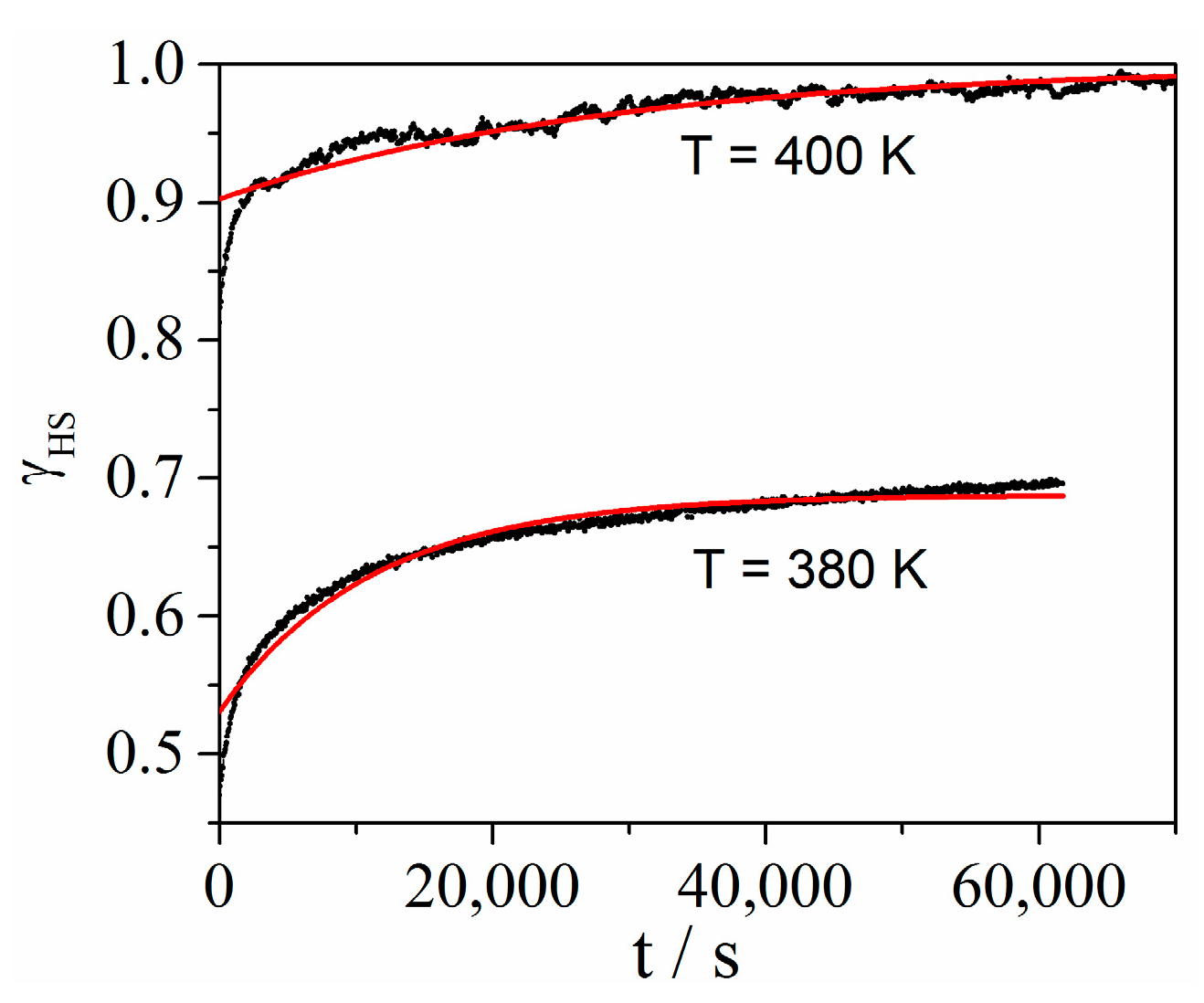
2. Results and Discussion
3. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds III; Springer-Verlag: Berlin Heidelberg, Germany, 2004.
