Building Up a Hexacopper(II)-Pyrazolate/Oxamate Magnetic Complex with Rare Ethane-1,2-Dioxide (–OCH2CH2O–) as a Bridge Between Copper(II) Units
Abstract
1. Introduction
2. Experimental Method
2.1. Chemical Characterizations
2.2. X-Ray Diffraction
2.3. Magnetic Measurements
3. Results
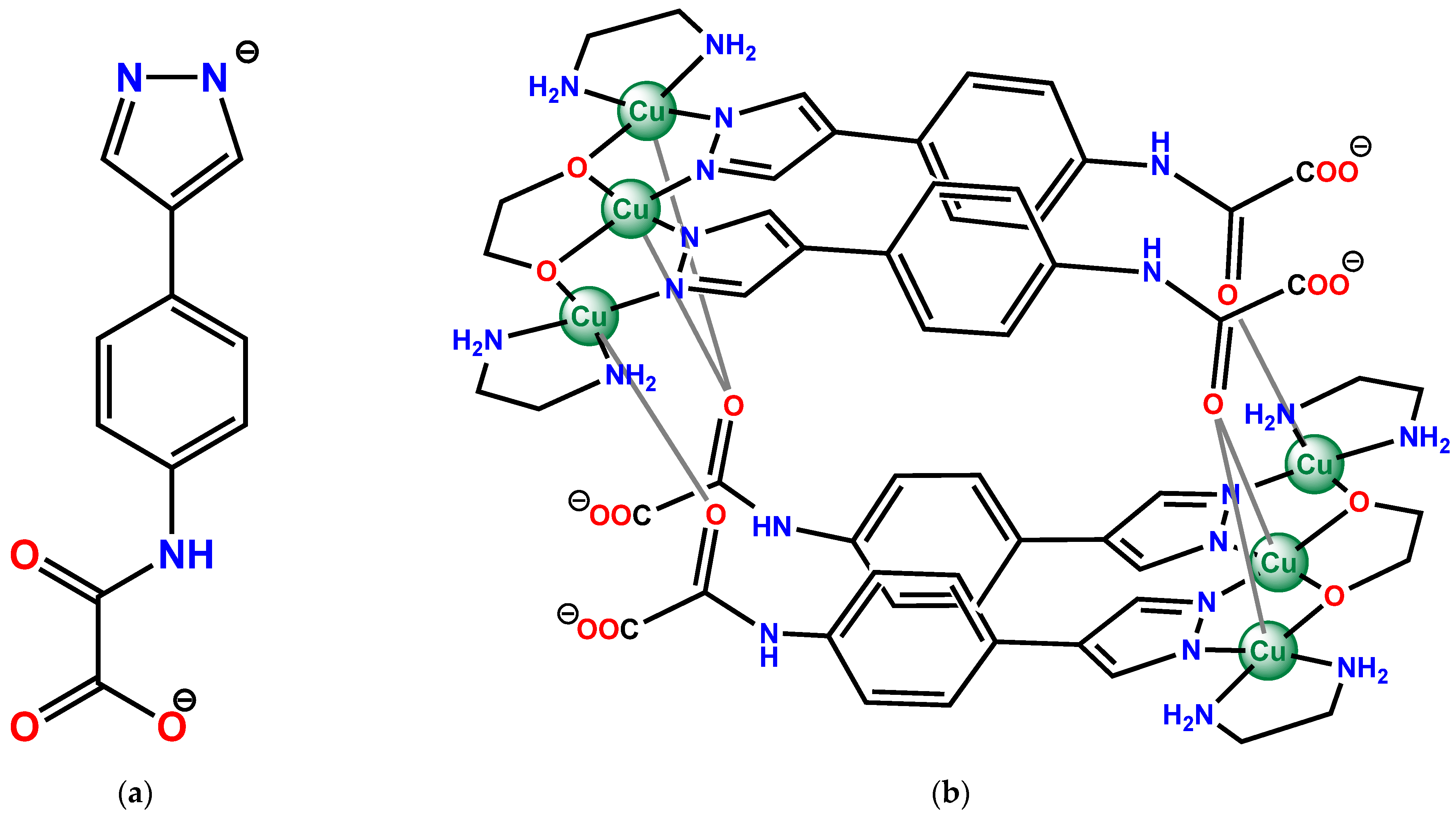
3.1. Synthesis and Product Formation Comments
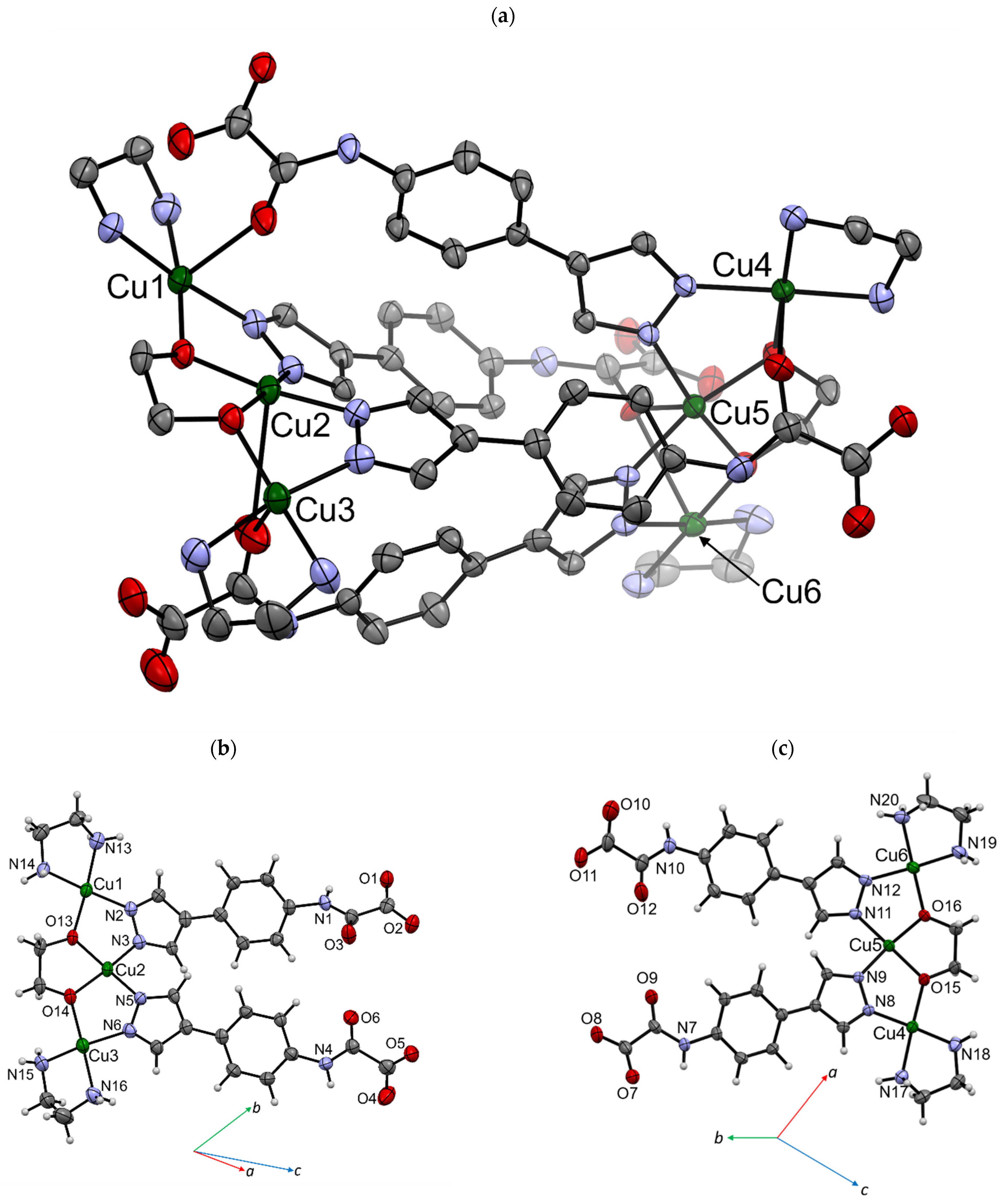
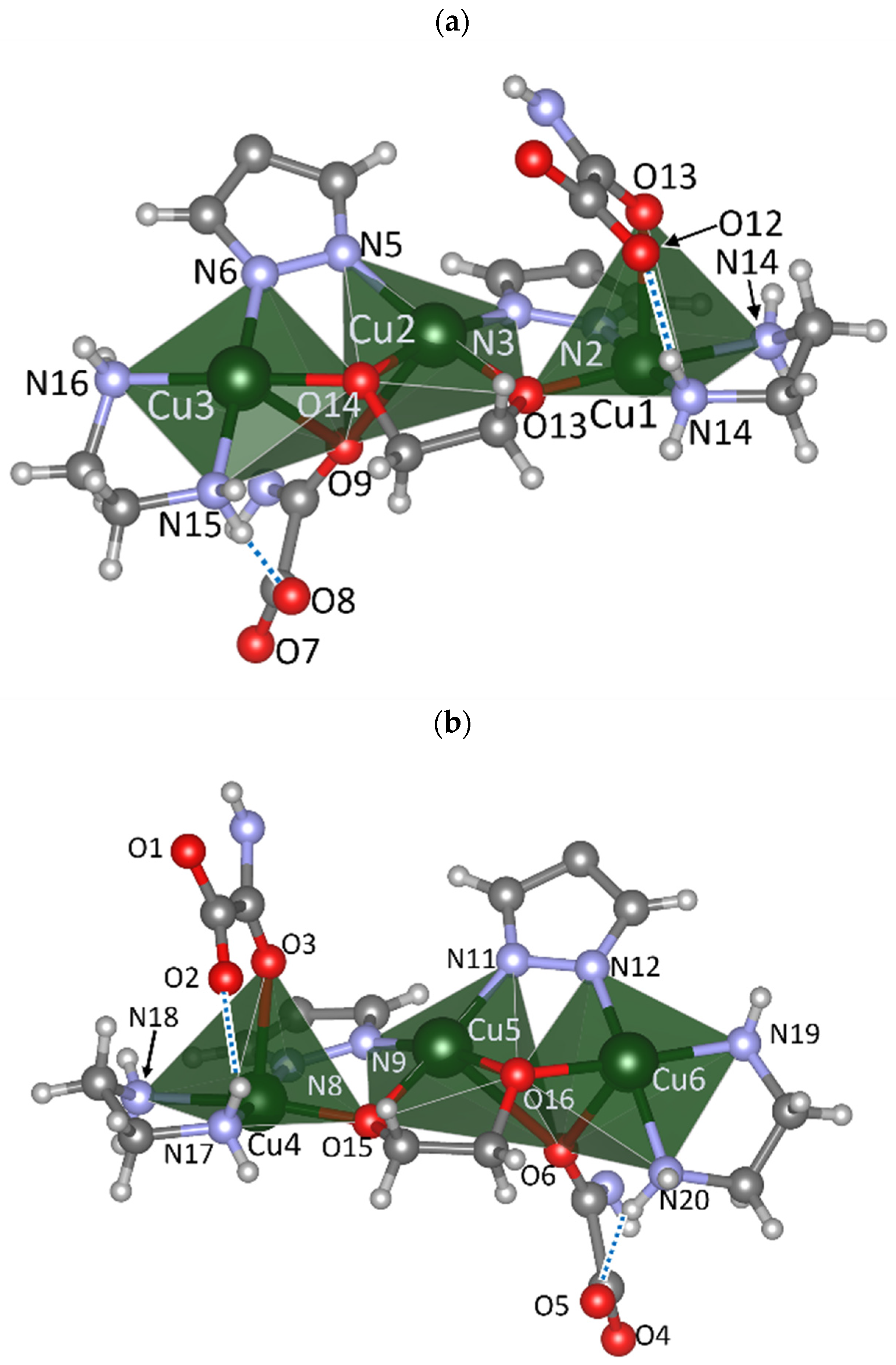
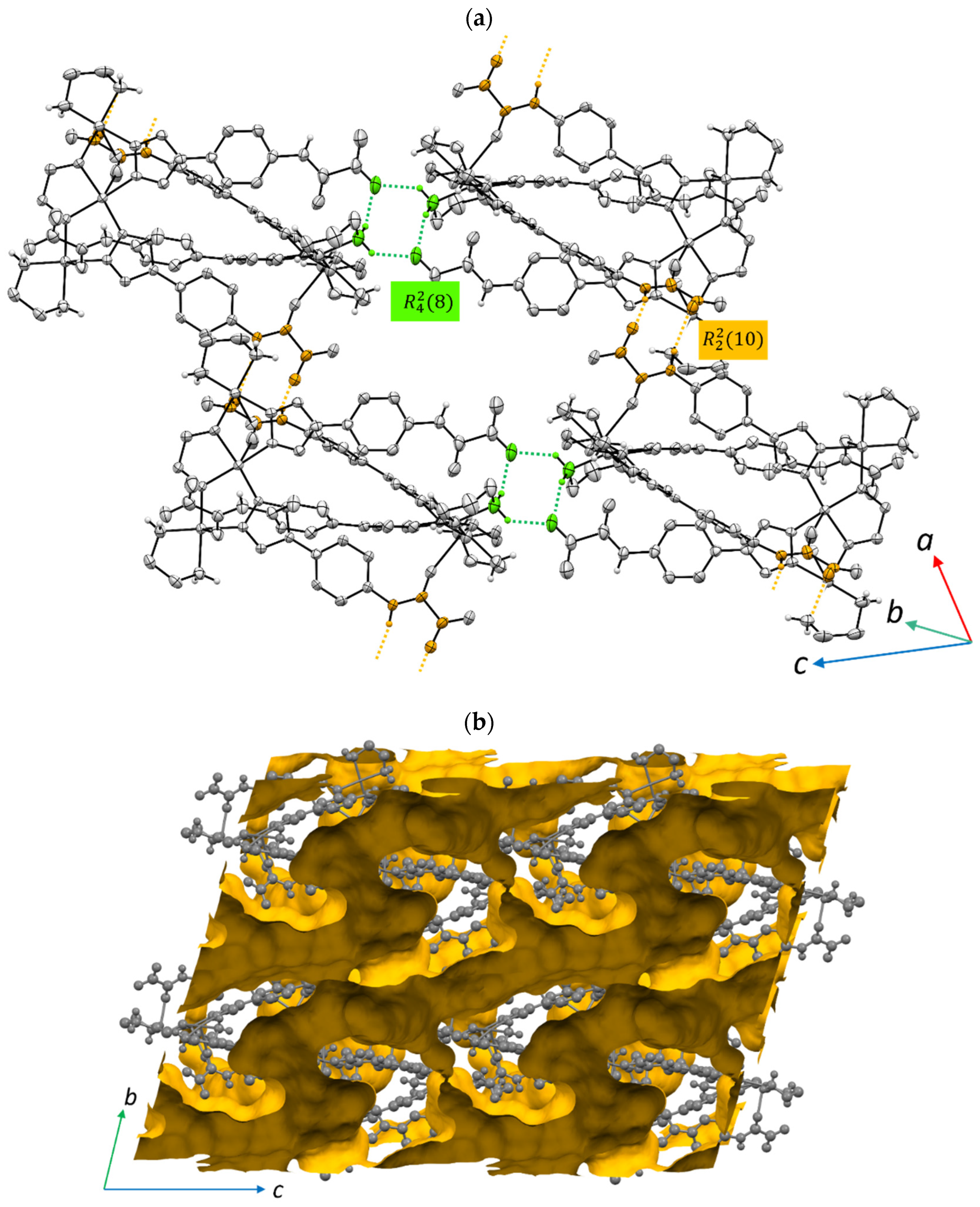
3.2. Crystal Structure Description
3.3. Magnetic Studies
| Compound | Cu–NN–Cu a/° | Cu–O–Cu b/° | Mean Planes Angle c/° | J/cm−1 | g | θ or zJ′/K | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | −4.1 (1.4)/−5.5 (1.2)/ 0.3 (1.4)/−0.43 (1.3) | 120.23(14)/115.20(14)/ 116.76(14)/110.96(14) | 30.9(4)/35.8(4)/37.8(5)/47.5(4) | −106.3 d | 2.12 d | −0.23 d | This work |
| −116.5 d | 2.12 d | −0.08 d | |||||
| [{Cu(bpca)}2Cu(opba)(H2O)]·H2O | --- e | --- e | 78.45/84.62 | −65.8 | 2.08 | --- | [50] |
| [{Cu(bpca)}2{Cu(dmopba)(H2O)}]2·4H2O | --- e | --- e | 78.09/81.67 | −31.96/+1.34 f | 2.12 | --- | [52] |
| [{Cu(Me4en)(H2O)}2Cu(pba)(ClO4)] | --- e | --- e | 11.29/18.50 | −334.4 | 2.08 | −0.36 | [53] |
| [{Cu(Me4en)(H2O)}2Cu(pba)(H2O)](PF6)2 | --- e | --- e | 7.94/10.75 | −342.1 | 2.14 | −0.61 | [54] |
| [Cu3(L1)2(BF4)(H2O)2]BF4 | 40.5(2)/33.0(1) | 116.23(7)/118.40(7) | 17.04 | −170 | 2.16 | --- | [59] |
| [Cu3(L1)2(NO3)2(H2O)2]·3H2O | 28.86 | 116.95 | 5.01 | −113.1 | 2.08 | --- | |
| [Cu3(L1)2Cl2(CH3OH)2]·2H2O | 30.54 | 116.89 | 4.64 | −98.3 | 2.07 | --- | |
| [Cu3(L1)2(ClO4)2(H2O)2] | 40.19/28.92 | 117.67/117.91 | 16.20 | −249.7 | 2.14 | --- | |
| {[Cu3(L2)2(H2O)2](ClO4)4}·4H2O | 17.2(8)/13.7(8) | 120.5(3)/116.6(3) | 8.19/18.36 | −179 | 2.0/2.2 | --- | [60] |
| [Cu3(HYDRAV)2Cl2] | 35.9 | 115 | 0.96/11.7 | −7.21 | 2.03 | --- | [61] |
| {[Cu3(L3)(L4)2(NO3)2(H2O)2]NO3·1.5H2O}n | −6.9(8)/8.0(8) | 119.3(3)/124.5(3) | 22.9/33.6 | −149/−175 | 2.20 | −0.7 | [62] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castellano, M.; Ruiz-García, R.; Cano, J.; Ferrando-Soria, J.; Pardo, E.; Fortea-Pérez, F.R.; Stiriba, S.