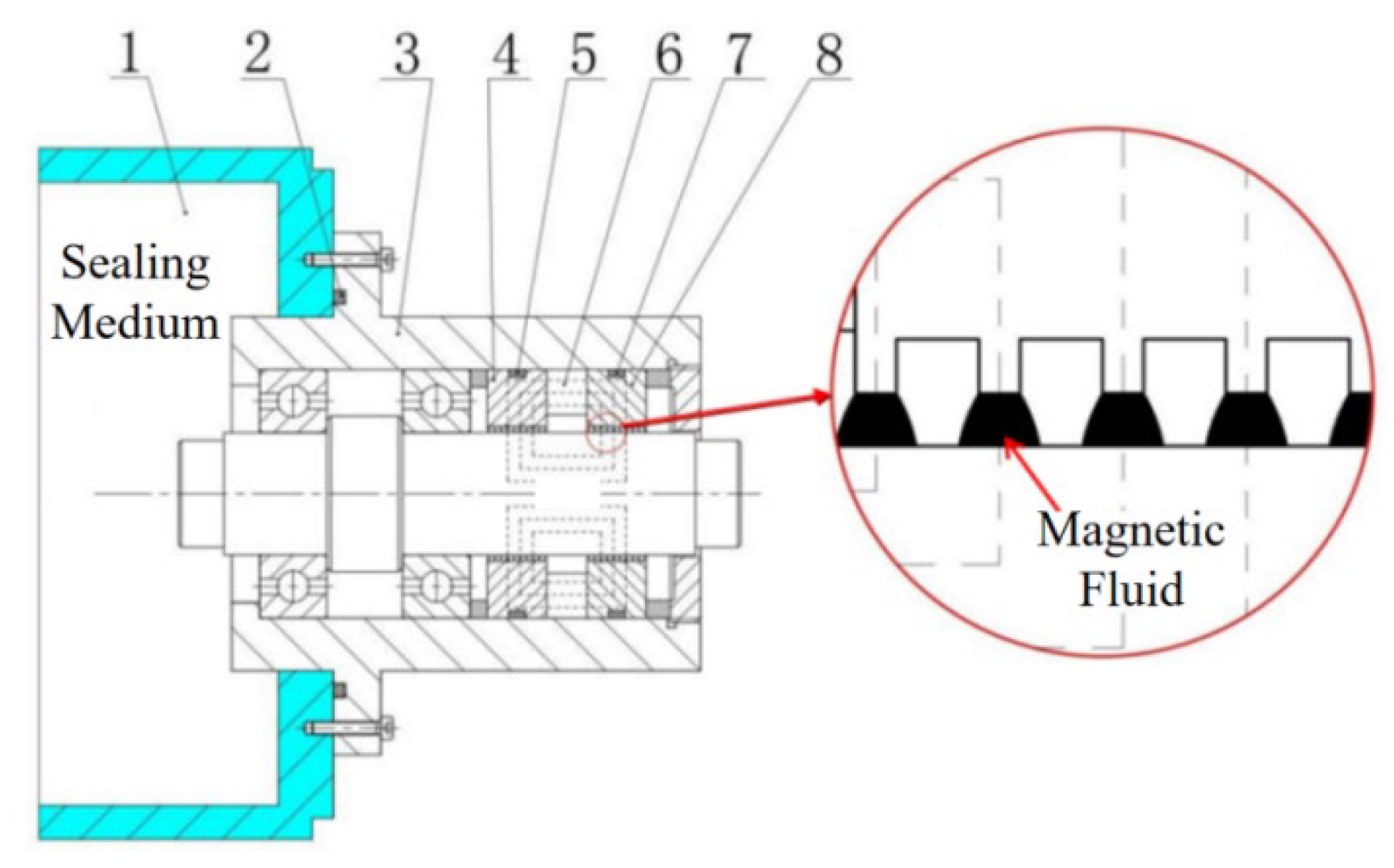
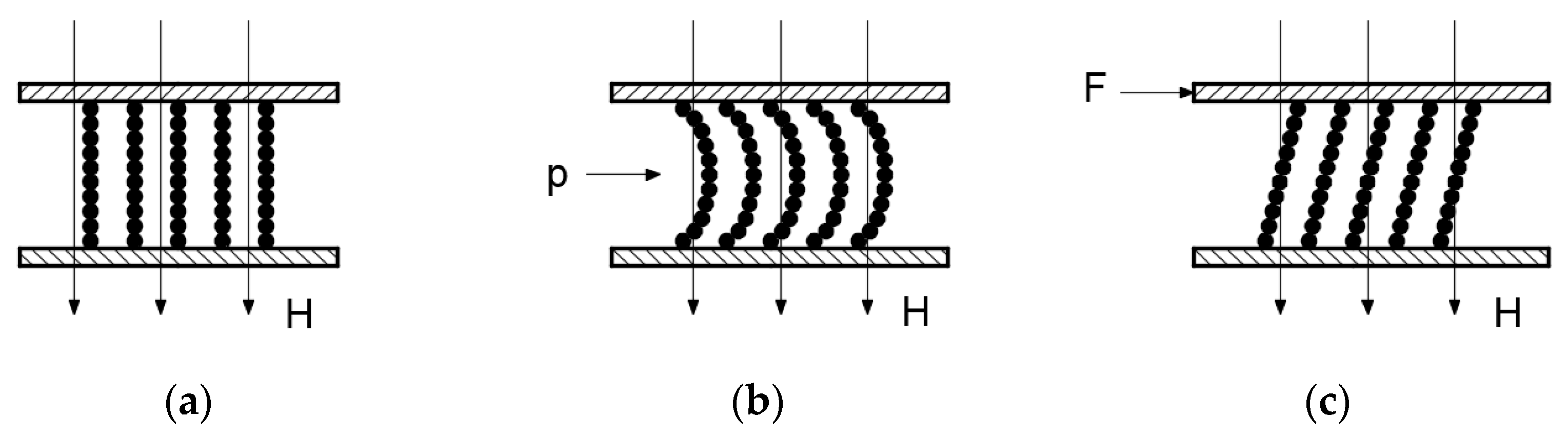
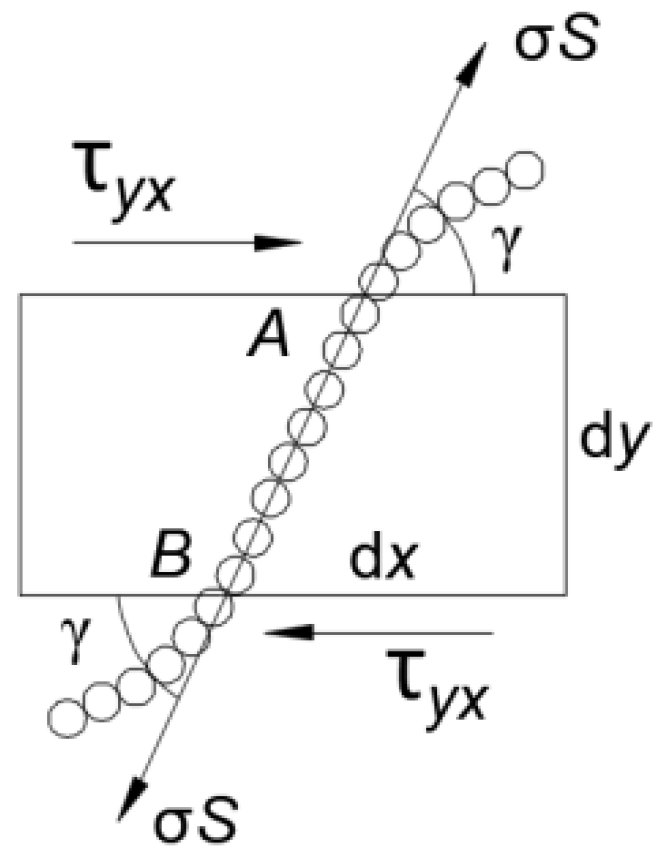
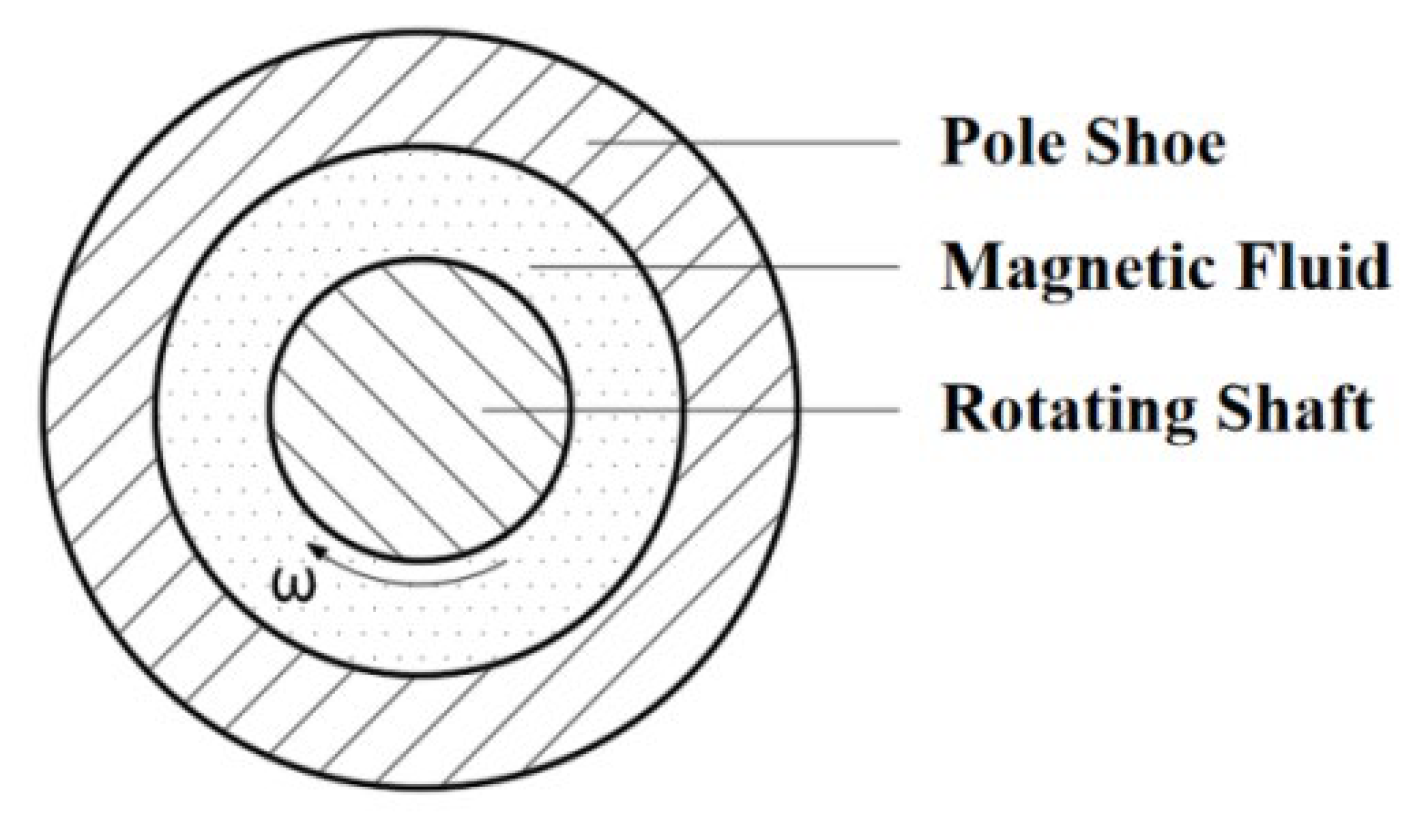
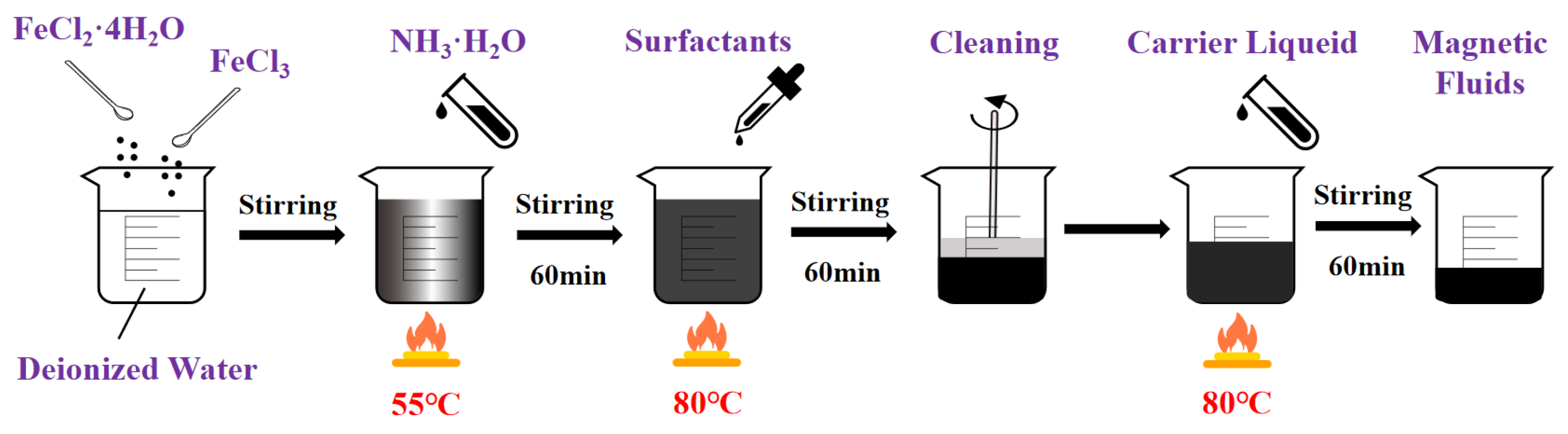
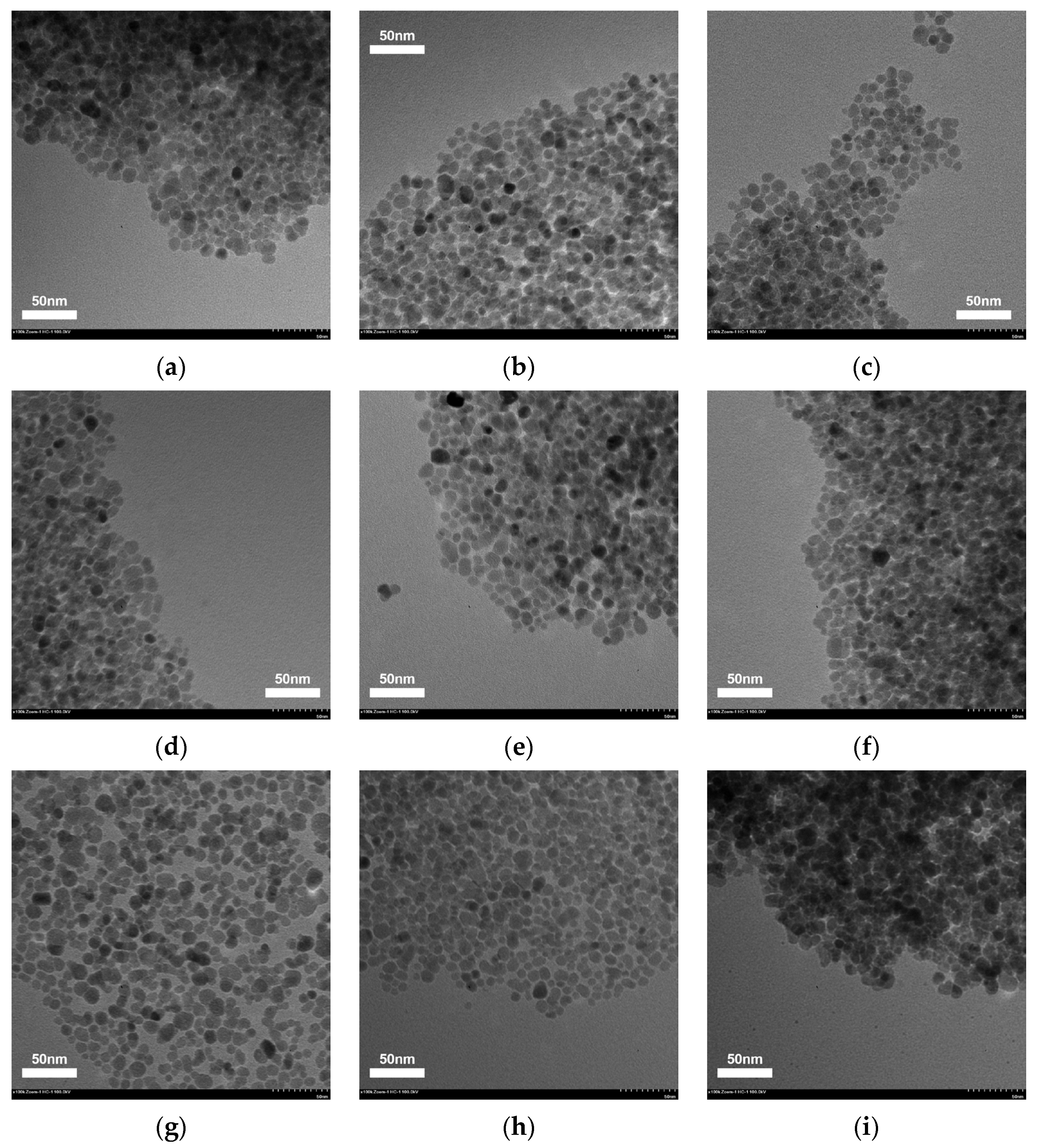
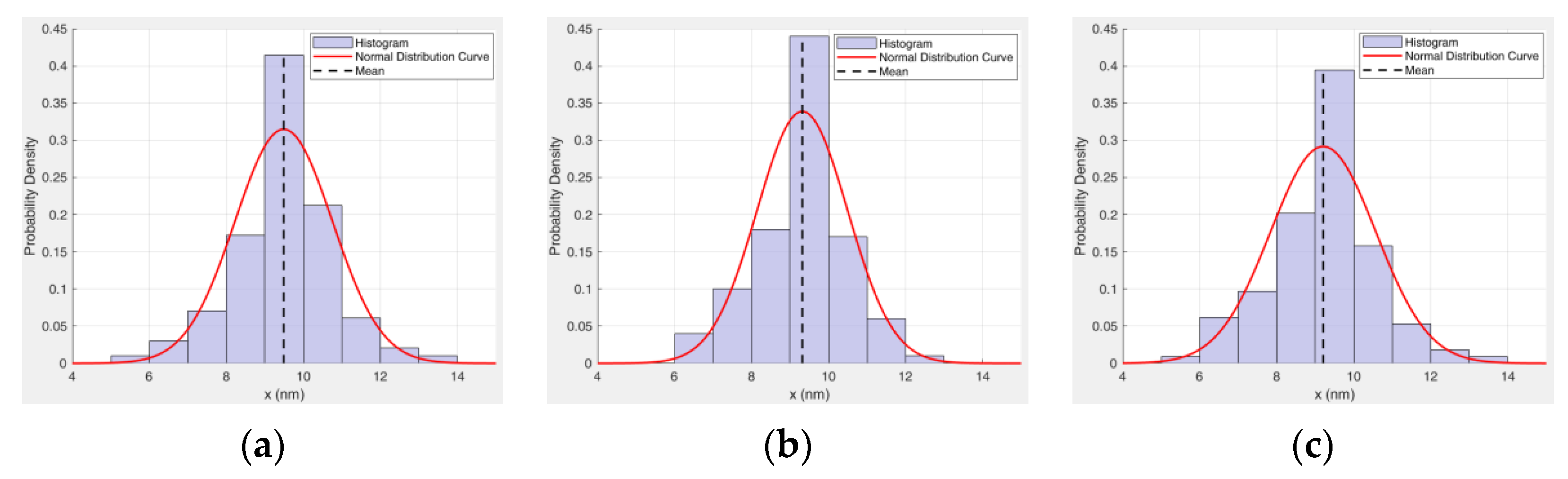
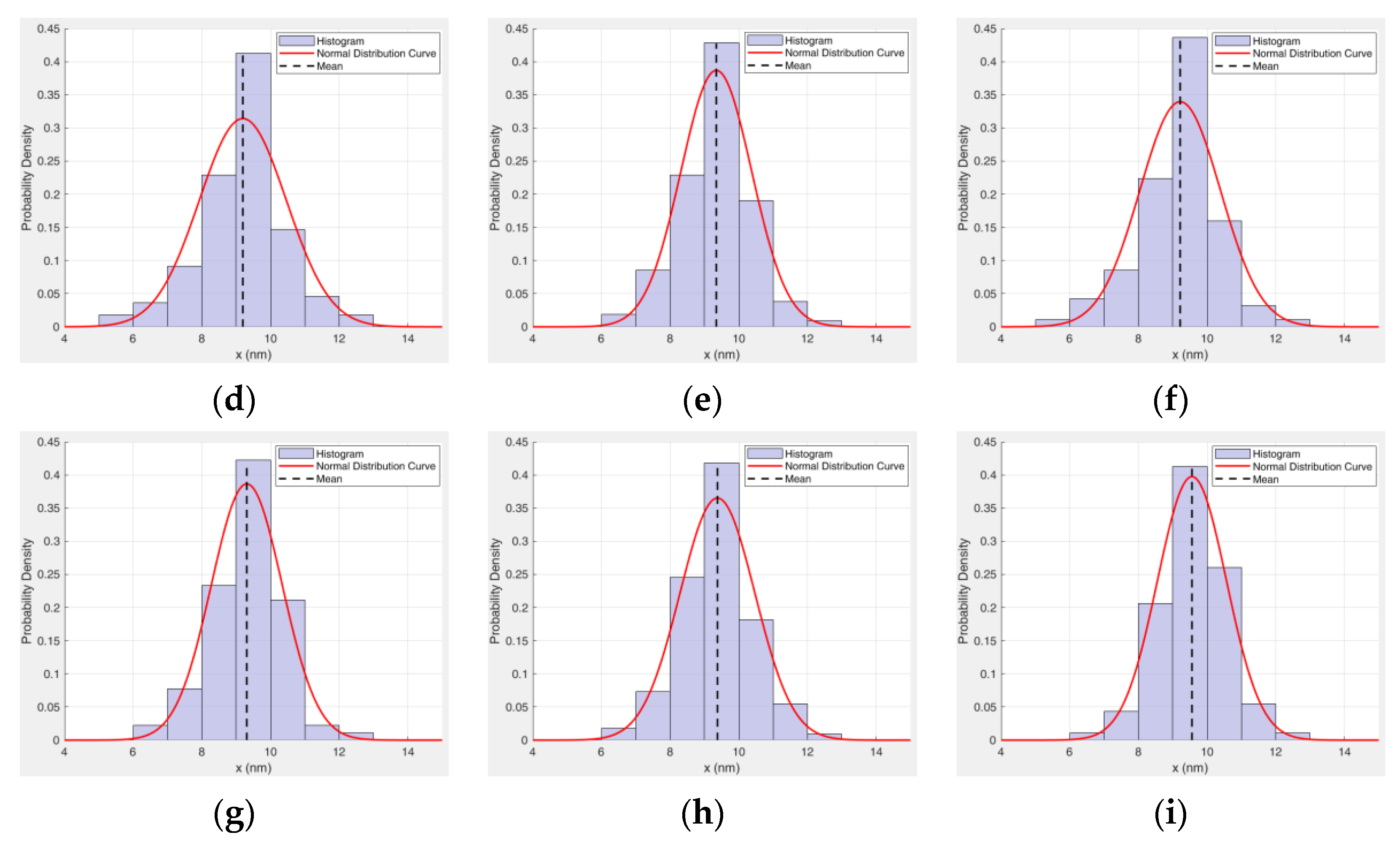
