Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals
Abstract
:1. Introduction
2. Mononuclear Iron(II) Complexes
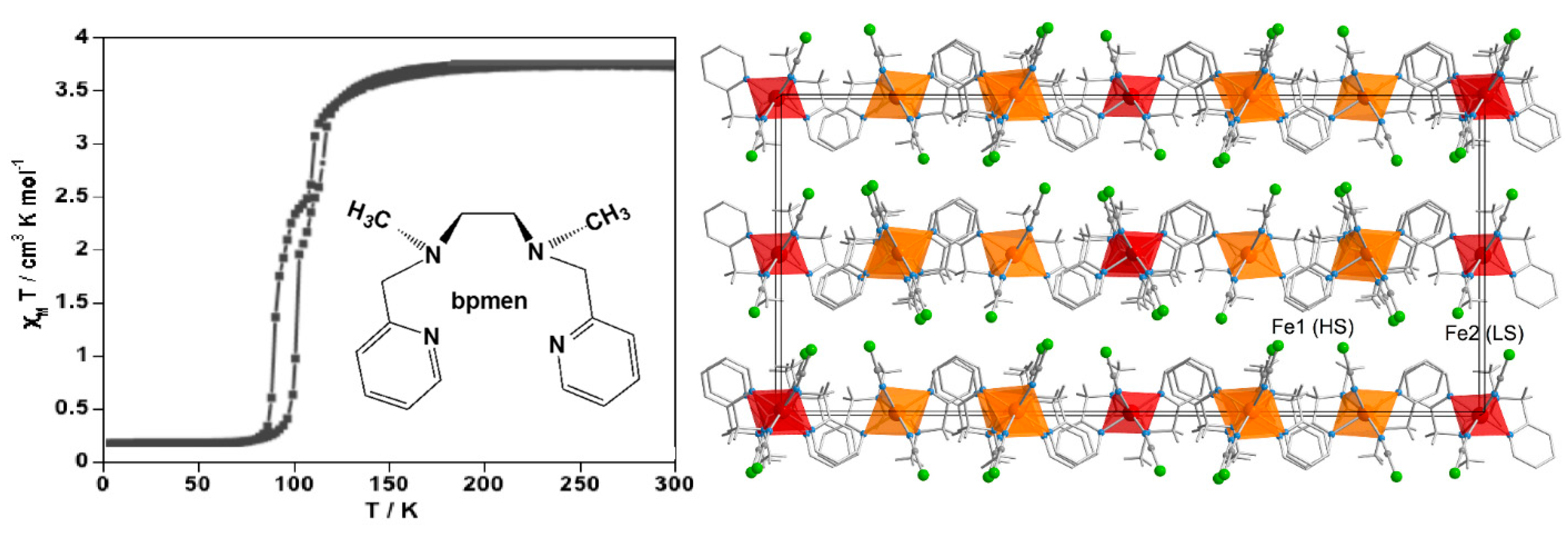
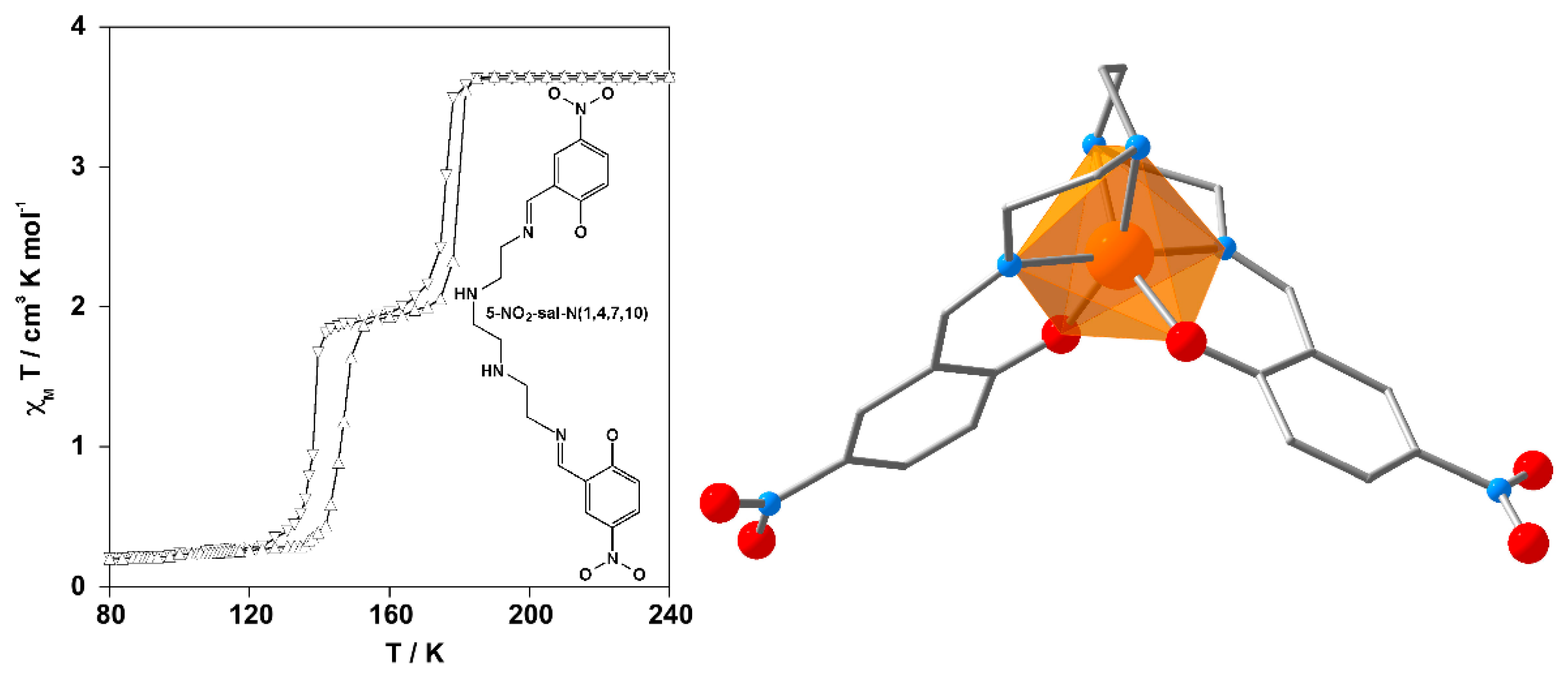
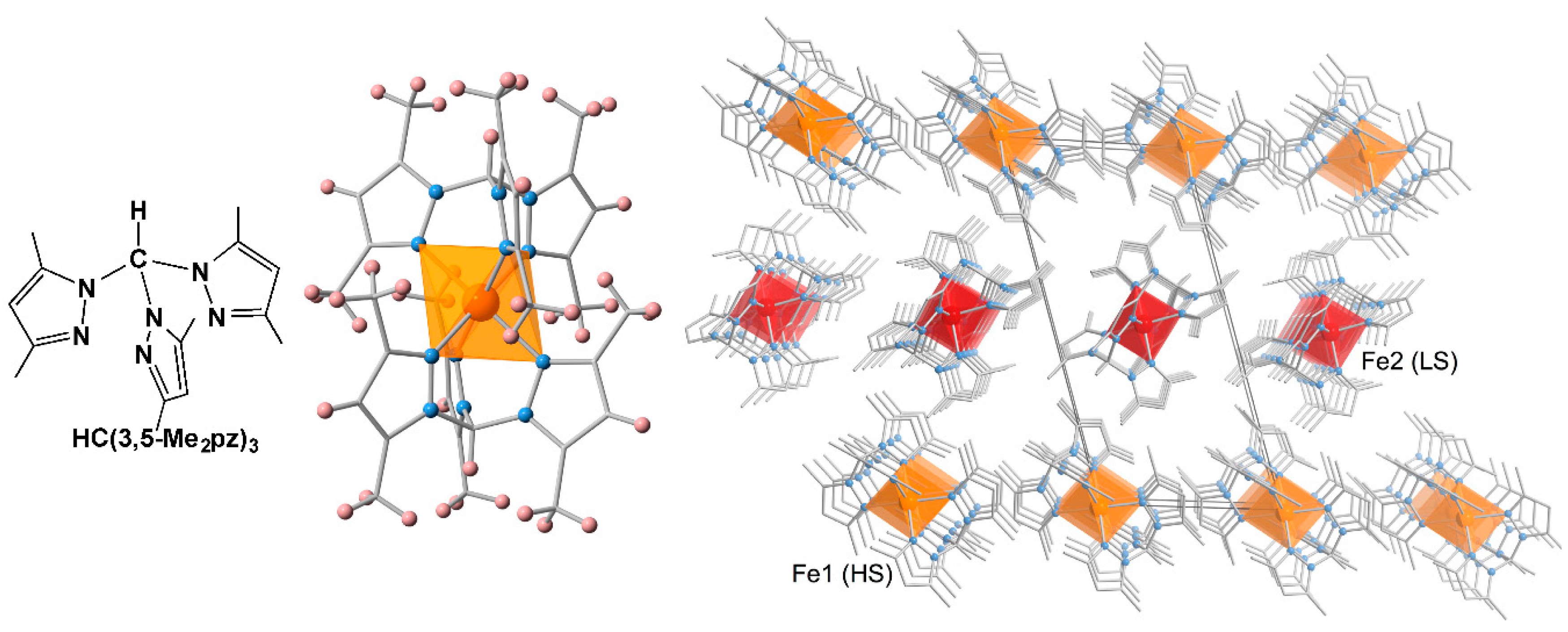
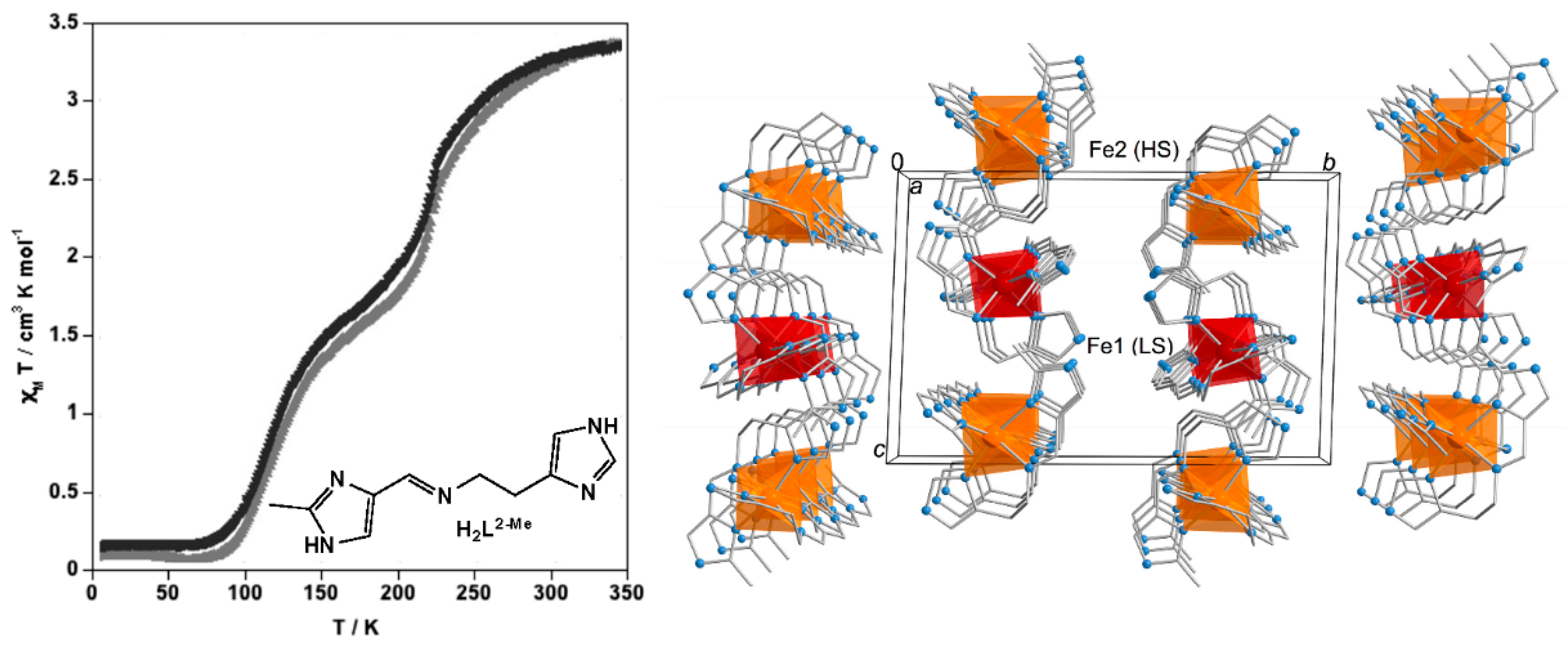
2.1. Stepwise SCO Behavior
2.2. One-Step SCO Behavior
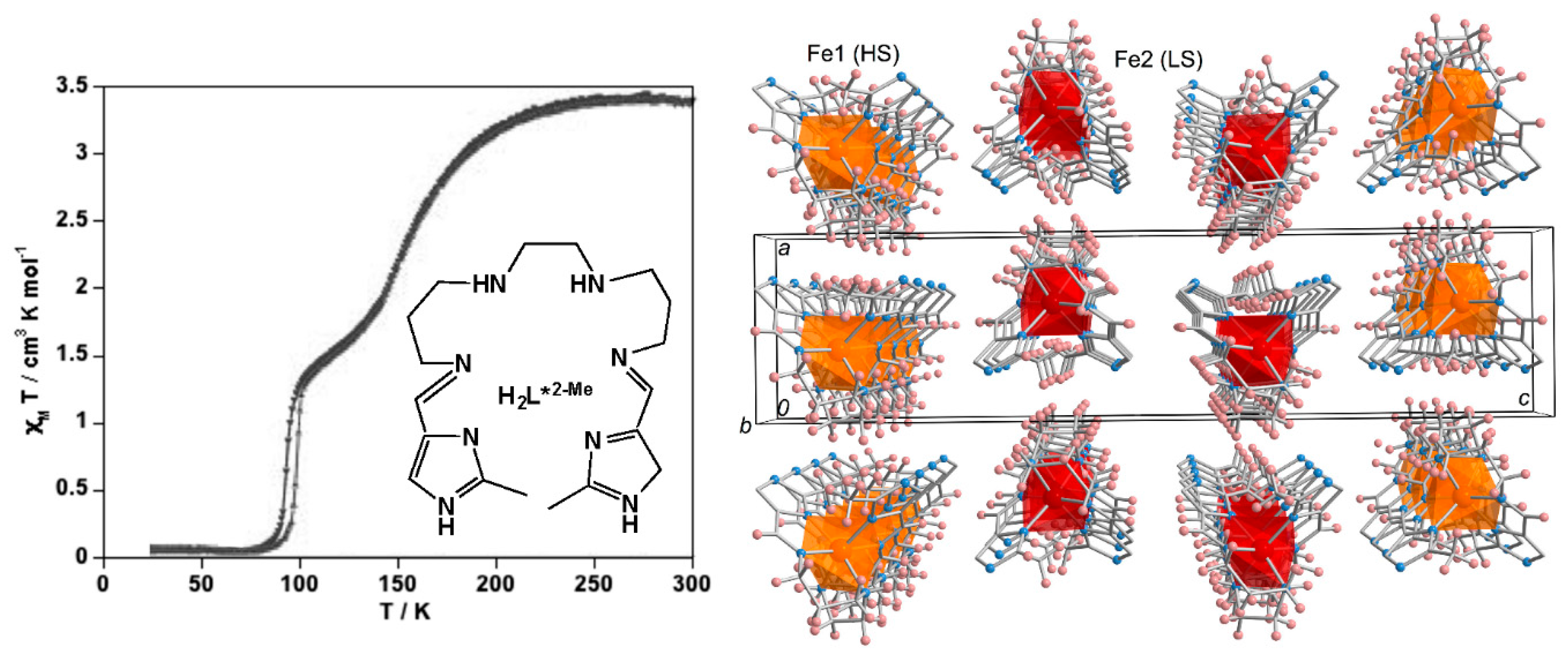
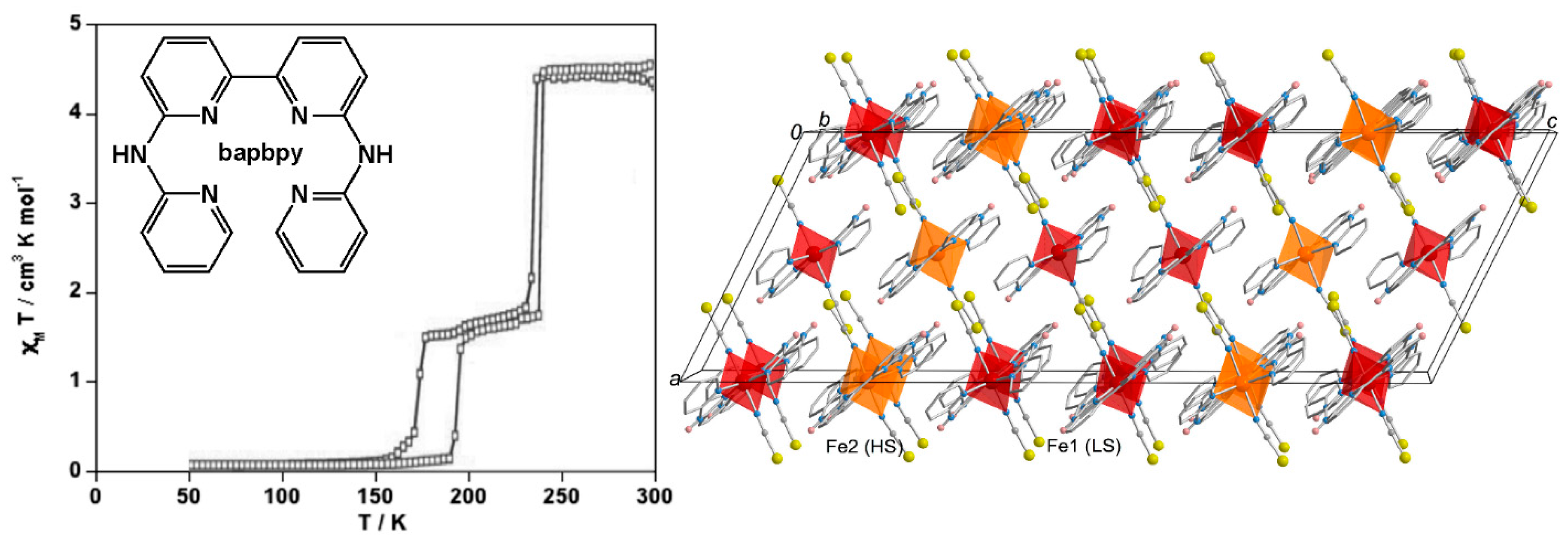
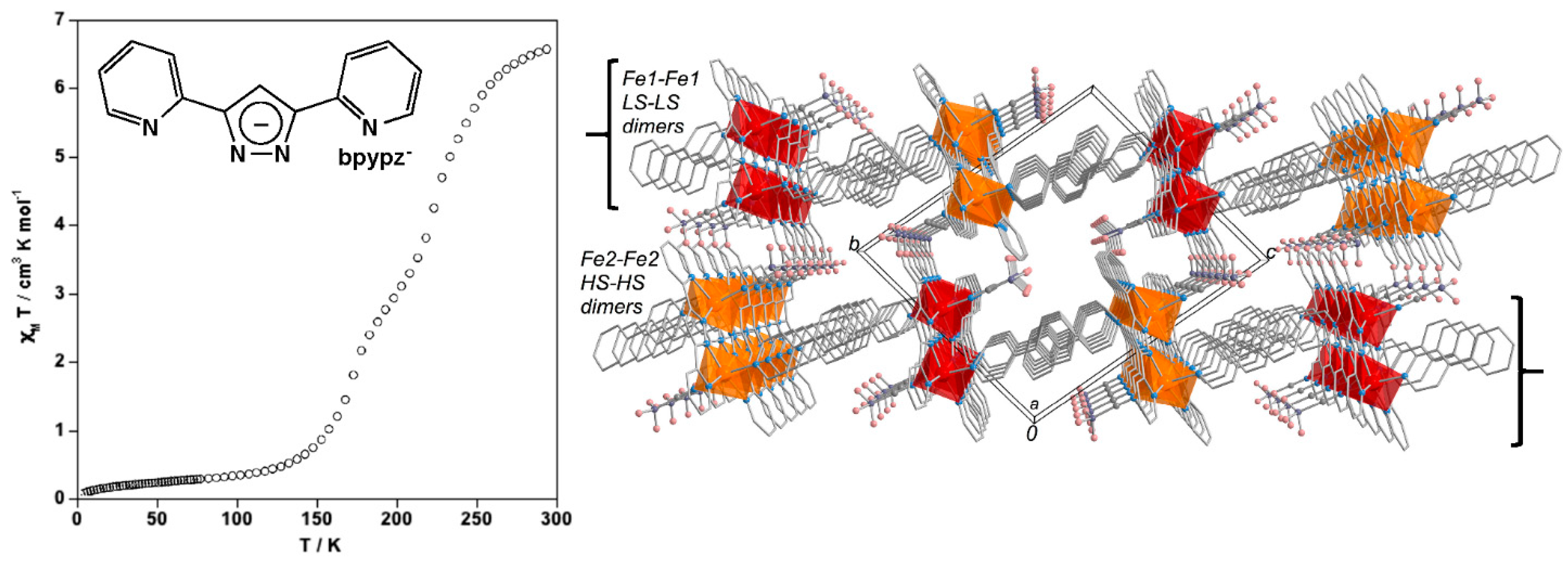
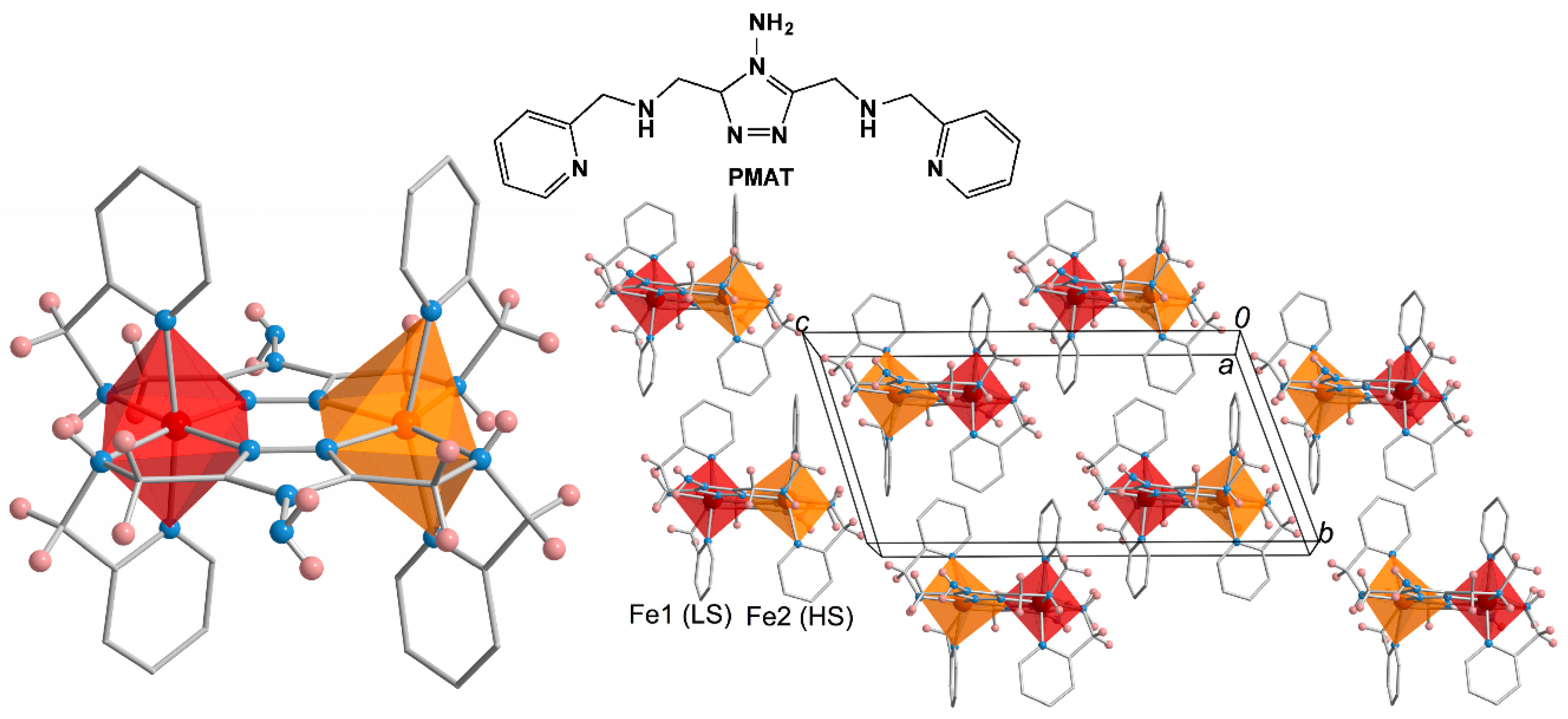
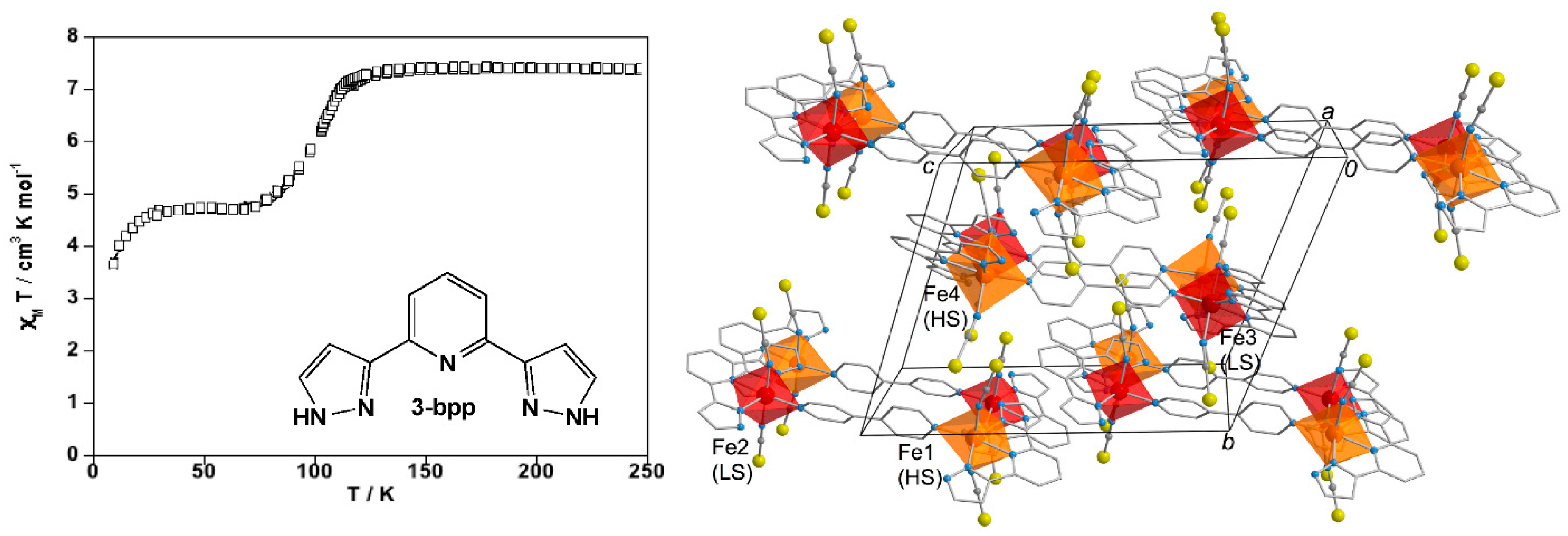
3. Polynuclear Iron(II) Complexes
4. Polymeric Iron(II) Complexes
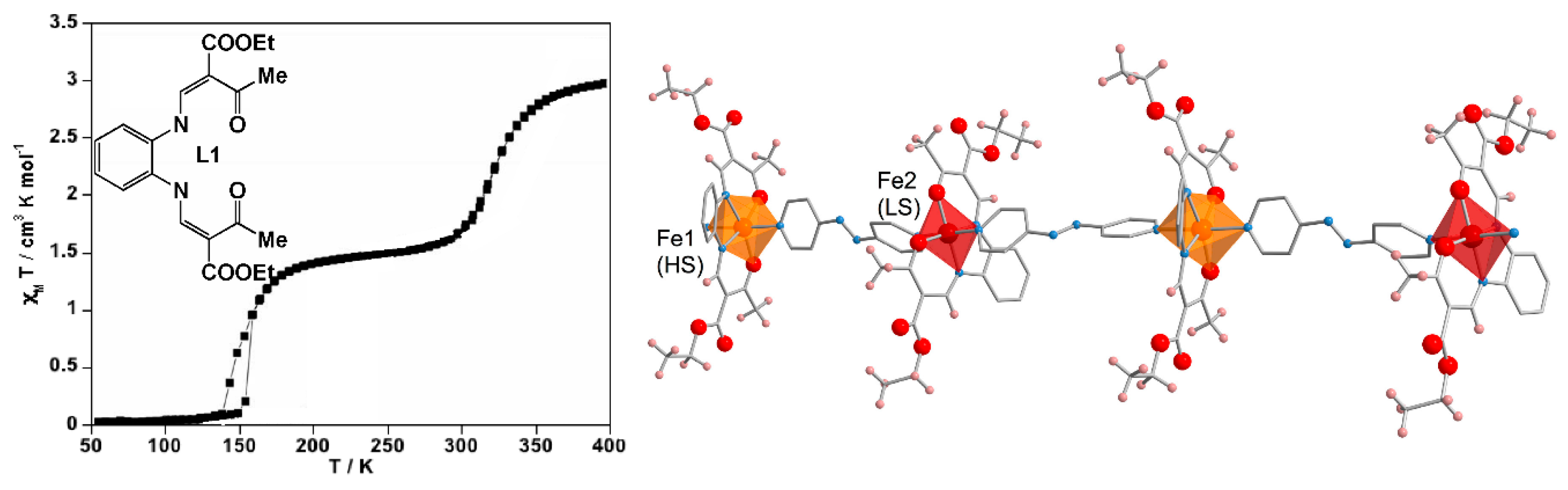
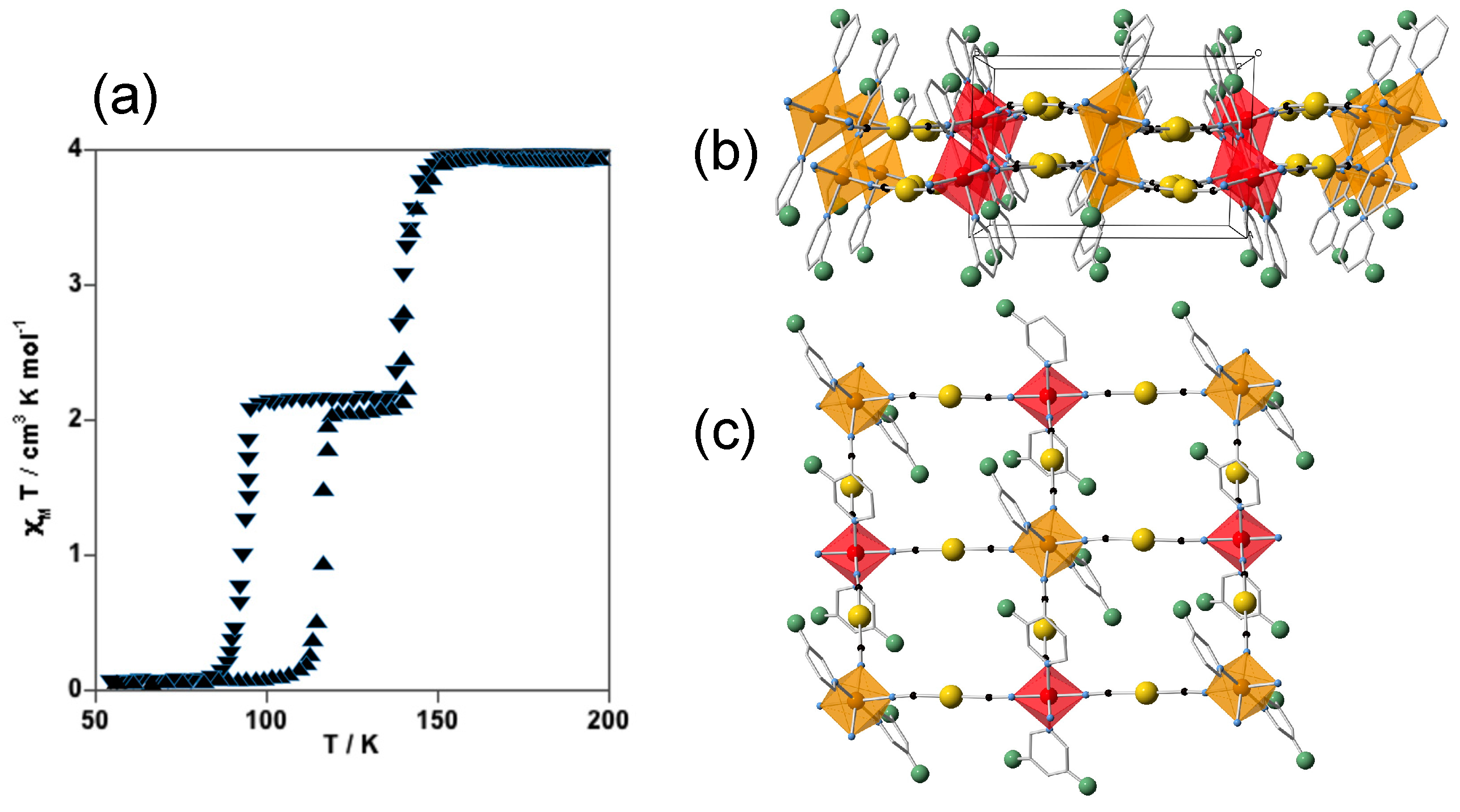
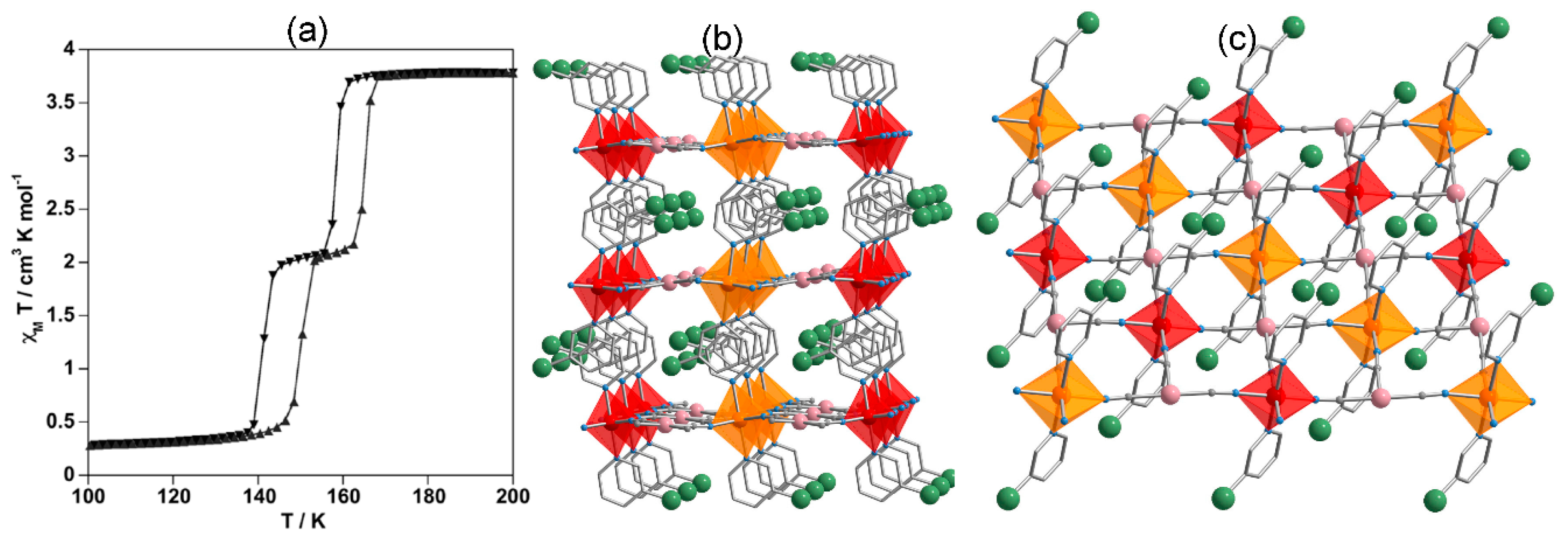
4.1. One-Dimensional Complexes
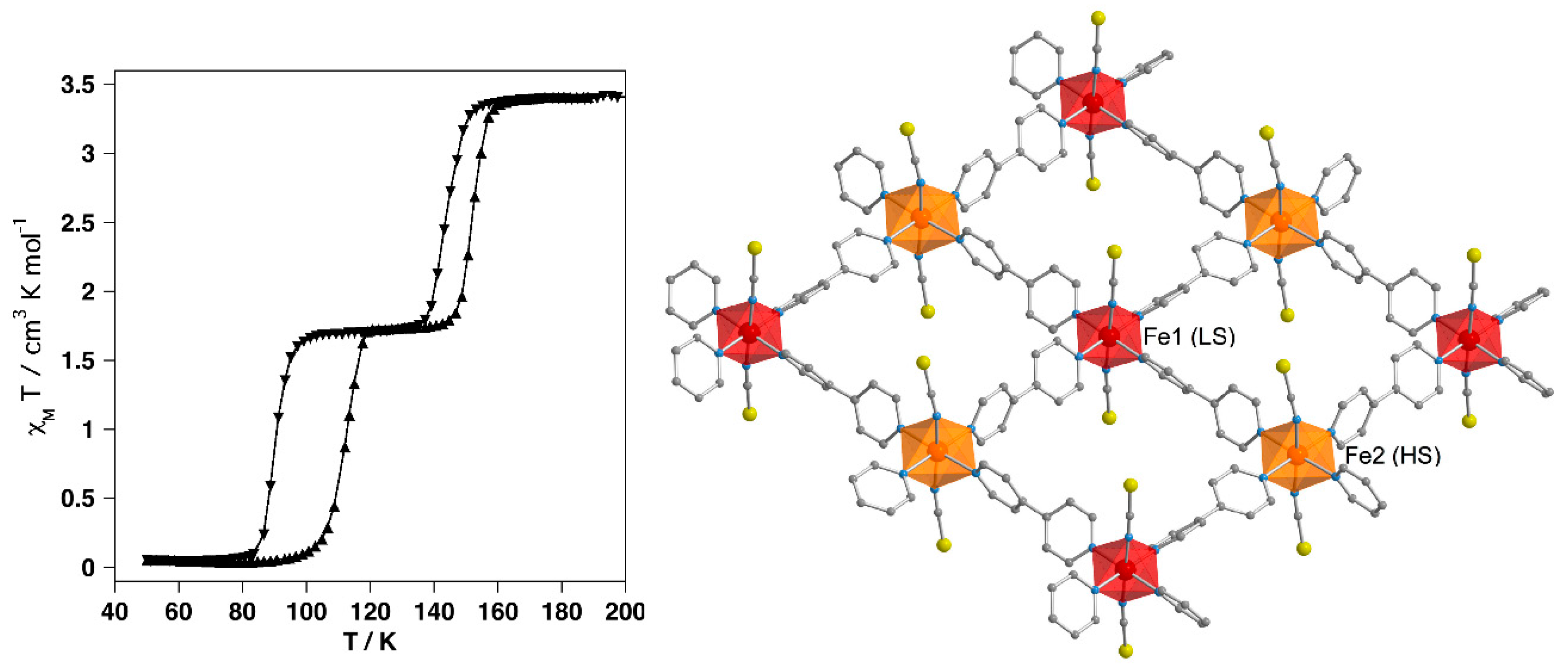
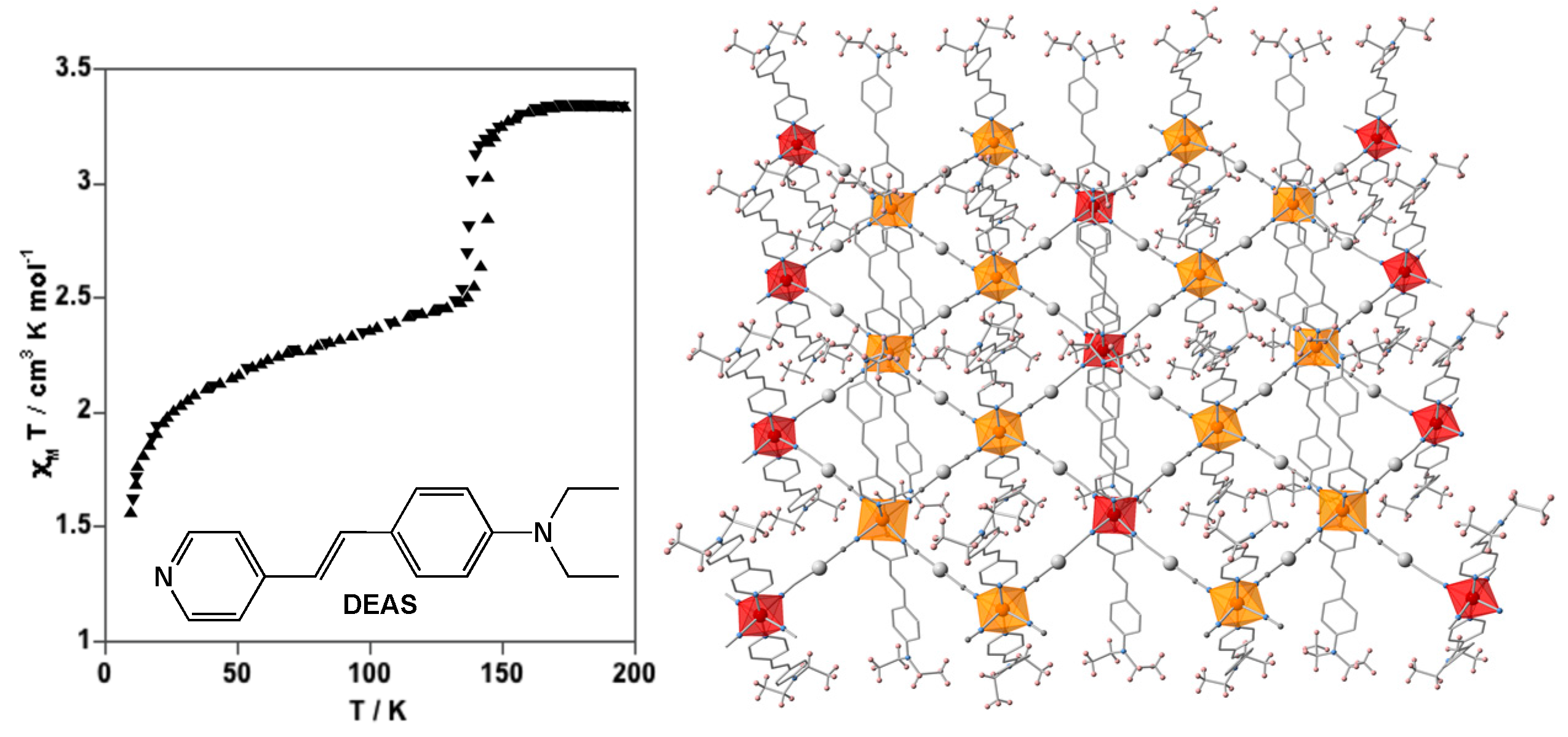
4.2. Two-Dimensional Coordination Polymers
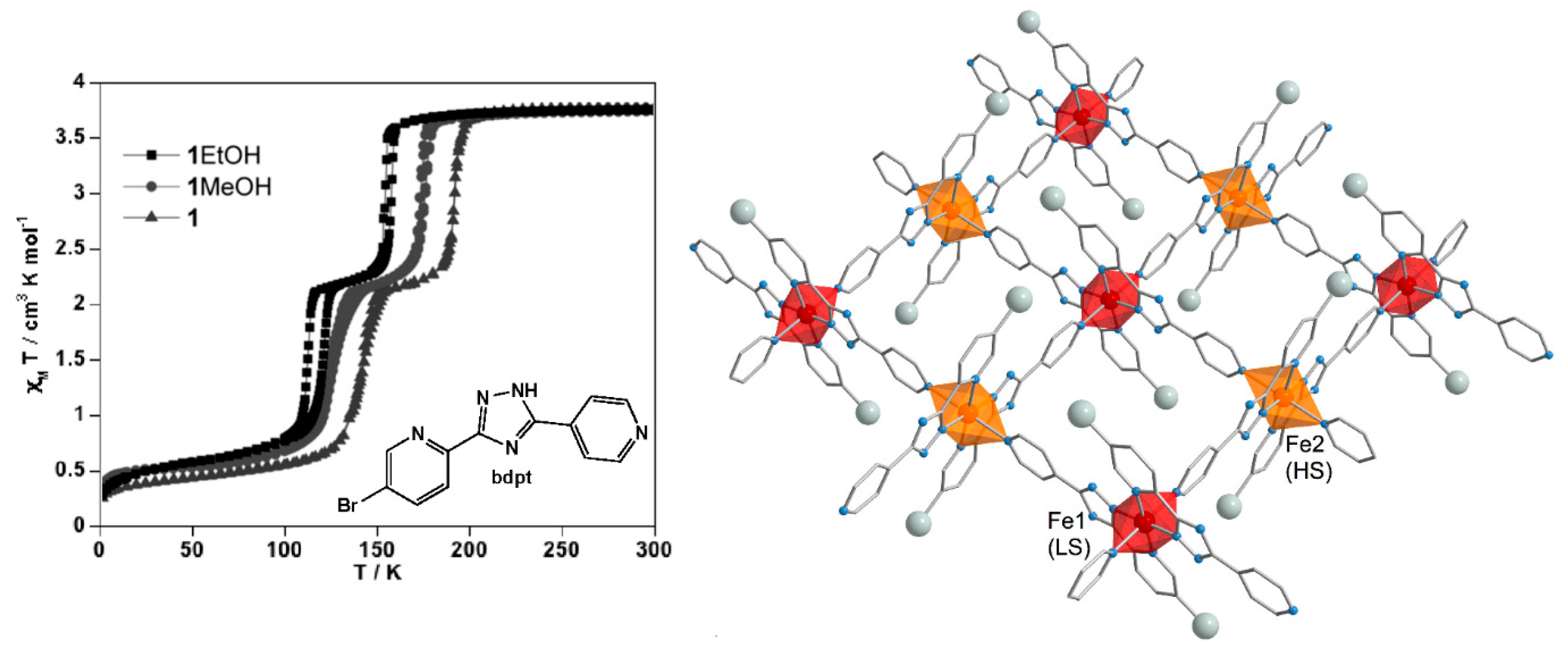
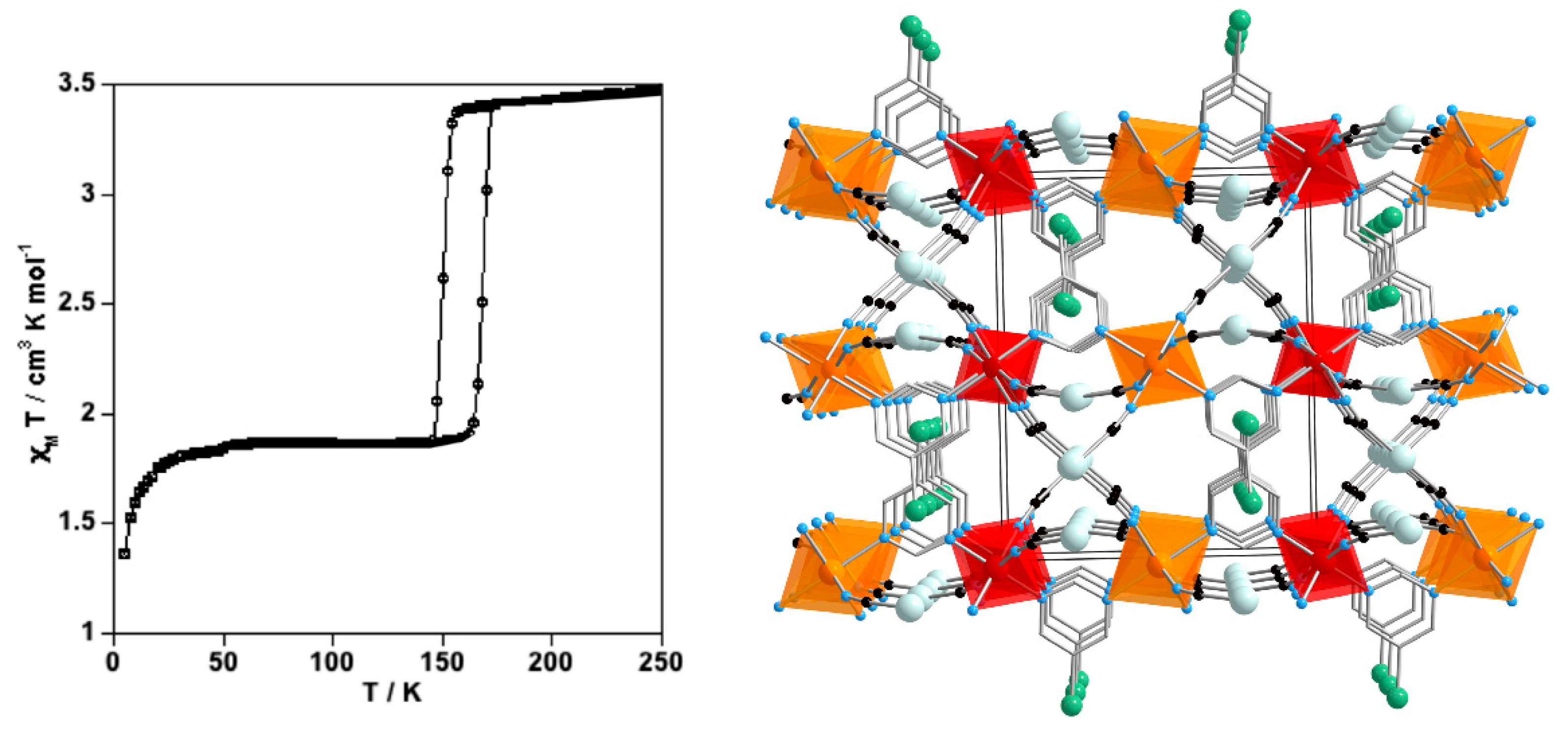
4.3. Three-Dimensional Coordination Polymers
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| SCO | Spin crossover |
| CSB | Crystallographic symmetry breaking |
| HS | High-spin |
| LS | Low-spin |
| IP | Intermediate phase |
Appendix
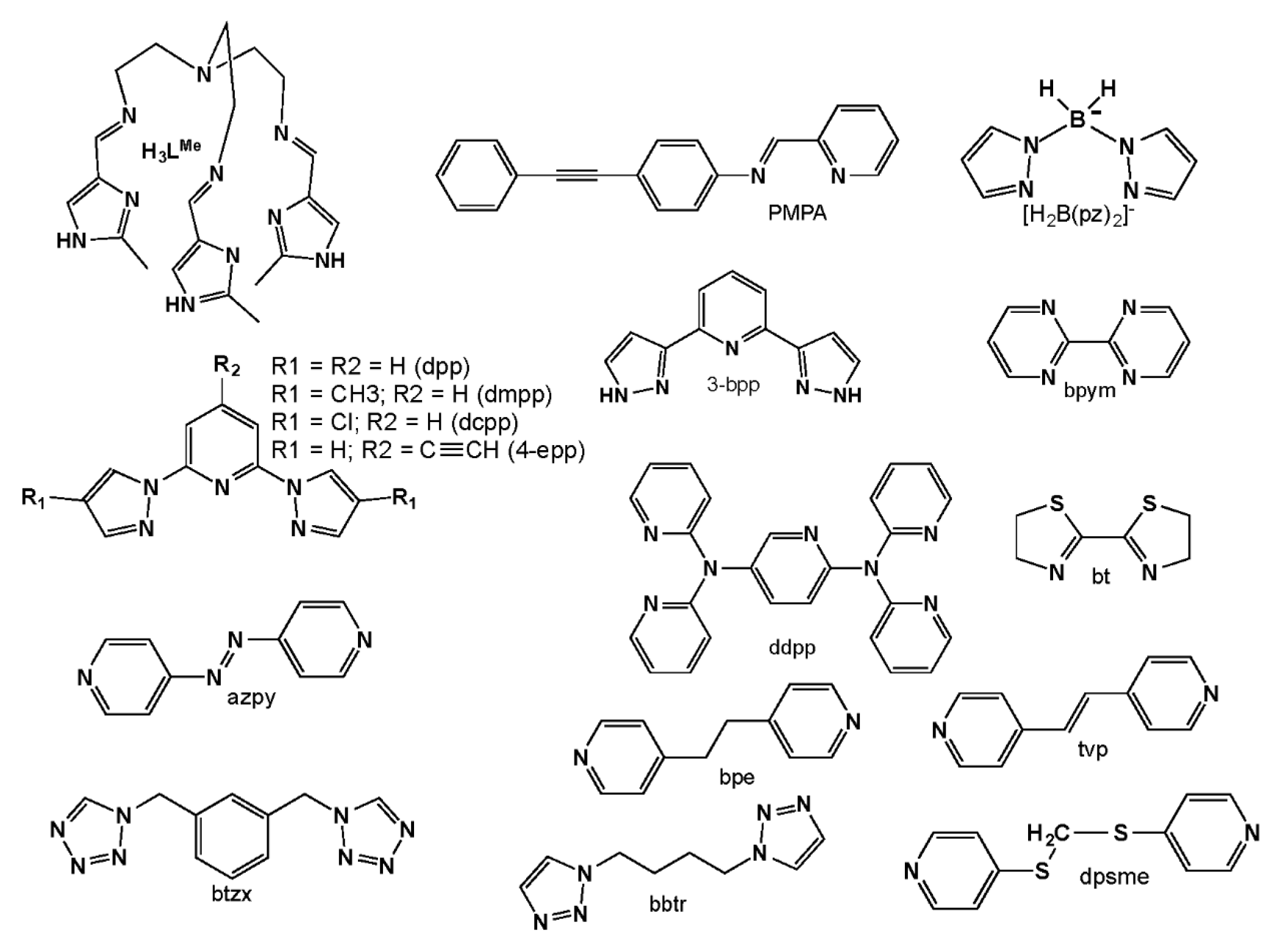
References
- Olguin, J.; Brooker, S. Spin crossover active iron(II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011, 255, 203–240. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Spin-crossover Materials: Properties and Applications; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Sasaki, N.; Kambara, T. Theory of the two-step spin conversion induced by the cooperative molecular distortions in spin-crossover compounds. Phys. Rev. B 1989, 40, 2442–2449. [Google Scholar] [CrossRef]
- Bousseksou, A.; Nasser, J.; Linares, J.; Boukheddaden, K.; Varret, F. Ising-like model for the two-step spin-crossover. J. Phys. I 1992, 2, 1381–1403. [Google Scholar] [CrossRef]
- Romstedt, H.; Spiering, H.; Gütlich, P. Modelling of two step high spin reversible arrow low spin transitions using the cluster variation method. J. Phys. Chem. Solids 1998, 59, 1353–1362. [Google Scholar] [CrossRef]
- Real, J.A.; Bolvin, H.; Bousseksou, A.; Dworkin, A.; Kahn, O.; Varret, F.; Zarembowitch, J. Two-step spin crossover in the new dinuclear compound [Fe(bt)(NCS)2]2bpym, with bt = 2,2′-Bi-2-thiazoline and bpym = 2,2′-Bipyrimidine: experimental investigation and theoretical approach. J. Am. Chem. Soc. 1992, 114, 4650–4658. [Google Scholar] [CrossRef]
- Cirera, J.; Ruiz, E. Theoretical modeling of two-step spin-crossover transitions in FeII dinuclear systems. J. Mater. Chem. C 2015, 3, 7954–7961. [Google Scholar] [CrossRef]