-E.; Barros, W.P.; Stumpf, H.O.; Cañadillas-Delgado, L.; et al. Metallosupramolecular Approach toward Multifunctional Magnetic Devices for Molecular Spintronics. Coord. Chem. Rev. 2015, 303, 110–138. [Google Scholar] [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular Magnetism, Quo Vadis? A Historical Perspective from a Coordination Chemist Viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Pereira, C.L.M.; Doriguetto, A.C.; Konzen, C.; Meira-Belo, L.C.; Leitão, U.A.; Fernandes, N.G.; Mascarenhas, Y.P.; Ellena, J.; Brandl, A.L.; Knobel, M.; et al. A Crystalline Phase Transition and Optical Properties in a CoIICuII Oxamato-Bridged Ferrimagnetic Chain. Eur. J. Inorg. Chem. 2005, 2005, 5018–5025. [Google Scholar] [CrossRef]
- Journaux, Y.; Sletten, J.; Kahn, O. Tunable Interaction in (μ-Oxamido)Copper(II) Binuclear Complexes. Inorg. Chem. 1985, 24, 4063–4069. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; Nunes, W.C.; Julve, M.; Journaux, Y.; Stumpf, H.O.; Pereira, C.L.M. Magneto-Structural Study of an Oxamato-Bridged PdIICoII Chain: X-ray Crystallographic Evidence of a Single-Crystal-to-Single-Crystal Phase Transition. Eur. J. Inorg. Chem. 2012, 2012, 5685–5693. [Google Scholar] [CrossRef]
- Das, A.K.; De, A.; Yadav, P.; Lloret, F.; Mukherjee, R. CuII2, CuII4 and CuII6 Complexes with 3-(2-Pyridyl)Pyrazolate. Structure, Magnetism and Core Interconversion. Polyhedron 2019, 171, 365–373. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Cano, J.; Lloret, F.; Julve, M. Relatively Strong Intramolecular Antiferromagnetic Coupling in a Neutral CrIII2NbV2 Heterobimetallic Molecular Square. Chem. Commun. 2015, 51, 11806–11809. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Grancha, T.; Pasán, J.; Ruiz-Pérez, C.; Cañadillas-Delgado, L.; Journaux, Y.; Julve, M.; Cano, J.; Lloret, F.; Pardo, E. Solid-State Aggregation of Metallacyclophane-Based MnIICuII One-Dimensional Ladders. Inorg. Chem. 2012, 51, 7019–7021. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.R.G.; do Pim, W.D.; Silva, I.F.; Oliveira, W.X.C.; Pinheiro, C.B.; Pereira, C.L.M.; Lloret, F.; Julve, M.; Stumpf, H.O. Solvent-Driven Dimensionality Control in Molecular Systems Containing CuII, 2,2′-Bipyridine and an Oxamato-Based Ligand. CrystEngComm 2013, 15, 10165. [Google Scholar] [CrossRef]
- Pereira, C.L.M.; Pedroso, E.F.; Stumpf, H.O.; Novak, M.A.; Ricard, L.; Ruiz-García, R.; Rivière, E.; Journaux, Y. A CuIICoII Metallacyclophane-Based Metamagnet with a Corrugated Brick-Wall Sheet Architecture. Angew. Chem. Int. Ed. 2004, 43, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Ferrando-Soria, J.; Grancha, T.; Fortea-Pérez, F.R.; Gascon, J.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Selective Gold Recovery and Catalysis in a Highly Flexible Methionine-Decorated Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 7864–7867. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Williams, P.A.; Bickley, J.F.; Steiner, A.; Davies, H.O.; Leedham, T.J.; Awaluddin, A.; Pemble, M.E.; Critchlow, G.W. Synthesis and Crystal Structures of Two New Titanium Alkoxy–Diolate Complexes. Potential Precursors for Oxide Ceramics. J. Mater. Chem. 2001, 11, 1428–1433. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal–Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Pajot, N.; Papiernik, R.; Hubert-Pfalzgraf, L.G.; Vaissermann, J.; Parraud, S. Metal-Assisted Activation of the C–O Bond of 2-Hydroxyethylmethacrylate. Synthesis and Molecular Structure of Ti5(OPri)9(µ-OPri)(µ,η2-OC2H4O)(µ3,η2-OC2H4O)3(µ4,η2-OC2H4O). J. Chem. Soc. Chem. Commun. 1995, 17, 1817–1819. [Google Scholar] [CrossRef]
- Hänninen, M.M.; Välivaara, J.; Mota, A.J.; Colacio, E.; Lloret, F.; Sillanpää, R. Ferromagnetic Dinuclear Mixed-Valence Mn(II)/Mn(III) Complexes: Building Blocks for the Higher Nuclearity Complexes. Structure, Magnetic Properties, and Density Functional Theory Calculations. Inorg. Chem. 2013, 52, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Gao, S.; Chui, S.S.; Shek, L.; Huang, J.; Che, C. Supramolecular Self-Assembly of 1D and 3D Heterometallic Coordination Polymers with Triruthenium Building Blocks. Chem. Eur. J. 2012, 18, 11228–11237. [Google Scholar] [CrossRef]
- Audhya, A.; Maity, M.; Abtab, S.M.T.; Mathonière, C.; Kalisz, M.; Clérac, R. Polyalcohols as Ancillary Ligands in Manganese–Oxime Chemistry: Syntheses, Structures and Magnetic Properties of a Series of Trinuclear Complexes Involving a Linear MnII–MnIV–MnII Core. Polyhedron 2012, 33, 353–359. [Google Scholar] [CrossRef]
- Teichert, J.; Doert, T.; Ruck, M. Mechanisms of the Polyol Reduction of Copper(II) Salts Depending on the Anion Type and Diol Chain Length. Dalton Trans. 2018, 47, 14085–14093. [Google Scholar] [CrossRef] [PubMed]
- George, T.; Brosseau, C.L.; Masuda, J.D. Electrochemical and X-Ray Structural Evidence of Multiple Molybdenum Precursor Candidates from a Reported Non-Aqueous Electrodeposition of Molybdenum Disulfide. RSC Adv. 2023, 13, 32199–32216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhou, J.; Hu, F. Two New Oxyiodoplumbates: The Unique 3-D Hybrid Oxyiodoplumbate Based on Neutral 2-D[Pb2I4]n Layers. Dalton Trans. 2018, 47, 8442–8447. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-F.; Wang, D.; Chen, G.-H.; Zhang, J.; Zhang, L. Ligands Hydrophobicity Dependent Electrocatalytic CO2 Reduction Activities of Sn5-Oxo Clusters. J. Solid State Chem. 2023, 321, 123918. [Google Scholar] [CrossRef]