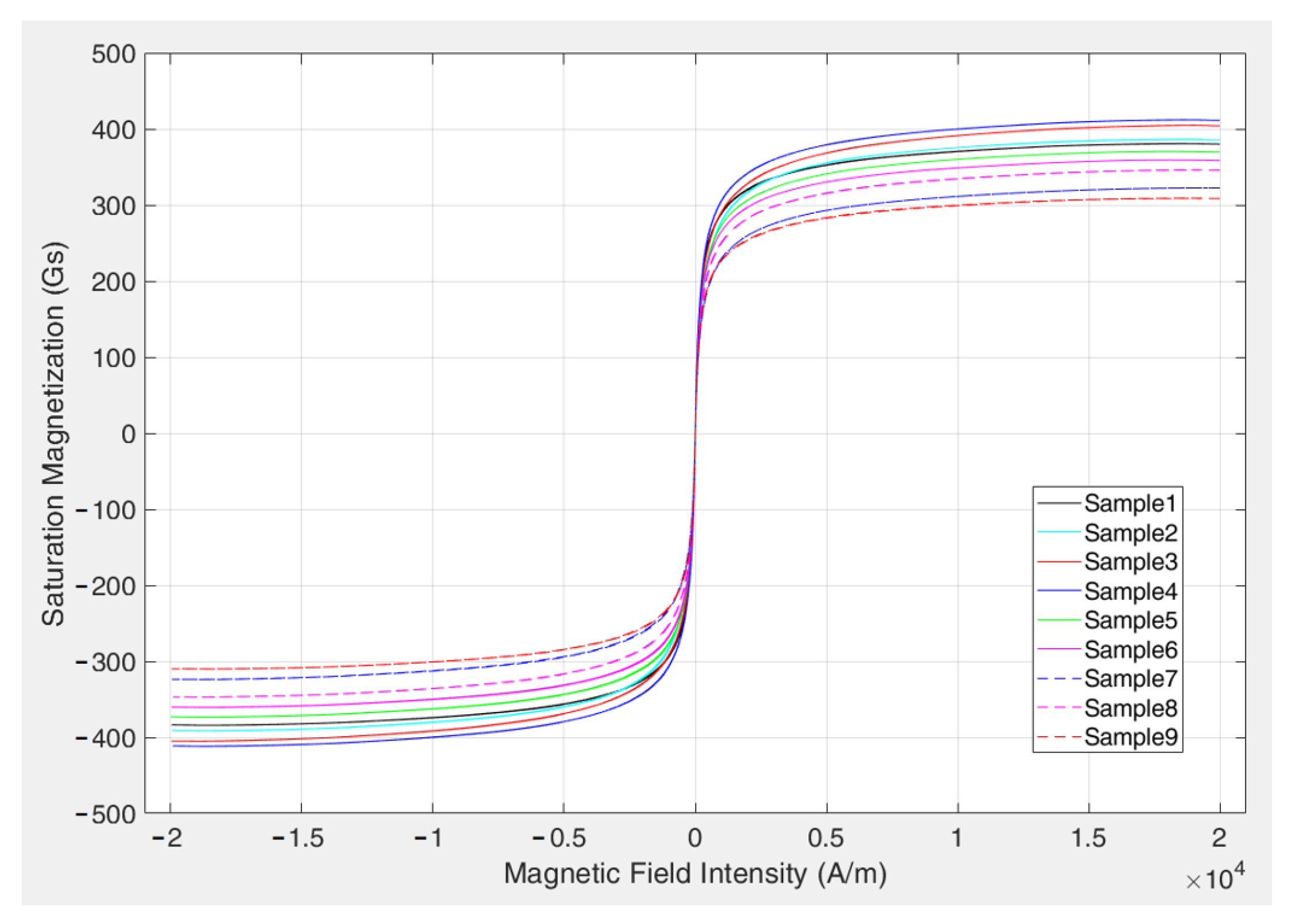
5.1. Sensitivity Analysis of Preparation Parameters Based on Range Analysis
The range analysis method employs mathematical statistics to calculate the range value for each column in an orthogonal array. By analyzing the mean values and the corresponding ranges of each factor, the optimal level combination and relative significance of the influencing parameters can be determined. The range of a factor is defined as the difference between its maximum and minimum mean values across all levels. A larger range indicates a more significant influence, and thus a primary order of importance, whereas a smaller range suggests a weaker influence and classifies the factor as secondary.
The first step in range analysis involves calculating the total value
for each specific level
j of a given factor
i. This value is obtained by summing all experimental results
corresponding to the
j-th level of the
i-th factor. Subsequently, the level mean is computed using the formula
where
n denotes the number of occurrences of level
j.
The range value
for each factor can then be calculated as
The value range for each factor was determined using the above equations. The range analysis was performed using the measured yield stress values of the kerosene-based magnetic fluids (
Table 9) to evaluate the preparation parameters. The calculation results are listed in
Table 10.
