Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
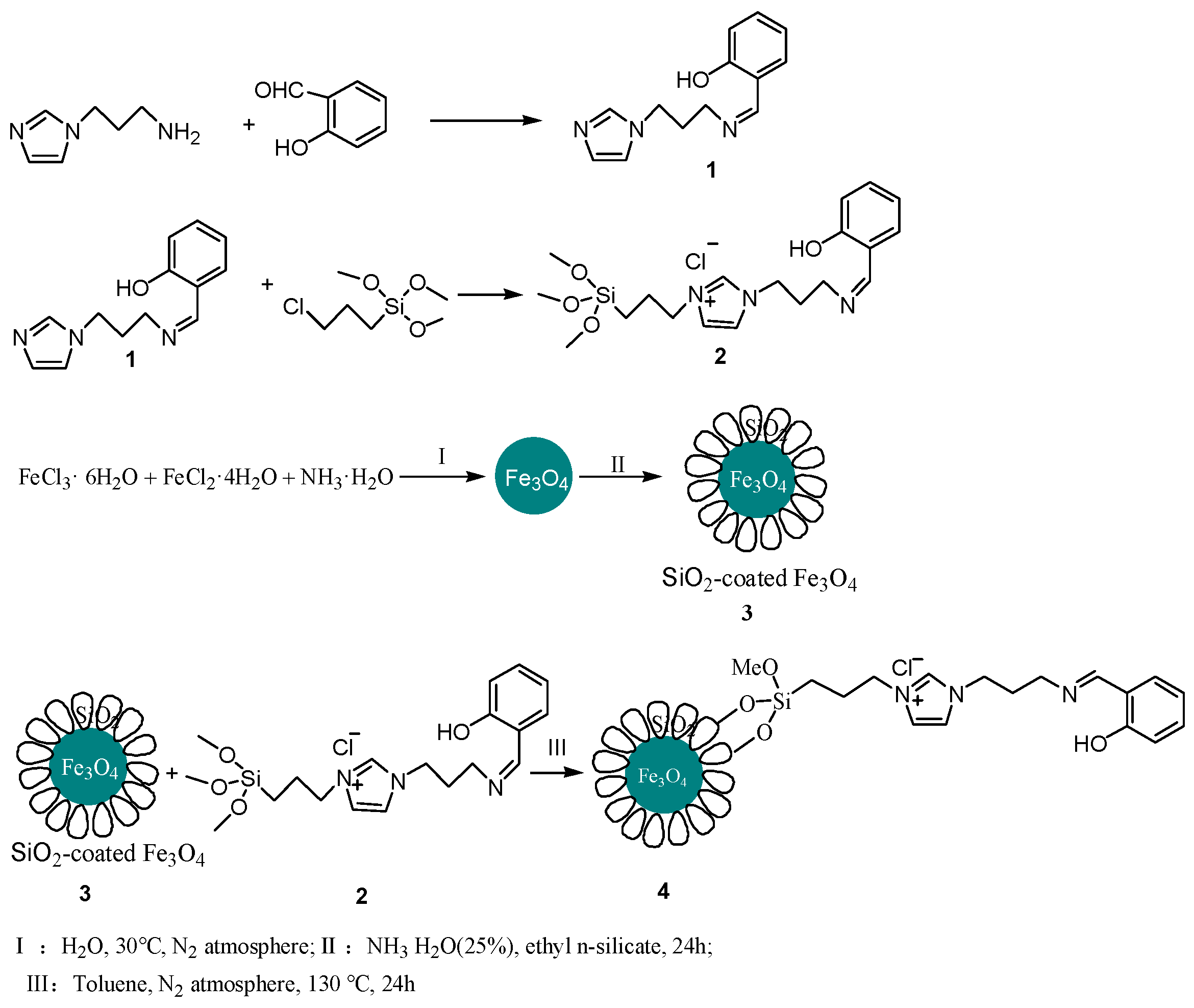
2.2. Synthesis of 2-(3-(1H-Imidazol-1-yl)propyliminomethyl)phenol (Schiff Base 1)
2.3. Synthesis of Ionic Liquid 2
2.4. Synthesis of Magnetic Nanoparticles Fe3O4
2.5. Synthesis of Magnetic Nanoparticles Fe3O4@SiO2
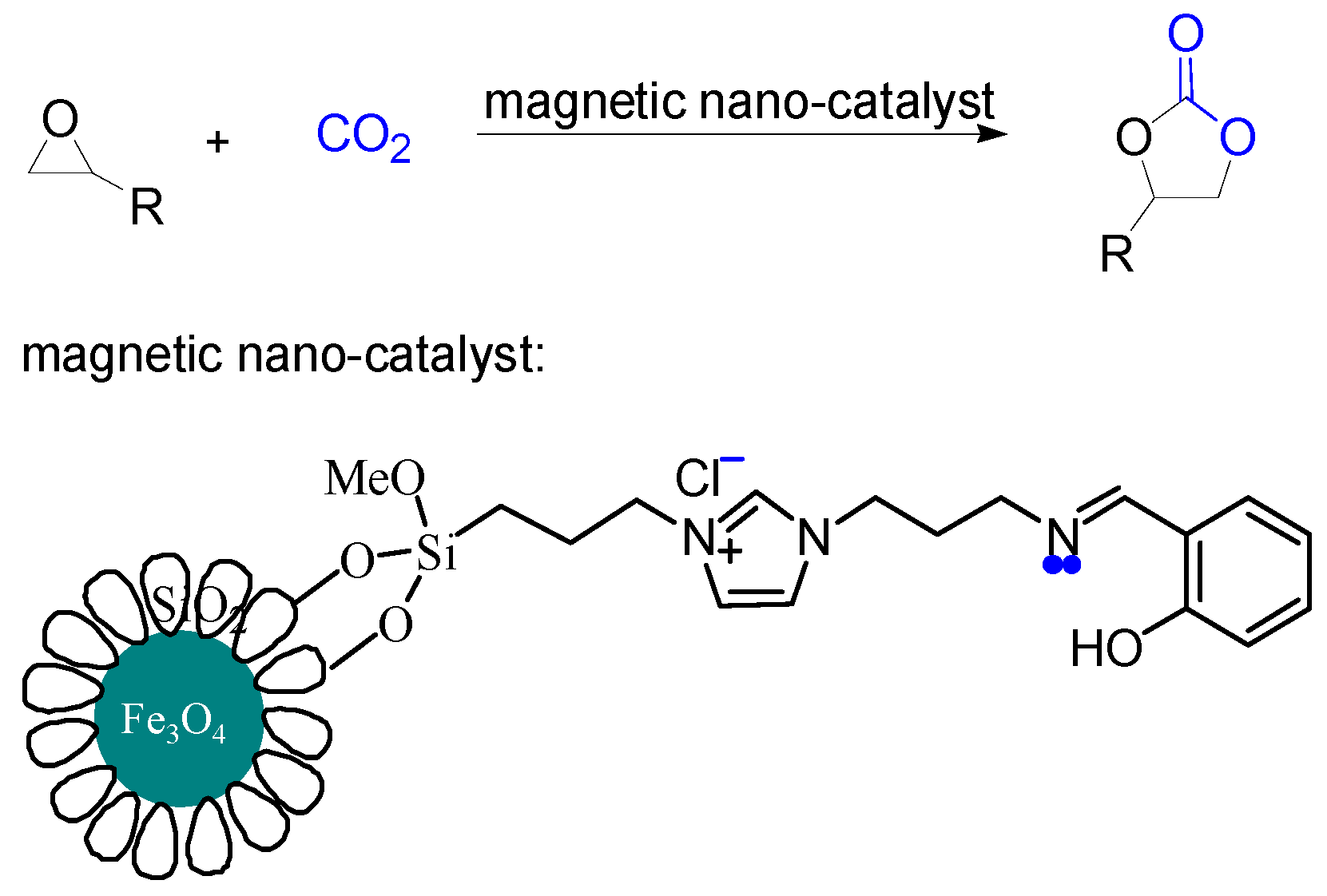
2.6. Synthesis of Magnetic Nano-Catalyst Containing Schiff Base Unit
2.7. General Method of Cycloaddition of Epoxides with CO2
- 4-Methyl-[1,3]dioxolan-2-one
- 4-Chloromethyl-[1,3]dioxolan-2-one
- 4-Phenyl-[1,3]dioxolan-2-one
- Hexahydro-benzo[1,3]dioxol-2-one
- 4-Butyl-1,3-dioxolan-2-one
2.8. Characterization Techniques
3. Results and Discussion
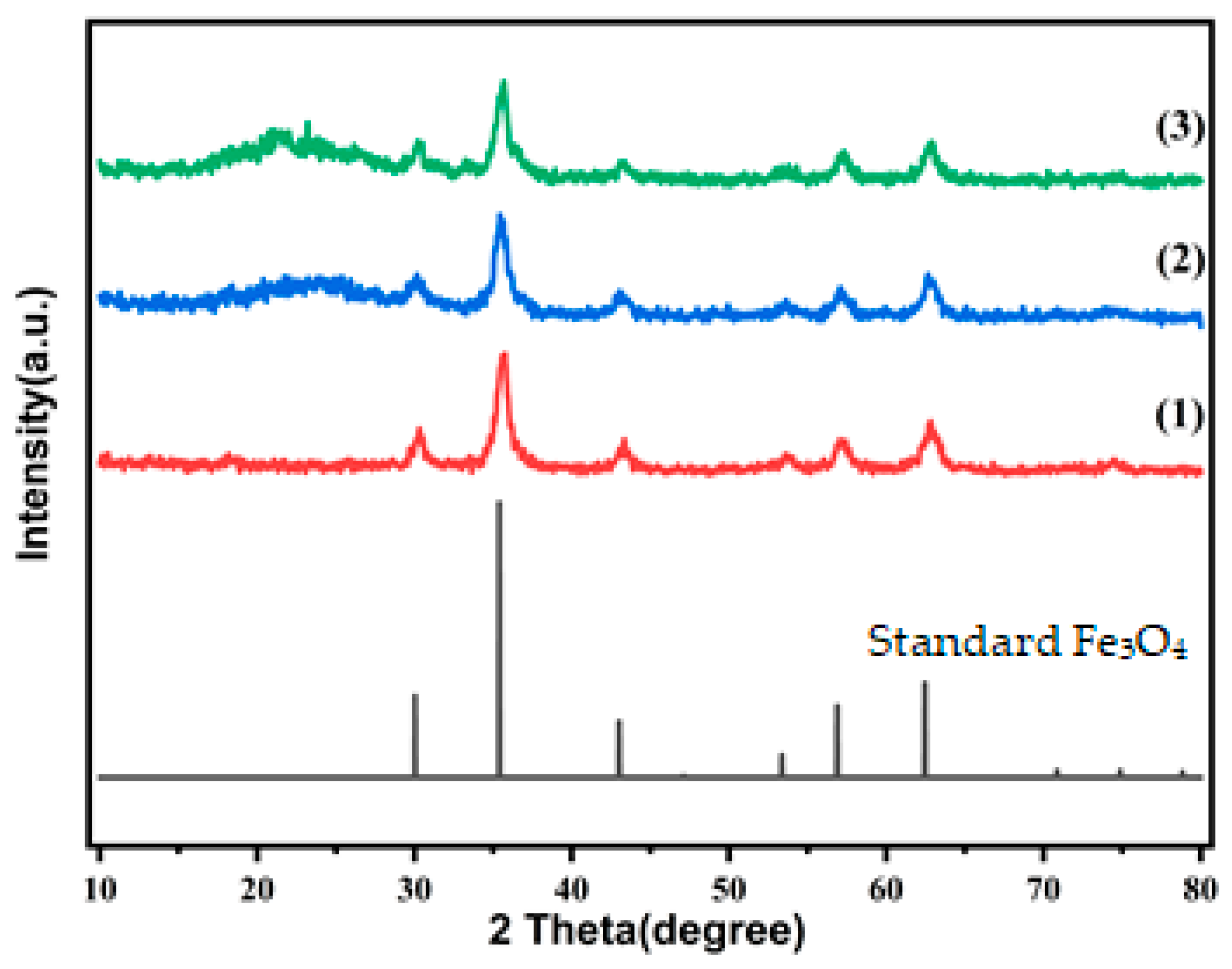
3.1. XRD Analysis
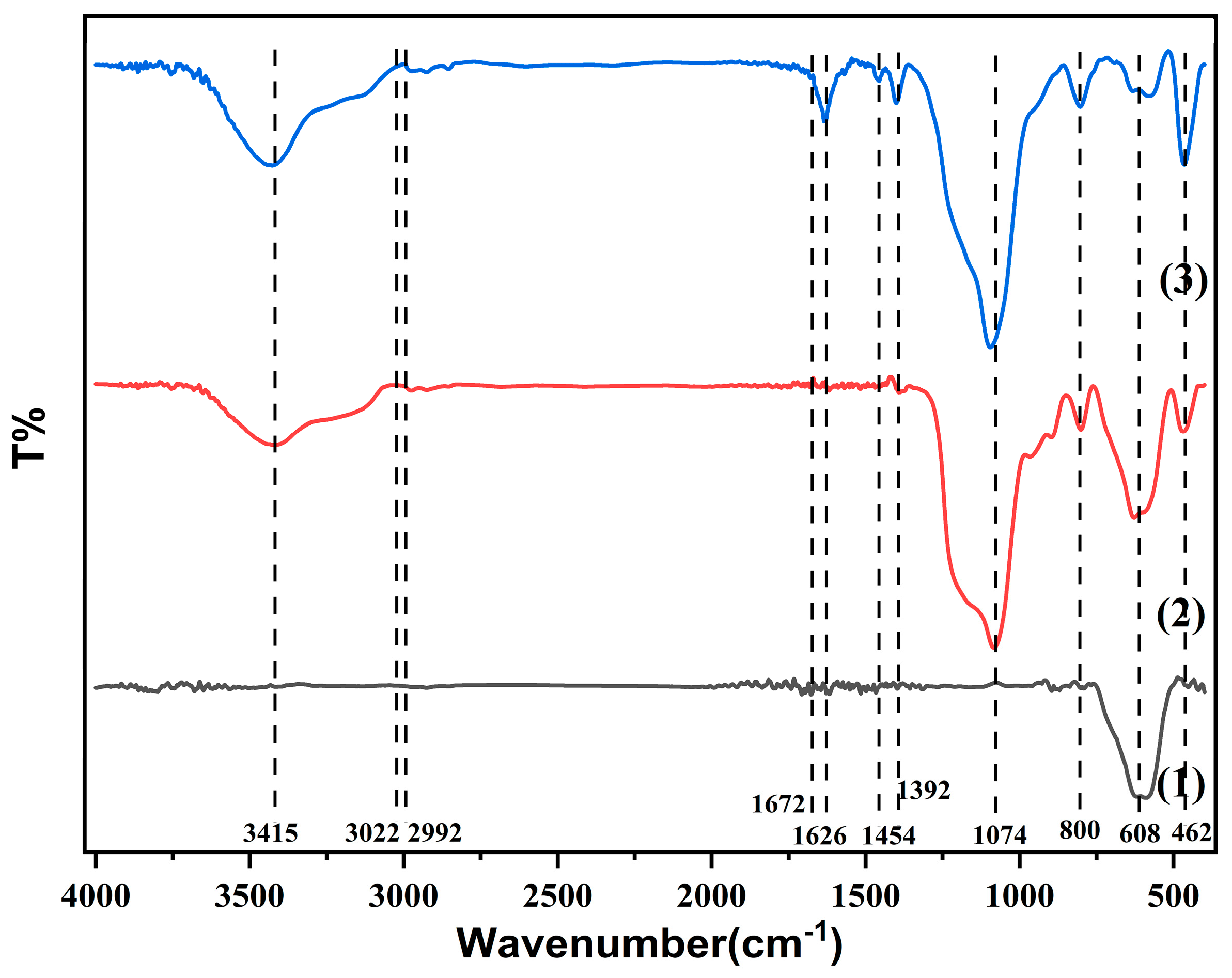
3.2. FTIR Analysis
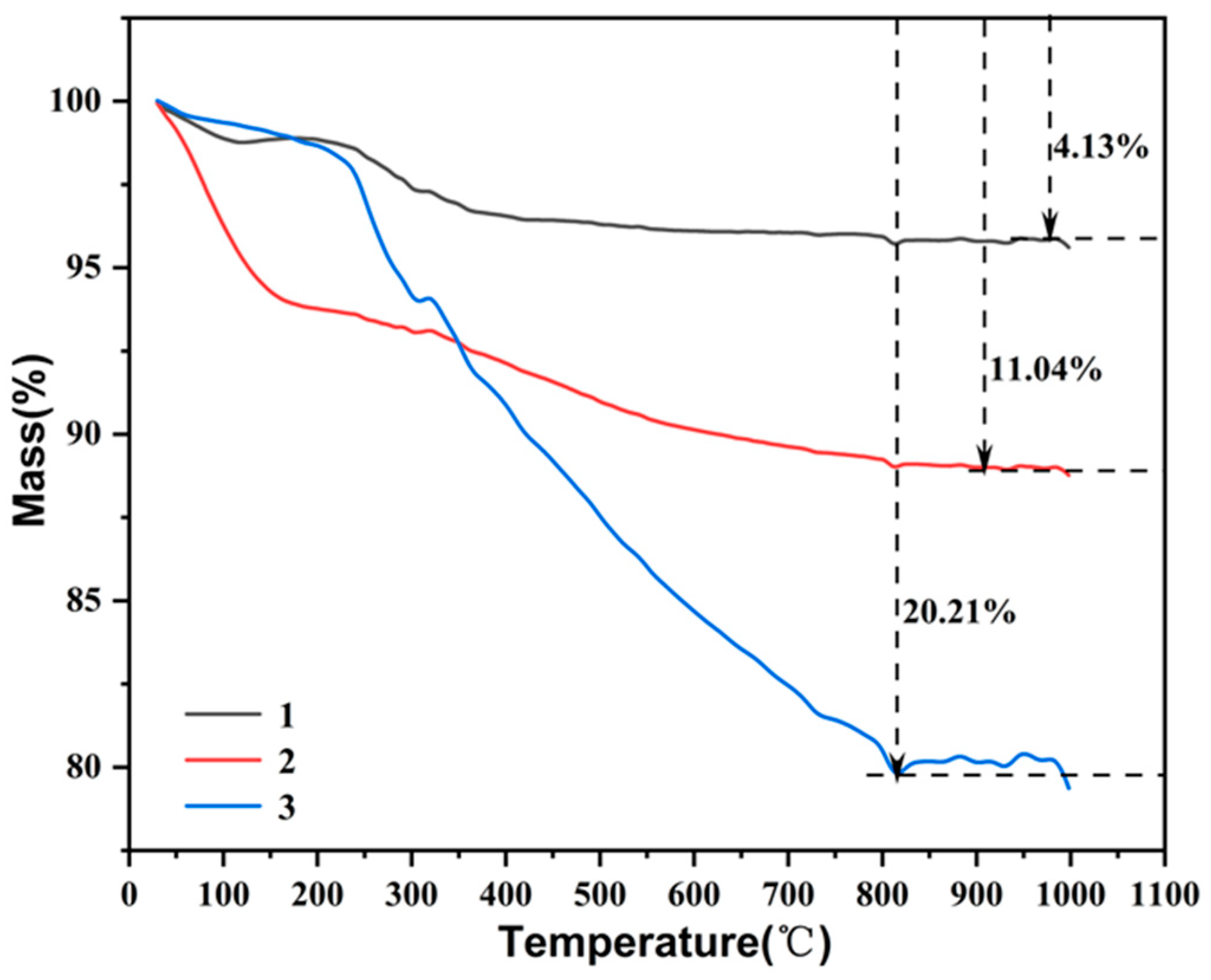
3.3. TG Analysis
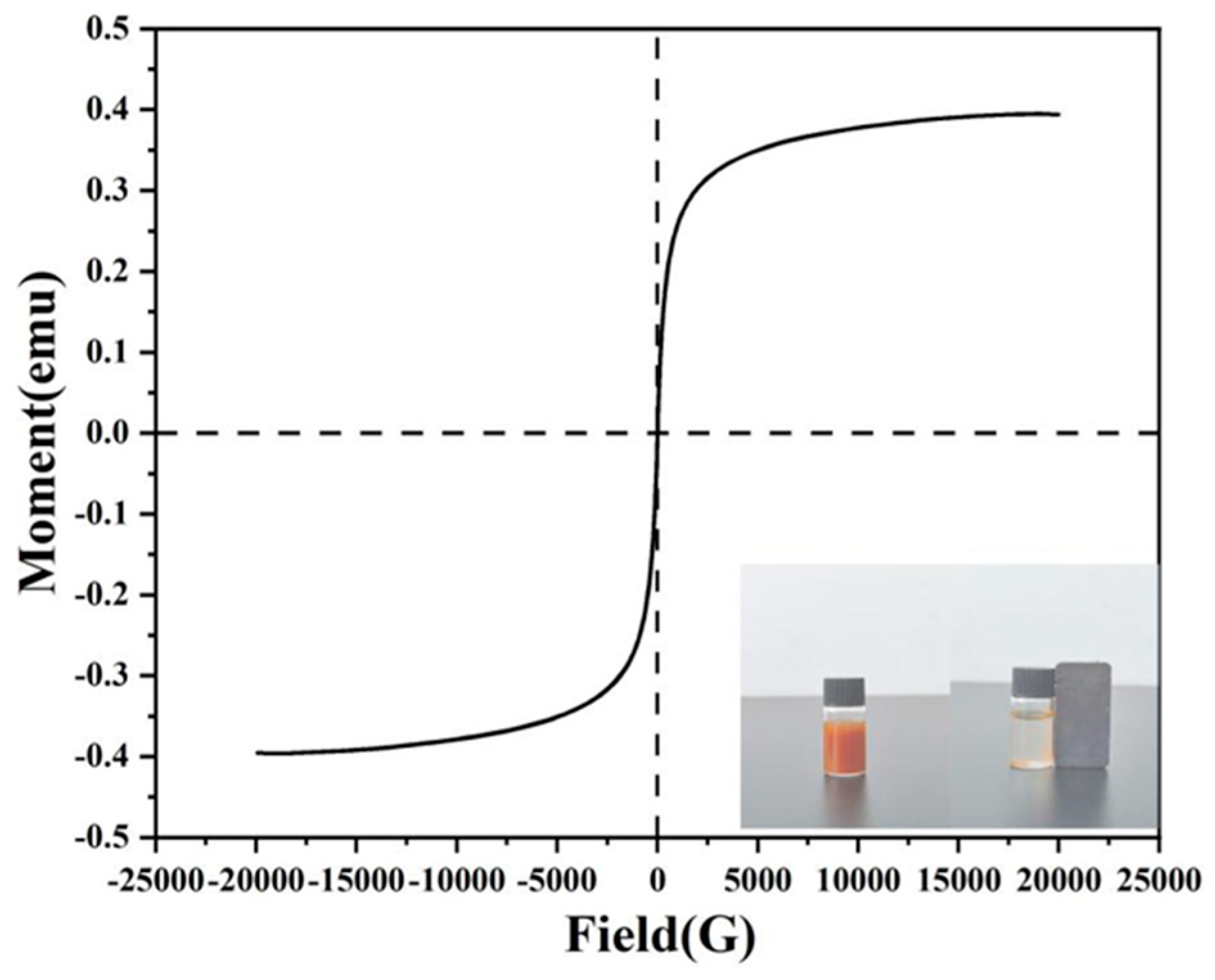
3.4. VSM Analysis
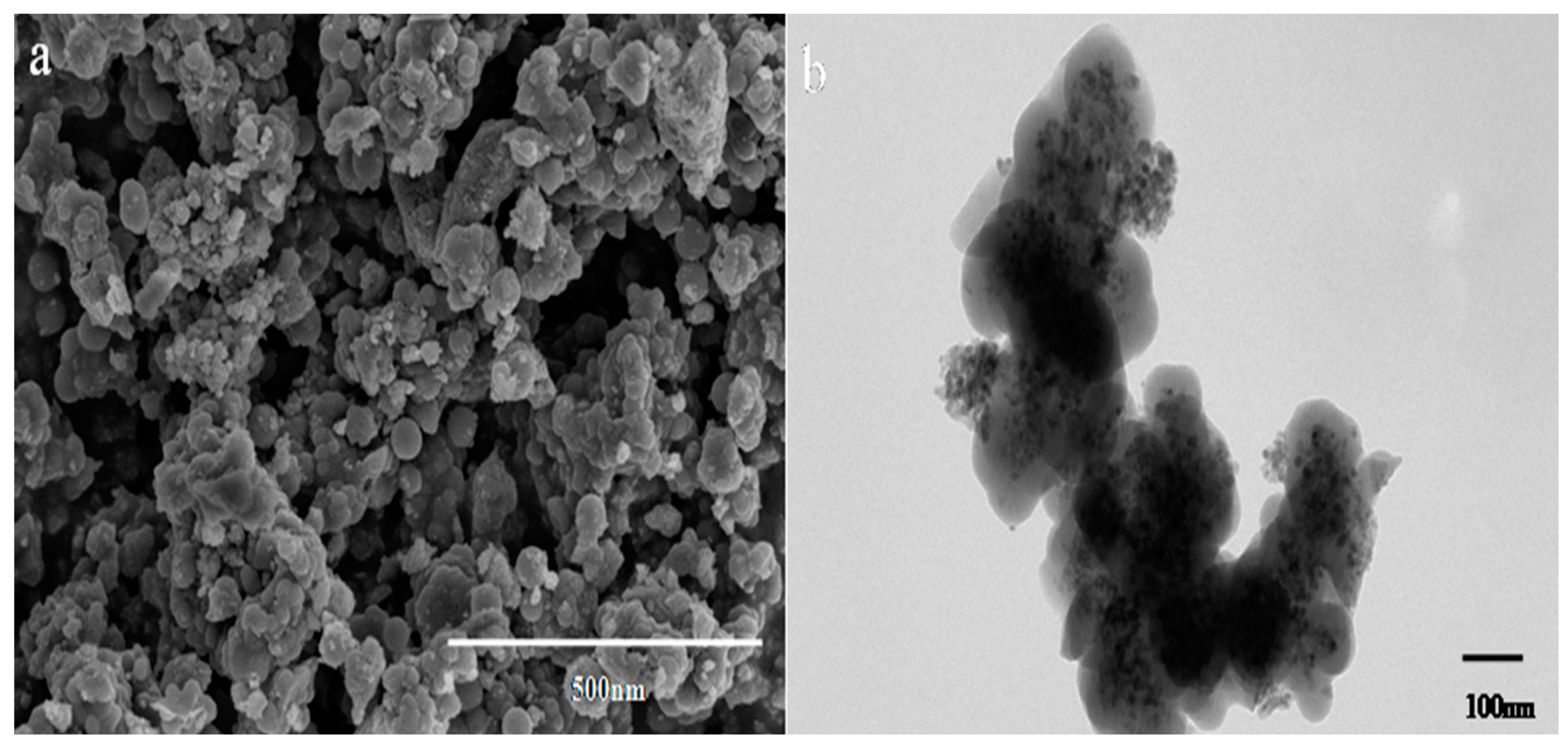
3.5. SEM and TEM Analysis
3.6. BET Analysis
3.7. Effects of Reaction Parameters on the Catalytic Activity of Magnetic Nano-Catalyst Containing Schiff Base Unit
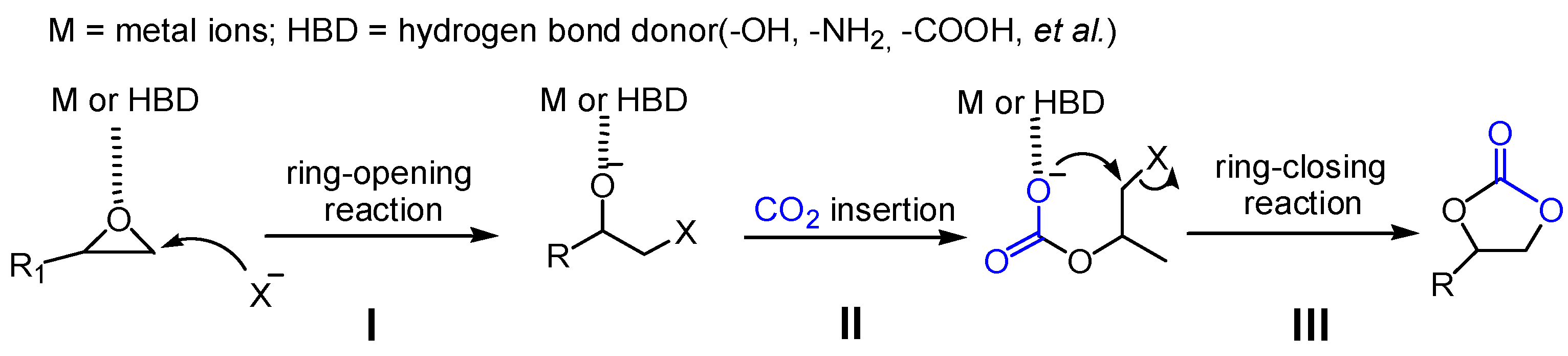
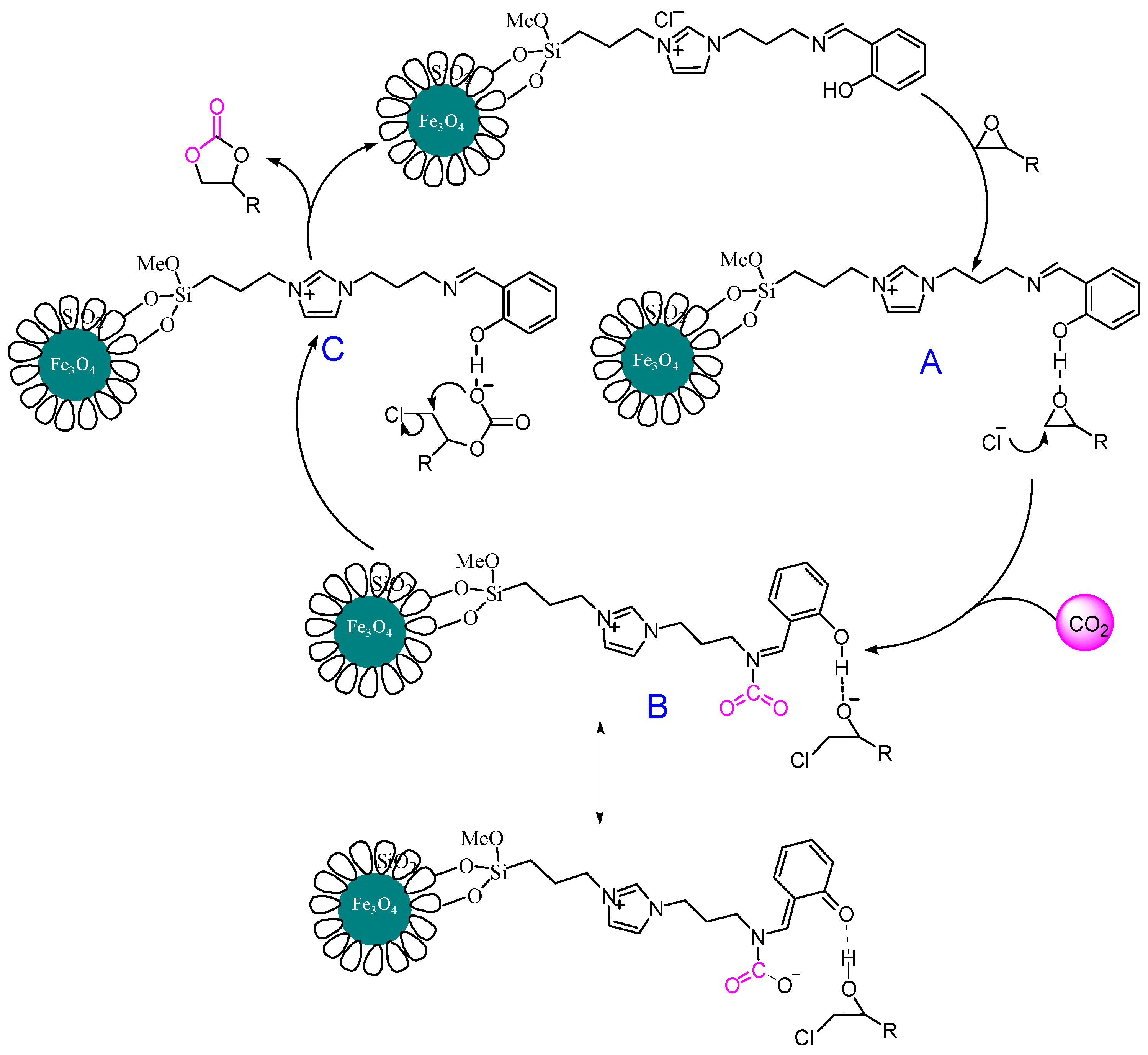
3.8. Proposed Mechanism
3.9. Recycling Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Grignard, B.; Méreau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. OrganoCatalyzed Coupling of Carbon Dioxide with Epoxides for the Synthesis of Cyclic Carbonates: Catalyst Design and Mechanisticstudies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active Sites for CO2 Hydrogenation to Methanol on Cu/ZnO Catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Khan, S.R.; Gour, N.K.; Deka, R.C.; Jain, S.L. Metal-free, Rdox-neutral, and Visible Light-triggered Coupling of CO2 with Epoxides to Cyclic Carbonates at Atmospheric Pressure. Green Chem. 2022, 24, 3644–3650. [Google Scholar] [CrossRef]
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Recent Advances in the Use of Catalysts Based on Natural Products for the Conversion of CO2 into Cyclic Carbonates. Green Chem. 2020, 22, 7665–7706. [Google Scholar] [CrossRef]
- Yu, W.; Maynard, E.; Chiaradia, V.; Arno, M.C.; Dove, A.P. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and their Application as Biomaterials. Chem. Rev. 2021, 121, 10865–10907. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, D. Density Functional Theory Study on the Cycloaddition of Carbon Dioxide with Propylene Oxide Catalyzed by Alkylmethylimidazolium Chlorine Ionic Liquids. J. Phys. Chem. A 2007, 111, 8036–8043. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg−Al Mixed Oxides as Highly Active Acid−base Catalysts for Cycloaddition of Carbon Dioxide to Epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Baalbaki, H.A.; Roshandel, H.; Hein, J.E.; Mehrkhodavandi, P. Conversion of Dilute CO2 to Cyclic Carbonates at Sub-atmospheric Pressures by a Simple Indium Catalyst. Catal. Sci. Technol. 2021, 11, 2119–2129. [Google Scholar] [CrossRef]