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo- switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure: Function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.; Martinez, C. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Letard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. In Spin Crossover in Transition Metal Compounds III; Springer-Verlag: Berlin Heidelberg, Germany, 2004; pp. 221–249. [Google Scholar]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Bertoni, R.; Cammarate, M.; Lorenc, M.; Matar, S.F.; Letard, J.-F.; Lemke, H.T.; Collet, E. Ultrafast light-induced spin-state trapping photophysics investigated in Fe(phen)2(NCS)2 spin-crossover crystal. Acc. Chem. Rev. 2015, 48, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Costa, J.; Balde, C.; Carbonera, C.; Denux, D.; Wattiaux, A.; Desplanches, C.; Ader, J.-P.; Gütlich, P.; Letard, J.-F. Photomagnetic properties of an iron(II) low-spin complex with an unusually long-lived metastable LIESST state. Inorg. Chem. 2007, 46, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, F.; Tobon, Y.A.; Bonhommeau, S.; Letard, J.-F.; Moulet, L.; Freysz, E. Photoswitching of the spin crossover polymeric material [Fe(Htrz)2(trz)](BF4) under continuous laser irradiation in a Raman scattering experiment. Chem. Phys. Lett. 2014, 604, 105–109. [Google Scholar] [CrossRef]
- Craig, G.A.; Sanchez-Costa, J.; Roubeau, O.; Teat, S.J.; Shepherd, H.J.; Lopes, M.; Molnar, G.; Bousseksou, A.; Aromi, G. High-temperature photo-induced switching and pressure-induced transition in a cooperative molecular spin-crossover material. Dalton Trans. 2013, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Murnaghan, K.D.; Carbonera, C.; Toupet, L.; Griffin, M.; Dirtu, M.M.; Desplanches, C.; Garcia, Y.; Collet, E.; Letard, J.-F.; Morgan, G.C. Spin-state ordering on one sub-lattice of a mononuclear iron(II) spin crossover complex exhibiting LIESST and TIESST. Chem. Eur. J. 2014, 20, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.S.; Galan-Mascaros, J.R. Spin crossover probes confer multistability to organic conducting polymers. Adv. Mater. 2014, 26, 6785–6789. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.; Benjamin, S.M.; Steve, E.; Brooks, J.S.; Shatruk, M. Photomagnetic response in highly conductive iron(II) spin-crossover complexes with TCNQ radicals. Angew. Chem. Int. Ed. 2015, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Sáenz de Pipaón, C.; Maldonado-Illescas, P.; Waerenborgh, J.C.; Martin, E.; Benet-Buchholz, J.; Galán-Mascarós, J.R. Easy excited-state trapping and record high TTIESST in a spin-crossover polyanionic FeII trimer. J. Am. Chem. Soc. 2015, 137, 11924–11927. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.; Enachescu, C.; Daku, M.L.; Vargas, A.; Amstutz, N. Low-temperature lifetimes of metastable high-spin states in spin-crossover and in low-spin iron(II) compounds: The rule and exceptions to the rule. Coord. Chem. Rev. 2006, 250, 1642–1652. [Google Scholar] [CrossRef]
- Wang, H.; Sinito, C.; Kaiba, A.; Costa, J.S.; Desplanches, C.; Dagault, P.; Guionneau, P.; Letard, J.-F.; Neǵrier, P.; Mondieig, D. Unusual solvent dependence of a molecule-based FeII macrocyclic spin-crossover complex. Eur. J. Inorg. Chem. 2014, 4927–4933. [Google Scholar]