- Niel, V.; Thompson, A.L.; Goeta, A.E.; Enachescu, C.; Hauser, A.; Galet, A.; Muñoz, M.C.; Real, J.A. Thermal- and photoinduced spin-state switching in an unprecedented three-dimensional bimetallic coordination polymer. Chem. Eur. J. 2005, 11, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Ui, M.; Yokota, M.; Han, L.; Maeda, A.; Kishida, H.; Okamoto, H.; Oshio, H. Two-step spin conversion in a cyanide-bridged ferrous square. Angew. Chem. Int. Ed. 2005, 44, 6484–6487. [Google Scholar] [CrossRef] [PubMed]
- Shongwe, M.S.; Al-Rashdi, B.A.; Adams, H.; Morris, M.J.; Mikuriya, M.; Hearne, G.R. Thermally induced two-step, two-site incomplete 6A1 ↔ 2T2 crossover in a mononuclear iron(III) phenolate-pyridyl Schiff-base complex: A rare crystallographic observation of the coexistence of pure S = 5/2 and 1/2 metal centers in the asymmetric unit. Inorg. Chem. 2007, 46, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Klingele, J.; Kaase, D.; Klingele, M.H.; Lach, J.; Demeshko, S. Two-step spin crossover in the mononuclear iron(II) complex [FeII(L)2(NCS)2] (L = 2,5-di-(2-pyridyl)-1,3,4-thiadiazole). Dalton Trans. 2010, 39, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Huo, Q.; Tao, J.; Huang, R.-B.; Zheng, L.-S. Spin-crossover FeII4 squares: Two-step complete spin transition and reversible single-crystal-to-single-crystal transformation. Angew. Chem. Int. Ed. 2011, 50, 8940–8943. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Guo, P.-H.; Liu, W.; Tucek, J.; Zhang, W.-X.; Leng, J.-D.; Chen, X.-M.; Gural’skiy, I.; Salmon, L.; Bousseksou, A.; et al. Remarkably high-temperature spin transition exhibited by new 2D metal-organic frameworks. Chem. Sci. 2012, 3, 1629–1633. [Google Scholar] [CrossRef]
- Klein, Y.M.; Sciortino, N.F.; Ragon, F.; Housecroft, C.E.; Kepert, C.J.; Neville, S.M. Spin crossover intermediate plateau stabilization in a flexible 2-D Hofmann-type coordination polymer. Chem. Commun. 2014, 50, 3838–3840. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-López, L.; Seredyuk, M.; Muñoz, M.C.; Real, J.A. Two- and one-step cooperative spin transitions in Hofmann-like clathrates with enhanced loading capacity. Chem. Commun. 2014, 50, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Bhar, K.; Khan, S.; Sánchez-Costa, J.; Ribas, J.; Roubeau, O.; Mitra, P.; Ghosh, B.K. Crystallographic Evidence for Reversible Symmetry Breaking in a Spin-Crossover d7 Cobalt(II) Coordination Polymer. Angew. Chem. Int. Ed. 2012, 51, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E.; Morgan, G.G. Electronic vs. structural ordering in a manganese(III) spin crossover complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Shakespeare, S.; Shepherd, H.J.; Harding, C.J.; Létard, J.F.; Desplanches, C.; Goeta, A.E.; Howard, J.A.K.; Powell, A.K.; Mereacre, V.; et al. A Symmetry-Breaking Spin-State Transition in Iron(III). Angew. Chem. Int. Ed. 2011, 50, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Dai, J.-W.; Shiota, Y.; Yoshizawa, K.; Kanegawa, S.; Sato, O. Multi-Step Spin Crossover Accompanied by Symmetry Breaking in an FeIII Complex: Crystallographic Evidence and DFT Studies. Chem. Eur. J. 2013, 19, 12948–12952. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.J.C.; Coutinho, J.T.; Santos, I.C.; Pereira, L.C.J.; Waerenborgh, J.C.; da Gama, V. [Fe(nsal2trien)]SCN, a new two-step iron(III) spin srossover compound, with symmetry breaking spin-state transition and an intermediate ordered state. Inorg. Chem. 2013, 52, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Murnaghan, K.D.; Carbonera, C.; Toupet, L.; Griffin, M.; Dîrtu, M.M.; Desplanches, C.; Garcia, Y.; Collet, E.; Létard, J.F.; Morgan, G.G. Spin-state ordering on one sub-lattice of a mononuclear iron(III) spin crossover complex exhibiting LIESST and TIESST. Chem. Eur. J. 2014, 20, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Phonsri, W.; Harding, P.; Murray, K.S.; Moubaraki, B.; Jameson, G.N.L. Abrupt two-step and symmetry breaking spin crossover in an iron(III) complex: An exceptionally wide [LS-HS] plateau. Dalton Trans. 2015, 44, 15079–15082. [Google Scholar] [CrossRef] [PubMed]
- Shatruk, M.; Phana, H.; Chrisostomoa, B.A.; Suleimenov, A. Symmetry-breaking structural phase transitions in spin crossover complexes. Coord. Chem. Rev. 2015, 289–290, 62–73. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(ii) complexes. Angew. Chem. Int. Ed. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
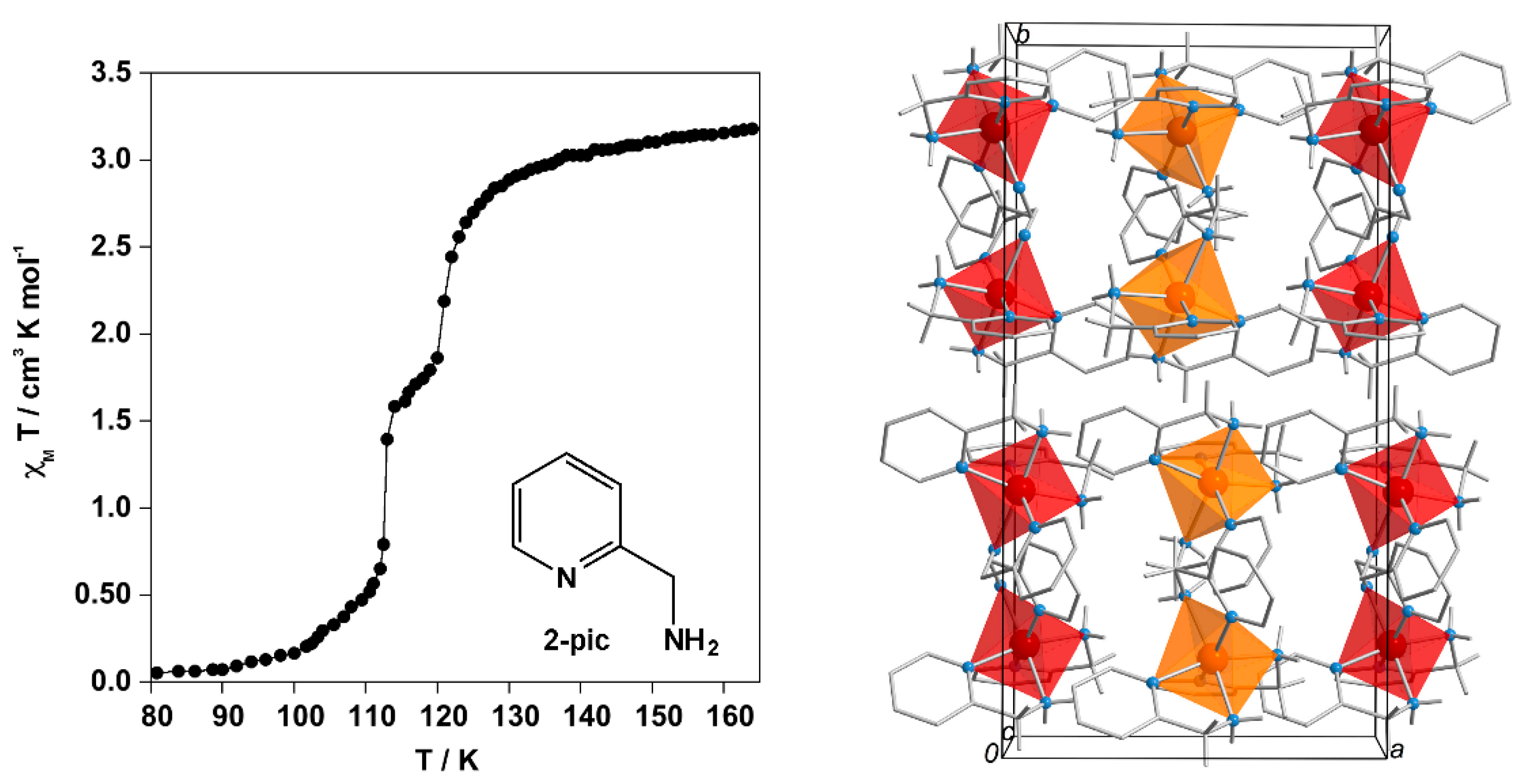
- Katz, B.A.; Strouse, C.E. Molecular-transformations in the solid-state. Crystallographic resolution of the spin isomers of tris(2-picolylamine)iron(II) dichloride and the structural relationship between the methanol and ethanol solvates. J. Am. Chem. Soc. 1979, 101, 6214–6221. [Google Scholar] [CrossRef]
- Mikami, M.; Konno, M.; Saito, Y. Structures of high-spin (298, 150 K) and low-spin (90 K) states and the spin phase-transition mechanism of a spin crossover complex; tris(α-picolylamine)iron(II) chloride-ethanol. Acta Cryst. 1980, B36, 275–287. [Google Scholar] [CrossRef]
- Köppen, H.; Müller, E.W.; Köhler, C.P.; Spiering, H.; Meissner, E.; Gütlich, P. Unusual spin-transition anomaly in the crossover system [Fe(2-pic)3]Cl2·EtOH. Chem. Phys. Lett. 1982, 91, 348–352. [Google Scholar] [CrossRef]
- Meissner, E.; Köppen, H.; Spiering, H.; Gütlich, P. The effect of low-pressure on a high-spin low-spin transition. Chem. Phys. Lett. 1983, 95, 163–166. [Google Scholar] [CrossRef]
- Kaji, K.; Sorai, M. Heat-capacity and dual spin-transitions in the crossover system [Fe(2-pic)3]Cl2·EtOH. Thermochim. Acta 1985, 88, 185–190. [Google Scholar] [CrossRef]
- Spiering, H.; Kohlhaas, T.; Romstedt, H.; Hauser, A.; Bruns-Yilmaz, C.; Gütlich, P. Correlations of the distribution of spin states in spin crossover compounds. Coord. Chem. Rev. 1999, 190–192, 629–647. [Google Scholar] [CrossRef]