- Deacy, A.C.; Phanopoulos, A.; Lindeboom, W.; Buchard, A.; Williams, C.K. Insights into the Mechanism of Carbon Dioxide and Propylene Oxide Ring-opening Copolymerization Using a Co(III)/K(I) Heterodinuclear Catalyst. J. Am. Chem. Soc. 2022, 144, 17929–17938. [Google Scholar] [CrossRef]
- Gu, Y.; Anjali, B.A.; Yoon, S.; Choe, Y.; Chung, Y.G.; Park, D.-W. Defect-engineered MOF-801 for Cycloaddition of CO2 with Epoxides. J. Mater. Chem. A 2022, 10, 10051–10061. [Google Scholar] [CrossRef]
- Qing, Y.; Liu, T.; Zhao, B.; Bao, X.; Yuan, D.; Yao, Y. Cycloaddition of Di-substituted Epoxides and CO2 under Ambient Conditions Catalysed by Rare-earth Poly(phenolate) Complexes. Inorg. Chem. Front. 2022, 9, 2969–2979. [Google Scholar] [CrossRef]
- Al Maksoud, W.; Saidi, A.; Samantaray, M.K.; Abou-Hamad, E.; Poater, A.; Ould-Chikh, S.; Guo, X.; Guan, E.; Ma, T.; Gates, B.C.; et al. Docking of Tetra-methyl Zirconium to the Surface of Silica: A Well-defined Pre-catalyst for Conversion of CO2 to Cyclic Carbonates. Chem. Commun. 2020, 56, 3528–3531. [Google Scholar] [CrossRef]
- Sani, R.; Dey, T.K.; Sarkar, M.; Basu, P.; Islam, S.M. A Study of Contemporary Progress Relating to COF Materials for CO2 Capture and Fixation Reactions. Mater. Adv. 2022, 3, 5575–5597. [Google Scholar] [CrossRef]
- Song, Q.W.; Ma, R.; Liu, P.; Zhang, K.; He, L.N. Recent Progress in CO2 Conversion into Organic Chemicals by Molecular Catalysis. Green Chem. 2023, 25, 6538–6560. [Google Scholar] [CrossRef]