- Bates, P.A.; Hursthouse, M.B.; Davies, A.G.; Slater, S.D. The Structure of 2,2-Di-t-Butyl-1,3,2-Dioxa-, -Oxathia-, and -Dithia-Stannolanes: A Study by Solution and Solid State NMR and Single Crystal X-Ray Diffraction. J. Organomet. Chem. 1989, 363, 45–60. [Google Scholar] [CrossRef]
- Biros, S.M.; Bridgewater, B.M.; Villeges-Estrada, A.; Tanski, J.M.; Parkin, G. Antimony Ethylene Glycolate and Catecholate Compounds: Structural Characterization of Polyesterification Catalysts. Inorg. Chem. 2002, 41, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Uhl, W.; Gerding, R.; Vester, A. The Decomposition Reaction of the Dialene Radical Anion [R2Al−AlR2]− in DME(II): Crystal Strctures of the Aluminum Glycolates [R2Al(OCH2)2]K(DME) and R2Al(OCH)2)2Al(R)(OCH2)2AlR2 (R = CH(SiMe3)2). J. Organomet. Chem. 1996, 513, 163–172. [Google Scholar] [CrossRef]
- Xiao, H.; Zhou, J.; Liu, X. Two New 3-D Cadmium Bromoplumbates: The Only Example of Heterometallic Bromoplumbate Based on Crown [Cd(Pb4O4)Br2] Clusters. Dalton Trans. 2018, 47, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.N.; Alemany, L.; Callender, R.L.; Bott, S.G.; Barron, A.R. Reaction of Al(tBu) 3 with Ethylene Glycol: Intermediates to Aluminum Alkoxide (Alucone) Preceramic Polymers. Chem. Mat. 1999, 11, 3181–3188. [Google Scholar] [CrossRef]
- Aguirre-Díaz, L.M.; Gándara, F.; Iglesias, M.; Snejko, N.; Gutiérrez-Puebla, E.; Monge, M.Á. Tunable Catalytic Activity of Solid Solution Metal–Organic Frameworks in One-Pot Multicomponent Reactions. J. Am. Chem. Soc. 2015, 137, 6132–6135. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, M.C.; Glasser, L.S.D. A Penta-Co-Ordinated Aluminate Dimer; X-Ray Crystal Structure. J. Chem. Soc. Chem. Commun. 1985, 2, 84. [Google Scholar] [CrossRef]
- Teichert, J.; Block, T.; Pöttgen, R.; Doert, T.; Ruck, M. Tin and Lead Alkoxides of Ethylene Glycol and Glycerol and Their Decomposition to Oxide Materials. Eur. J. Inorg. Chem. 2019, 2019, 3820–3831. [Google Scholar] [CrossRef]
- Rabelo, R.; Stiriba, S.-E.; Cangussu, D.; Pereira, C.; Moliner, N.; Ruiz-García, R.; Cano, J.; Faus, J.; Journaux, Y.; Julve, M. When Molecular Magnetism Meets Supramolecular Chemistry: Multifunctional and Multiresponsive Dicopper(II) Metallacyclophanes as Proof-of-Concept for Single-Molecule Spintronics and Quantum Computing Technologies? Magnetochemistry 2020, 6, 69. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pinheiro, C.B.; Journaux, Y.; Julve, M.; Pereira, C.L.M. Effects on the Magnetic Interaction Caused by Molecular Recognition in Complexes of 1,2-Azole-Based Oxamate and [Cu(Bpca)]+ Units. CrystEngComm 2024, 26, 647–665. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; do Pim, W.D.; Pinheiro, C.B.; Journaux, Y.; Julve, M.; Pereira, C.L.M. Monitoring the Hydrogen Bond Net Configuration and the Dimensionality of Aniline and Phenyloxamate by Adding 1H-Pyrazole and Isoxazole as Substituents for Molecular Self-Recognition. CrystEngComm 2019, 21, 2818–2833. [Google Scholar] [CrossRef]
- Rafat, F.; Siddiqi, K.S. Lutfullah Synthesis and Characterization of Trinuclear Complexes Containing Cu(II) and Ti(IV). Synth. React. Inorg. Met. Org. Chem. 2004, 34, 763–774. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlis PRO; Rigaku Corporation: Oxford, UK, 2022. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA: A Three-Dimensional Visualization System for Electronic and Structural Analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Xue, J.-P.; Xie, J.; Yao, Z.-S.; Tao, J. Giant Single-Crystal-to-Single-Crystal Transformations Associated with Chiral Interconversion Induced by Elimination of Chelating Ligands. Nat. Commun. 2021, 12, 6908. [Google Scholar] [CrossRef] [PubMed]
- Labadi, I.; Parkanyi, L.; Grobelny, R.; Mrozinski, J. Redetermination of Crystal Structure, EPR and Magnetic Data of Tris(1,2-Ethanediol) Copper(II) Sulphate. Polyhedron 1994, 13, 2767–2774. [Google Scholar] [CrossRef]
- Antti, B.-M.; Røst, E.; Valde, G.; Andresen, A.F.; Sandström, M. The Molecular and Crystal Structures of [CuCl2(C2H6O2)] and [CuCl2(C2H6O2)].½H2O; Two Compounds Containing Neutral Dichloro(1,2-Ethanediol)Copper(II) Molecules. Acta Chem. Scand. 1976, 30a, 405–410. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Llunell, P.M.; Alemany, S. Alvarez SHAPE 2013, Version 2.1; The Electronic Structure Groups: Barcelona, Spain, 2013. [Google Scholar]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.R.G.; Mambrini, R.V.; Reis, D.O.; Marinho, M.V.; Ribeiro, M.A.; Pinheiro, C.B.; Ferrando-Soria, J.; Déniz, M.; Ruiz-Pérez, C.; Cangussu, D.; et al. Copper(II) Assembling with Bis(2-Pyridylcarbonyl)Amidate and N,N′-2,2-Phenylenebis(Oxamate). Dalton Trans. 2013, 42, 5778. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-coupled Polynuclear d- and f-block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Lage, A.L.A.; Ribeiro, L.A.; Doriguetto, A.C.; Pinheiro, C.B.; Nunes, W.C.; Pedroso, E.F.; Pereira, C.L.M. Solid-State Self-Assembly of a Linear Hexanuclear Copper(II) Oxamate Complex with Alternating Antiferro- and Ferromagnetic Coupling. Magnetochemistry 2022, 8, 116. [Google Scholar] [CrossRef]
