Microstructural, Mechanical, Thermal, and Magnetic Properties of the Mechanically Alloyed and Consolidated Al–16 wt. % Mn–7 wt. % Cu Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanically Alloyed Powders
3.1.1. Structural Characterisation
3.1.2. Morphology and EDS Analysis
3.1.3. Magnetic Properties
3.1.4. Approach to Magnetic Saturation
3.2. Heat-Treated Powders
3.3. Sintered Powders
3.3.1. Structural Analysis
3.3.2. Morphology
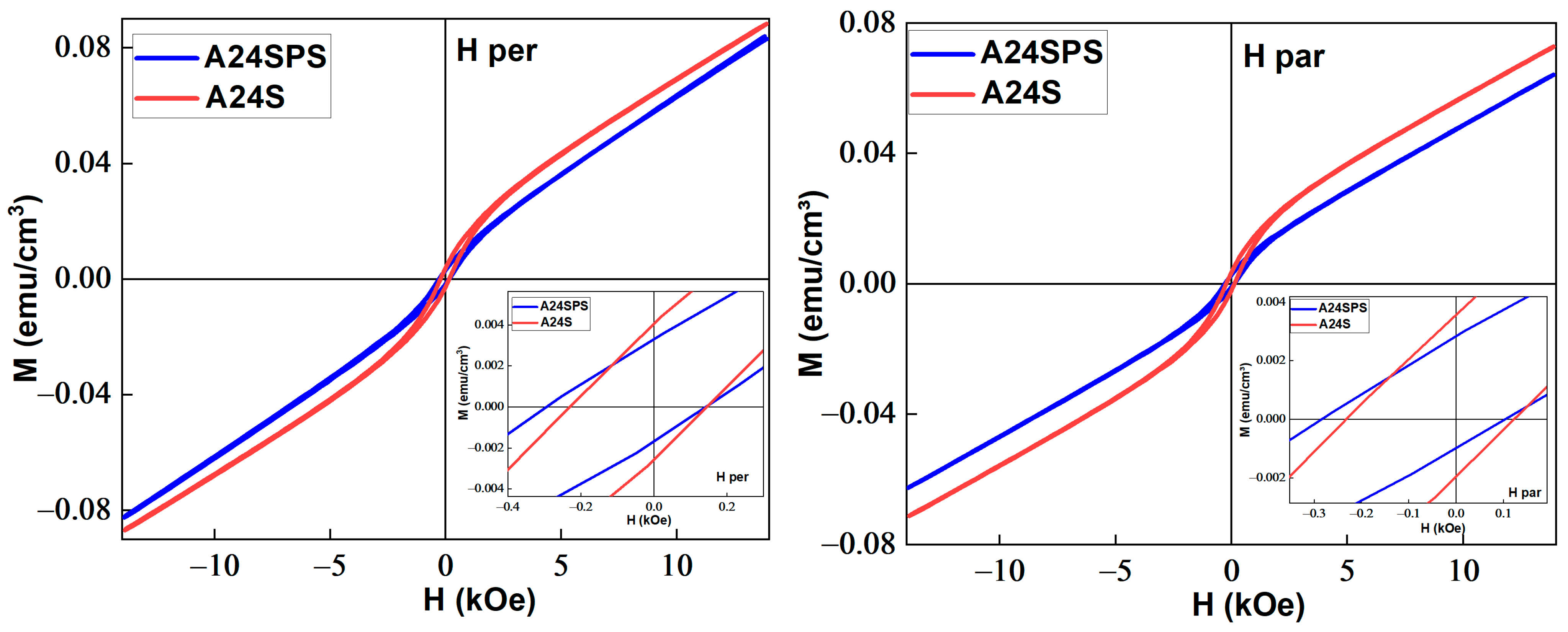
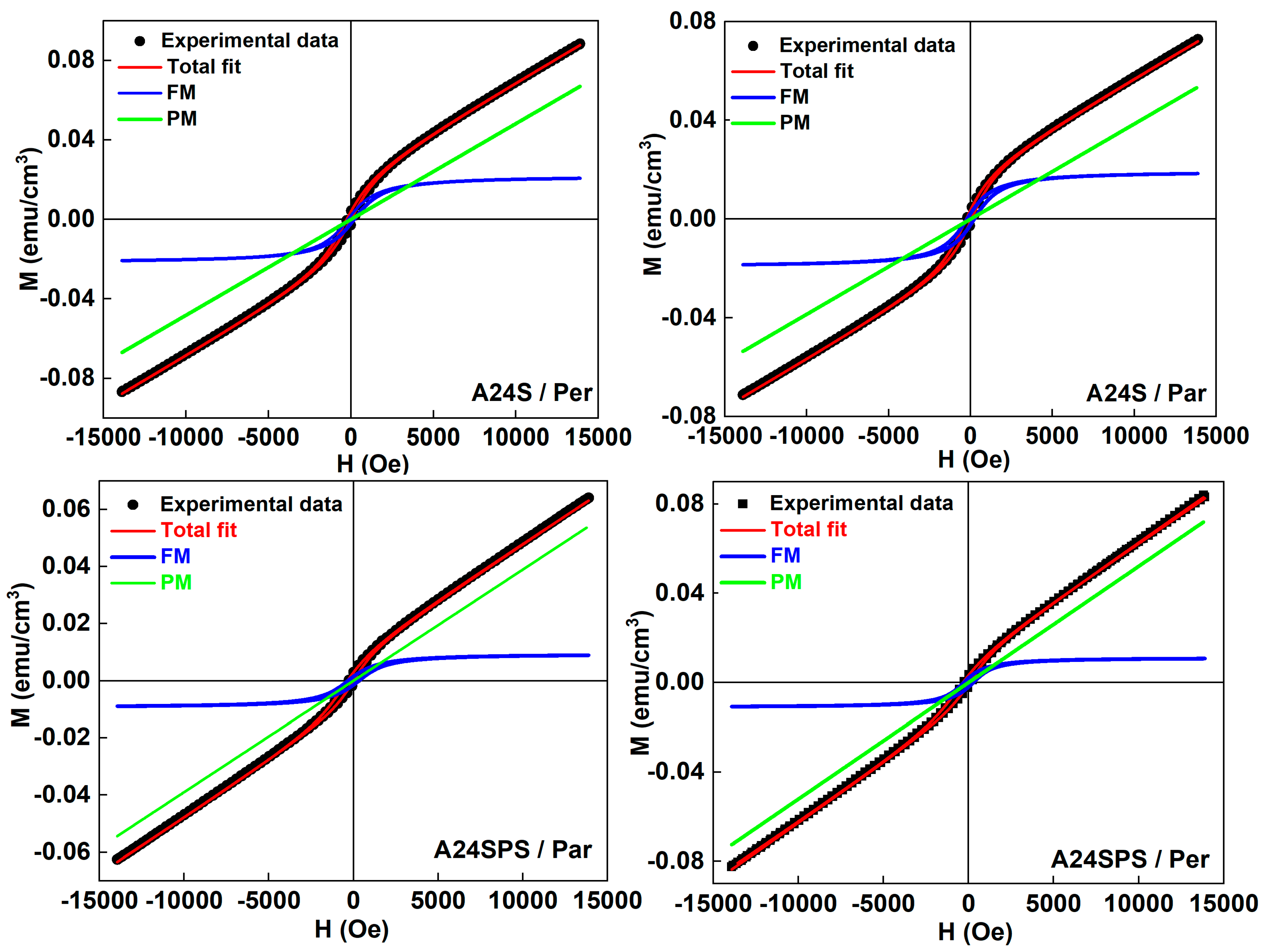
3.3.3. Magnetic Properties
3.4. Mechanical Properties
4. Conclusions
- The milling process, up to 24 h (A24), refines the microstructure and forms a nanocomposite structure where nanosized Mn (77 nm, 17.55%) and Cu (62 nm, 11.25%) precipitates are dispersed within the Al matrix (175 nm, 71.2%). The EDS analysis reveals the presence of Cu-Al-, Mn-Al-, and Al-Mn-enriched particles.
- The presence of several exothermic and endothermic peaks in the DSC scans during heating and cooling indicates the occurrence of different phase transformations. The XRD analysis and EDS mapping of the heat-treated A24_900 °C powder reveal a nanocomposite structure where the intermetallic Al20Cu2Mn3 nanoprecipitates are dispersed into the Al matrix.
- The milled powders exhibit a weak ferromagnetism with an EB behaviour. Hc reaches 101 Oe after 24 h of milling. The magnetisation increases and the EB drops with increasing milling time.
- The fitted Ms values using the LAS are comparable to the experimental values.