- Li, D.; Clerac, R.; Roubeau, O.; Harte, E.; Mathoniere, C.; Le Bris, R.; Holmes, S.M. Magnetic and optical bistability driven by thermally and photoinduced intramolecular electron transfer in a molecular cobalt-iron prussian blue analogue. J. Am. Chem. Soc. 2008, 130, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kulmaczewski, R.; Olguin, J.; Kitchen, J.A.; Feltham, H.L.C.; Jameson, G.N.L.; Tallon, J.L.; Brooker, S. Remarkable scan rate dependence for a highly constrained dinuclear iron(II) spin crossover complex with a wide thermal hysteresis loop. J. Am. Chem. Soc. 2014, 136, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Carbonera, C.; Dei, A.; Sangregorio, C.; Létard, J.-F. Optically switchable behaviour of a dioxolene adduct of a cobalt-macrocycle complex. Chem. Phys. Lett. 2004, 396, 198–201. [Google Scholar] [CrossRef]
- Hauser, A.; Jeftić, J.; Romstedt, H.; Hinek, R.; Spiering, H. Cooperative phenomena and light-induced bistability in iron(II) spin-crossover compounds. Coord. Chem. Rev. 1999, 190–192, 471–491. [Google Scholar] [CrossRef]
- Paradis, N.; Chastanet, G.; Letard, J.-F. When stable and metastable HS states meet in spin-crossover compounds. Eur. J. Inorg. Chem. 2012, 2012, 3618–3624. [Google Scholar] [CrossRef]
- Letard, J.-F. Photomagnetism of iron(II) spin crossover complexes-the TLIESST approach. J. Mater. Chem. 2006, 16, 2550–2559. [Google Scholar] [CrossRef]
- Paradis, N.; Chastanet, G.; Palamarciuc, T.; Rosa, P.; Varret, F.; Boukheddaden, K.; Letard, J.-F. Detailed investigation of the interplay between the thermal decay of the low temperature metastable HS state and the thermal hysteresis of spin-crossover solids. J. Phys. Chem. C 2015, 119, 20039–20050. [Google Scholar] [CrossRef]
- Gómez, V.; Benet-Buchholz, J.; Martin, E.; Galán-Mascarós, J.R. Hysteretic spin crossover above room temperature and magnetic coupling in trinuclear transition-metal complexes with anionic 1,2,4-triazole ligands. Chem. Eur. J. 2014, 20, 5369–5379. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáenz de Pipaón, C.; Maldonado-Illescas, P.; Gómez, V.; Galán-Mascarós, J.R. Spin Transition Kinetics in the Salt [H2N(CH3)2]6[Fe3(L)6(H2O)6] (L = 4-(1,2,4-triazol-4-yl)ethanedisulfonate). Magnetochemistry 2016, 2, 20. https://doi.org/10.3390/magnetochemistry2020020
Sáenz de Pipaón C, Maldonado-Illescas P, Gómez V, Galán-Mascarós JR. Spin Transition Kinetics in the Salt [H2N(CH3)2]6[Fe3(L)6(H2O)6] (L = 4-(1,2,4-triazol-4-yl)ethanedisulfonate). Magnetochemistry. 2016; 2(2):20. https://doi.org/10.3390/magnetochemistry2020020
Chicago/Turabian StyleSáenz de Pipaón, Cristina, Pilar Maldonado-Illescas, Verónica Gómez, and José Ramón Galán-Mascarós. 2016. "Spin Transition Kinetics in the Salt [H2N(CH3)2]6[Fe3(L)6(H2O)6] (L = 4-(1,2,4-triazol-4-yl)ethanedisulfonate)" Magnetochemistry 2, no. 2: 20. https://doi.org/10.3390/magnetochemistry2020020
APA StyleSáenz de Pipaón, C., Maldonado-Illescas, P., Gómez, V., & Galán-Mascarós, J. R. (2016). Spin Transition Kinetics in the Salt [H2N(CH3)2]6[Fe3(L)6(H2O)6] (L = 4-(1,2,4-triazol-4-yl)ethanedisulfonate). Magnetochemistry, 2(2), 20. https://doi.org/10.3390/magnetochemistry2020020