- Costa, R.; Garcia, A.; Ribas, J.; Mallah, T.; Journaux, Y.; Sletten, J.; Solans, X.; Rodriguez, V. Tailored Magnetic Properties in Trinuclear Copper(II) Complexes: Synthesis, Structure, and Magnetic Properties of Complexes Derived from [1,3-Propanediylbis(Oxamato)]Cuprate(II) ([Cu(Pba)]2-). Inorg. Chem. 1993, 32, 3733–3742. [Google Scholar] [CrossRef]
- Tercero, J.; Diaz, C.; Ribas, J.; Mahía, J.; Maestro, M.; Solans, X. Synthesis, Characterization, and Magnetic Properties of New Complexes Based on Self-Assembled Homotrinuclear Units CuII–CuII–CuII. J. Chem. Soc. Dalton Trans. 2002, 9, 2040–2046. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yan, C.-W.; Guan, H.-S. Synthesis and Magnetic Studies of Oxalato-Bridged Copper(II)–Chromium(III)–Copper(II) and Copper(II)–Iron(III)–Copper(II) Heterotrinuclear Complexes. Polyhedron 2003, 22, 3223–3230. [Google Scholar] [CrossRef]
- Cortes, R.; Urtiaga, M.K.; Lezama, L.; Arriortua, M.I.; Rojo, T. A Ferromagnetic Copper(II)-Vanadium(IV) Oxide .Mu.-Oxalato Complex: Crystallographic Structure and Spectroscopic and Magnetic Properties. Inorg. Chem. 1994, 33, 829–832. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Lloret, F.; Julve, M.; Pereira, C.L.M. Crystal Engineering Applied to Modulate the Structure and Magnetic Properties of Oxamate Complexes Containing the [Cu(bpca)]+ Cation. Cryst. Growth Des. 2016, 16, 4094–4107. [Google Scholar] [CrossRef]
- da Cunha, T.T.; da Silveira, C.O.C.; Barbosa, V.M.M.; Oliveira, W.X.C.; da Silva Júnior, E.N.; Ferreira, F.F.; Pedroso, E.F.; Pereira, C.L.M. Ferromagnetic Coupling in a Dicopper(II) Oxamate Complex Bridged by Carboxylate Groups. CrystEngComm 2021, 23, 1885–1897. [Google Scholar] [CrossRef]
- Adhikary, A.; Ghosh, K. Fine Tuning of Coordination Environments by Anions on a Series of Cu(II) Dihydrazide Complexes: Syntheses, Structures, Magnetic Properties and Solution Phase Anion Exchange. Polyhedron 2019, 168, 37–47. [Google Scholar] [CrossRef]
- Cruz, C.; Audebrand, N.; Páez-Hernández, D.; Paredes-García, V. Novel Linear Trinuclear CuII Compound with Trapped Chiral Hemiaminal Ligand: Magnetostructural Study. Magnetochemistry 2023, 9, 175. [Google Scholar] [CrossRef]