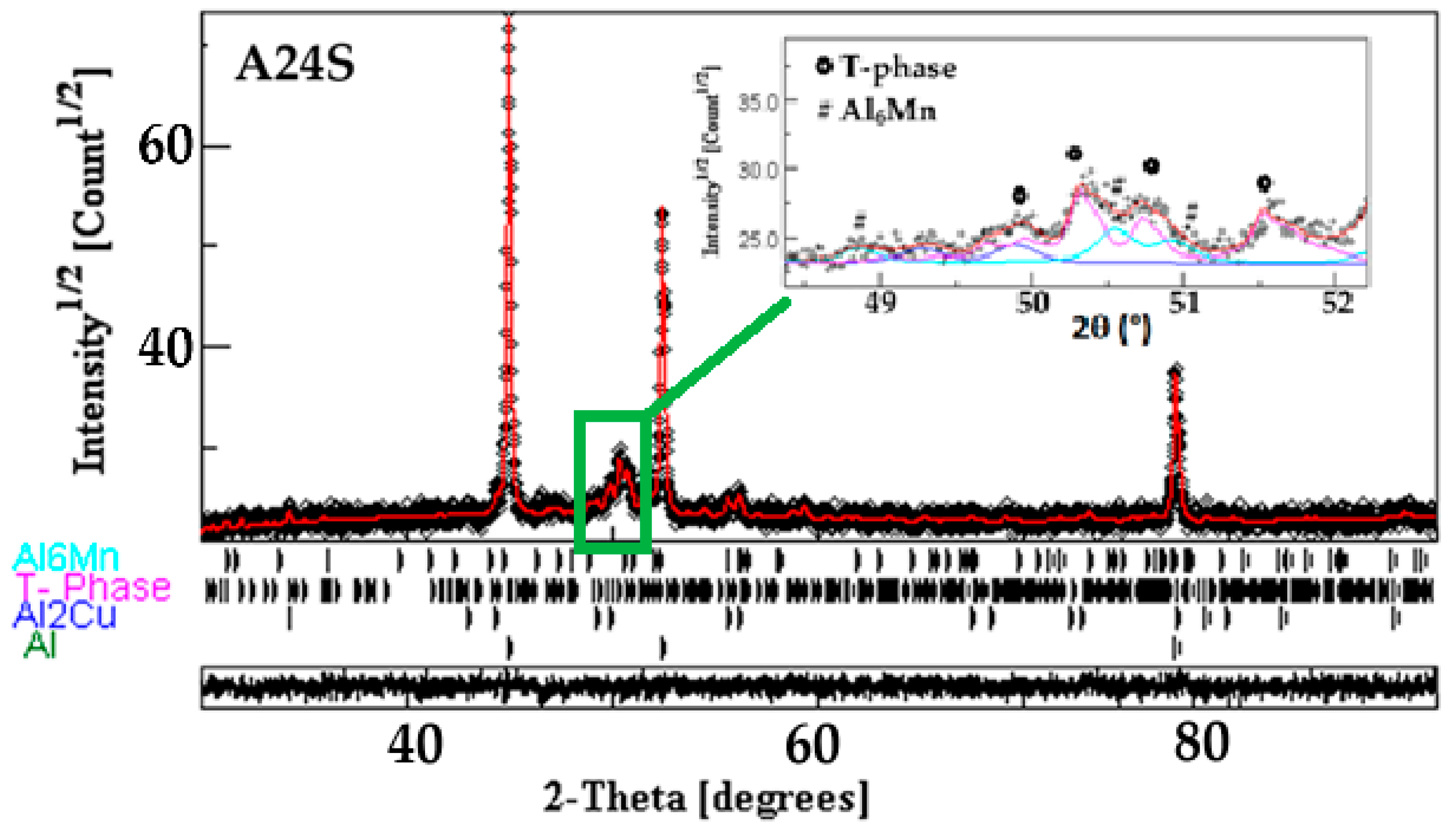
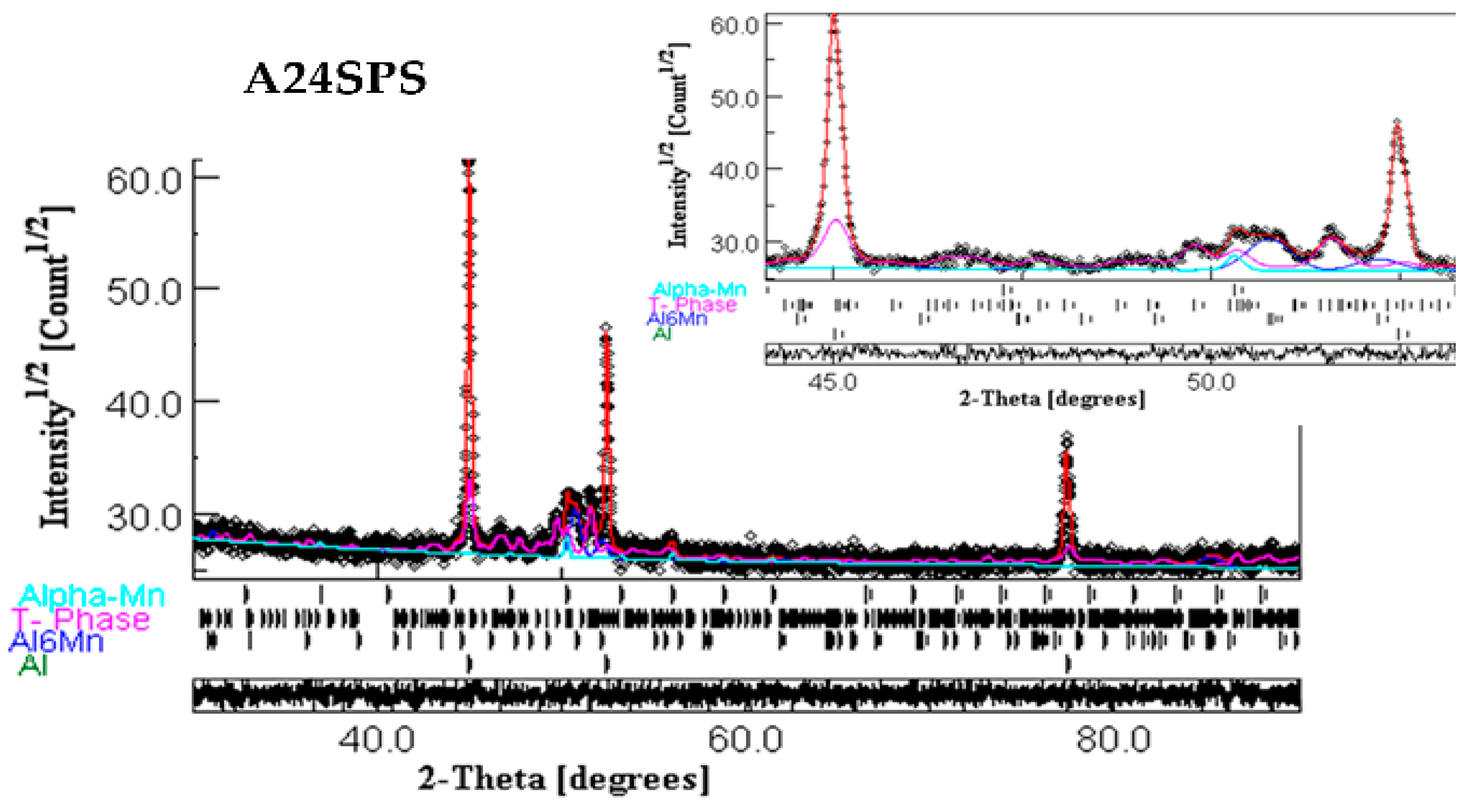
- Conventional sintering of the A24 powder (A24S) at 500 °C for one hour leads to the precipitation of Al6Mn, Al2Cu, and Al20Cu2Mn3 T-phase into the Al-enriched matrix. In contrast, the crystal structure of the consolidated powders by SPS (A24SPS) consists of an Al solid solution, Al6Mn, T-phase, and α-Mn.
- The heat treatment and sintering affect the lattice parameters (a, b, and c), cell volume, crystallite size, weight fraction, atom site occupancy, and fractional coordinates of the Al20Cu2Mn3 T-phase.
- The sintered samples exhibit the coexistence of a significant PM/AFM contribution to the M-H curves, in addition to the FM contribution, with increasing Hc and decreasing EB.