- Chernyshov, D.; Hostettler, M.; Törnroos, K.W.; Bürgi, H.B. Ordering phenomena and phase transitions in a spin-crossover compound-uncovering the nature of the intermediate phase of [Fe(2-pic)3]Cl2·EtOH. Angew. Chem. Int. Ed. 2003, 42, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Money, V.A.; Carbonera, C.; Elhaïk, J.; Halcrow, M.A.; Howard, J.A.K.; Létard, J.F. Interplay between kinetically slow thermal spin-crossover and metastable high-spin state relaxation in an iron(II) complex with similar T1/2 and T(LIESST). Chem. Eur. J. 2007, 13, 5503–5514. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zhou, J.; Liu, Z.; Zhu, B.; Wang, H.; Bao, X.; Liu, W.; Tong, M.L.; Peng, G.; Peng, H.; et al. Polymorphism-dependent spin-crossover: Hysteretic two-step spin transition with an ordered [HS-HS-LS] intermediate phase. Inorg. Chem. 2015, 54, 5145–5147. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Tahira, H.; Takahashi, N.; Otake, Y.; Yamamura, Y.; Saito, K.; Oshio, H. Multiple bistability and tristability with dual spin-state conversions in [Fe(dpp)2][Ni(mnt)2]2·MeNO2. J. Am. Chem. Soc. 2010, 132, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Boinnard, D.; Bousseksou, A.; Dworkin, A.; Savariault, J.M.; Varret, F.; Tuchagues, J.P. Two-step spin conversion of [FeII(5-NO2-sal-N(1,4,7,10))]: 292, 153, and 103 K. X-ray crystal and molecular-structure and infrared, magnetic, mossbauer, calorimetric, and theoretical-studies. Inorg. Chem. 1994, 33, 271–281. [Google Scholar] [CrossRef]
- Reger, D.L.; Little, C.A.; Young, V.G., Jr.; Pink, M. Variable-temperature X-ray structural investigation of {Fe[HC(3,5-Me2pz)3]2}(BF4)2 (pz = pyrazolyl ring): Observation of a thermally induced spin state change from all high spin to an equal high spin-low spin mixture, concomitant with the onset of nonmerohedral twinning. Inorg. Chem. 2001, 40, 2870–2874. [Google Scholar] [PubMed]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, M.; Dahan, F.; Tuchagues, J.P.; Re, N.; Iijima, S. A variety of spin-crossover behaviors depending on the counter anion: Two-dimensional complexes constructed by NH···Cl− hydrogen bonds, [(FeIIH3LMe)]Cl·X (X = PF6−, AsF6−, SbF6−, CF3SO3−; H3LMe = tris[2-{[(2-methylimidazol-4-yl)methylidene]amino}ethyl]amine). Chem. Eur. J. 2006, 12, 4536–4549. [Google Scholar] [PubMed]
- Sato, T.; Nishi, K.; Iijima, S.; Kojima, M.; Matsumoto, N. One-step and two-step spin-crossover iron(II) complexes of ((2-methylimidazol-4-yl)methylidene)histamine. Inorg. Chem. 2009, 48, 7211–7229. [Google Scholar] [CrossRef] [PubMed]
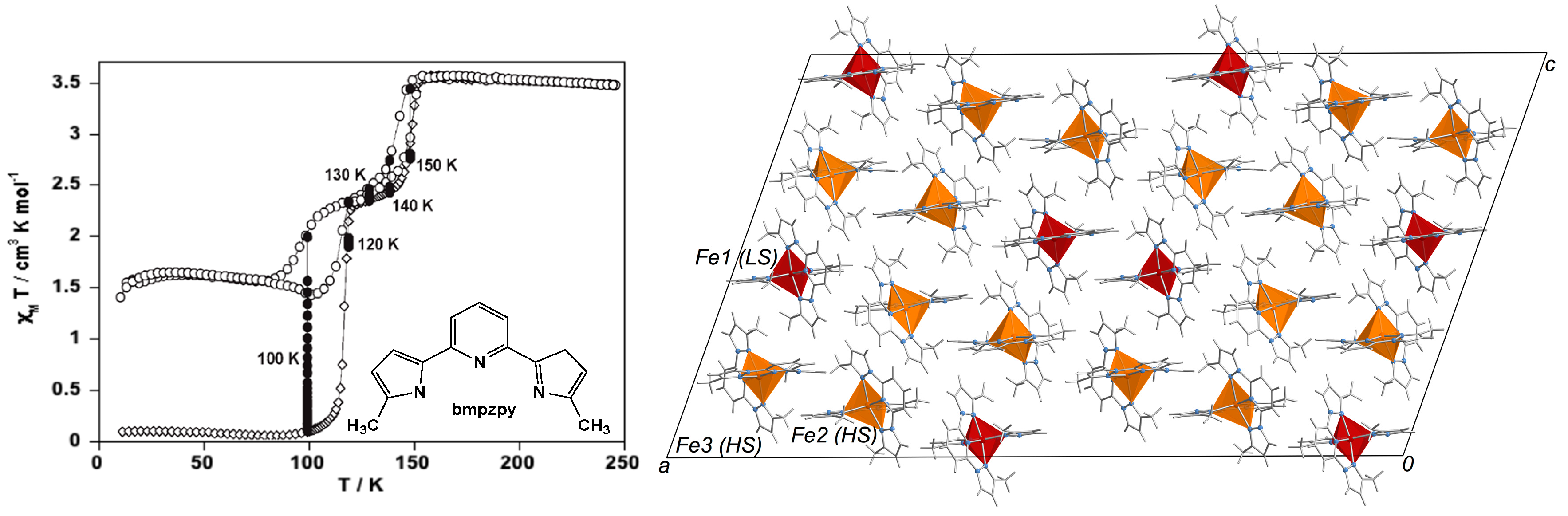
- Bréfuel, N.; Collet, E.; Watanabe, H.; Kojima, M.; Matsumoto, N.; Toupet, L.; Tanaka, K.; Tuchagues, J.P. Nanoscale self-hosting of molecular spin-states in the intermediate phase of a spin-crossover material. Chem. Eur. J. 2010, 16, 14060–14068. [Google Scholar] [CrossRef] [PubMed]