- Gholizadeh Dogaheh, S.; Khanmohammadi, H.; Sañudo, E.C. A New Trinuclear N–N Bridged Cu(II) Complex with an Asymmetric Schiff Base Ligand Derived from Hydrazine. Polyhedron 2017, 133, 48–53. [Google Scholar] [CrossRef][Green Version]
- Bazhina, E.S.; Bovkunova, A.A.; Medved’ko, A.V.; Efimov, N.N.; Kiskin, M.A.; Eremenko, I.L. Unusual Polynuclear Copper(II) Complexes with a Schiff-Base Ligand Containing Pyridyl and 1,2,4-Triazolyl Rings. J. Clust. Sci. 2019, 30, 1267–1275. [Google Scholar] [CrossRef]

| Compound | 1 |
|---|---|
| Chemical Formula | C56H68N20O16Cu6 |
| M/g mol−1 | 1658.54 * |
| λ/Å | 0.71073 |
| Crystal System | Triclinic |
| Space Group | |
| a/Å | 13.9356(4) |
| b/Å | 15.5338(5) |
| c/Å | 22.5074(7) |
| α/° | 72.610(3) |
| β/° | 76.845(3) |
| γ/° | 67.435(3) |
| Volume/Å3 | 4258.2(3) |
| T/K | 120(2) |
| Z | 2 |
| ρcalc/g cm−3 | 1.294 |
| Crystal size/mm3 | 0.131 × 0.279 × 0.435 |
| μ/mm−1 | 1.535 |
| Reflections collected (Rint) | 51,218 (0.0525) |
| Independent reflections | 17,441 |
| Reflections with I ≥ 2σ (I) | 11,551 |
| R (R total) a | 0.0566 (0.1427) |
| wR (wR total) b | 0.0849 (0.1568) |
| S c | 1.048 |
| ρmax, ρmin/e Å−3 | +0.988; −0.520 |
| CCDC number | 2,389,741 |
| Cu1 | Cu2 | Cu3 | |||||||||
| Cu1–L/Å | L–Cu1–L/° | Cu2–L/Å | L–Cu2–L/° | Cu3–L/Å | L–Cu3–L/° | ||||||
| Cu1–N2 | 1.981(3) | N2–Cu1–N13 | 94.94(14) | Cu2–N3 | 1.952(3) | N3–Cu2–N5 | 100.11(13) | Cu3–N6 | 1.964(3) | N6–Cu3–N15 | 173.16(16) |
| Cu1–N13 | 2.029(4) | N2–Cu1–N14 | 176.47(14) | Cu2–N5 | 1.961(3) | N3–Cu2–O9 | 88.8(3) | Cu3–N15 | 1.992(4) | N6–Cu3–N16 | 93.04(15) |
| Cu1–N14 | 1.993(3) | N2–Cu1–O12 | 92.24(10) | Cu2–O9 | 2.71(1) | N3–Cu2–O13 | 87.13(12) | Cu3–N16 | 2.019(4) | N6–Cu3–O9 | 78.0(7) |
| Cu1–O12 | 2.527(4) | N2–Cu1–O13 | 85.56(12) | Cu2–O13 | 1.926(3) | N3–Cu2–O14 | 169.07(13) | Cu3–O9 | 2.64(2) | N6–Cu3–O14 | 87.95(13) |
| Cu1–O13 | 1.918(3) | N13–Cu1–N14 | 83.87(15) | Cu2–O14 | 1.921(3) | N5–Cu2–O9 | 86.2(3) | Cu3–O14 | 1.940(3) | N15–Cu3–N16 | 84.25(16) |
| N13–Cu1–O12 | 84.61(10) | N5–Cu2–O13 | 163.58(13) | N15–Cu3–O9 | 96.3(7) | ||||||
| N13–Cu1–O13 | 176.10(13) | N5–Cu2–O14 | 88.53(13) | N15–Cu3–O14 | 94.58(14) | ||||||
| N14–Cu1–O12 | 85.35(10) | O9–Cu2–O13 | 108.9(3) | N16–Cu3–O9 | 100.8(7) | ||||||
| N14–Cu1–O13 | 95.86(13) | O9–Cu2–O14 | 85.1(3) | N16–Cu3–O14 | 178.08(14) | ||||||
| O12–Cu1–O13 | 99.24(10) | O13–Cu2–O14 | 86.30(12) | O9–Cu3–O14 | 77.8(7) | ||||||
| Cu4 | Cu5 | Cu6 | |||||||||