- A higher microhardness value of about 581 HV is achieved for the A24SPS sample compared to those of the A24 (68 HV) and A24S (80 HV) samples. The improvement in microhardness can be related to the decrease in the T-phase’s crystallite size and the increase in its weight fraction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torralba, J.D.; Da Costa, C.; Velasco, F. P/M aluminum matrix composites: An overview. J. Mater. Process. Technol. 2003, 133, 203–206. [Google Scholar] [CrossRef]
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A review on the production of metal matrix composites through stir casting—Furnace design, properties, challenges, and research opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Ravikumar, K.S.; Ghanaraja, S.; Ramesh, M.R. Synthesis and characterization of nano-alumina powder by milling of Al and MnO2 powder mixture. Mater. Today Proc. 2021, 46, 9021–9026. [Google Scholar] [CrossRef]
- Nokhrin, A.; Malekhonova, N.; Chuvil’deev, V.; Melekhin, N.; Bragov, A.; Filippov, A.; Boldin, M.; Lantsev, E.; Sakharov, N. Effect of High-Energy Ball Milling Time on the Density and Mechanical Properties of W-7%Ni-3%Fe Alloy. Metals 2023, 13, 1432. [Google Scholar] [CrossRef]
- Wei, L.K.; Abd Rahim, S.Z.; Al Bakri Abdullah, M.M.; Yin, A.T.M.; Ghazali, M.F.; Omar, M.F.; Nemes, O.; Sandu, A.V.; Vizureanu, P.; Abdellah, A.E. Producing Metal Powder from Machining Chips Using Ball Milling Process: A Review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Nayak, K.; Han, J.; Jung, C.; Joo, M.; Lee, K.; Bae, D.; Choi, H. Synergetic effect of milling speed and duration on particle morphology and mechanical properties of nanocrystalline Al matrix containing SiC. Powder Metall. 2023, 66, 519–529. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Influence of Pre-Milling on the Mn Solid Solubility in the Al-Mn-Cu Alloy during Mechanical Alloying. Metals 2023, 13, 756. [Google Scholar] [CrossRef]
- Prosviryakov, A.; Shcherbachev, K.; Tabachkova, N.Y. Investigation of nanostructured Al-10 wt. % Zr material prepared by ball milling for high temperature applications. Mater. Charact. 2017, 123, 173–177. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Esquivel, J.; Murdoch, H.; Darling, K.; Gupta, R. Excellent corrosion resistance and hardness in Al alloys by extended solid solubility and nanocrystalline structure. Mater. Res. Lett. 2018, 6, 79–83. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Li, Y.; Hu, A.; Fu, Y.; Liu, S.; Shen, W.; Hu, H.; Nie, X. Al Alloys and Casting Processes for Induction Motor Applications in Battery-Powered Electric Vehicles: A Review. Metals 2022, 12, 216. [Google Scholar] [CrossRef]
- Murashkin, M.Y.; Sabirov, I.; Sauvage, X.; Valiev, R.Z. Nanostructured Al and Cu alloys with superior strength and electrical conductivity. J. Mater. Sci. 2015, 51, 33–49. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Bazlov, A.I.; Prosviryakov, A.S.; Emelina, N.B.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The influence of the Al2O3 particles on the microstructure of the mechanically alloyed Al-Mn-Cu alloy. J. Alloys Compd. 2023, 930, 167452. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Armstrong, L.; Kapoor, D.; Tschopp, M.A.; Kecskes, L.J.; Mathaudhu, S.N. Influence of Mn solute content on grain size reduction and improved strength in mechanically alloyed Al–Mn alloys. Mater. Sci. Eng. A 2014, 589, 57–65. [Google Scholar] [CrossRef]
- Ruan, S.; Schuh, C.A. Electrodeposited Al–Mn alloys with microcrystalline, nanocrystalline, amorphous and nano-quasicrystalline structures. Acta Mater. 2009, 57, 3810–3822. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. Nanodispersoids of the quasicrystalline I-phase in Mn- and Mg-bearing aluminum-based alloys. Mater. Lett. 2021, 284, 128945. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation crystallography of plate-shaped Al6(Mn, Fe) dispersoids in AA5182 alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Asgar-Khan, M.; Medraj, M. Thermodynamic Description of the Mg-Mn, Al-Mn and Mg-Al-Mn Systems Using the Modified Quasichemical Model for the Liquid Phases. Mater. Trans. 2009, 50, 1113–1122. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Catalano, J.E.; Tschopp, M.A.; Kecskes, L.J. Effect of Processing Parameters on the Microstructure of Mechanically Alloyed Nanostructured Al-Mn Alloys. In Advanced Composites for Aerospace, Marine, and Land Applications II; Springer: Cham, Switzerland, 2016; pp. 3–11. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M.; Abomostafa, H. Fabrication of Al/Al2O3/SiC/graphene hybrid nanocomposites from Al-dross by powder metallurgy: Sinterability, mechanical and electrical properties. Ceram. Int. 2022, 48, 20923–20932. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Robert, M.H.; Torralba, J.M. Mechanically alloyed AlN particle-reinforced Al-6061 matrix composites: Powder processing, consolidation and mechanical strength and hardness of the as-extruded materials. Mater. Sci. Eng. A 2006, 426, 85–94. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Mochugovskiy, A.G.; Prosviryakov, A.S.; Bazlov, A.I.; Emelina, N.B.; Mikhaylovskaya, A.V. The Microstructure and Properties of Al–Mn–Cu–Zr Alloy after High-Energy Ball Milling and Hot-Press Sintering. Metals 2024, 14, 310. [Google Scholar] [CrossRef]
- Soares, E.; Bouchonneau, N.; Alves, E.; Alves, K.; Araujo Filho, O.; Mesguich, D.; Chevallier, G.; Laurent, C.; Estournes, C. Microstructure and Mechanical Properties of AA7075 Aluminum Alloy Fabricated by Spark Plasma Sintering (SPS). Materials 2021, 14, 430. [Google Scholar] [CrossRef]
- Aliyu, I.; Saheb, N.; Hassan, S.; Al-Aqeeli, N. Microstructure and Properties of Spark Plasma Sintered Aluminum Containing 1 wt. % SiC Nanoparticles. Metals 2015, 5, 70–83. [Google Scholar] [CrossRef]
- Korznikova, G.F.; Nazarov, K.S.; Khisamov, R.K.; Sergeev, S.N.; Shayachmetov, R.U.; Khalikova, G.R.; Baimova, J.A.; Glezer, A.M.; Mulyukov, R.R. Intermetallic growth kinetics and microstructure evolution in Al-Cu-Al metal-matrix composite processed by high pressure torsion. Mater. Lett. 2019, 253, 412–415. [Google Scholar] [CrossRef]
- Belov, N.; Belov, V.; Alabin, A.; Mishurov, S. New generation of economically alloyed aluminum alloys. Metallurgist 2010, 54, 311–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD Program Version 2.992. Available online: https://luttero.github.io/maud/ (accessed on 28 November 2021).