- He, J.; Jin, Z.; Gan, F.; Xie, L.; Guo, J.; Zhang, S.; Jia, C.Q.; Ma, D.; Dai, Z.; Jiang, X. Liquefiable Biomass-derived Porous Carbons and their Applications in CO2 Capture and Conversion. Green Chem. 2022, 24, 3376–3415. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Kosina, J.; Kolska, Z.; Trelin, A.; Svorcik, V.; Lyutakov, O. A Breath of Fresh Air for Atmospheric CO2 Utilisation: A Plas Mon-assisted Preparation of Cyclic Carbonate at Ambient Conditions. J. Mater. Chem. A 2021, 9, 8462–8469. [Google Scholar] [CrossRef]
- Chen, J.; Chiarioni, G.; Euverink, G.-J.W.; Pescarmona, P.P. Dyes as Efficient and Reusable Organocatalysts for the Synthesis of Cyclic Carbonates from Epoxides and CO2. Green Chem. 2023, 25, 9744–9759. [Google Scholar] [CrossRef]
- Wilhelm, M.E.; Anthofer, M.H.; Cokoja, M.; Markovits, I.I.E.; Herrmann, W.A.; Kühn, F.E. Cycloaddition of Carbon Dioxide and Epoxides Using Pentaerythritol and Halides as Dual Catalyst System. Chem. Sus. Chem. 2014, 7, 1357–1360. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Han, B.; Hu, S.; Li, W.; Xie, Y. Synthesis of Cyclic Carbonates from Epoxides and CO2 Catalyzed by Potassium Halide in the Presence of β-cyclodextrin. Green Chem. 2008, 10, 1337–1341. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, H.; Yu, X.; Liu, E. Lignin-based Green Catalyst for the Chemical Fixation of Carbon Dioxide with Epoxides to form Cyclic Carbonates under Solvent-free Conditions. Chem. Cat. Chem. 2013, 5, 1328–1333. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Yang, Z.; Wang, J.; Xu, T.; Xin, J.; Zhang, S. Super base/Cellulose: An Environmentally Benign Catalyst for Chemical Fixation of Carbon Dioxide into Cyclic Carbonates. Green Chem. 2014, 16, 3071–3078. [Google Scholar] [CrossRef]
- Guo, L.; Lamb, K.J.; North, M. Recent Developments in Organocatalysed Transformations of Epoxides and Carbon Dioxide into Cyclic Carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Kodama, K.; Hirose, T. An Efficient Metal- and Solvent-free Organocatalytic System for Chemical Fixation of CO2 into Cyclic Carbonates under Mild Conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Zhao, S. Preparation of a Novel Fe3O4@SiO2@propyl@DBU Magnetic Core-shell Nanocatalyst for Knoevenagel Reaction in Aqueous Medium. Green Chem. Lett. Rev. 2021, 14, 85–98. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Wang, Z.; Zhao, S. Tertiary Amine Ionic Liquid Incorporated Fe3O4 Nanoparticles as a Versatile Catalyst for the Knoevenagel Reaction. Synthetic Commun. 2022, 52, 774–786. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, Y.; Wang, X.; Wen, W.; Zhao, S. DABCO-catalyzed Knoevenagel Condensation of Aldehydes with Ethyl Cyanoacetate Using Hydroxyl Ionic Liquid as a Promoter. RSC Adv. 2018, 8, 30180–30185. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, D.; Wei, L.; Qiao, Y.; Xi, F. Novel DBU-based Hydroxyl Ionic Liquid for Efficient Knoevenagel Reaction in Water. Green Chem. Lett. Rev. 2019, 12, 271–277. [Google Scholar] [CrossRef]