| Cu4–L/Å | L–Cu4–L/° | Cu5–L/Å | L–Cu5–L/° | Cu6–L/Å | L–Cu6–L/° | ||||||
| Cu4–N8 | 1.983(3) | N8–Cu4–N17 | 95.70(15) | Cu5–N9 | 1.952(3) | N9–Cu5–N12 | 99.68(14) | Cu6–N11 | 1.963(4) | N11–Cu6–N19 | 163.27(16) |
| Cu4–N17 | 2.016(4) | N8–Cu4–N18 | 173.99(16) | Cu5–N12 | 1.956(3) | N9–Cu5–O6 | 97.18(10) | Cu6–N19 | 2.018(4) | N11–Cu6–N20 | 93.10(15) |
| Cu4–N18 | 2.021(3) | N8–Cu4–O3 | 87.02(10) | Cu6–O6 | 2.934(4) | N9–Cu5–O15 | 86.11(13) | Cu6–N20 | 2.018(4) | N11–Cu6–O6 | 85.25(10) |
| Cu4–O3 | 2.442(3) | N8–Cu4–O15 | 85.74(13) | Cu5–O15 | 1.930(3) | N9–Cu5–O16 | 168.82(14) | Cu6–O6 | 2.846(4) | N11–Cu6–O16 | 87.19(13) |
| Cu4–O15 | 1.921(3) | N17–Cu4–N18 | 83.63(15) | Cu5–O16 | 1.951(3) | N12–Cu5–O6 | 84.66(10) | Cu6–O16 | 1.964(3) | N19–Cu6–N20 | 84.26(16) |
| N17–Cu4–O3 | 95.48(19) | N12–Cu5–O15 | 168.58(14) | N19–Cu6–O6 | 79.74(19) | ||||||
| N17–Cu4–O15 | 171.64(15) | N12–Cu5–O16 | 88.78(13) | N19–Cu6–O16 | 96.89(14) | ||||||
| N18–Cu4–O3 | 87.11(19) | O6–Cu5–O15 | 104.49(10) | N20–Cu6–O16 | 174.90(16) | ||||||
| N18–Cu4–O15 | 95.79(13) | O6–Cu5–O16 | 76.22(10) | N20–Cu6–O6 | 106.82(19) | ||||||
| O3–Cu4–O15 | 92.81(10) | O15–Cu5–O16 | 86.86(12) | O6–Cu6–O16 | 78.29(10) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, W.X.C.; Araújo, V.G.; Pinheiro, C.B.; Julve, M.; Pereira, C.L.M. Building Up a Hexacopper(II)-Pyrazolate/Oxamate Magnetic Complex with Rare Ethane-1,2-Dioxide (–OCH2CH2O–) as a Bridge Between Copper(II) Units. Magnetochemistry 2024, 10, 94. https://doi.org/10.3390/magnetochemistry10120094
Oliveira WXC, Araújo VG, Pinheiro CB, Julve M, Pereira CLM. Building Up a Hexacopper(II)-Pyrazolate/Oxamate Magnetic Complex with Rare Ethane-1,2-Dioxide (–OCH2CH2O–) as a Bridge Between Copper(II) Units. Magnetochemistry. 2024; 10(12):94. https://doi.org/10.3390/magnetochemistry10120094
Chicago/Turabian StyleOliveira, Willian X. C., Victor G. Araújo, Carlos B. Pinheiro, Miguel Julve, and Cynthia L. M. Pereira. 2024. "Building Up a Hexacopper(II)-Pyrazolate/Oxamate Magnetic Complex with Rare Ethane-1,2-Dioxide (–OCH2CH2O–) as a Bridge Between Copper(II) Units" Magnetochemistry 10, no. 12: 94. https://doi.org/10.3390/magnetochemistry10120094
APA StyleOliveira, W. X. C., Araújo, V. G., Pinheiro, C. B., Julve, M., & Pereira, C. L. M. (2024). Building Up a Hexacopper(II)-Pyrazolate/Oxamate Magnetic Complex with Rare Ethane-1,2-Dioxide (–OCH2CH2O–) as a Bridge Between Copper(II) Units. Magnetochemistry, 10(12), 94. https://doi.org/10.3390/magnetochemistry10120094