- Nam, S.W.; Lee, D.H. The effect of Mn on the mechanical behavior of Al alloys. Met. Mater. 2000, 6, 13–16. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.; Kacprzak, L.; Scott, W.W. Binary Alloy Phase Diagrams; American Society for Metals: Metals Park, OH, USA, 1986; pp. 106–107. [Google Scholar]
- Li, F.; Ishihara, K.; Shingu, P. The formation of metastable phases by mechanical alloying in the aluminum and copper system. Metall. Trans. A 1991, 22, 2849–2854. [Google Scholar] [CrossRef]
- Sivakova, A.; Karpov, A.; Busurina, M.; Lazarev, P.; Sytschev, G.; Morozov, Y.G.; Sytschev, A. Self-Propagating High-Temperature Synthesis of Cu–Mn–Al Alloys: Thermoelectric and Magnetic Properties. Int. J. Self-Propag. High-Temp Synth. 2024, 33, 237–244. [Google Scholar] [CrossRef]
- Boutabia, N.; Bouzabata, B.; Alleg, S.; Suñol, J.-J.; Greneche, J.-M. Mechanochemical reaction and phase transformations in the α-Fe2O3/Mg mixture. Mater. Chem. Phys. 2025, 337, 130570. [Google Scholar] [CrossRef]
- Dadda, K.; Djerad, S.; Alleg, S.; Dadda, N.; Rabhi, S.; Hlil, E.-K. Structural, magnetic, and electronic structure of the nanostructured (CoMn)50Ni50 powders used in dye discoloration via a heterogeneous Fenton-like process. Transit. Met. Chem. 2024, 49, 275–283. [Google Scholar] [CrossRef]
- Alleg, S.; Bekhouche, A.; Hachache, H.; Suñol Martínez, J.J. Effect of aluminum addition on the microstructure, magnetic, and mechanical properties of FeCrCoNiMn high-entropy alloy. Crystals 2023, 13, 1483. [Google Scholar] [CrossRef]
- Alleg, S.; Chabi, T.; Bensebaa, N.; Saurina, J.; Escoda, L.; Hlil, E.-K.; Suñol, J.-J. Investigation of the Critical Behavior, Magnetocaloric Effect and Hyperfine Structure in the Fe72Nb8B20 Powders. Materials 2020, 13, 4476. [Google Scholar] [CrossRef]
- Bekhouche, A.; Alleg, S.; Dadda, K.; Daoudi, M.I.; Saurina, J.; Suñol, J.-J. Microstructure, martensitic transformation kinetics, and magnetic properties of (Ni50Mn40In10)100−xCox melt-spun ribbons. J. Therm. Anal. Calorim. 2024, 150, 953–964. [Google Scholar] [CrossRef]
- Bekhouche, A.; Alleg, S.; Dadda, K.; Costa, B.F.; Wederni, A.; Suñol, J.-J. Ni50Mn37.5Sn12.5 Heusler Alloy: Influence of Co Addition on the Structure, Martensitic Transition, and Magnetic Properties. Magnetochemistry 2024, 10, 59. [Google Scholar] [CrossRef]
- Souza, J.S.; Silva, R.A.G. Microstructure Changes in Sn- or Gd-Modified Cu–11%Al–10%Mn Alloy. Metallogr. Microstruct. Anal. 2021, 10, 116–126. [Google Scholar] [CrossRef]