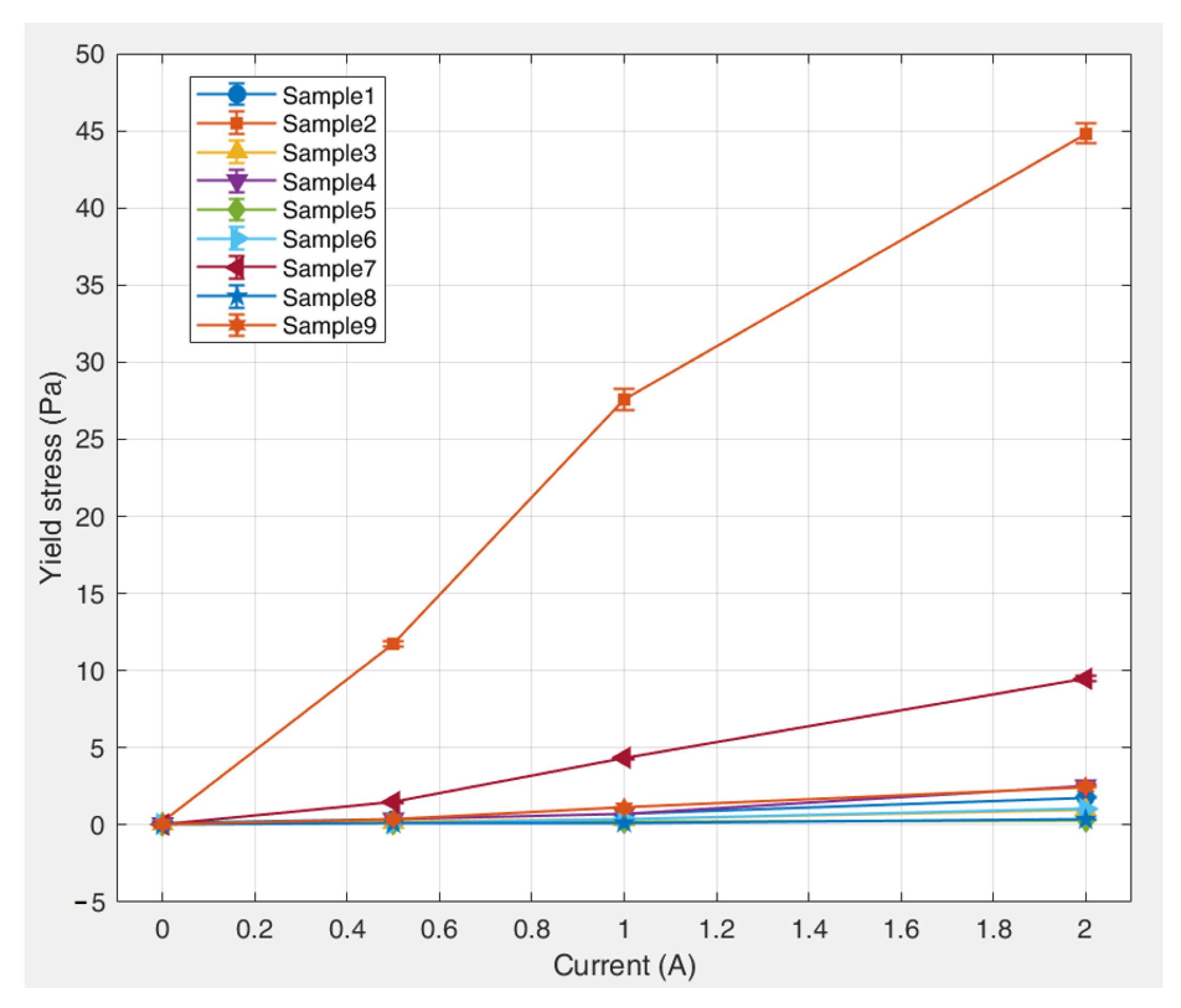
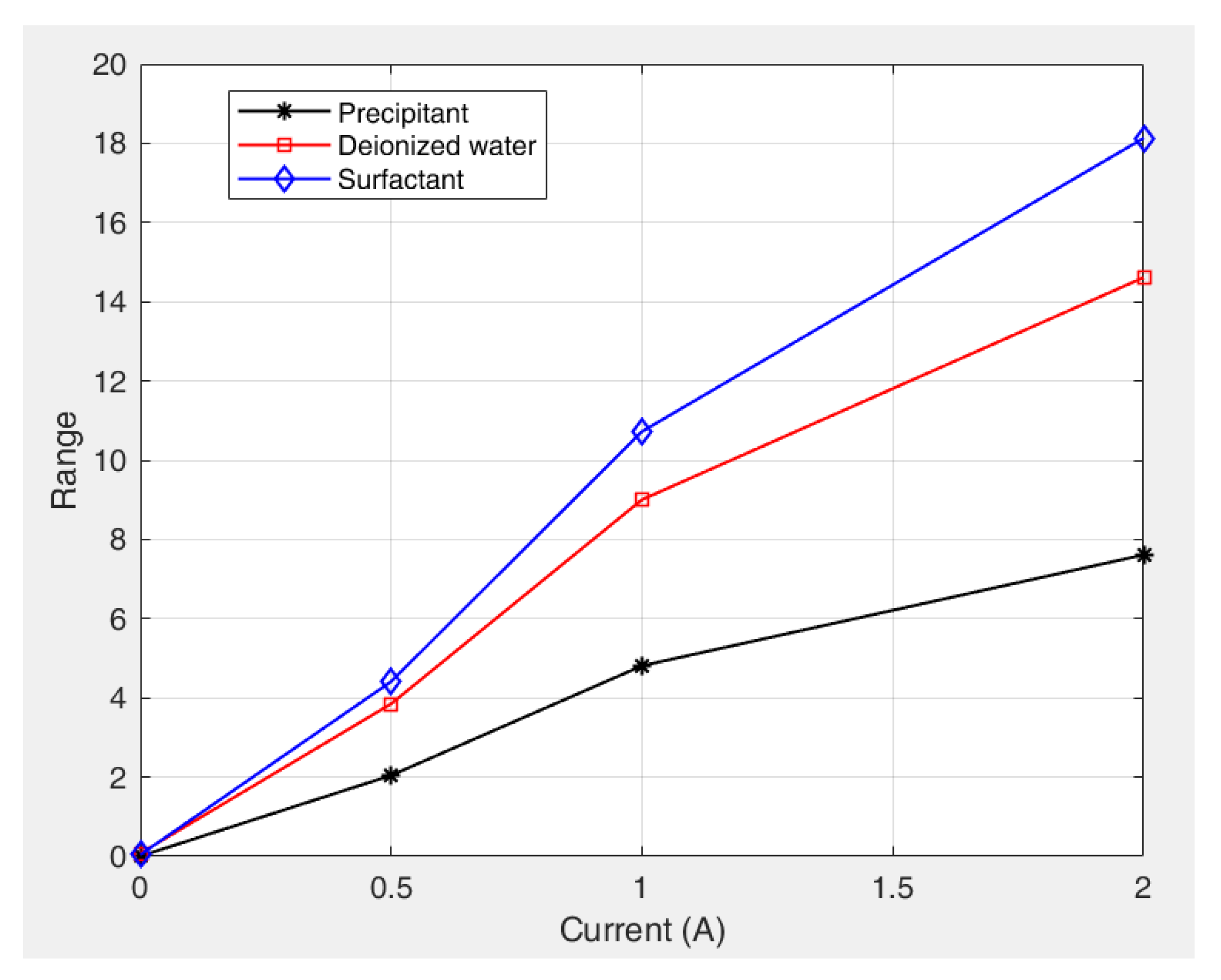
The results indicate that under an applied magnetic field, the preparation parameters of the kerosene-based magnetic fluids exhibited the following descending order of influence on the yield stress based on their range values: surfactant dosage > deionized water volume > precipitant dosage. This quantitative ranking suggests that the surfactant dosage (largest range value) is the most dominant factor governing the yield stress behavior, the deionized water volume exerts a secondary but measurable influence, and the precipitant dosage (smallest range value) exerts the least significant effect.