- Bréfuel, N.; Watanabe, H.; Toupet, L.; Come, J.; Matsumoto, N.; Collet, E.; Tanaka, K.; Tuchagues, J.P. Concerted spin crossover and symmetry breaking yield three thermally and one light-induced crystallographic phases of a molecular material. Angew. Chem. Int. Ed. 2009, 48, 9304–9307. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Siegler, M.A.; Sánchez-Costa, J.; Molnár, G.; Bousseksou, A.; Spek, A.L.; Gamez, P.; Reedijk, J. A two-step spin crossover mononuclear iron(II) complex with a [HS-LS-LS] intermediate phase. Chem. Commun. 2008, 5619–5621. [Google Scholar] [CrossRef] [PubMed]
- Létard, J.F.; Guionneau, P.; Codjovi, E.; Lavastre, O.; Bravic, G.; Chasseau, D.; Kahn, O. Wide thermal hysteresis for the mononuclear spin-crossover compound cis-bis(thiocyanato)bis[N-(2′-pyridylmethylene)-4-(phenylethynyl)anilino]iron(II). J. Am. Chem. Soc. 1997, 119, 10861–10862. [Google Scholar] [CrossRef]
- Thompson, A.L.; Goeta, A.E.; Real, J.A.; Galet, A.; Muñoz, M.C. Thermal and light induced polymorphism in iron(II) spin crossover compounds. Chem. Commun. 2004, 1390–1391. [Google Scholar] [CrossRef] [PubMed]
- Carbonera, C.; Saánchez-Costa, J.; A. Money, V.; Elhaïk, J.; Howard, J.A. K.; Halcrow, M.A.; Létard, J.F. Photomagnetic properties of iron(II) spin crossover complexes of 2,6-dipyrazolylpyridine and 2,6-dipyrazolylpyrazine ligands. Dalton Trans. 2006, 3058–3066. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, R.; Kilner, C.A.; Halcrow, M.A. Iron(II) complexes with a terpyridine embrace packing motif show remarkably consistent cooperative spin-transitions. Chem. Commun. 2007, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Salitros, I.; Fuhr, O.; Eichhöfer, A.; Kruk, R.; Pavlik, J.; Dlhán, L.; Boca, R.; Ruben, M. The interplay of iron(II) spin transition and polymorphism. Dalton Trans. 2012, 41, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Sugiyarto, K.H.; McHale, W.A.; Craig, D.C.; Rae, A.D.; Scudder, M.L.; Goodwin, H.A. Spin transition centres linked by the nitroprusside ion. The cooperative transition in bis(2,6-bis(pyrazol-3-yl)-pyridine)iron(II) nitroprusside. Dalton Trans. 2003, 2443–2448. [Google Scholar] [CrossRef]
- Bréfuel, N.; Imatomi, S.; Torigoe, H.; Hagiwara, H.; Shova, S.; Meunier, J.F.; Bonhommeau, S.; Tuchagues, J.P.; Matsumoto, N. Structural-electronic correlation in the first-order phase transition of [FeH2L2−Me](ClO4)2 (H2L2−Me = bis[((2-methylimidazol-4-yl)methylidene)-3-aminopropyl]ethylenediamine). Inorg. Chem. 2006, 45, 8126–8135. [Google Scholar] [CrossRef] [PubMed]
- Tafili-Kryeziu, M.; Weil, M.; Muranaka, T.; Bousseksou, A.; Hasegawa, M.; Junc, A.; Linert, W. Effect of the counter-anion on the spin-transition properties of a family of Fe(II) tetrazole complexes, [Fe(i4tz)6]X2 (X = ClO4−, PF6−, SbF6−, BF4−). Dalton Trans. 2013, 42, 15796–15804. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Zarembowitch, J.; Kahn, O.; Solans, X. Magnetic interaction and spin transition in iron(II) dinuclear compounds. Crystal-structure of (µ-2,2′-bipyrimidine)bis[(2,2′-bipyrimidine) bis(thiocyanato)iron(II)]. Inorg. Chem. 1987, 26, 2939–2943. [Google Scholar] [CrossRef]