- McGinley, J.; McCann, M.; Ni, K.; Tallon, T.; Kavanagh, K.; Devereux, M.; Ma, X.; McKee, V. Imidazole Schiff Base Ligands: Synthesis, Coordination Complexes and Biological Activities. Polyhedron 2013, 55, 169–178. [Google Scholar] [CrossRef]
- Qian, H.; Dai, Y.; Geng, J.; Wang, L.; Wang, C.; Huang, W. A Flexible Multidentate Schiff-base Ligand Having Multifarious Coordination modes in Its Copper(II) and Cadmium(II) Complexes. Polyhedron 2014, 67, 314–320. [Google Scholar] [CrossRef]
- Bu, D.L.; Li, N.; Zhou, Y.; Feng, H.Y.; Yu, F.; Cheng, C.X.; Li, M.; Xiao, L.H.; Ao, Y.H. Enhanced UV Stability of N-Halamine-Immobilized Fe3O4@SiO2@TiO2 Nanoparticles: Synthesis, Characteristics and Antibacterial Property. New J. Chem. 2020, 44, 10352–10358. [Google Scholar] [CrossRef]
- Choi, H.J.; Piao, S.H.; Kim, S.D.; Chae, H.S. Core-shell Structured Fe3O4@SiO2 Nanoparticles Fabricated by Sol-gel Method and their Magnetorheology. Colloid Polym. Sci. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Chen, X.; Du, S.; Hong, R.; Chen, H. Preparation of RGO/Fe3O4 Nanocomposites as a Microwave Absorbing Material. Inorganics 2023, 11, 143. [Google Scholar] [CrossRef]
- Peng, J.; Deng, Y. Cycloaddition of Carbon Dioxide to Propylene Oxide Catalyzed by Ionic Liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
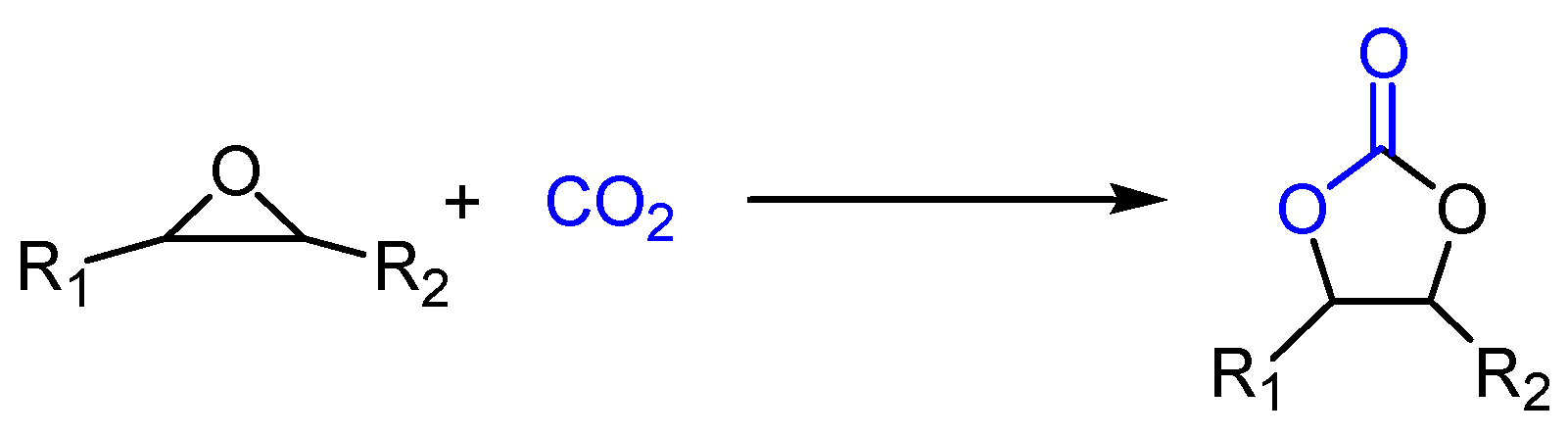