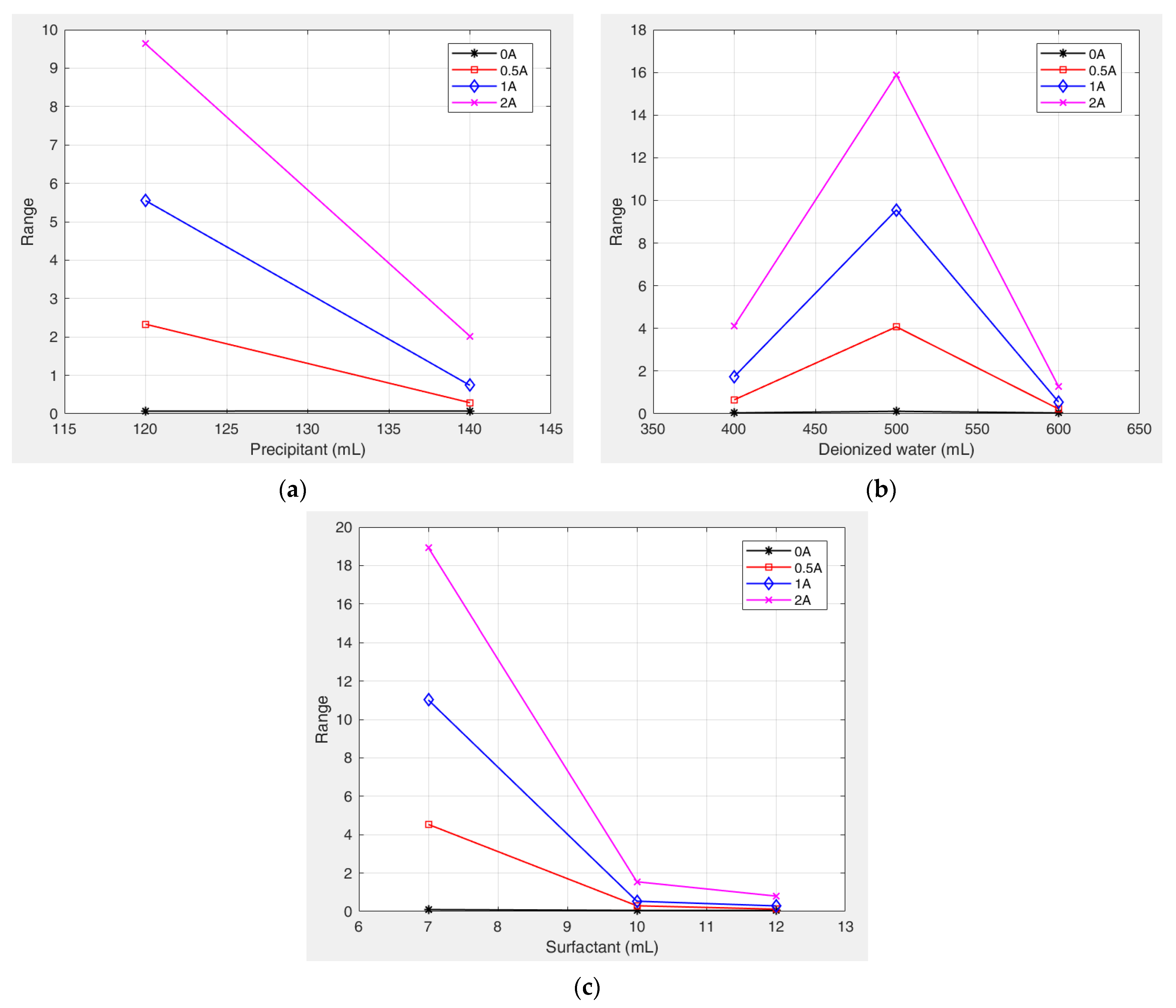
Figure 12 shows the variation in the range values (R) for the different factors with increasing current (magnetic field strength). The results indicate that the range values for all the factors positively correlate with the magnetic field intensity. This proportional relationship leads to more pronounced parameter effects under strong magnetic fields. Surfactant dosage remained the dominant factor and was particularly significant at higher field strengths. These trends confirm that an increase in the magnetic field strength increases the sensitivity of the yield stress to preparation parameters, especially the surfactant concentration.