- Real, J.A.; Castro, I.; Bousseksou, A.; Verdaguer, M.; Burriel, R.; Castro, M.; Linares, J.; Varret, F. Spin crossover in the 2,2′-bipyrimidine-(bpym-) bridged iron(II) complexes [Fe(L)(NCX)2]2(bpym) (L = 2,2′-bithiazoline (bt) and bpym; X = S, Se). X-ray absorption spectroscopy, magnetic susceptibility, calorimetric, and Mossbauer spectroscopy studies. Inorg. Chem. 1997, 36, 455–464. [Google Scholar] [CrossRef]
- Létard, J.F.; Real, J.A.; Moliner, N.; Gaspar, A.B.; Capes, L.; Cador, O.; Kahn, O. Light induced excited pair spin state in an iron(II) binuclear spin-crossover compound. J. Am. Chem. Soc. 1999, 121, 10630–10631. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C.; Gütlich, P.; Ksenofontov, V.; Spiering, H. Bipyrimidine-bridged dinuclear iron(II) spin crossover compounds. Top. Curr. Chem. 2004, 233, 167–193. [Google Scholar]
- Bousseksou, A.; Molnár, G.; Real, J.A.; Tanaka, K. Spin crossover and photomagnetism in dinuclear iron(II) compounds. Coord. Chem. Rev. 2007, 251, 1822–1833. [Google Scholar] [CrossRef]
- Moussa, N.O.; Molnár, G.; Bonhommeau, S.; Zwick, A.; Mouri, S.; Tanaka, K.; Real, J.A.; Bousseksou, A. Selective photoswitching of the binuclear spin crossover compound {[Fe(bt)(NCS)2]2(bpm)} into two distinct macroscopic phases. Phys. Rev. Lett. 2005, 94. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Ksenofontov, V.; Reiman, S.; Gütlich, P.; Thompson, A.L.; Goeta, A.E.; Muñoz, M.C.; Real, J.A. Mossbauer investigation of the photoexcited spin states and crystal structure analysis of the spin-crossover dinuclear complex [{Fe(bt)(NCS)2)2bpym] (bt = 2,2′-bithiazoline, bpym = 2,2′-bipyrimidine). Chem. Eur. J. 2006, 12, 9289–9298. [Google Scholar] [CrossRef] [PubMed]
- Trzop, E.; Buron-Le Cointe, M.; Cailleau, H.; Toupet, L.; Molnár, G.; Bousseksou, A.; Gaspar, A.B.; Real, J.A.; Collet, E. Structural investigation of the photoinduced spin conversion in the dinuclear compound {[Fe(bt)(NCS)2]2(bpym)}: Toward controlled multi-stepped molecular switches. J. Appl. Cryst. 2007, 40, 158–164. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Muñoz, M.C.; Real, J.A. Dinuclear iron(II) spin crossover compounds: Singular molecular materials for electronics. J. Mater. Chem. 2006, 16, 2522–2533. [Google Scholar] [CrossRef]
- Nakano, K.; Kawata, S.; Yoneda, K.; Fuyuhiro, A.; Yagi, T.; Nasu, S.; Morimoto, S.; Kaizaki, S. Direct two-step spin-crossover through [HS-HS]···[LS-LS] at the plateau in dinuclear diiron(II) complex [{Fe(NCBH3)(4phpy)}2(µ-bpypz)2]. Chem. Commun. 2004, 2892–2893. [Google Scholar] [CrossRef] [PubMed]
- Klingele, M.H.; Moubaraki, B.; Cashion, J.D.; Murray, K.S.; Brooker, S. The first X-ray crystal structure determination of a dinuclear complex trapped in the [low spin-high spin] state: [FeII2(PMAT)2](BF4)·DMF. Chem. Commun. 2005, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Amoore, J.J.M.; Kepert, C.J.; Cashion, J.D.; Moubaraki, B.; Neville, S.M.; Murray, K.S. Structural and magnetic resolution of a two-step full spin-crossover transition in a dinuclear iron(II) pyridyl-bridged compound. Chem. Eur. J. 2006, 12, 8220–8227. [Google Scholar] [CrossRef] [PubMed]