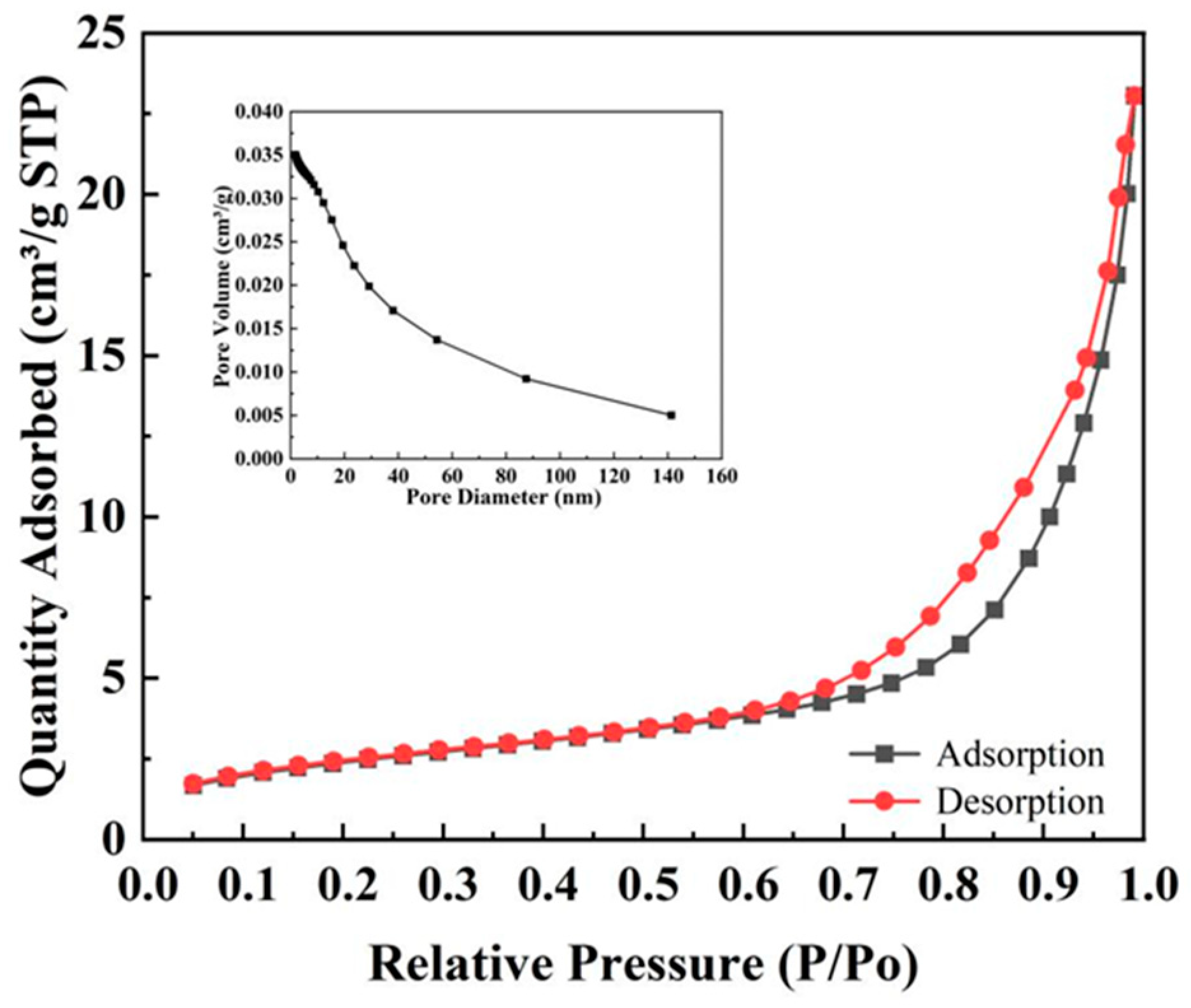
| Parameters | Magnetic Nano-Catalyst Containing Schiff Base Unit | |
|---|---|---|
| Surface Area | Single point surface area at P/Po = 0.294306215 | 8.3206 m2/g |
| BET Surface Area | 8.6415 m2/g | |
| Langmuir Surface Area | 13.0942 m2/g | |
| t-Plot External Surface Area | 8.7656 m2/g | |
| Pore Volume | Single point adsorption total pore volume of pores less than 181.2138 nm diameter at P/Po = 0.989213975 | 0.034517 cm3/g |
| BJH Adsorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter | 0.035029 cm3/g | |
| BJH Desorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter | 0.035171 cm3/g | |
| Pore Size | Adsorption average pore diameter (4V/A using BET) | 15.97728 nm |
| BJH Adsorption average pore diameter (4V/A) | 16.7619 nm | |
| BJH Desorption average pore diameter (4V/A) | 15.1738 nm |
| Entry | Magnetic Nano-Catalyst (g) | CO2 (atm) | Time (h) | Temperature (°C) | Yield 2 (%) |
|---|---|---|---|---|---|
| 1 | 1.25 | 3 | 15 | 120 | 99 |
| 2 | 1.25 | 2 | 15 | 120 | 99 |
| 3 | 1.00 | 2 | 15 | 120 | 99 |
| 4 | 1.00 | 2 | 10 | 120 | 99 |
| 5 | 0.75 | 2 | 10 | 120 | 99 |
| 6 | 0.75 | 1 | 10 | 120 | 99 |
| 7 | 0.75 | 1 | 10 | 100 | 99 |
| 8 | 0.50 | 1 | 10 | 100 | 94 |
| 9 | 0.75 | 1 | 8 | 100 | 99 |
| 10 | 0.75 | 1 | 6 | 100 | 93 |
| 11 3 | 0.75 | 1 | 8 | 100 | 0 |
| 12 4 | 0.75 | 1 | 8 | 100 | 82 |
| Entry 1 | Substrate | Product | Yield (%) 2 |
|---|---|---|---|