Figure 13 shows the variation trends of yield stress with different factor levels. Under the combined influence of multiple factor levels, the deionized water content exhibited an initial positive and subsequent negative correlation with the yield stress. Specifically, as the deionized water content increased, the degree of its influence on the yield stress first increased and then decreased. In contrast, both the precipitant and surfactant dosages exhibited an overall negative correlation with the yield stress of the kerosene-based magnetic fluids. With increasing dosages of these components, their influence on the yield stress decreased significantly.
5.2. Sensitivity Analysis of Preparation Parameters Based on Variance Analysis
Although range analysis can identify the relative magnitude of influence of each factor on the yield stress, it cannot assess the statistical significance of these effects or quantify the degree of significance. In contrast, ANOVA can be used to eliminate the effect of experimental errors, evaluate the effects of factor-level variations, and determine the sensitivity of different influencing factors. Thus, ANOVA enables a rigorous investigation into the statistical significance of the effects of these factors on the yield stress of kerosene-based magnetic fluids.
Accordingly, ANOVA was applied to the preparation parameter data to examine the significance of the three key factors—the precipitant dosage, deionized water volume, and surfactant dosage—on the yield stress. The parameters can be systematically adjusted based on the results to achieve targeted control over the rheological properties of the magnetic fluid.
The total value
for each factor level was first calculated. Subsequently, the sum of squares
and the corresponding degrees of freedom
were computed as follows:
where
denotes the number of horizontal replicates, and
denotes the sum of the orthogonal experimental measurement data.
Next, the error sum of squares , defined as the sum of squares from the blank columns and several relatively small column sums of squares, was determined. The corresponding degrees of freedom were obtained as the sum of the degrees of freedom of these columns. In the absence of repeated trials and blank columns, the column with the smallest sums of squares may be selected to represent the error sum of squares.
The results are summarized in
Table 11, where the mean square
and F-ratio are computed. Here,
.
The rejection region corresponding to a given significance level α was determined, and a parameter sensitivity analysis was conducted. Generally, when F-ratio < F
0.05 (5% confidence level), the factor is considered to have no significant effect on the evaluation index. When F
0.05 < F-ratio < F
0.01 (1% confidence level), the factor is considered as a sensitive parameter with a significant influence on the evaluation index. When F-ratio > F
0.01 (1% confidence level), the factor is identified as a highly sensitive parameter that has a significant effect on the evaluation index [
21]. The ANOVA results obtained from the orthogonal experimental data are presented in
Table 12.
The F-distribution table indicates that F
0.05 (2,6) = 19.33 and F
0.01 (2,6) = 99.33. An analysis of the data shown in
Table 10 indicated that, for magnetic flux densities at 0 and 0.5 A, all three factors yield F-ratios below 19.33, suggesting no significant effect on the yield stress. At 1 A, the F-ratio for the surfactant dosage lies between 19.33 and 99.33, suggesting a significant influence on the yield stress, thereby identifying it as a sensitive parameter. For a higher magnetic flux density at 2 A, the F-ratio for the surfactant dosage exceeds 99.33, indicating a highly significant effect, identifying it as an extremely sensitive parameter. Moreover, the significance of the surfactant dosage increases rapidly with increasing magnetic field strength.
Based on the magnitude of the F-ratios, the sensitivity of the parameters affecting the yield stress of the kerosene-based magnetic fluids follows the order surfactant dosage > precipitant dosage > deionized water content.
Therefore, these results are consistent with those of range analysis.
Theoretical analysis revealed that the yield stress of magnetic fluids can be determined based on the number of chains per unit area, interparticle interactions, and the size distribution of magnetic particles. Based on the experimental results, when no external magnetic field is applied or when the magnetic field strength is relatively low, the three preparation factors exhibit comparable levels of influence on the yield stress of the kerosene-based magnetic fluid. However, as the magnetic field strength increases, their impacts diverge: the surfactant demonstrates the most pronounced effect on the yield stress, followed by the precipitating agent, while the influence of deionized water remains relatively minor.
During magnetic fluid preparation, deionized water helps maintain product purity primarily by controlling the ionic strength within the system. This modulates the electrostatic interactions between particles, thereby influencing the yield stress of the magnetic fluid. Nevertheless, deionized water content exerts a relatively minor influence on the yield stress.
In this study, ammonium hydroxide (NH3·H2O) was used as the precipitating agent for the chemical reaction. The OH− ions it provides promote the coprecipitation of metal salt solutions to form ultrafine magnetic particles, exerting a decisive influence on their size, morphology, and size distribution. Larger magnetic particles exhibit stronger magnetic attraction, facilitating the formation of robust chain-like structures, which leads to an increase in yield stress. Conversely, the uniformity of narrow particle size distributions enables the formation of highly ordered chain-like structures under a magnetic field. In such cases, the interparticle forces are more consistent, generally resulting in a higher yield stress under magnetic fields. Therefore, the precipitating agent exerts a more substantial influence on the yield stress of magnetic fluids.
Surfactants serve primarily as dispersing agents during the preparation of magnetic fluids, preventing particle agglomeration and playing a critical role in maintaining the stability of magnetic fluids in practical applications. The steric hindrance effect provided by the surfactant enables uniform and stable dispersion of magnetic particles within the carrier liquid, attenuates the van der Waals forces between particles, and suppresses magnetic dipole interactions. When the surfactant quantity is sufficient to form a complete, dense, and stable coating on the particles, each particle is effectively isolated by surfactant molecules, resulting in the lowest yield stress. If the surfactant amount is insufficient, incomplete particle coating occurs, which leads to agglomeration and the formation of robust chain-like or columnar network structures, which significantly increase the yield stress. Conversely, if an excessive amount of surfactant is used, the surplus surfactant molecules disperse within the magnetic fluid in the form of micelles or free molecules, increasing the viscosity of the magnetic fluid and consequently raising the yield stress. Therefore, the amount of surfactant exserts a more pronounced influence on the yield stress of magnetic fluids.