- Kok, M.; Qadir, R.A.; Mohammed, S.S.; Qader, I.N. Effect of transition metals (Zr and Hf) on microstructure, thermodynamic parameters, electrical resistivity, and magnetization of CuAlMn-based shape memory alloy. Eur. Phys. J. Plus 2022, 137, 62. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Fallot, M. Ferromagnétisme des alliages de fer. Ann. Phys. 1936, 11, 305–387. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Zhang, H.; Zeng, D.; Liu, Z. The law of approach to saturation in ferromagnets originating from the magnetocrystalline anisotropy. J. Magn. Magn. Mater. 2010, 322, 2375–2380. [Google Scholar] [CrossRef]
- Andreev, S.V.; Bartashevich, M.I.; Pushkarsky, V.I.; Maltsev, V.N.; Pamyatnykh, L.A.; Tarasov, E.N.; Kudrevatykh, N.V.; Goto, T. Law of approach to saturation in highly anisotropic ferromagnets application to Nd Fe B melt-spun ribbons. J. Alloys Compd. 1997, 260, 196–200. [Google Scholar] [CrossRef]
- Bradley, A.J.; Thewlis, J. The crystal structure of α-manganese. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1927, 115, 456–471. [Google Scholar] [CrossRef]
- Grushko, B.; Mi, S. Al-rich region of Al–Cu–Mn. J. Alloys Compd. 2016, 688, 957–963. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Yan, M. Determination of structure of Al20Cu2Mn3 phase in Al-Cu-Mn alloys. Mater. Res. Bull. 1989, 24, 1267–1270. [Google Scholar] [CrossRef]
- Robinson, K. LXXIII. The unit cell and Brillouin Zones of Ni4Mn11Al60 and related compounds. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1952, 43, 775–782. [Google Scholar] [CrossRef]
- Cheng, J.; Cai, Q.; Zhao, B.; Yang, S.; Chen, F.; Li, B. Microstructure and mechanical properties of nanocrystalline Al-Zn-Mg-Cu alloy prepared by mechanical alloying and spark plasma sintering. Materials 2019, 12, 1255. [Google Scholar] [CrossRef]
- Bobel, A.; Kim, K.; Wolverton, C.; Walker, M.; Olson, G.B. Equilibrium composition variation of Q-phase precipitates in aluminum alloys. Acta Mater. 2017, 138, 150–160. [Google Scholar] [CrossRef]
- Chen, J.; Liao, H.; Wu, Y.; Li, H. Contributions to high temperature strengthening from three types of heat-resistant phases formed during solidification, solution treatment, and ageing treatment of Al-Cu-Mn-Ni alloys, respectively. Mater. Sci. Eng. A 2020, 772, 138819. [Google Scholar] [CrossRef]
- Su, X.; Lei, Y.; Chen, Y.; Qu, H.; Qi, Z.; Zheng, G.; Liu, X.; Xiang, H.; Chen, G. Precipitating thermally reinforcement phase in aluminum alloys for enhanced strength at 400 °C. J. Mater. Sci. Technol. 2024, 172, 71–82. [Google Scholar] [CrossRef]
- Dar, S.M.; Liao, H.; Xu, A. Effect of Cu and Mn content on solidification microstructure, T-phase formation and mechanical property of Al Cu Mn alloys. J. Alloys Compd. 2019, 774, 758–767. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Bazlov, A.I.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum. Metals 2023, 13, 1765. [Google Scholar] [CrossRef]