- Fedaoui, D.; Bouhadja, Y.; Kaiba, A.; Guionneau, P.; Létard, J.F.; Rosa, P. Complexation of 2,6-bis(3-pyrazolyl)pyridine-bis(thiocyanato)iron(II) with a bridging 4,4′-bipyridine: A new example of a dinuclear spin crossover complex. Eur. J. Inorg. Chem. 2008, 2008, 1022–1026. [Google Scholar] [CrossRef]
- Kaiba, A.; Shepherd, H.J.; Fedaoui, D.; Rosa, P.; Goeta, A.E.; Rebbani, N.; Létard, J.F.; Guionneau, P. Crystallographic elucidation of purely structural, thermal and light-induced spin transitions in an iron(II) binuclear complex. Dalton Trans. 2010, 39, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.; Pfaffeneder, T.; Achterhold, K.; Weber, B. Complete two-step spin-transition in a 1D chain iron(II) complex with a 110-K wide intermediate plateau. Eur. J. Inorg. Chem. 2011, 2011, 3183–3192. [Google Scholar] [CrossRef]
- Quesada, M.; Prins, F.; Bill, E.; Kooijman, H.; Gamez, P.; Roubeau, O.; Spek, A.L.; Haasnoot, J.G.; Reedijk, J. Counterion effect on the spin-transition properties of the cation [Fe(btzx)3]2+ (btzx = µ-xylylenebis(tetrazole)). Chem. Eur. J. 2008, 14, 8486–8499. [Google Scholar] [CrossRef] [PubMed]
- Patrick, B.O.; Reiff, W.M.; Sanchez, V.; Storr, A.; Thompson, R.C. Poly(2,2-bipyridine)tetrakis(imidazolato)diiron(II): Structural and spin-state phase transitions and low-temperature magnetic ordering in a unique 2-dimensional material. Inorg. Chem. 2004, 43, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.J.; Chapman, K.W.; Neville, S.M.; Moubaraki, B.; Murray, K.S.; Létard, J.F.; Kepert, C.J. Elucidating the mechanism of a two-step spin transition in a nanoporous metal-organic framework. J. Am. Chem. Soc. 2008, 130, 17552–17562. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morcillo, T.; de la Pinta, N.; Callejo, L.M.; Piñeiro-López, L.; Muñoz, M.C.; Madariaga, G.; Ferrer, S.; Breczewski, T.; Cortés, R.; Real, J.A. Nanoporosity, inclusion chemistry, and spin crossover in orthogonally interlocked two-dimensional metal-organic frameworks. Chem. Eur. J. 2015, 21, 12112–12120. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Muñoz, M.C.; Waddington, R.E.; Real, J.A. Cooperative spin transition in the two-dimensional coordination polymer [Fe(4,4′-bipyridine)2(NCX)2)·4CHCl3 (X = S, Se). Inorg. Chem. 2011, 50, 10633–10642. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Xue, W.; Wang, B.-Y.; Tao, J.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Chemical/physical pressure tunable spin-transition temperature and hysteresis in a two-step spin crossover porous coordination framework. Inorg. Chem. 2012, 51, 9423–9430. [Google Scholar] [CrossRef] [PubMed]