| 1 | 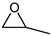 | 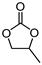 | 99 |
| 2 | 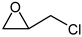 | 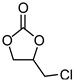 | 99 |
| 3 | 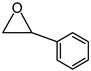 | 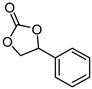 | 40 |
| 4 | 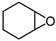 | 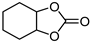 | 5 |
| 5 | 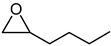 | 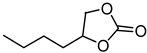 | 82 |
| Run | Time (h) | Isolated Yield/% |
|---|---|---|
| 1 | 8 | 99 |
| 2 | 8 | 99 |
| 3 | 8 | 98 |
| 4 | 8 | 98 |
| 5 | 8 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.; Fan, Y.; Li, D.; Jia, X.; Zhao, S. Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates. Magnetochemistry 2024, 10, 33. https://doi.org/10.3390/magnetochemistry10050033
Kang N, Fan Y, Li D, Jia X, Zhao S. Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates. Magnetochemistry. 2024; 10(5):33. https://doi.org/10.3390/magnetochemistry10050033
Chicago/Turabian StyleKang, Na, Yindi Fan, Dan Li, Xiaoli Jia, and Sanhu Zhao. 2024. "Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates" Magnetochemistry 10, no. 5: 33. https://doi.org/10.3390/magnetochemistry10050033
APA StyleKang, N., Fan, Y., Li, D., Jia, X., & Zhao, S. (2024). Preparation of Magnetic Nano-Catalyst Containing Schiff Base Unit and Its Application in the Chemical Fixation of CO2 into Cyclic Carbonates. Magnetochemistry, 10(5), 33. https://doi.org/10.3390/magnetochemistry10050033