- Onuki, J.; Koizumi, M.; Araki, I. Investigation of the reliability of copper ball bonds to aluminum electrodes. IEEE Trans. Compon. Hybrids Manuf. Technol. 1987, 10, 550–555. [Google Scholar] [CrossRef]
- Grössinger, R.; Sato, R.; Holzer, D.; Dahlgren, M. Properties, Benefits, and Application of Nanocrystalline Structures in Magnetic Materials. Adv. Eng. Mater. 2003, 5, 285–290. [Google Scholar] [CrossRef]
- Makhlouf, M.B.; Bachaga, T.; Sunol, J.J.; Dammak, M.; Khitouni, M. Synthesis and characterization of nanocrystalline Al-20 at.% Cu powders produced by mechanical alloying. Metals 2016, 6, 145. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wu, X.L. Ductility and plasticity of nanostructured metals: Differences and issues. Mater. Today Nano 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Valiev, R.; Estrin, Y.; Horita, Z.; Langdon, T.; Zehetbauer, M.; Zhu, Y. Fundamentals of superior properties in bulk nanoSPD materials. Mater. Res. Lett. 2016, 4, 1–21. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Prosviryakov, A.S.; Cheverikin, V.V.; Zanaeva, E.N.; Mikhaylovskaya, A.V. Influence of High-Energy Ball Milling on the Microstructure, Phase Composition, and Microhardness of the Al–Mn–Cu Alloy. Russ. J. Non-Ferrous Met. 2022, 63, 426–433. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Wu, H.; Wang, W.; Zhai, S.; Xu, J.; Niu, Z.; Wang, Y. Microstructure and property characterization of Al-based composites reinforced with CuZrAl particles fabricated by mechanical alloying and spark plasma sintering. Adv. Powder Technol. 2018, 29, 1695–1702. [Google Scholar] [CrossRef]

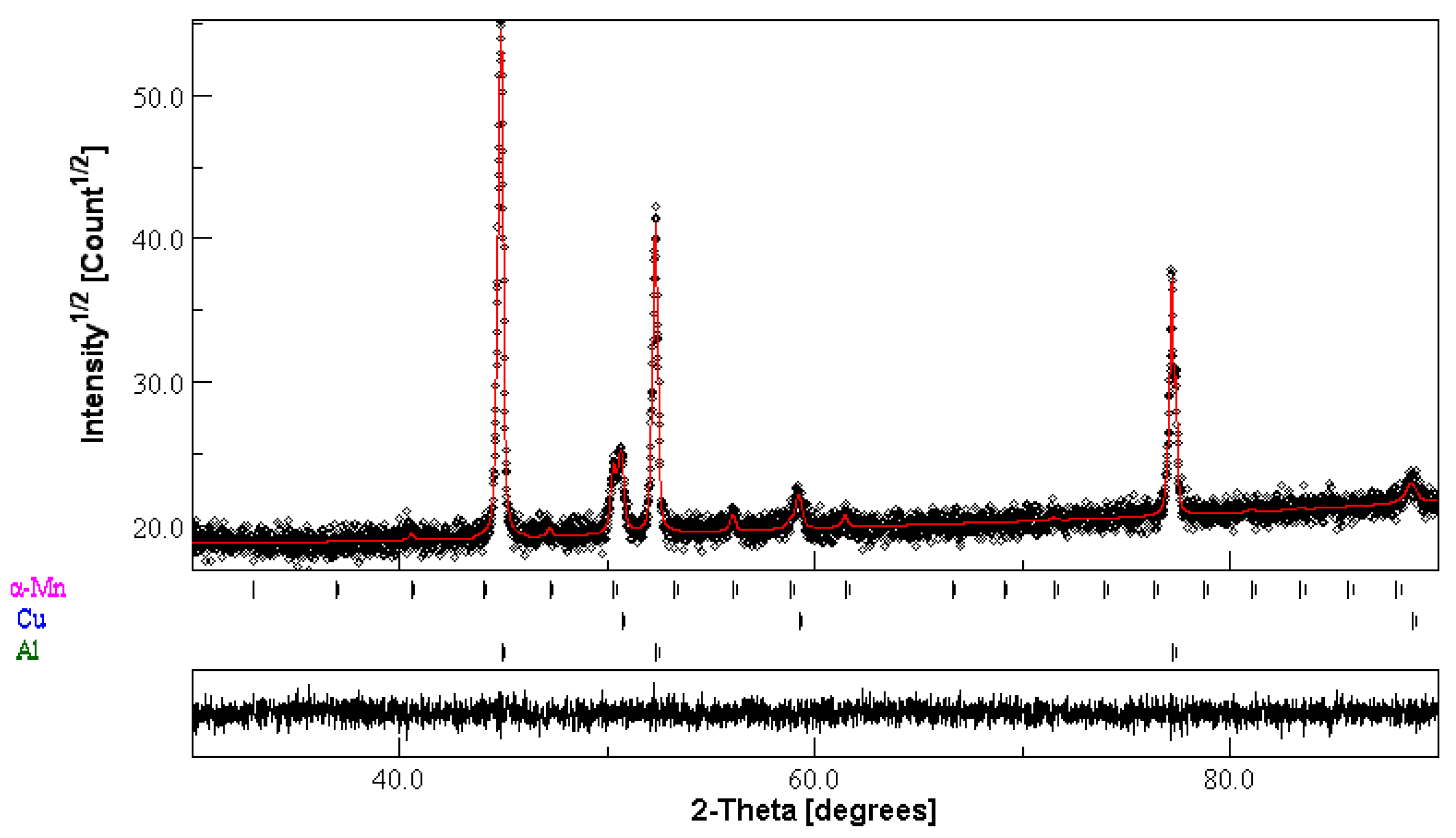
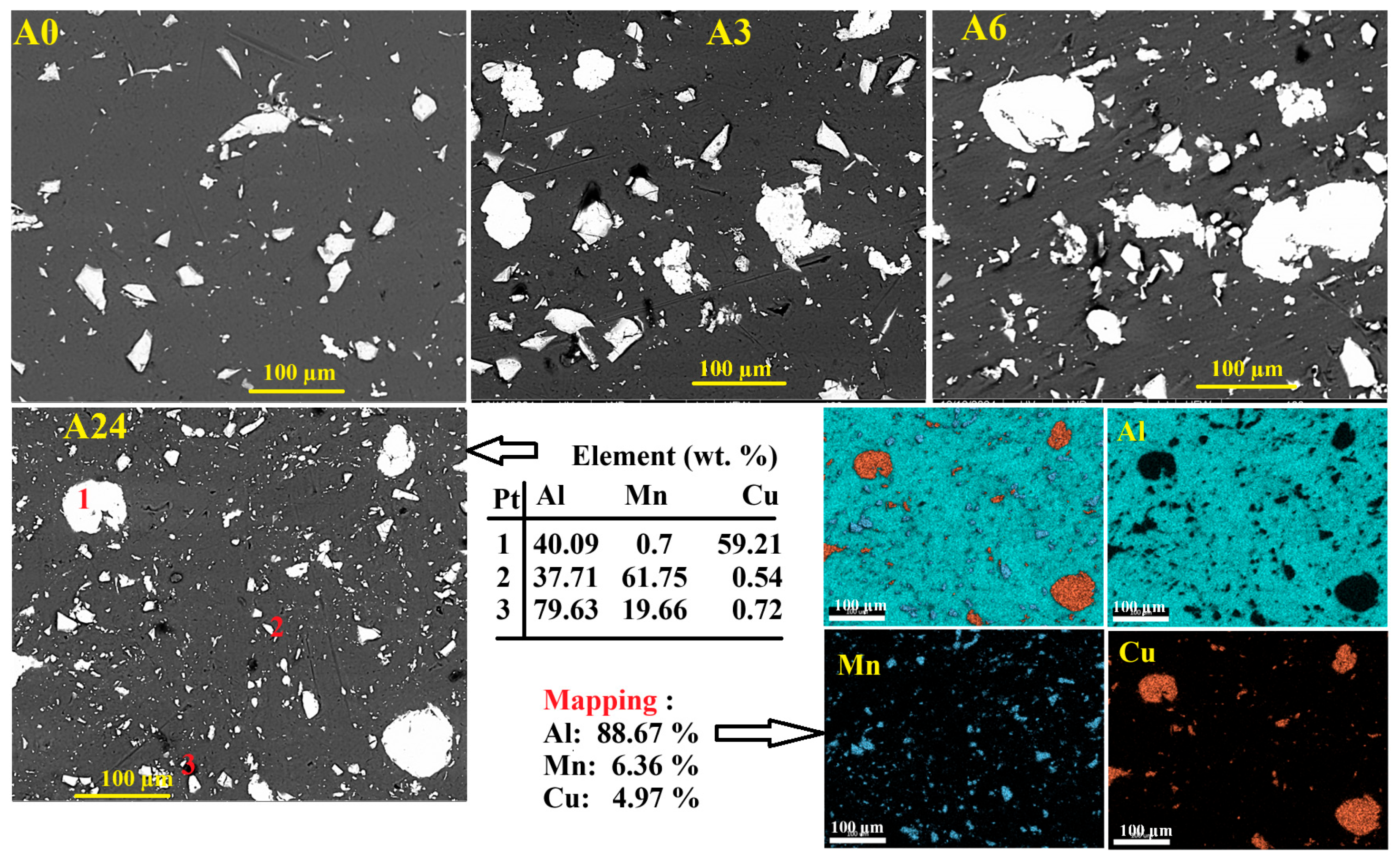
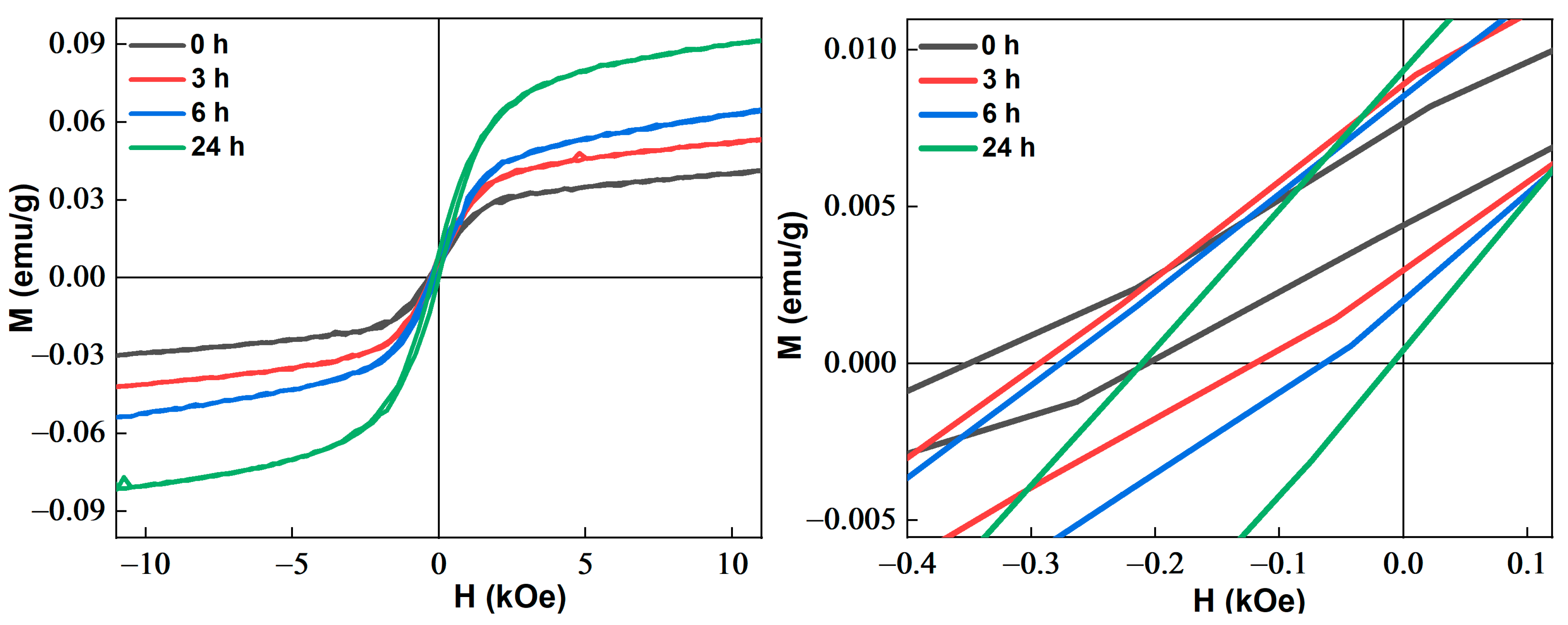


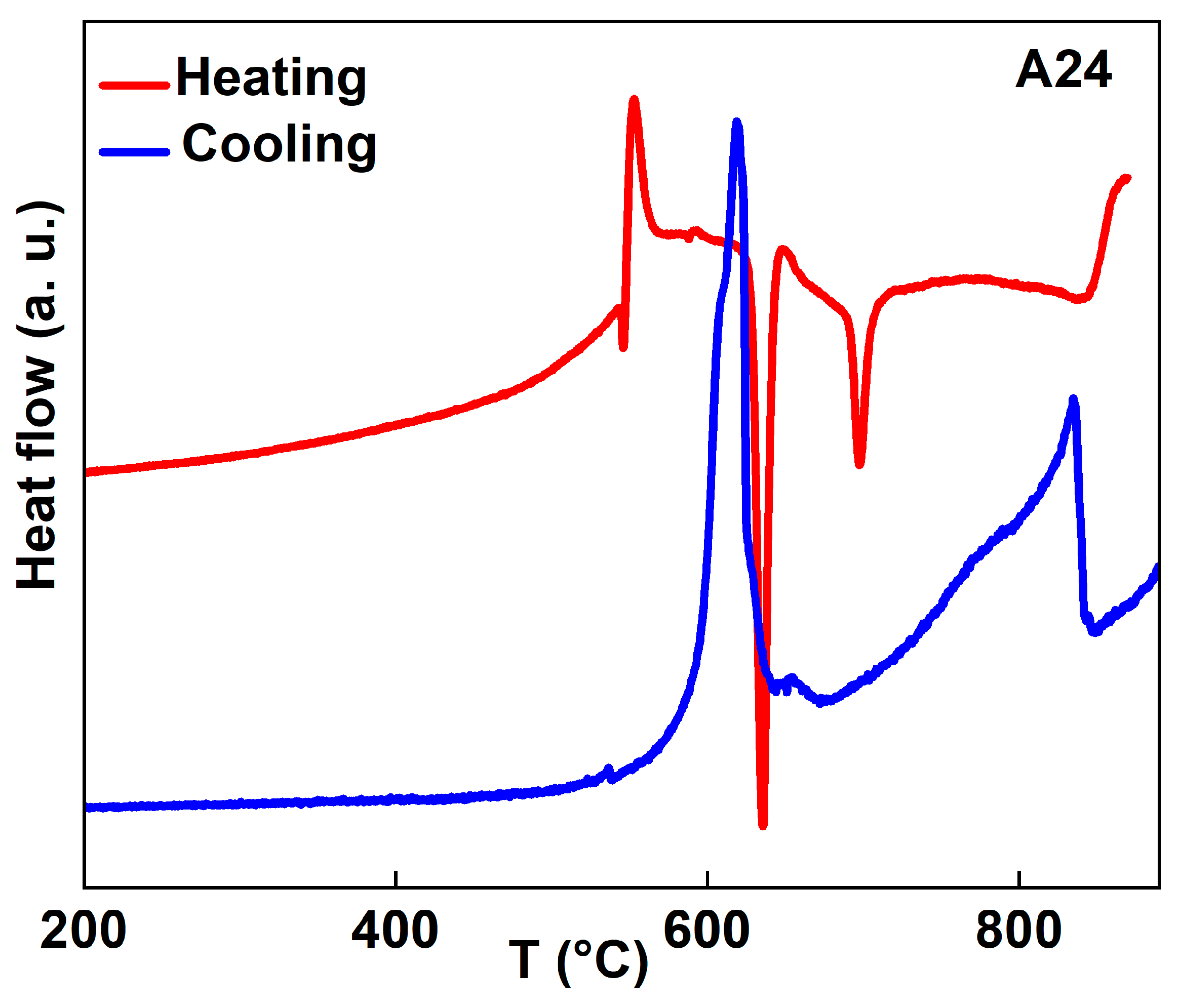
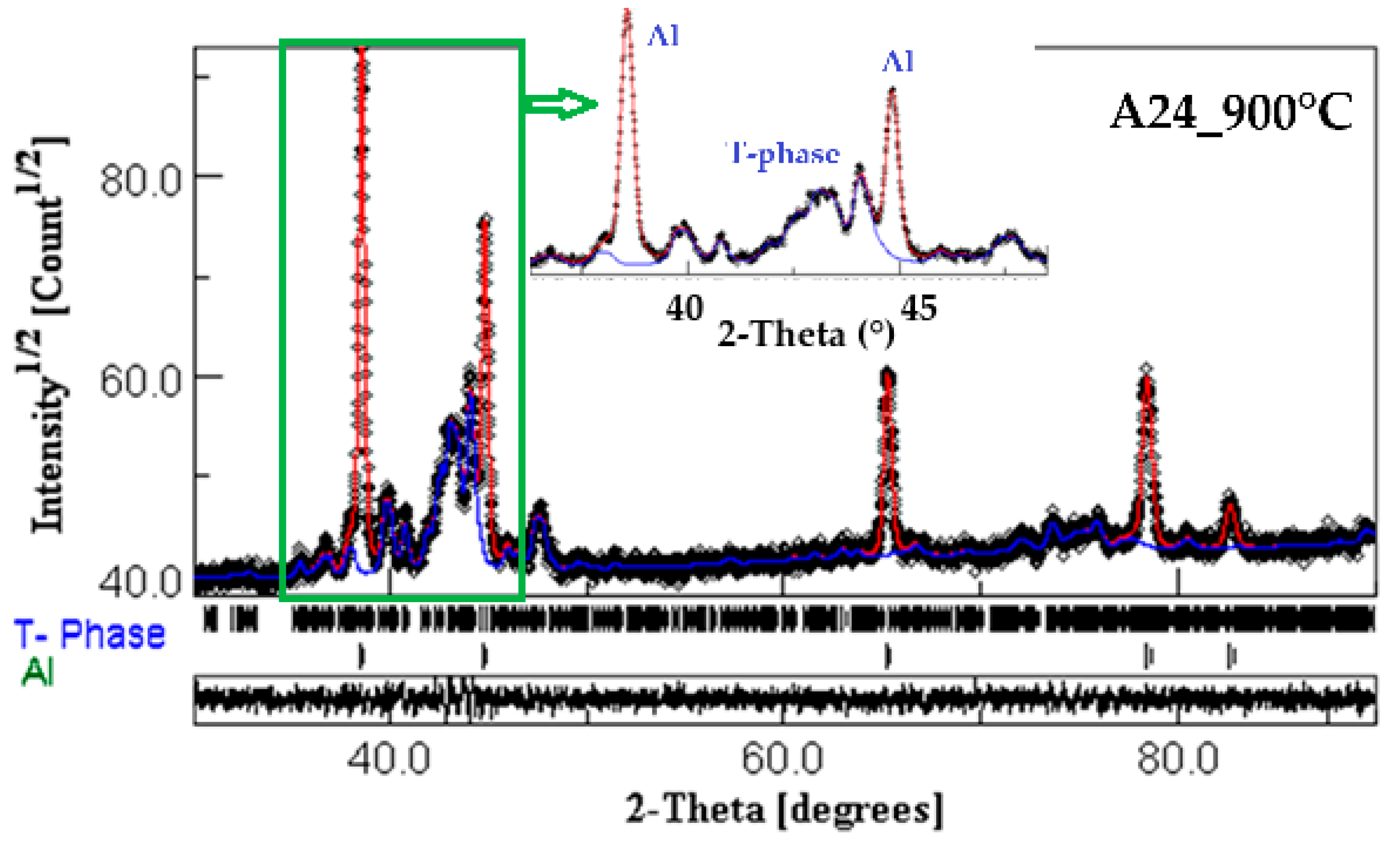
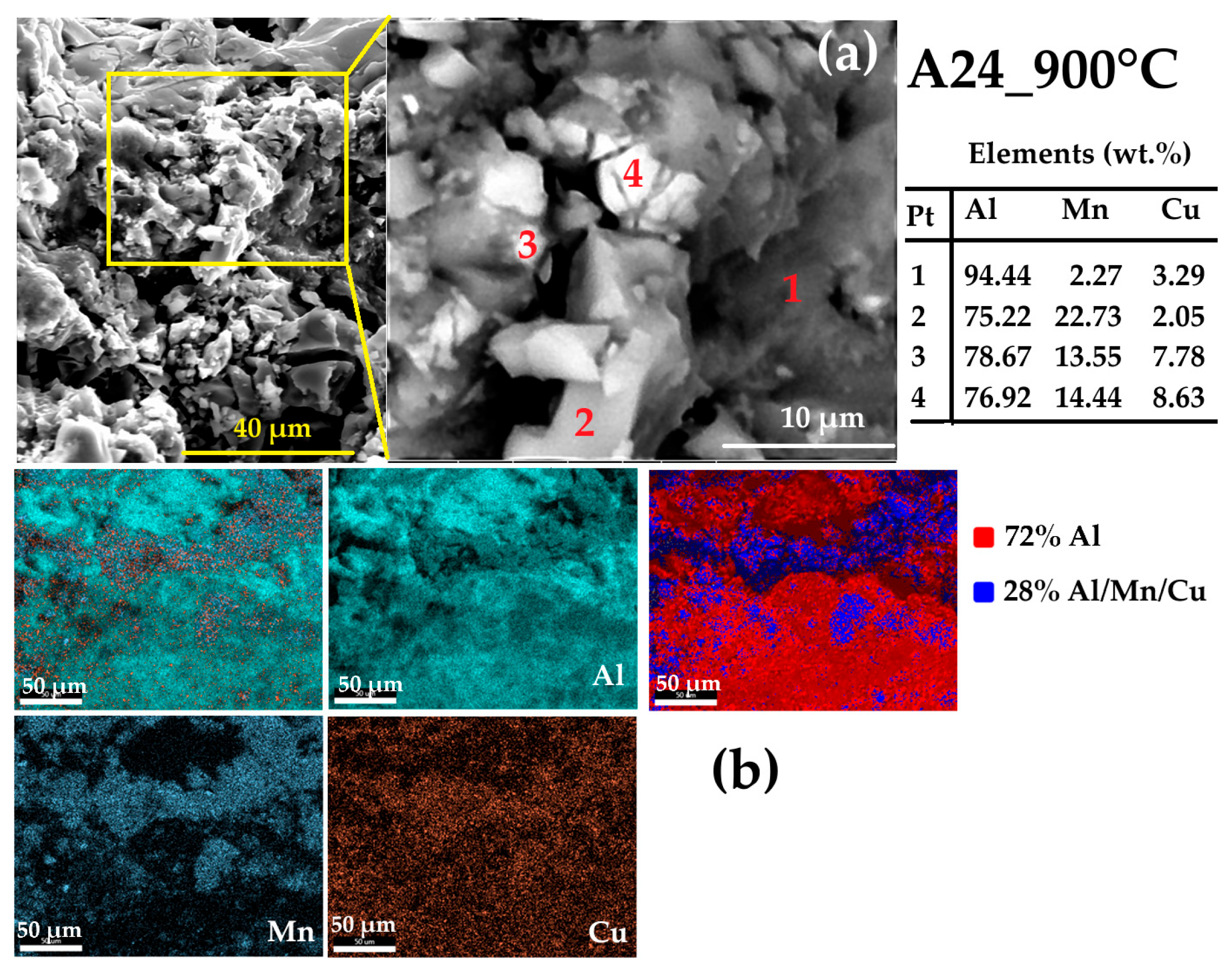

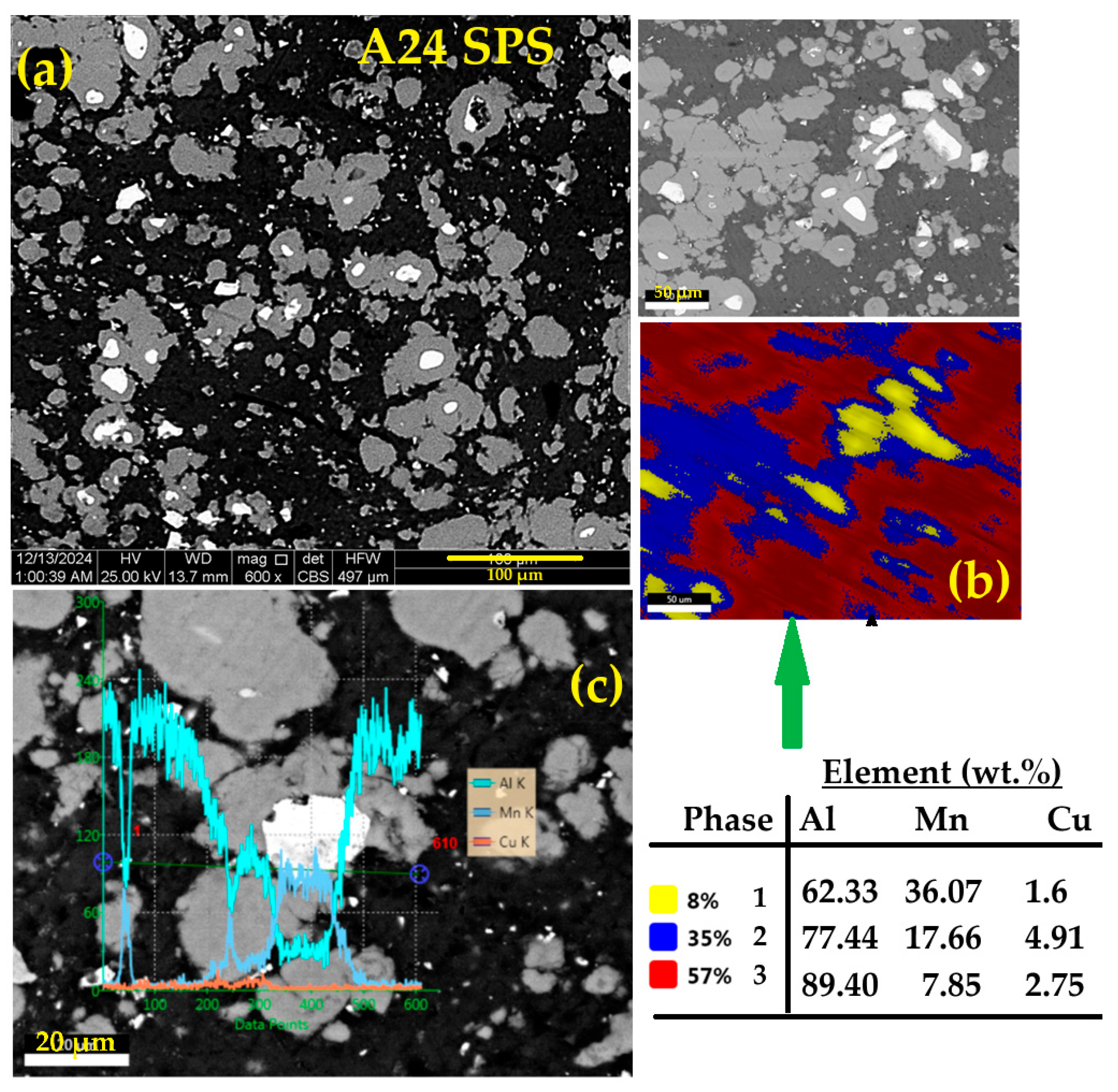
| Phase | a (Å) ± 10−4 | <L> (nm) | Weight Fraction (%) | Occupancy | Fractional Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Al | 4.0480 | 175 ± 2 | 71.20 ± 0.69 | 0.602 | 0 | 0 | 0 |
| Cu | 3.6140 | 62 ± 1 | 11.25 ± 0.28 | 1 | 0 | 0 | 0 |
| α-Mn | 8.9110 | 77 ± 1 | 17.55 ± 0.56 | Mn4: 0.974 Mn3: 0.975 Mn2: 0.971 Mn1: 0.967 | 0.1081 0.3584 0.3011 0 | 0.1081 0.3584 0.3011 0 | 0.280 0.0335 0.3011 0 |
| Sample | Ms (emu/g) ± 4 × 10−4 | A (Oe) | B × 104 (Oe2) | χ × 10−7 ± 0.3 (emu/g Oe) | R2 | M/Ms |
|---|---|---|---|---|---|---|
| A0 | 0.033 | 364 ± 4 | −2.34 | 7.78 | 0.995 | 0.564 |
| A3 | 0.045 | 419 ± 5 | −3.20 | 8.76 | 0.998 | 0.496 |
| A6 | 0.050 | 384 ± 4 | 3.79 | 14.9 | 0.998 | 0.428 |
| A24 | 0.088 | 722 ± 9 | −21.65 | 8.44 | 0.999 | 0.423 |
| Phase | a (Å) ± 10−4 | b (Å) ± 10−4 | c (Å) ± 10−4 | <L> ± 1 (nm) | Weight Fraction (%) |