- Kusz, J.; Bronisz, R.; Zubko, M.; Bednarek, G. On the role of intermolecular interactions on structural and spin-crossover properties of 2D coordination networks [Fe(bbtr)3]A2 (bbtr = 1,4-bis(1,2,3-triazol-1-yl)butane; A = ClO4−, BF4−). Chem. Eur. J. 2011, 17, 6807–6820. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Spin-crossover behavior in cyanide-bridged Iron(II)-Gold(I) bimetallic 2D Hofmann-like metal-organic frameworks. Inorg. Chem. 2008, 47, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Kosone, T.; Kachi-Terajima, C.; Kanadani, C.; Saito, T.; Kitazawa, T. A two-step and hysteretic spin-crossover transition in new cyano-bridged hetero-metal FeIIAuI 2-dimensional assemblage. Chem. Lett. 2008, 37, 422–423. [Google Scholar] [CrossRef]
- Kosone, T.; Kanadani, C.; Saito, T.; Kitazawa, T. Synthesis, crystal structures, magnetic properties and fluorescent emissions of two-dimensional bimetallic coordination frameworks FeII(3-fluoropyridine)2[AuI(CN)2]2 and MnII(3-fluoropyridine)2[AuI(CN)2]2. Polyhedron 2009, 28, 1930–1934. [Google Scholar] [CrossRef]
- Martínez, V.; Gaspar, A.B.; Muñoz, M.C.; Bukin, G.V.; Levchenko, G.; Real, J.A. Synthesis and characterisation of a new series of bistable iron(II) spin-crossover 2D metal-organic frameworks. Chem. Eur. J. 2009, 15, 10960–10971. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Gaspar, A.B.; Muñoz, M.C.; Lacroix, P.G.; Real, J.A. Spin crossover and paramagnetic behaviour in two-dimensional iron(II) coordination polymers with stilbazole push-pull ligands. Aust. J. Chem. 2009, 62, 1155–1165. [Google Scholar] [CrossRef]
- Agustí, G.; Gaspar, A.B.; Muñoz, M.C.; Real, J.A. Thermal- and pressure-induced cooperative spin transition in the 2D and 3D coordination polymers {Fe(5-Br-pmd)z]M(CN)x]y} (M = AgI, AuI, NiII, PdII, PtII). Inorg. Chem. 2007, 46, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, N.F.; Scherl-Gruenwald, K.R.; Chastanet, G.; Halder, G.J.; Chapman, K.W.; Létard, J.F.; Kepert, C.J. Hysteretic three-step spin crossover in a thermo- and photochromic 3D pillared Hofmann-type metal-organic framework. Angew. Chem. Int. Ed. 2012, 51, 10154–10158. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Villar, N.; Muñoz, M.C.; Real, J.A. Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals. Magnetochemistry 2016, 2, 16. https://doi.org/10.3390/magnetochemistry2010016
Ortega-Villar N, Muñoz MC, Real JA. Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals. Magnetochemistry. 2016; 2(1):16. https://doi.org/10.3390/magnetochemistry2010016
Chicago/Turabian StyleOrtega-Villar, Norma, M. Carmen Muñoz, and José A. Real. 2016. "Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals" Magnetochemistry 2, no. 1: 16. https://doi.org/10.3390/magnetochemistry2010016
APA StyleOrtega-Villar, N., Muñoz, M. C., & Real, J. A. (2016). Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals. Magnetochemistry, 2(1), 16. https://doi.org/10.3390/magnetochemistry2010016