|---|---|---|---|---|---|
| Al | 4.0438 | 62 | 72.3 ± 1 | ||
| T-phase | 24.3702 | 12.3035 | 7.6068 | 38 | 27.7 ± 0.6 |
| Site Label | Atom | Wyckoff Symbol/Multiplicity | Coordinates | Occupancy | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Al | 8f | 0.6385 | 0.6481 | 0.0000 | 1.0 |
| 2 | Al | 8f | 0.7438 | 0.5605 | 0.0000 | 1.0 |
| 3 | Al | 8f | 0.5463 | 0.3852 | 0.0000 | 0.981 |
| 4 | Al | 8f | 0.9836 | 0.5994 | 0.0000 | 1.0 |
| 5 | Al | 8g | 0.5286 | 0.2500 | 0.2663 | 1.0 |
| 6 | Al | 8g | 0.3987 | 0.2500 | 0.3093 | 1.0 |
| 7 | Al | 4c | 0.1982 | 0.2500 | 0.0000 | 1.0 |
| 8 | Al | 4c | 0.7939 | 0.2500 | 0.0000 | 1.0 |
| 9 | Al | 4c | 0.6366 | 0.2504 | 0.0000 | 1.0 |
| 10 | Al | 16h | 0.5504 | 0.5705 | 0.8132 | 1.0 |
| 11 | Al | 16h | 0.3253 | 0.4332 | 0.30400 | 1.0 |
| 12 | Al | 16h | 0.7106 | 0.3680 | 0.8055 | 1.0 |
| 13 | Al | 16h | 0.3901 | 0.6079 | 0.2957 | 1.0 |
| 1 | Cu | 8f | 0.9131 | 0.4345 | 0.0000 | 1.0 |
| 2 | Cu | 4c | 0.0951 | 0.2500 | 0.0000 | 1.0 |
| 1 | Mn | 8f | 0.6412 | 0.4444 | 0.0000 | 1.0 |
| 2 | Mn | 8g | 0.2895 | 0.2500 | 0.8017 | 1.0 |
| 3 | Mn | 4c | 0.4604 | 0.2500 | 0.0000 | 0.441 |
| Phase | a(Å) ± 10−4 | b(Å) ± 10−4 | c (Å) ± 10−4 | <L>, (nm) | Weight Fraction (%) |
|---|---|---|---|---|---|
| Al | 4.0471 | ---- | ---- | 394 ± 4 | 70.50 ± 1 |
| T-phase | 23.9536 | 12.5249 | 7.6644 | 202 ± 2 | 18.50 ± 0.31 |
| Al6Mn | 7.5477 | 6.5059 | 9.0330 | 99 ± 1 | 3.60 ± 0.07 |
| Al2Cu | 6.0665 | ---- | 4.8757 | 132 ± 1 | 7.40 ± 0.15 |
| Site Label | Atom | Wyckoff Symbol/Multiplicity | Coordinates | Occupancy | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Al | 8f | 0.6364 | 0.6499 | 0.0 | 1.0 |
| 2 | Al | 8f | 0.7377 | 0.5634 | 0.0 | 0.916 |
| 3 | Al | 8f | 0.5380 | 0.3841 | 0.0 | 1.0 |
| 4 | Al | 8f | 0.9829 | 0.5979 | 0.0 | 0.884 |
| 5 | Al | 8g | 0.5148 | 0.2504 | 0.2676 | 1.0 |
| 6 | Al | 8g | 0.3951 | 0.2495 | 0.3088 | 1.0 |
| 7 | Al | 4c | 0.1979 | 0.2503 | 0.0 | 0.929 |
| 8 | Al | 4c | 0.7927 | 0.2497 | 0.0 | 0.989 |
| 9 | Al | 4c | 0.6348 | 0.2495 | 0.0 | 1.0 |
| 10 | Al | 16h | 0.5504 | 0.5701 | 0.8143 | 1.0 |
| 11 | Al | 16h | 0.3261 | 0.4336 | 0.3027 | 0.859 |
| 12 | Al | 16h | 0.7175 | 0.3707 | 0.8103 | 1.0 |
| 13 | Al | 16h | 0.3893 | 0.6094 | 0.2956 | 0.929 |
| 1 | Cu | 8f | 0.9121 | 0.4334 | 0.0 | 0.787 |
| 2 | Cu | 4c | 0.0952 | 0.2504 | 0.0 | 0.967 |
| 1 | Mn | 8f | 0.6432 | 0.4469 | 0.0 | 1.0 |
| 2 | Mn | 8g | 0.2886 | 0.2497 | 0.8115 | 1.0 |
| 3 | Mn | 4c | 0.4576 | 0.2495 | 0.0 | 1.0 |
| Phase | a (Å) ± 10−4 | b (Å) ± 10−4 | c (Å) ± 10−4 | <L> (nm) | Weight Fraction (%) |
|---|---|---|---|---|---|
| Al | 4.0472 | ---- | ---- | 326 ± 4 | 51.13 ± 0.21 |
| T-phase | 24.1144 | 12.4815 | 7.6236 8.8946 ---- | 53 ± 1 | 31.51 ± 0.14 |
| Al6Mn | 7.5646 | 6.7176 | 621 ± 6 | 11.67 ± 0.06 | |
| α-Mn | 8.9191 | ---- | 226 ± 2 | 5.70 ± 0.18 |
| Site Label | Atom | Wyckoff Symbol/Multiplicity | Coordinates | Occupancy | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Al | 8f | 0.6254 | 0.6615 | 0.0 | 0.918 |
| 2 | Al | 8f | 0.7397 | 0.5695 | 0.0 | 1.0 |
| 3 | Al | 8f | 0.5468 | 0.3698 | 0.0 | 1.0 |
| 4 | Al | 8f | 0.9763 | 0.5981 | 0.0 | 1.0 |
| 5 | Al | 8g | 0.5244 | 0.25 | 0.2842 | 0.900 |
| 6 | Al | 8g | 0.3954 | 0.25 | 0.3048 | 0.877 |
| 7 | Al | 4c | 0.1866 | 0.25 | 0.0 | 1.0 |
| 8 | Al | 4c | 0.7917 | 0.25 | 0.0 | 1.0 |
| 9 | Al | 4c | 0.6201 | 0.25 | 0.0 | 1.0 |
| 10 | Al | 16h | 0.5462 | 0.5628 | 0.8253 | 1.0 |
| 11 | Al | 16h | 0.3269 | 0.4210 | 0.3055 | 1.0 |
| 12 | Al | 16h | 0.7176 | 0.3851 | 0.8213 | 0.996 |
| 13 | Al | 16h | 0.3882 | 0.6045 | 0.2826 | 0.945 |
| 1 | Cu | 8f | 0.9142 | 0.4426 | 0.0 | 0.817 |
| 2 | Cu | 4c | 0.0842 | 0.25 | 0.0 | 0.530 |
| 1 | Mn | 8f | 0.6430 | 0.4615 | 0.0 | 1.0 |
| 2 | Mn | 8g | 0.2840 | 0.25 | 0.8116 | 1.0 |
| 3 | Mn | 4c | 0.4461 | 0.25 | 0.0 | 0.854 |
| Sample | Field Direction | PM | FM Contribution | ||||
|---|---|---|---|---|---|---|---|
| χ × 10−6 emu/g Oe | Ms (emu/cm3) | Mr (emu/cm3) | Hc (Oe) | EB (Oe) | Mr/Ms | ||
| A24S | Par | 3.85 | 0.019 | 0.0022 | 141 | 216 | 0.11 |
| Per | 4.82 | 0.022 | 0.0019 | 187 | 187 | 0.08 | |
| A24SPS | Par | 3.89 | 0.009 | 0.0015 | 315 | 323 | 0.16 |
| Per | 5.22 | 0.011 | 0.0019 | 276 | 391 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad Bekhouche, A.; Alleg, S.; Bouasla, A.; Hachache, H.; Sunol, J.J. Microstructural, Mechanical, Thermal, and Magnetic Properties of the Mechanically Alloyed and Consolidated Al–16 wt. % Mn–7 wt. % Cu Alloy. Magnetochemistry 2025, 11, 59. https://doi.org/10.3390/magnetochemistry11070059
Saad Bekhouche A, Alleg S, Bouasla A, Hachache H, Sunol JJ. Microstructural, Mechanical, Thermal, and Magnetic Properties of the Mechanically Alloyed and Consolidated Al–16 wt. % Mn–7 wt. % Cu Alloy. Magnetochemistry. 2025; 11(7):59. https://doi.org/10.3390/magnetochemistry11070059
Chicago/Turabian StyleSaad Bekhouche, Ahlem, Safia Alleg, Abdelaziz Bouasla, Hacene Hachache, and Joan José Sunol. 2025. "Microstructural, Mechanical, Thermal, and Magnetic Properties of the Mechanically Alloyed and Consolidated Al–16 wt. % Mn–7 wt. % Cu Alloy" Magnetochemistry 11, no. 7: 59. https://doi.org/10.3390/magnetochemistry11070059
APA StyleSaad Bekhouche, A., Alleg, S., Bouasla, A., Hachache, H., & Sunol, J. J. (2025). Microstructural, Mechanical, Thermal, and Magnetic Properties of the Mechanically Alloyed and Consolidated Al–16 wt. % Mn–7 wt. % Cu Alloy. Magnetochemistry, 11(7), 59. https://doi.org/10.3390/magnetochemistry11070059








